Abstract
Introduction
Recent experimental data has revealed that the course of alveolar echinococcosis (AE) depends on adaptive immunity. For this study, we aimed to analyze the incidence and outcome of AE in immunocompromised humans.
Material and methods
Retrospective analysis of 131 patients with a median age of 54 years treated for AE between 1971 and 2017 at a Swiss tertiary referral Centre. Fifty-two percent were females and 65 patients (50%) were diagnosed incidentally. Fourteen patients (16%) were operated on laparoscopically. Overall, median follow-up was 48 months.
Results
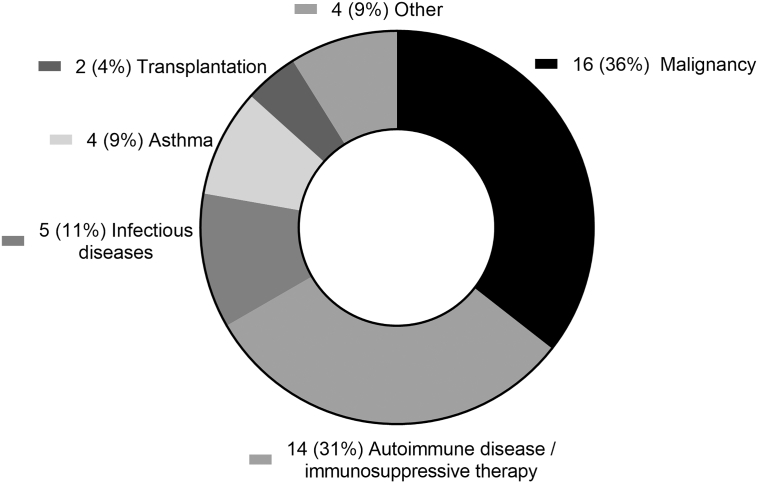
New diagnoses have increased fourfold in immunocompetent and tenfold in immunocompromised patients in the past decade (p ≤ 0.005). Forty-one patients (31.3%) had co-existing or previous immunosuppressive conditions including 16 malignancies (36%), 11 auto-immune diseases or immunosuppressive therapies (31%), 5 infectious diseases (11%), 4 chronic asthma conditions (9%), 2 previous transplantations (4%) and 4 other immunocompromising conditions (9%). Serum levels of anti-Em18, −Em2 and -EgHF antibodies were neither associated with immunocompetence at diagnosis nor during follow-up, but significantly decreased after treatment with benzimidazole (n = 43) or surgery (n = 88) in all patients. Adjuvant therapy for ≥1 year (p = 0.007) with benzimidazole and resection status (R0) (p = 0.002) were both correlated with recurrence-free survival. Survival at 5 and 10 years after surgery was 97% and 94%, respectively, and after conservative treatment 91% and 73%, respectively. Curative surgery (p = 0.014) and immunocompetence (p = 0.048) correlated significantly with overall survival.
Conclusion
The incidence of human AE has increased over the last 2 decades with surgical interventions resulting in excellent outcomes. We have observed an association of immunosuppressive conditions with both incidence and survival of AE eventually justifying the implementation of a screening program for patients at risk in endemic regions.
Abbreviations: Alveolar echinococcosis, (AE); benzimidazole, (BZM); immunocompetent, (ICT); immunocompromised, (ICR); Echinococcus multilocularis, (E. multilocularis)
Keywords: Echinoccocosis, alveolar_echinococcosis, Echinococcus multilocularis, Immunosuppression, Benzimidazole
Highlights
-
•
Alveolar echinococcosis incidence increased significantly in Switzerland.
-
•
Immunosuppression may lead to an increased susceptibility for the disease.
-
•
Coexisting immunosuppressive conditions lead to worse survival of AE.
-
•
Adjuvant treatment with benzimidazole increases recurrence-free survival.
-
•
Resections with sufficient safety margin improve recurrence-free survival.
1. Introduction
Human alveolar echinococcosis (AE) is a zoonotic infection caused by the larval forms of Echinococcus multilocularis tapeworms, also known as metacestodes (Eckert and Deplazes, 2004). Within endemic regions of Europe a growing incidence of human infections has been reported, which is most likely associated with the increase in the infected urban and rural fox populations (Craig et al., 2017; Schweiger et al., 2007) and also with increased diagnosis due to modern imaging techniques (Bresson-Hadni et al., 2000). In humans, metacestodes develop mainly within the liver, with a tumor-like and locally invasive growth pattern (Eckert and Deplazes, 2004). While resection is still the only curative treatment available, survival of unresectable patients has been significantly improved with the development of the anthelmintic benzimidazoles (BZM) in the 1970`s (Torgerson et al., 2008). The latest expert consensus of the World Health Organization (WHO) recommends a radical tumor resection whenever technically feasible, followed by a 2 year adjuvant treatment with BZM to avoid disease recurrence (Brunetti et al., 2010).
