Abstract
Leishmaniasis is a neglected disease caused by the protozoa Leishmania ssp. Environmental differences found by the parasites in the vector and the host are translated into cellular stress, leading to the production of heat shock proteins (Hsp). These are molecular chaperones involved in the folding of nascent proteins as well as in the regulation of gene expression, signalling events and proteostasis. Since Leishmania spp. use Hsp90 to trigger important transitions between their different stages of the life cycle, this protein family becomes a profitable target in anti-parasite drug discovery. In this work, we implemented a multidisciplinary strategy coupling molecular modelling with in vitro assays to identify small molecules able to inhibit Hsp90 from L. braziliensis (LbHsp90). Overall, we identified some compounds able to kill the promastigote form of the L. braziliensis, and to inhibit LbHsp90 ATPase activity.
Keywords: Hsp90, inhibitors, fluorescence, leishmaniasis, molecular modelling
Introduction
Therapeutic regimens for the treatment of neglected tropical diseases (NTDs) are limited, have high toxicity, restrictions on use by some patients, and are difficult to adhere because they generally require parenteral and long-term administration. This scenario becomes even more complex given the low investment in research and development aimed at treating NTDs1.
The aetiological agents of NTDs may be protozoa, bacteria, helminths and viruses. Specifically, leishmaniasis is a neglected disease endemic in 98 countries that is caused by the protozoa Leishmania ssp., whose transmission in humans occurs through the bite of an insect belonging to the subfamily Phlebotominae2. According to the World Health Organization, leishmaniasis causes every year more than one million new cases and 20,000–30,000 deaths worldwide2,3. Current drugs approved for the treatment of leishmaniasis include pentavalent antimony, Amphotericin B, Miltefosine, Paromomycin and, in some specific cases, ketoconazole, itraconazole and fluconazole2. However, an increasing number of cases of acquired resistance to these drugs, coupled with toxicity, the lack of efficacy, and the need for parenteral administration (except Miltefosine) highlight the need of novel treatment options for leishmaniasis2,4.
The environmental differences found by the parasites from their existence in the insect vector until the mammalian host are translated into a form of cellular stress, leading to the production of heat shock proteins (Hsp)5. Indeed, for some parasites thermal stress is one of the signals responsible for cell differentiation to which the parasite must resist for adapting to the new environment6,7. Among the Hsp family, Hsp90s are evolutionarily conserved ATP-dependent chaperones that participate in the stabilisation and enhancement of different client proteins, many of which are essential for constitutive cell signalling and adaptive stress response8,9. Hsp90 chaperone function is not limited to assist the folding of nascent proteins, but extends to the regulation of gene expression, signalling events and proteostasis7,10. The ability of Hsp90 to affect important cellular transformations is greatly explored by intracellular protozoa such as Trypanosoma, Leishmania, Toxoplasma, and Plasmodium spp., which use Hsp90 to trigger important transitions between their different life-cycle stages7,11. Accordingly, Hsp90 is a multi-potential target in anti-parasite drug discovery and development12–15.
It is estimated that Hsp90 constitutes 2.8% of the total protein content of L. donovani promastigotes, and that the levels of this proteins increase in parasites subjected to thermal stress16. It was also indicated that Hsp90 from L. major (LmHsp90) plays a key role in the differentiation from the amastigote to the promastigote stage. Treatment of promastigotes with the Hsp90 inhibitor geldanamycin (GA) have induced the synthesis of specific proteins of the amastigote form in the promastigotes17,18. In addition, morphological changes of the parasites under treatment with GA have been very similar to those induced by thermal shock, which is also capable of inducing differentiation17. These data suggest that modulation of Hsp90 activity by heat shock-induced sequestration, or Hsp90 inhibition by GA, triggers the differentiation between the different stages of the parasite, thus highlighting the relevance of this protein in measuring environmental changes and controlling parasite growth19.
Based on above evidences, in this work we focussed our efforts in the identification of small molecule inhibitors of Hsp90 from L. braziliensis (LbHsp90). To this aim, we established a multidisciplinary approach combining molecular modelling with in vitro evaluations. Starting from a two-step structure-based virtual screening, 28 chemically diverse compounds were selected and tested in vitro for their ability to bind and inhibit recombinant LbHsp90, as well as to impair parasite growth and replication. Overall, three molecules were highlighted in this study based on their ability to kill the promastigote form of L. braziliensis, and to inhibit the ATPase activity of LbHsp90 at low micromolar concentration, thus becoming worth of further investigations as anti-leishmaniasis candidates.
Materials and methods
Homology modelling
Sequences of LbHsp90 and LmHsp90 N-terminal domain (LbHsp90N and LmHsp90, respectively) were retrieved from the UniProt Knowledgebase (UniProtKB – http://www.uniprot.org/) under the accession codes A4HL70 and Q4Q4I6, respectively, and were aligned using Clustal X20, obtaining 94% of sequence identity (supplementary Figure S1). The crystallographic structure of LmHsp90N in complex with ADP (PDB ID 3U6721) was selected as the structural template to model the LbHsp90N. The 3 D model of LbHsp90N was obtained using the Prime software, version 3.522, by keeping ADP and three conserved water molecules (thereafter referred as W1, W2, and W3) in the model. The homology model was optimised through energy minimisation in explicit solvent by means of AMBER12 programme23. The ff12SB and GAFF force fields were used for the protein and the small molecule, respectively. In details, LbHsp90N/ADP complex was solved in a rectilinear box of TIP3P3 water molecules buffering 6 Å from the macromolecular system. Energy minimisation was performed in two consecutive steps: (i) the solvent alone was energy minimised for 1000 steps with the Steepest Descent algorithm (SD) and subsequent 200 steps with the Conjugate Gradient algorithm (CG); (ii) the whole solvated complex was energy minimised for 1500 steps SD and subsequent 6000 steps CG. Pymol software (The PyMOL Molecular Graphics System, version 1.7.1.0, Schrödinger, LLC) was used to visualise the model and to prepare graphics.
First-round virtual screening
A multi-step structure-based virtual screening composed of an initial similarity search with ROCS (Rapid Overlay of Chemical Structures, OpenEye software)24, followed by molecular docking, visual inspection, and chemical diversity analysis was carried out. The ROCS query was generated on the binding conformation of ADP in the relaxed homology model of LbHsp90N (supplementary Figure S2) by means of the vROCS graphic interface (OpenEye, Santa Fe, NM)25. The MolPort database (MolPort – https://www.molport.com/shop/index), consisting of around six million commercially available compounds, was screened in this work. Ligand conformational analysis was performed with OMEGA version 2.5.1.4 (OpenEye, Santa Fe, NM)26 generating up to 600 conformers for each molecule. Default parameters were used, except for strictstereo that was set as ‘false’, and fromCT that was set to ‘true’.
The MolPort database was then screened on the query using ROCS25. A total of 66,294 compounds endowed with a Tanimoto Combo value greater than 0.8 (arbitrary threshold value) were retained, and were docked towards the LbHsp90N homology model. The receptor for molecular docking was prepared by the make_receptor utility of OEDoking (OpenEye, Santa Fe, NM) version 3.0.0, with default settings. The potential shape was identified by molecular probes, and a constraint on Asp78 as hydrogen bond donor was set. Receptor box volume was 10720 Å3 with the dimensions of 24.00 * 23.33 * 20.00 Å. Molecular docking was performed with FRED docking programme (OpenEye, Santa Fe, NM)27, setting the resolution value as High.
Second-round virtual screening
The moieties of Glb08 and Glb11 that were found to interact with Asp78 and W1, W2, and W3 of LbHsp90N were drawn in MarvinSketch software (ChemAxon – https://chemaxon.com/) (supplementary Figure S3) and were used to generate in the corresponding SMARTS notation of the substructure of Glb08 and Glb11, namely [H]N = C(N([H][H])N([H])[$([#1,*])] and [H]N = C(S[$([#1,*])]N([H])[H], respectively. The Ligand Filtering tool of LigPrep (Schrödinger Maestro suite)28 was used to filter the MolPort database, using the above SMARTS notations as queries. Overall, 2348 compounds were filtered and moved to the further docking study. Ligand energy minimisation was carried out by Szybki version 1.8.0.1 (OpenEye Scientific Software, Santa Fe, NM)29, with the MMFF94S force field and default parameters, while the most probable ionisation form of each molecule at pH 7.4 was generated by Fixpka (OpenEye Scientific Software, Santa Fe, NM)30. Conformational analysis and molecular docking were performed by using the same procedure already described above. Finally, the binding mode of 15% top ranking compounds was visually analysed.
Protein expression and purification
The LbHsp90 and LbHsp90N (amino acid residues 1–221) recombinant proteins were expressed and purified as previously described31. Hsp90β and its N-terminal domain construct (Hsp90βN – residues 1–223) were produced as described in Minari et al.32. These plasmids were used to transform cells of Escherichia coli BL21(DE3) strain, which were grown in LB medium at 37 °C until reaching an OD600 nm about 0.6–0.8, in the presence of the appropriate antibiotic. Protein expression was induced by the addition of 0.4 mM IPTG, and kept at constant temperature for 18 h at 18 °C for hHsp90β, and 4 h at 37 °C for hHsp90βN. Induced cells were then harvested by centrifugation, and the bacterial pellet was disrupted by sonication in 20 mM sodium phosphate (pH 7.4), 20 mM imidazole, and 500 mM NaCl (20 mL of buffer/L of culture medium), after incubation with 5 U of DNAse and 30 μg/mL of lysozyme for 40 min on ice. The supernatant of the lysed cells, obtained by centrifugation at 11,000 rpm for 30 min at 4 °C, was filtered using a 0.45 μm membrane filter and subjected to protein purification protocol as described for LbHsp90 recombinant protein31. Both N-terminal constructions were incubated with 1 U of thrombin/mg of protein for 12–14 h at 4 °C for His-tag cleavage. The purification efficacy was attested by 12% SDS-PAGE. Proteins concentrations were spectroscopically determined (at 280 nm) using the molar extinction coefficient predicted by the protein amino acid sequences at water conditions.
Interaction screening by differential scanning fluorimetry
The interaction of Hsp90 proteins with different compounds was monitored through the melting temperature (Tm) of each protein in the presence of a fixed concentration of the target compound by differential scanning fluorimetry (DSF) also referred as thermal shift assay33. A final concentration of 10 µM of dimeric LbHsp90 and 20 µM of monomeric LbHsp90N in 40 mM Hepes buffer (pH 7.5) containing 100 mM KCl were used. The DMSO concentration in each sample was fixed at 2.5% (v/v). Prior to the readings, the samples were incubated for 30 min on ice. The fluorophore Sypro Orange (Life Technologies) was used as fluorescent probe. The experiments were performed at a thermocycler CFX96 Touch Real Time PCR Detection System (BioRad) and the data were analysed using the CFX Manager software (BioRad). As positive controls, 100 µM of GA and Radicicol (RDC) were used.