Experimental studies in mice suggest that the immune system of the intermediate host may control the human infection with E.multilocularis (Vuitton and Gottstein, 2010; Wang et al., 2018a; Wang et al., 2018b; Wang et al., 2018c) and it is possible that the same may be true for human AE. Previous studies have suggested that immunocompromised (ICR) individuals may present with rapid disease progression in comparison with immunocompetent patients who seem to be able to control the disease and present with a slowly growing tumor years after the initial infection (Vuitton and Gottstein, 2010). With the increasing incidence of AE in endemic regions and the rising use of immunomodulating therapies, a better understanding of the underlying pathophysiology of the immune response to the infection is needed in addition to the development of new surveillance and treatment strategies.
In the current study, we performed a comprehensive retrospective analysis of 131 patients treated for AE at our institution, in order to detect a potential correlation of immunosuppressive conditions with the incidence of disease, recurrence and survival.
2. Material and methods
We retrospectively analyzed clinical and histopathological data of 131 patients with AE who were treated at the Department of Visceral Surgery and Medicine of the University Hospital Bern, in Bern, Switzerland, between 1971 and 2017. The study protocol was approved by the local ethics committee (2017-01534). Patients with AE were diagnosed according to the WHO guidelines, which requires at least one of the following four diagnostic criteria: 1) typical organ lesion in radiological examination (abdominal ultrasound, computed tomography (CT) or magnetic resonance tomography (MRT)), 2) detection of Echinococcus spp. specific serum antibodies 3) detection of parasitic vesicles and laminated layer in histopathology 4) detection of E. multilocularis nucleic acid sequences (Brunetti et al., 2010). In line with the WHO guidelines, the PNM staging system was applied to our cohort in order to classify the patients according to the localization of the tumor, the involvement of neighbouring organs and the presence or absence of metastases (Brunetti et al., 2010). For the histopathological data analysis, tumor size, resection margin and parasite activity in form of hematoxylin-eosin (HE) positive germinal layer was used (Gottstein et al., 2014).
Farmers were considered as patients with increased risk for infection (Conraths et al., 2017). We also included patients who reported close contact to foxes, those who had significant dog bites and patients who repeatedly and knowingly ate unwashed berries from the ground in an endemic region, as patients with a potential increased risk of infection to AE. In our cohort, a patient was considered immunocompromised when previously diagnosed with an autoimmune disease or immunosuppressive therapy, a malignancy, infectious diseases, asthma with chronic high dose corticoid treatment, a previous transplantation or other conditions such as recurrent infectious problems.
2.1. Statistical analyses
Descriptive statistics were used to present of patient characteristics and outcome data. Continuous data is shown using mean and standard deviation or median and range where appropriate. The two-way Anova was used to analyze the incidence of AE over time, the Wilcoxon Test as a non-parametric test for grouped values, and the Mann-Whitney-U as a non-parametric test for independent values. The Kaplan-Meier method, log rank test, logistic regression and cox regression were used to analyze the association of variables with local recurrence, progression-free and overall survival rates. The threshold for statistical significance was set to p ≤ 0.05. Descriptive statistics and graphs were analyzed and produced using SPSS Version 25 and GraphPad Prism 8, respectively.
3. Results
3.1. Patient characteristics
Patient characteristics are shown in Table 1. Potential risk factors included professions with elevated risk (6 farmers, 3 rangers and 1 veterinarian, 2 patients who reported close contact to foxes, 2 patients who had significant dog bites and 2 patients who repeatedly and knowingly ate unwashed berries from the ground). For 23 patients there was no information regarding occupation, 43 patients were registered as retired without any information about their previous occupation and 49 patients had professions with no obvious risk situation for an AE infection. The overall median follow-up after initial diagnosis was 48 months (0–520) for all patients.
Table 1.
Clinical data.
| n (%) | |
|---|---|
| Patients | 131 |
| Female gender | 68 (52%) |
| Mean age at diagnosis (range) | 54 (18–88) |
| Patients with incidental diagnosis | 65 (50%) |
| Patients with potential risk factorsa | 16/65 (25%) |
| Immunocompromised patients | 41 (31%) |
Information was only available for 65 patients.
3.2. Distribution of immunosuppressive conditions
In 41 patients, 45 different immunosuppressive conditions were identified (Fig. 1); among these 41 patients, 4 patients had a malignancy plus an additional autoimmune disease or chronic infection.
Fig. 1.
Distribution of the different immune system compromising conditions of the patients.
Fig. 2 shows incidences of new diagnoses of AE in respect to co-existing immunosuppressive conditions over time, organized by decades. In the last 2 decades new AE diagnoses of ICT and ICR patients have increased significantly over time (Fig. 2, p = 0.0273, 2-way Anova). The increase from the second last (1998–2007) to the last decade (2008–2017) (84 new cases versus 22 in ICT patients and 38 versus 3 in ICR patients) were also statistically significant (Fig. 2, p = 0.0004 for ICT, p = 0.005 for ICR patients, Student's t-test).
Fig. 2.