Cell viability assay
The leishmanicidal activity of the compounds was tested in vitro against the promastigote form of L. braziliensis applying 200 μM/well, (with a total of 0.5% DMSO) into 90 μL of culture. The concentration of L. braziliensis (MHOM/BR/1973/M2269) was adjusted to 107 cells/mL. Amphotericin (100 μM/well) was used as positive control, and 0.5% DMSO as negative control.
The plates were incubated at room temperature (RT) for 72 h. After this period, the viability of the promastigotes was verified via MTT (3–(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide) colorimetric assay. MTT/PMS 11.1 μL/well solution (MTT = 5 mg/mL; Phenazine methosulfate-PMS = 0.22 mg/mL) was added, and the plate was incubated at RT for 4 h, at 150 RPM, protected from light. Formazan crystals were then solubilised by adding 100 μL/well of a solubilisation solution containing 7% SDS, 10 mM HCl and 30% DMSO. The mixture was incubated at RT for 90 min at 150 RPM protected from light. Wells containing the medium and the compounds or DMSO without MTT were used as blank controls. The absorbance was quantified in a plate reader (Biotek Synergy HT) at 570 nm.
The cell viability percentages were calculated by the following equation:
| (1) |
where AT was an averaged absorbance from treated samples, AB blank absorbance and AU was an averaged absorbance from negative control.
Cytotoxic assay
Compounds cytotoxicity was evaluated in 100 μL of Balb-c 3T3 cone A31 mouse fibroblasts (105 cells/mL) into a 96-well plate, incubated for 24 h at 37 °C to become adhered. After this period, the medium was washed and 100 μL of each compound was added to the culture medium (concentration ranging from 1 to 200 μM/well, with a total of 0.5% DMSO) in quadruplicate. Rapamycin was used as a positive control, and 0.5% DMSO was used as negative control. The plates were incubated for 72 h at 37 °C. After this, the viability of Balb-c 3T3 cells was evaluated by MTT colorimetric assay, as described previously.
In vitro IC50 determination
For determination of the inhibitory concentration able of killing 50% of the parasites (IC50), an aliquot of 10 μL of each compound (concentration ranging from 1 to 200 μM/well, with a total of 0.5% DMSO) was added in quadruplicate on a 96-well plate containing 90 μL of culture with the promastigote forms of L. braziliensis, previously adjusted to 107 cells/mL. Amphotericin 100 μM/well and 0.5% DMSO were used as positive and negative control, respectively. The plates were incubated at RT for 72 h. After this, the viability of the promastigotes was verified by MTT/PMS colorimetric assay, as described above. In vitro leishmanicidal activity, expressed as IC50, was determined using GraphPad Prism 5 software according to the following equation:
| (2) |
where Amin and Amax are, respectively, the minimum and maximum activities obtained, x is the ligand concentration and Hill Slope is the parameter that describes the steepness of the curve.
The selectivity index parameter was obtained by the ratio of compound IC50 against mammalian cells and compound IC50 against promastigote cells.
In vitro interaction assay
Two µM of protein in 40 mM Hepes buffer (pH 7.5), 100 mM KCl, was incubated for 30 min on ice with increasing concentrations of the compounds diluted in DMSO. The DMSO concentration did not exceed 2% of the total reaction volume (v/v). The samples were excited at 280 nm and the emission was collected in the 300–400 nm range, in a 1.0 × 0.2 cm optical path cuvette, on an F-4500 Fluorescence Spectrophotometer (Hitachi). The fluorescence quenching was analysed at the maximum emission wavelength in the absence of ligand. The determination of the apparent dissociation constant (KDapp) was performed by fitting the fluorescence values obtained for each compound concentration according to a dose-response model through the Equation (3):
| (3) |
where Fmin and Fmax are the minimum and maximum fluorescence intensity and [ligand] is the compound concentration used in the assay, according to the GraphPad Prism 5 software. The fluorescence intensities were corrected by inner filter effects as previously described31.
Spectrophotometric ATPase activity assays
The ATPase activity of LbHsp90 and hHsp90β was evaluated spectrophotometrically through inorganic phosphate (Pi) quantification following ATP hydrolysis, by the EnzChek™ assay kit (Invitrogen®) as previous described31,34. Briefly, a 2 µM solution of LbHsp90 and hHsp90β enzymes was prepared in 150 mM NaCl, 5 mM MgCl2 and 20 mM Tris-HCl, pH 7.5, and incubated with ATP (concentration range 0–5 mM) for 60 min at 37 °C. Then, the chromogenic substrate 2-amino-6-mercapto 7-methyl purine riboside (MESG) and the purine nucleoside phosphorylase (PNP) were added and the resulting reaction mixture was incubated for 30 min at 37 °C. The assays were performed in a 96-well plate and kinetic parameters determined by Michaelis–Menten fitting using GraphPad Prism 6 software.
ATPase activity inhibition assays
The inhibitory activity against LbHsp90 and hHsp90β of compounds showing interaction in DSF assays (namely Glb08, Glb15, Gl23, Glb25 and Glb27) was determined using spectrophotometric ATPase activity assays as described above. Initial screenings were performed using 50 μM compound concentration in a reaction mixture consisting of 2 μM enzyme and 2.5 mM ATP. After 1 h incubation at 37 °C, MESG and PNP were added. The amount of Pi was estimated after 30 min incubation at 37 °C. GA was used as reference compound, reporting 100% ATPase activity inhibition (at 50 μM). Compounds having percentage inhibition higher than 80% (namely compounds Glb08, Glb15 and Glb23 towards LbHsp90) were selected for IC50 determination. A compound concentration ranging from 1 to 75 μM was included in the reaction mixture and Pi quantification performed as previously described (measurements were performed in triplicate). IC50 values were determined by non-linear regression data fitting (using GraphPad Prism 6 software).
Results
Homology modelling
The lack of structural details on LbHsp90N seriously limited the rational design of specific small molecule inhibitors. We thus generated a homology model thanks to the availability of high quality structural templates from L. major, i.e. the LmHsp90N that shares 94% sequence identity with LbHsp90N, particularly within the ATP binding site (supplementary Figure S1). The structure of LmHsp90N in complex with ADP (PDB ID 3U6721) was selected as template in homology modelling, because it describes an almost physiological state of the parasite’s Hsp90. Structurally conserved water molecules W1, W2 and W3 were kept in the homology model because they are known from multiple Hsp90/ligand complexes to be crucial in mediating the interactions of the ADP, particularly with Asp7835. Active site composition of the two proteins differs at position 63, where isoleucine of LmHsp90N is replaced by valine in LbHsp90N. Although these residues share similar physicochemical features, they differ slightly in steric hindrance. Thus, energy minimisation in explicit solvent was performed to relax the homology model and to account for the Ile/Val replacement near the ligand binding site. In the relaxed model, the adenine moiety of ADP establishes a hydrogen bond with the carboxylate ion of Asp78, and is further involved in water-mediated interactions with the same Asp78, Leu33, and Gly82. The ADP ligand establishes additional H-bonds with Asn36, Asn91, Gly122, and Phe123. Overall, the LbHsp90N homology model was structurally similar to the template (Cα RMSD = 0.420 Å, supplementary Figure S2) as expected by the very high sequence identity between the two proteins (supplementary Figure S4), and it was subsequently used for structure-based molecular modelling studies.
First-round virtual screening
The 3D representation of chemical features that are relevant in ligand/protein interaction is a powerful tool to screen in silico large chemical libraries. In this work, a pharmacophore-like ROCS query was generated on the binding mode of ADP into the homology model of LbHsp90N (supplementary Figure S3), and was used to filter the MolPort database. Molecules with a Tanimoto Combo value higher than 0.8 (around 60,000 molecules that correspond to 1.2% of the initial database) were selected for molecular docking studies towards the LbHsp90N homology model. The first 500 top-scoring compounds were visually inspected and further analysed according to multiple parameters, such as chemical diversity, scoring values, and visual inspection of the docking pose. Finally, 13 molecules (namely Glb01–Glb13, supplementary Figure S4), which are different from known Hsp90 inhibitors15, were selected and submitted to in vitro assays.
Second-round virtual screening
To obtain insights into the structure-activity relationships of compounds identified in the first-round of virtual screening (see below), the scaffold of active compounds Glb08 and Glb11 was further expanded through a substructure search. Indeed, analysis of the docking-based binding modes of Glb08 and Glb11 highlighted two substructure patterns that account for the interaction with Asp78 and W1, W2, and W3. These are a guanidine in Glb08, and an imidothiocarbamate in Glb11 (supplementary Figure S4). Then, 2,348 compounds bearing these substructures were retrieved from the MolPort database by using a SMARTS filter, and submitted to molecular docking against the homology model of LbHsp90N using the same settings as in the first-round virtual screening. Docking poses for the 15% top ranking virtual hits were examined through visual inspection, and 15 molecules (Glb14–Glb28, supplementary Figure S6) were submitted to in vitro assays.
In vitro evaluations
Compound selected by virtual screening were tested for their capacity to interact with LbHsp90 and hHsp90β (N-terminal and full-length constructs), as well as for their capacity to impair the viability of the promastigote form of L. braziliensis through different in vitro approaches. Results are summarised in Table 1 and are described in deeper details below.
Table 1.
Summary of in vitro results obtained in parasite and cell-based assays, as well as against the N-terminal and full-length constructs of LbHsp90 and hHsp90β for the 28 selected compounds.