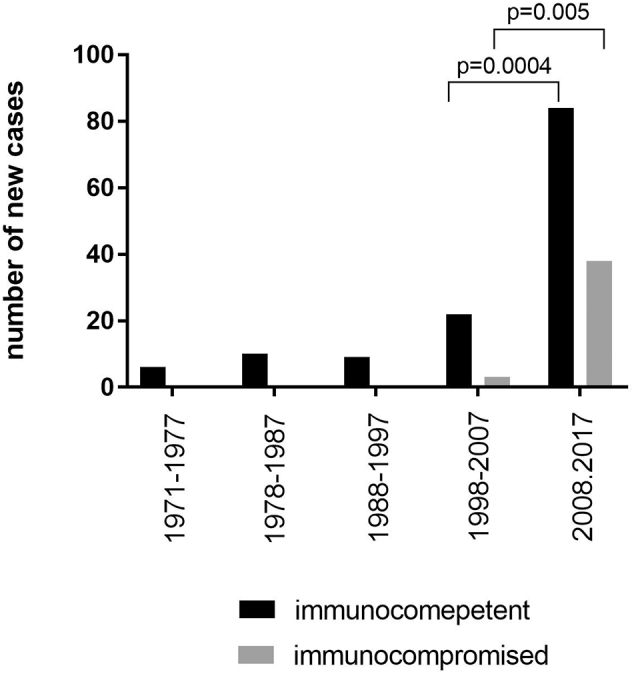
Incidence of new cases of immunocompetent (ICT) and immunocompromised (ICR) patients organized by decades showing a significant increase of new cases over time (p = 0.0273) and from the second last to the last decade for ICT patients (p = 0.0004) and ICR patients (p = 0.005).
3.3. Serology
In the last decade, serum antibody levels against Em18-, Em2- and EgHF-antigens were routinely analyzed at initial diagnosis and during follow-up (Fig. 3). There was no difference in the antibody response against any of the 3 antigens between ICR and ICT patients (Mann-Whitney Test, p = 0.0921 for EgHF, p = 0.6745 for Em2, p = 0.5783 for Em18, data not shown). In all patients we observed a significant decrease in the antibody levels against all 3 antigens after treatment (Wilcoxon Test, p < 0.001 for EgHF, Em2 and Em18) for, ICR patients only (Fig. 3, p ≤ 0.001, Wilcoxon-Test) and for ICT patients only (Fig. 3, p ≤ 0.001, Wilcoxon-Test).
Fig. 3.
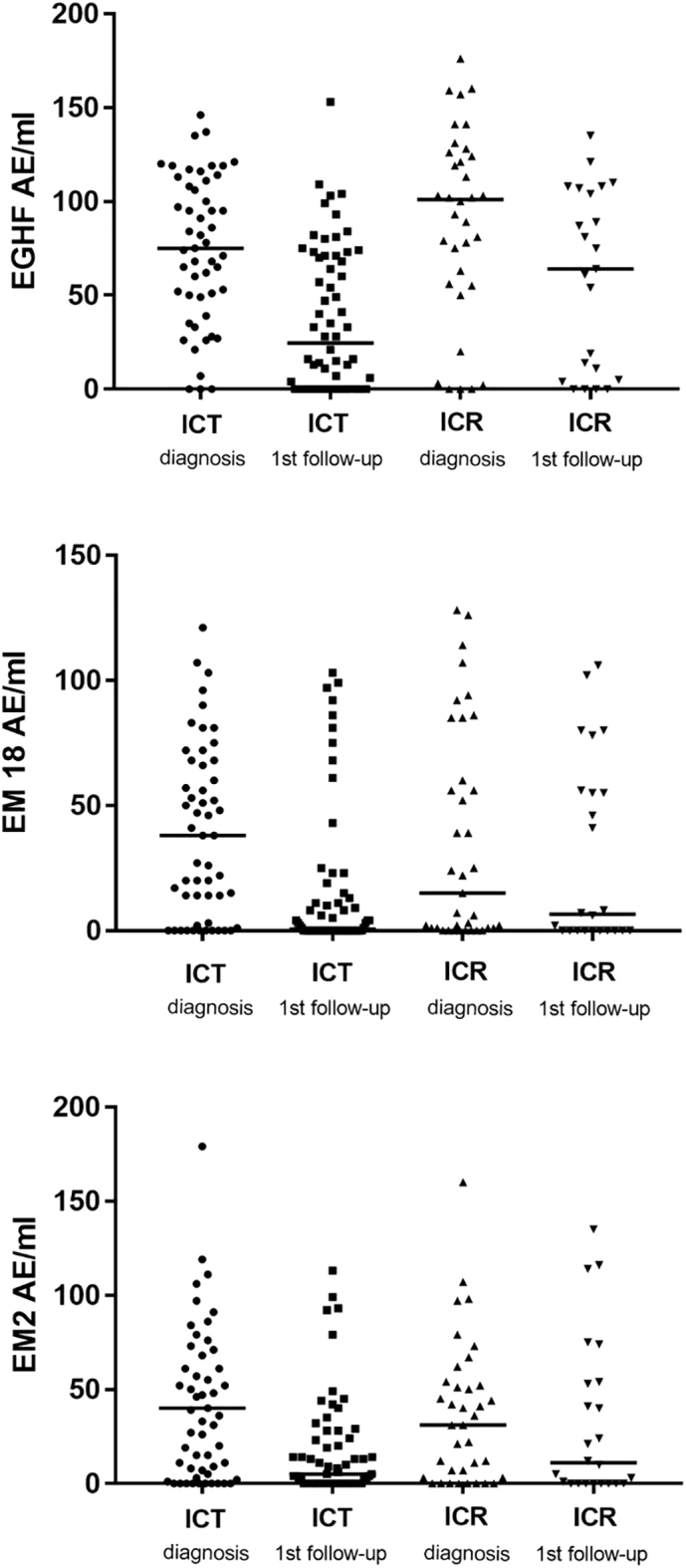
Levels of antibodies against EgHF, Em2 and Em18 at initial diagnosis and 1st follow-up in immunocompetent (ICT) and immunocompromised (ICR) patients. Horizontal bars represent the median. The figure shows a significant decrease of the antibody levels against all 3 antigens after treatment for ICR and for ICT patients (Fig. 3, p ≤ 0.001, Wilcoxon sign-ranktest).