| Ligand | Thermal shift | Cell viability |
IC50 (μM) |
KDapp (μM) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| L. braziliensis | Balb-c | Selectivity index | LbHsp90 | LbHsp90N | hHsp90β | hHsp90βN | |||
| Glb01 | No | No | – | – | – | – | – | – | – |
| Glb02 | Yes | No | – | – | – | – | – | – | – |
| Glb03 | No | No | – | – | – | – | – | – | – |
| Glb04 | No | No | – | – | – | – | – | – | – |
| Glb05 | No | No | – | – | – | – | – | – | – |
| Glb06 | No | No | – | – | – | – | – | – | – |
| Glb07 | No | No | – | – | – | – | – | – | – |
| Glb08 | Yes | Yes | 43 ± 2 | 75 ± 3 | 1.7 | 13 ± 4 | 17 ± 6 | 20 ± 5 | 29 ± 3 |
| Glb09 | No | No | – | – | – | – | – | – | – |
| Glb10 | No | No | – | – | – | – | – | – | – |
| Glb11 | Yes | No | – | – | – | – | – | – | – |
| Glb12 | No | No | – | – | – | – | – | – | – |
| Glb13 | No | No | – | – | – | – | – | – | – |
| Glb14 | No | Yes | 30 ± 1 | >200 | >6.7 | – | – | – | – |
| Glb15 | No | Yes | 71 ± 2 | >200 | >2.8 | 14 ± 4 | 13 ± 3 | 14 ± 6 | 12 ± 3 |
| Glb16 | Yes | Yes | 12 ± 2 | 23 ± 1 | 1.9 | – | – | – | – |
| Glb17 | No | Yes | 4 ± 1 | 58 ± 1 | 14.5 | 120 ± 20 | 100 ± 70 | Ambiguous# | Ambiguous# |
| Glb18 | No | No | – | – | – | – | – | – | – |
| Glb19 | No | No | – | – | – | – | – | – | – |
| Glb20 | No | No | – | – | – | – | – | – | – |
| Glb21 | No | No | – | – | – | – | – | – | – |
| Glb22 | No | No | – | – | – | – | – | – | – |
| Glb23 | No | Yes | >100 | 55 ± 1 | <0.5 | 26 ± 7 | 20 ± 10 | 21 ± 9 | 20 ± 30 |
| Glb24 | No | No | – | – | – | – | – | – | – |
| Glb25 | Yes | Yes | >200 | 11 ± 1 | <0.05 | 7 ± 3 | 12 ± 2 | 15 ± 9 | 14 ± 3 |
| Glb26 | No | No | – | – | – | – | – | – | – |
| Glb27 | Yes | Yes | 11 ± 1 | 13 ± 2 | 1.2 | 7 ± 1 | 7 ± 1 | 5 ± 4 | 6 ± 2 |
| Glb28 | No | Yes | – | – | – | – | – | – | – |
| GA | Yes | Yes | 0.19* | – | – | 7.6 ± 0.2** | 1.8 ± 0.3** | 1.2*** | 0.77*** |
First, the 28 compounds selected in silico were preliminarily submitted to DSF analysis to test their capacity to interact with recombinant LbHsp90N and LbHsp90 proteins.
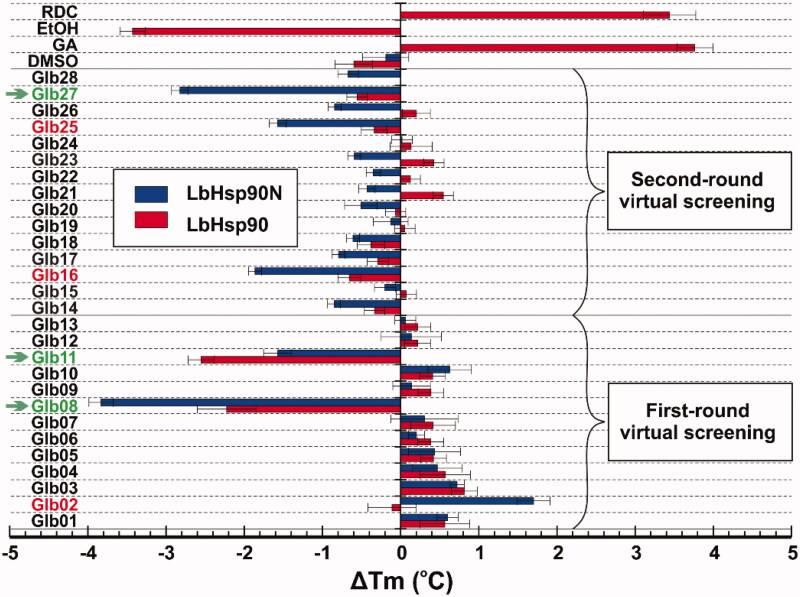
Once obtained the Tm for the above proteins in the presence of each compound, ΔTm values were calculated as the difference with Tm of isolated proteins (Figure 1). As expected, reference Hsp90 inhibitors RDC and GA led to protein stabilisation with a ΔTm > 2.0 °C (Figure S7). While testing selected compounds, we established a Tm variation greater or equal to ± 2 °C as inclusion criteria to consider potential Hsp90 binders21. Among the 28 tested compounds, Glb02, Glb08, Glb11, Glb16, Glb25 and Glb27 showed positive outcomes by DSF technique (Figure 1). Specifically, comparison of results obtained against LbHsp90N and the full length LbHsp90 clearly highlighted that all compounds might bind preferentially within the N-terminal domain of the protein. Glb11 seems to have additional binding sites than that at the N-terminal, since the ΔTm against the full length LbHsp90 is higher than that observed for LbHsp90N.
Figure 1.
ΔTm obtained from DSF experiments against LbHsp90 and LbHsp90N recombinant proteins in the presence of known Hsp90 inhibitors RDC and GA, as well as the test set Glb01–Glb28. Compounds marked in green satisfied the inclusion criteria, whereas those marked in red induced borderline ΔTm changes.
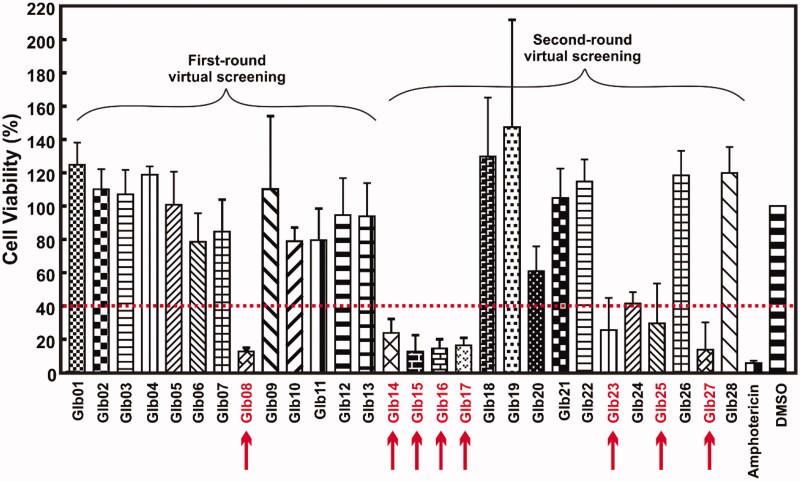
Subsequently, we tested the compounds against L. braziliensis promastigote cells. Based on the results showed in the Figure 2, compounds Glb08, Glb14, Glb15, Glb16, Glb17, Glb23, Glb25 and Glb27 exhibited leishmanicidal activity as they reduced cell viability more than 60% after 72 h. Amphotericin was used as positive control, showing more than 95% of killing activity (Figure 2).
Figure 2.
Cell viability experiments. Viability of L. braziliensis promastigote cells in the presence of each compound, after 72 h of incubation, assessed by MTT/PMS colorimetric methods. The arrows indicate the compounds impairing more than 50% of cells viability.
Eight compounds showed promising results against L. braziliensis promastigote cells. However, combining DSF screening with in vitro assays led to discontinue compounds Glb02 and Glb11, because they lack leishmanicidal activity although they proved positive in binding to LbHsp90. On the other hand, despite compounds Glb14, Glb15 and Glb17 were not highlighted as potential LbHsp90 binders by DSF, they exhibited leishmanicidal activity towards the parasite and were therefore included in following tests.
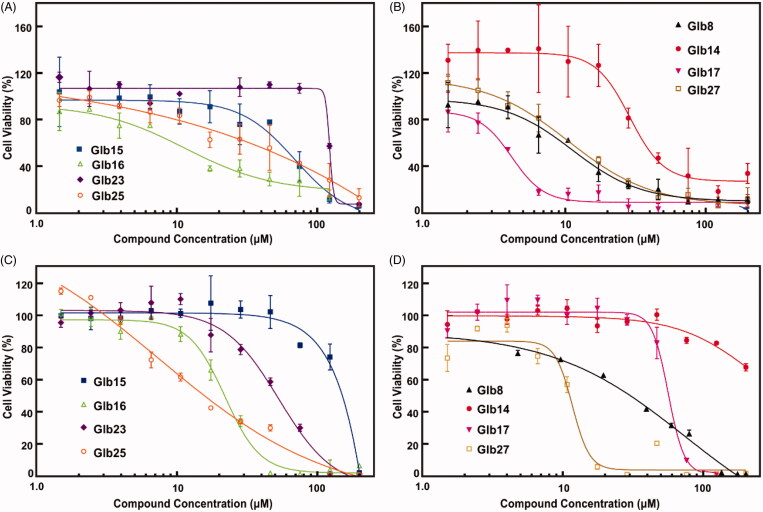
The effect of compounds Glb08, Glb14–Glb17, Glb23, Glb25 and Glb27 on the L. braziliensis promastigote cell viability was demonstrated to be concentration-dependent (Figure 3(A,B). Data reported in Table 1 show that Glb08, Glb14, Glb16, Glb17 and Glb27 presented the lowest IC50 values, with Glb16, Glb17 and Glb27 being the most effective against L. braziliensis, whereas Glb15, Glb23 and Glb25 proved active although to a lesser extent.
Figure 3.
Compounds IC50 determination against L. braziliensis promastigotes cells and their cytotoxic effect against mammalian cells. (A, B) L. braziliensis promastigotes cell viability was determined as described in Material and Methods section (Equation (2). (C, D) Compounds cytotoxic activity against Balb/C 3T3 clone A31 mouse fibroblast cells. IC50 for each compound concentration was fitted by a concentration-dependent equation.
Given the high degree of structural similarity between LbHsp90 and hHsp90, to monitor the possible selectivity for L. brazilienzis with respect to the host, cytotoxicity assays were carried out on Balb-c 3T3 mouse fibroblasts cells. Specifically, compounds showing impairment of L. braziliensis promastigotes cell viability (see above) were tested. Results are reported in Figure 3(C,D) and in Table 1 and clearly substantiate that Glb23 and Glb25, endowed with a moderate leishmanicidal activity, also experienced the weakest selectivity. In contrast, Glb27 also showed a weak selectivity for L. braziliensis promastigotes. Similarly, Glb08 and Glb16 showed comparable IC50 values against L. braziliensis promastigotes and Balb-c cells, which lead to a low selectivity index. Although showing generally a slightly weaker leishmanicidal activity compared to other compounds, Glb14 and Glb15 seem to not interfere with cell viability of Balb-c cells, which leads to an interesting selectivity index (Table 1). Finally, Glb17 emerged as the most selective compound, also showing the strongest leishmanicidal activity against L. braziliensis promastigotes (IC50 of 4 and 58 µM against L. braziliensis promastigotes and Balb-c cells, respectively).