3.4. Treatment
3.4.1. Surgical treatment
Eighty-eight patients (67%) underwent surgical procedures, including radical major surgical resections (47/88; 53%), radical minor liver resections with curative intent (34/88; 39%), and palliative surgeries in 7/88 patients (8%) (R2 liver resections in 5 patients). One patient had additional pancreatic lesions that were not resected and was therefore only treated with palliative care. Fourteen patients (17%) were treated curatively by laparoscopic liver resection.
The duration of surgery ranged between 45 and 630 min. Major complications occurred in 5 patients (4%), who needed re-operation for bile leaks (3×), bleeding (1×) and paralytic ileus (1×). Minor complications were documented for 23 patients (26%): 2 Dindo grade I (2%), 7 grade II (8%) and 14 grade IIIa (16%). The latter complications were a result of postoperative fluid collections or bile leaks in 10 patients that were treated by the minimal-invasive insertion of percutaneous drainages. Three patients with cholestasis required a percutaneous transhepatic cholangio-drainage (PTCD) or endoscopic retrograde cholangiopancreatography (ERCP) and 1 patient with post-operative pain received local infiltration therapy. Laparoscopic resections had no major complications (≥ Dindo IIIb). The median hospital stay for resected patients was 10 (1–143) days, which was reduced to 7 (2–25) days over the past 5 years. Patients after laparoscopic resection stayed median 5 (1–9) days.
3.4.2. Medical treatment
Forty-three non-resected patients (33%) in our cohort received a life-long conservative treatment with benzimadozole (BZM). The majority of resected patients (n = 77, 88%) received adjuvant therapy with BZM for a median time of 24 (1–328) months. Sixteen patients were treated for <1 year and 9 patients for more than one year but <2 years postoperatively. Neoadjuvant therapy (BZM treatment before planned curative surgery) was prescribed to 59 patients (67%) for a median duration of 7.5 (1–127) weeks. Most patients were treated with albendazole (400–800 mg/d), 11 patients received mebendazole (2.5 - 6 g/day).
3.5. Histopathological data
The histopathological data of the resected patients is summarized in Table 2, showing the wide range of tumor sizes (0.6–20 cm) and the high accumulation of early parasite stages (P1 51%) with a relatively high incidence of N1 (regional involvement of contiguous organs or tissues) stages of 30%. Eighteen patients (29%) did not have a safety margin of 10 mm and were classified having a positive resection margin (R1). Thirty-seven patients (57%) had positive parasite activity in the histologic examination. There was no statistically significant correlation of the immune status with positive parasite activity ((11/18 (61%) in immunocompromised patients vs. 25/50 (50%) of immunocompetent patients)), positive N stage ((4/19 (21%) in immunocompromised patients vs. 21/65 (32%) in immunocompetent patients)) or tumor size ((mean 6.5 cm in immunocompromised vs. mean 8 cm in immunocompetent patients)). In addition, the R- and P-status showed no difference between groups.
Table 2.
Histopathological data.
| Immunocompetent patients | Immunocompromised patients | |
|---|---|---|
| Median tumor diameter (range) | 8 ± 4.5 (0.6–20) cm | 7 ± 3.7 (1–15.2) cm |
| P1 | 40/59 (68%) | 14/19 (74%) |
| P2 | 21/59 (36%) | 3/19 (16%) |
| P3 | 10/59 (17%) | 2/19 (11%) |
| P4 | 7/59 (12%) | 0/19 (0%) |
| N0 | 44/65 (68%) | 15/19 (79%) |
| N1 | 21/65 (32%) | 4/19 (21%) |
| R0 | 45/63 (71%) | 17/18 (94%) |
| R1 | 17/63 (29%) | 1/18 (6%) |
| Parasite activity | 25/50 (50%) | 11/18 (61%) |
(P = parasitic mass in the liver, N = involvement of neighbouring organs, and R = resection status).