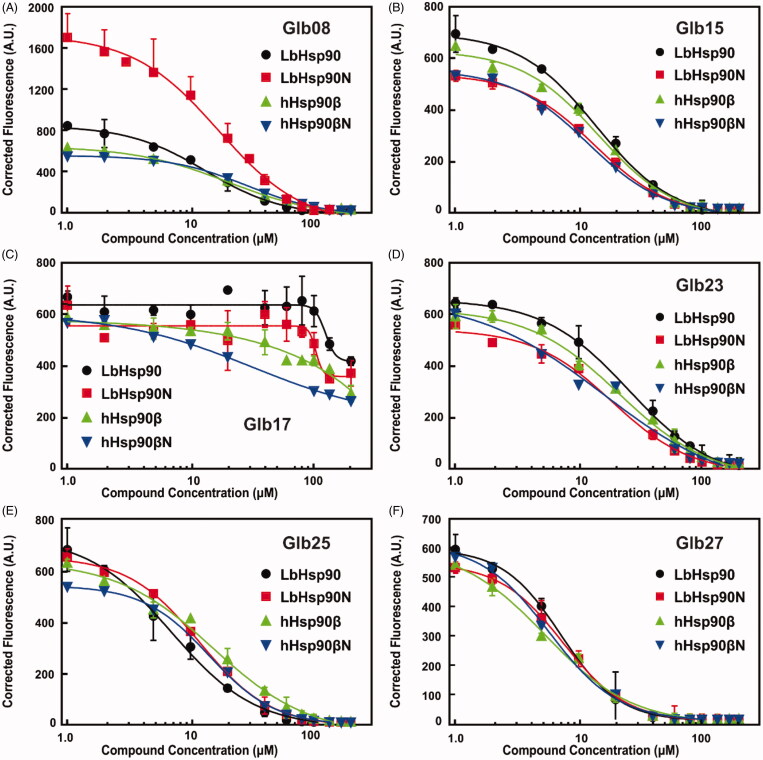
To confirm whether leishmanicidal activity is due to LbHsp90 inhibition, or might be triggered by different mechanisms of action, the direct binding between compounds Glb08, Glb14–Glb17, Glb23, Glb25 and Glb27 and recombinant LbHsp90, hHsp90β and their N-terminal constructs was monitored by fluorescence quenching. This methodology requires that the ligands have a no intrinsic fluorescence (although a low fluorescence might be tolerated) in the same region of the Tryptophan residue (∼300–400 nm). Unfortunately, Glb14 and Glb16 do not match with this requirement, and were not able to be evaluated by the fluorescence quenching assay even after inner filter effect corrections (data not shown).
Figure 4(A) indicates that all tested compounds, with the exception of the Glb17, are able to quench fluorescence of both LbHsp90 and hHsp90β constructs. Notably, KDapp values observed for the interaction with full-length constructions were highly comparable to those monitored for the interaction with the N-terminal domains, which suggests that the preferred binding site of tested compounds is located in the N-terminal region of Hsp90 (Table 1). Glb17 did not induce tryptophan fluorescence quenching neither in the LbHsp90 nor in the hHsp90β constructions (Figure 4(C)), indicating that Glb17 is unable to bind LbHsp90, in agreement with DSF data (Figure 1(B)). Therefore, leishmanicidal activity of Glb17 might be due to the interference with a target that is not LbHsp90, whose identification will be the subject of a further study.
Figure 4.
Protein interaction with ligands monitored by the Tryptophan Fluorescence Quenching. The protein fluorescence was monitored in the presence of increasing ligand concentrations and the KDapp was determined as described in Material and Methods section (Equation (3). (A) GLb08, (B) GLb15, (C) Glb17, (D) Glb23, (E) Glb25 and F) Glb27. The continuous line represents the non-linear fitting adjustment with Equation (3) performed by the GrapPrism 6 software.
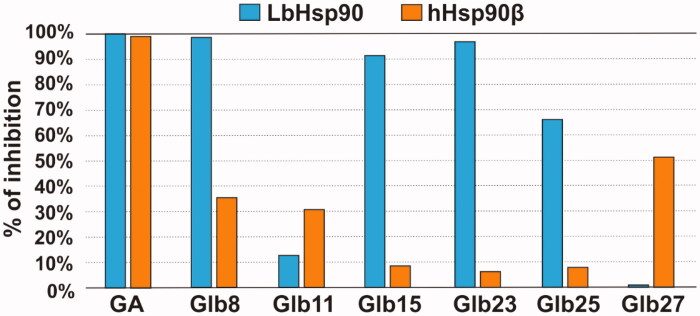
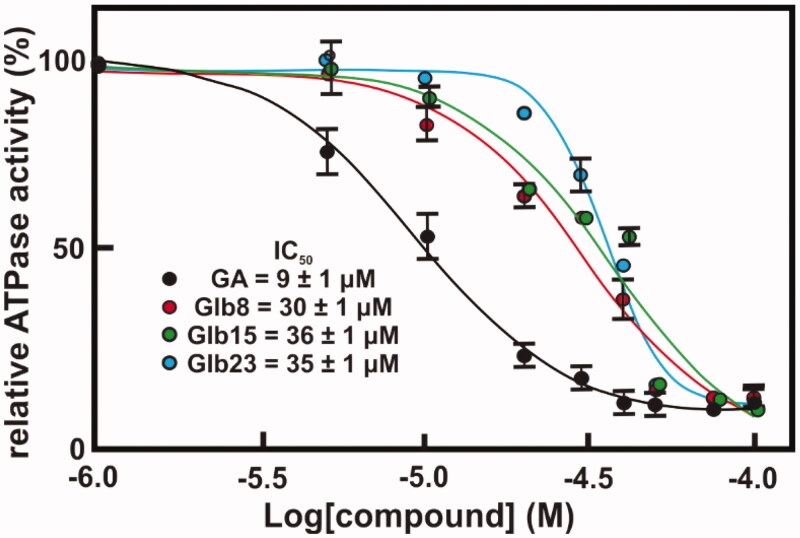
Since our data suggested that the compounds bind preferentially the N-terminal domain of Hsp90, which exploit its functions through binding and hydrolysing ATP, the effect of compounds Glb08, Glb15, Gl23, Glb25 and Glb27 on the ATPase activity of both recombinant LbHsp90 and hHsp90β was evaluated. All compounds showed a percentage inhibition lower than 60% against hHsp90β, with the only exception of the reference GA that fully inhibits enzyme activity with an IC50 of 702 nM11. On the other hand, compounds Glb08, Glb15 and Glb23 displayed more than 90% inhibition of LbHsp90 at 50 μM (Figure 5) and were submitted to IC50 determination (Figure 6), showing inhibition at micromolar concentration, and comparable to the value observed for the positive control GA (IC50 of 9 μM).