3.6. Disease recurrence and survival
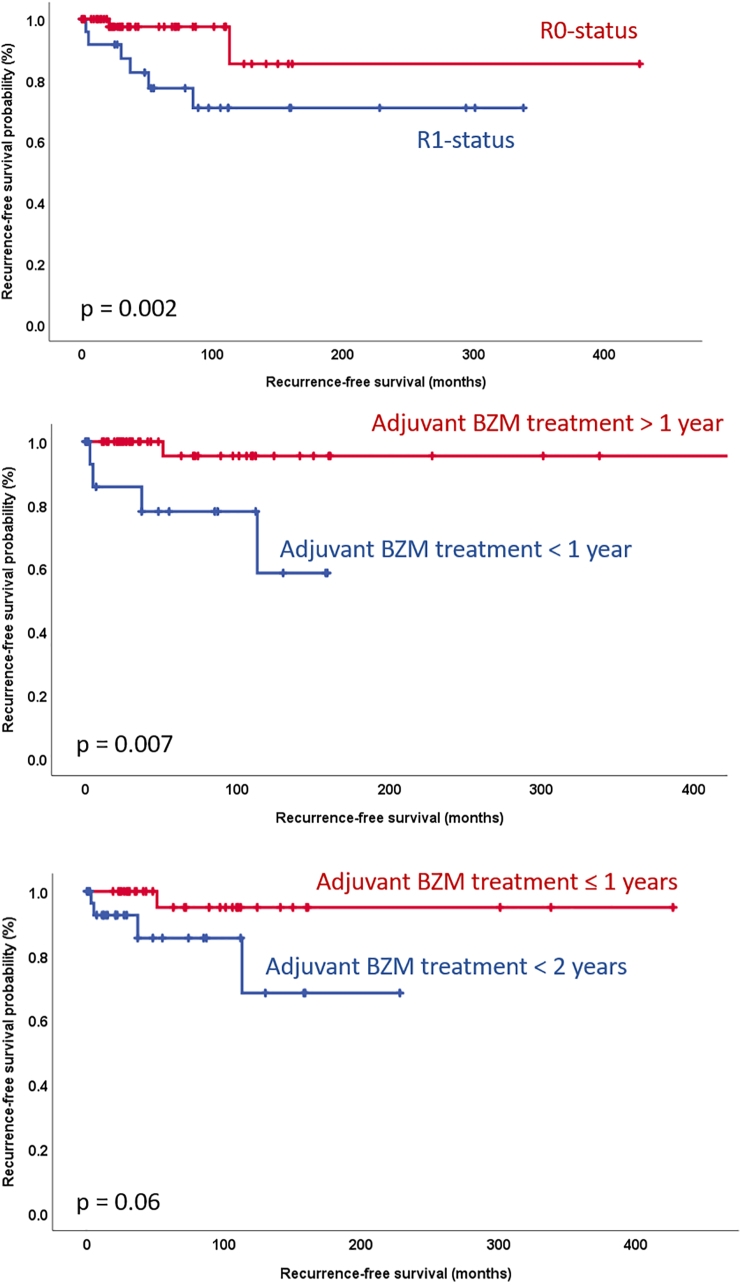
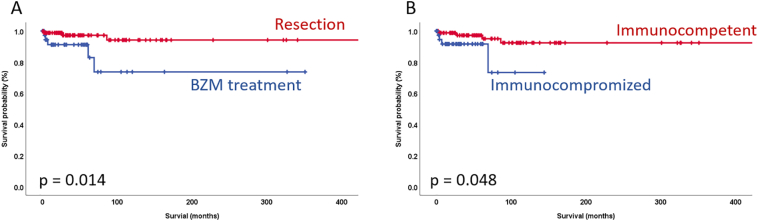
Disease recurrence occurred in 11 out of 80 curatively treated patients (14%). Seven of these 11 patients (64%) were initially operated upon in an external hospital and were referred to our Center for further treatment. Four of these patients could successfully be re-resected and remained recurrence-free for 89–102 months. The other 3 patients received medical therapy with BZM. In the time-to-recurrence analysis, the resection status (R0 vs. R1) and the administration of adjuvant BZM therapy for >1 year were significantly correlated with recurrence-free survival rates (Fig. 4) in the univariate, but not in the multivariate analysis (Suppl. Table 1). Neither immunosuppression, parasite activity, P-, N-, R-status, neoadjuvant or adjuvant therapy, or laparoscopic resection could be correlated to disease recurrence in the logistic regression analysis in our cohort. Surgical treatment and an immunocompetent condition were identified as significant prognostic factors associated with longer survival rates in the univariate, but not in the multivariate analysis (Fig. 5, Suppl. Table 2). The 5-year and 10-year survival rates after surgical treatment were 97% and 94%, respectively. The 5-year and 10-year survival rates in the non-surgery group were 91% and 74%, respectively.
Fig. 4.
Recurrence-free survival Kaplan-Meier curves comparing (A) resection status RO with R1 (B) adjuvant treatment with BZM for <1 year with treatment ≥2 years and, (C) adjuvant treatment with BZM <2 years with treatment ≥2 years post resection. Statistical differences were calculated by the log rank test.
Fig. 5.
Overall survival Kaplan-Meier curves comparing (A) surgical treatment with conservative treatment with BZM and (B) immunocompetent with IC conditions. Statistical differences were calculated by the log rank test.
4. Discussion
In this large retrospective study of patients treated for AE in the liver, we were able to detect a significant increase of new diagnoses within the last decade accompanied by a significant increase in co- or pre-existing immunosuppressive conditions. The current study therefore confirms reports of increased incidence of AE in its endemic regions including Switzerland (Vuitton et al., 2015). Despite increased incidences, the overall treatment success has generally improved due to earlier diagnosis, advanced surgical techniques and the possibility of livelong medical BZM treatment (Beldi et al., 2019; Du et al., 2016). Although the influence of the host's immune system on the infection, tolerance or development of a resistance has been studied in several animal models, clinical data of large patient cohorts is limited (Vuitton and Gottstein, 2010; Wang and Gottstein, 2016). In our cohort, the incidence of AE was mainly associated with an impaired immune system but not the clinical course. Conversely, others have observed a rather progressive course of the disease in ICR patients (Chauchet et al., 2014; Vuitton et al., 2016). Data from one large French registry showed that almost 10% of the patients with AE had some kind of immunosuppressive condition including different malignancies, autoimmune diseases or the intake of immunosuppressive drugs (Chauchet et al., 2014). However, type and extent of immune deficiency are difficult to compare between the different series. Given the long observation period and provided that the documentation of patient data improved over the years, the actual number of co-existing immunosuppressive conditions might be even higher for the entire cohort. In addition, it is well known that the use of immunosuppressive medications has in general increased over the past years (Allison, 2000), probably contributing to the observed increase of AE in ICR patients. Current data on the involvement of the immune system on the clinical course of AE is rather conflicting (Craig et al., 2017; Dentan et al., 2012; Geyer et al., 2011; Sulima et al., 2016). For example, in contrast to the French series, we neither identified an association of immunocompetence with the clinical course nor with the serology (Chauchet et al., 2014). This may be related to the fact that most of our patients received life-long BZM treatment starting immediately after diagnosis or adjuvant BZM therapy starting after their resection, assuming that these therapies themselves lead to the observed treatment success irrespective of the immune status of the patient.
It remains unclear though with which mechanism a functional immune system is required to prevent or delay AE. Theoretically, humans may be able to either develop a resistance towards the metacestode or an induced tolerance (Gottstein et al., 2015). Optimally, immunocompetent patients might present with just a seroconversion to parasite-specific antigens or with non-active or aborted metacestodes as a result of a previous contact with infectious E. multilocularis material (Gottstein et al., 2017). Mechanistic studies revealed that this is based on a Th2-type and anti-inflammatory immune response that is at least partially involved in the maintenance of tolerance and inhibition of cytotoxicity (Vuitton and Gottstein, 2010). However, the exact mechanisms of how different alterations of the immune system because of immunosuppressive drugs, immune deficiencies or autoimmune diseases contribute to a higher susceptibility or a faster disease progression still needs to be evaluated.
In agreement with the published literature (Hillenbrand et al., 2017), we also identified that a negative resection margin and adjuvant BZM treatment for more than one year significantly improved recurrence-free survival in all resected patients in the univariate analyses, with a 5-year and 10-year survival of 97% and 94% after surgery, respectively. Our long-term outcomes are better or comparable to what is known from the few large patient series in the literature (Chen et al., 2018; Du et al., 2016; Joliat et al., 2015; Kawamura et al., 2011). Although surgical treatment is clearly associated with improved overall survival in our cohort in the univariate analysis and should be performed whenever technically feasible, it is also important to note that the 5- and 10 year survival after conservative BZM treatment is 91% and 73%, respectively, offering a valid treatment alternative in advanced and unresectable patients. This should always be taken into account when extensive surgical procedures are considered for patients with AE. In addition, the treatment success of aggressive and extensive liver resections is often limited by high morbidity and mortality rates (Aji et al., 2018). Survival rates after liver transplantation for AE have been reported as approximately 77% and 49% after 1 and 10 years, respectively, hampered by high and aggressive recurrences potentially because of associated immunosuppression (Koch et al., 2003).
Our study is limited as a result of the methodological issues of using a retrospective design and may not have captured the full picture of AE in ICR patients. This is why a potential causal relationship between overall survival and AE progression in patients with either immunosuppressive conditions or co-morbidities cannot be properly assessed. However, it does provide a current method to address such intriguing clinical associations in a rare disease.
Nevertheless, patients and physicians in endemic regions should be aware of this correlation in order to detect potential infections early enough for a curative treatment and in order to advise the patients about the risk and how to avoid an infection. Whether or not systemic surveillance in the form of serological testing should be performed in all ICR patients in endemic regions, should be addressed in an independent prospective study.
5. Conclusion
We show a significant increase of AE together with a significant increase of coexisting immunosuppressive conditions, eventually leading to worse outcomes for patients. While resection clearly remains the treatment of choice, offering potential cure and the best overall survival, the exact role and duration of peri-operative BZM treatment still needs to be examined. Specific surveillance strategies for ICR patients at risk should be evaluated and potentially introduced in endemic regions.
The following are the supplementary data related to this article.
Multivariate regression analysis recurrence.
Multivariate regression analysis survival.
References
- Aji T., Dong J.H., Shao Y.M., Zhao J.M., Li T., Tuxun T., Shalayiadang P., Ran B., Jiang T.M., Zhang R.Q., He Y.B., Huang J.F., Wen H. Ex vivo liver resection and autotransplantation as alternative to allotransplantation for end-stage hepatic alveolar echinococcosis. J. Hepatol. 2018 doi: 10.1016/j.jhep.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Allison A.C. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47:63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- Beldi G., Vuitton D., Lachenmayer A., Heyd B., Dufour J.F., Richou C., Candinas D., Bresson-Hadni S. Is ex vivo liver resection and autotransplantation a valid alternative treatment for end-stage hepatic alveolar echinococcosis in Europe? J. Hepatol. 2019 doi: 10.1016/j.jhep.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Bresson-Hadni S., Vuitton D.A., Bartholomot B., Heyd B., Godart D., Meyer J.P., Hrusovsky S., Becker M.C., Mantion G., Lenys D., Miguet J.P. A twenty-year history of alveolar echinococcosis: analysis of a series of 117 patients from eastern France. Eur. J. Gastroenterol. Hepatol. 2000;12:327–336. doi: 10.1097/00042737-200012030-00011. [DOI] [PubMed] [Google Scholar]
- Brunetti E., Kern P., Vuitton D.A., Writing Panel for the, W.-I Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Chauchet A., Grenouillet F., Knapp J., Richou C., Delabrousse E., Dentan C., Millon L., Di Martino V., Contreras R., Deconinck E., Blagosklonov O., Vuitton D.A., Bresson-Hadni S., FrancEchino N. Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Clin. Infect. Dis. 2014;59:1095–1104. doi: 10.1093/cid/ciu520. [DOI] [PubMed] [Google Scholar]
- Chen K.F., Tang Y.Y., Wang R., Fang D., Chen J.H., Zeng Y., Li B., Wen T.F., Wang W.T., Wu H., Xu M.Q., Yang J.Y., Wei Y.G., Huang J.W., Li J.X., Zhang H.Z., Feng X., Yan L.N., Chen Z.Y. The choose of different surgical therapies of hepatic alveolar echinococcosis: a single-center retrospective case-control study. Medicine. 2018;97 doi: 10.1097/MD.0000000000010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conraths F.J., Probst C., Possenti A., Boufana B., Saulle R., La Torre G., Busani L., Casulli A. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P.S., Hegglin D., Lightowlers M.W., Torgerson P.R., Wang Q. Echinococcosis: control and prevention. Adv. Parasitol. 2017;96:55–158. doi: 10.1016/bs.apar.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Dentan C., Mazet R., Gilson M., Marchou-Lopez S., Gaudin P. Rheumatoid arthritis, alveolar echinococcosis, and rituximab: a case report. Joint Bone Spine. 2012;79:325–327. doi: 10.1016/j.jbspin.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Du C., Liu Z., Yang X., Yan L., Li B., Wen T., Yang J., Xu M., Chen Z., Wang W. Hepatectomy for patients with alveolar echinococcosis: long-term follow-up observations of 144 cases. Int. J. Surg. 2016;35:147–152. doi: 10.1016/j.ijsu.2016.09.094. [DOI] [PubMed] [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M., Wilpert J., Wiech T., Theilacker C., Stubanus M., Kramer-Zucker A., Fischer K.G., Drognitz O., Frydrychowicz A., Kern W., Walz G., Pisarski P. Rapidly progressive hepatic alveolar echinococcosis in an ABO-incompatible renal transplant recipient. Transpl. Infect. Dis. 2011;13:278–284. doi: 10.1111/j.1399-3062.2010.00583.x. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Wang J., Blagosklonov O., Grenouillet F., Millon L., Vuitton D.A., Muller N. Echinococcus metacestode: in search of viability markers. Parasite. 2014;21:63. doi: 10.1051/parasite/2014063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B., Wang J., Boubaker G., Marinova I., Spiliotis M., Muller N., Hemphill A. Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis) Vet. Parasitol. 2015;213:103–109. doi: 10.1016/j.vetpar.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Soboslay P., Ortona E., Wang J., Siracusano A., Vuitton D. Immunology of alveolar and cystic echinococcosis (AE and CE) Adv. Parasitol. 2017;96:1–54. doi: 10.1016/bs.apar.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Hillenbrand A., Gruener B., Kratzer W., Kern P., Graeter T., Barth T.F., Buttenschoen K., Henne-Bruns D. Impact of safe distance on long-term outcome after surgical therapy of alveolar echinococcosis. World J. Surg. 2017;41:1012–1018. doi: 10.1007/s00268-016-3813-6. [DOI] [PubMed] [Google Scholar]
- Joliat G.R., Melloul E., Petermann D., Demartines N., Gillet M., Uldry E., Halkic N. Outcomes after liver resection for hepatic alveolar echinococcosis: a single-center cohort study. World J. Surg. 2015;39:2529–2534. doi: 10.1007/s00268-015-3109-2. [DOI] [PubMed] [Google Scholar]
- Kawamura N., Kamiyama T., Sato N., Nakanishi K., Yokoo H., Kamachi H., Tahara M., Yamaga S., Matsushita M., Todo S. Long-term results of hepatectomy for patients with alveolar echinococcosis: a single-center experience. J. Am. Coll. Surg. 2011;212:804–812. doi: 10.1016/j.jamcollsurg.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Koch S., Bresson-Hadni S., Miguet J.P., Crumbach J.P., Gillet M., Mantion G.A., Heyd B., Vuitton D.A., Minello A., Kurtz S., European Collaborating C. Experience of liver transplantation for incurable alveolar echinococcosis: a 45-case European collaborative report. Transplantation. 2003;75:856–863. doi: 10.1097/01.TP.0000054230.63568.79. [DOI] [PubMed] [Google Scholar]
- Schweiger A., Ammann R.W., Candinas D., Clavien P.A., Eckert J., Gottstein B., Halkic N., Muellhaupt B., Prinz B.M., Reichen J., Tarr P.E., Torgerson P.R., Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulima M., Wolyniec W., Oladakowska-Jedynak U., Patkowski W., Wasielak N., Witczak-Malinowska K., Borys S., Nahorski W., Wroczynska A., Szostakowska B., Lass A., Krawczyk M. Liver transplantation for incurable alveolar echinococcosis: an analysis of patients hospitalized in Department of Tropical and Parasitic Diseases in Gdynia. Transplant. Proc. 2016;48:1708–1712. doi: 10.1016/j.transproceed.2016.01.087. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Schweiger A., Deplazes P., Pohar M., Reichen J., Ammann R.W., Tarr P.E., Halkik N., Mullhaupt B. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008;49:72–77. doi: 10.1016/j.jhep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Vuitton D.A., Gottstein B. Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay. J Biomed Biotechnol. 2010;2010:923193. doi: 10.1155/2010/923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuitton D.A., Demonmerot F., Knapp J., Richou C., Grenouillet F., Chauchet A., Vuitton L., Bresson-Hadni S., Millon L. Clinical epidemiology of human AE in Europe. Vet. Parasitol. 2015;213:110–120. doi: 10.1016/j.vetpar.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Vuitton D.A., Azizi A., Richou C., Vuitton L., Blagosklonov O., Delabrousse E., Mantion G.A., Bresson-Hadni S. Current interventional strategy for the treatment of hepatic alveolar echinococcosis. Expert Rev. Anti-Infect. Ther. 2016;14:1179–1194. doi: 10.1080/14787210.2016.1240030. [DOI] [PubMed] [Google Scholar]
- Wang J., Gottstein B. Immunoregulation in larval Echinococcus multilocularis infection. Parasite Immunol. 2016;38:182–192. doi: 10.1111/pim.12292. [DOI] [PubMed] [Google Scholar]
- Wang J., Cardoso R., Marreros N., Muller N., Lundstrom-Stadelmann B., Siffert M., Vuitton D.A., Boue F., Lin R., Wen H., Gottstein B. Foxp3(+) T regulatory cells as a potential target for immunotherapy against primary infection with echinococcus multilocularis eggs. Infect. Immun. 2018;86 doi: 10.1128/IAI.00542-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Goepfert C., Mueller N., Piersigilli A., Lin R., Wen H., Vuitton D.A., Vuitton L., Mueller C., Gottstein B. Larval Echinococcus multilocularis infection reduces dextran sulphate sodium-induced colitis in mice by attenuating T helper type 1/type 17-mediated immune reactions. Immunology. 2018;154:76–88. doi: 10.1111/imm.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jebbawi F., Bellanger A.P., Beldi G., Millon L., Gottstein B. Immunotherapy of alveolar echinococcosis via PD-1/PD-L1 immune checkpoint blockade in mice. Parasite Immunol. 2018;40 doi: 10.1111/pim.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariate regression analysis recurrence.
Multivariate regression analysis survival.