Figure 5.
LbHsp90 and hHsp90β ATPase inhibition assay. Percentage of inhibition of GA, Glb8, Glb11, Glb15, Glb23, Glb25 and Glb27 towards LbHsp90 and hHsp90β at 50 µM concentration.
Figure 6.
Concentration–response curves of compounds against LbHsp90 ATPase activity inhibition. The ATPase activity inhibition of LbHsp90 was determined as described in Material and Methods section. Concentration-response curve of four compounds, namely (A) Glb08, (B) Glb15, (C) Glb23 and (D) GA. The absolute IC50 value was determined by nonlinear regression analysis employing GraphPad Prism 6 software.
Overall, 28 compounds selected by virtual screening (Glb01–Glb28) were tested in vitro by different methods. Among them, 8 compounds exhibited leishmanicidal activity (Glb08, Glb14–Glb17, Glb23, Glb25, and Glb27), and 6 were highlighted as potential Hsp90 binders by DSF (Glb08, Glb15, Glb17, Glb23, Glb25, and Glb27). Finally, Glb08, Glb15, and Glb23 were considered as the most promising hits of this series, particularly based on their selective inhibition of ATP hydrolysis in LbHsp90 with respect to the human hHsp90 orthologue.
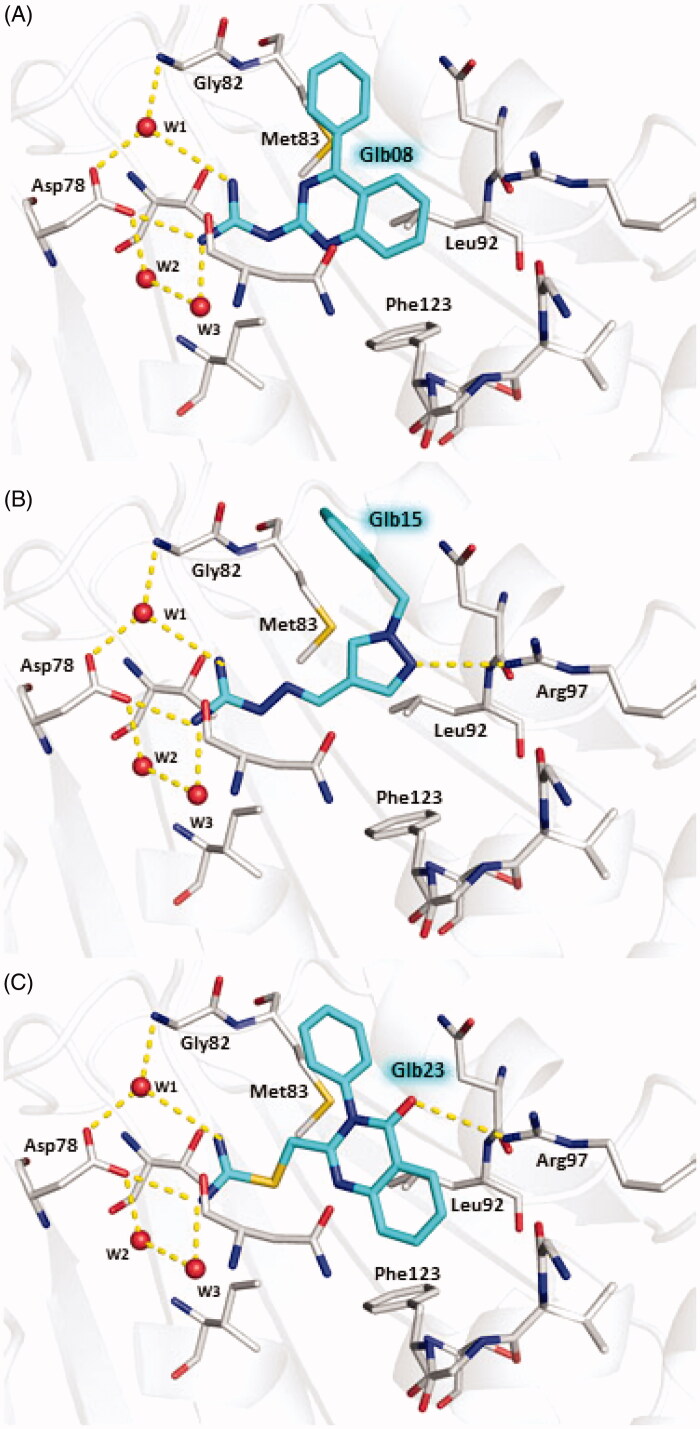
Predicted binding modes of Glb08, Glb15 and Glb23 towards LbHsp90N
The predicted binding modes of Glb08, Glb15 and Glb23 within the ATP binding site of LbHsp90 are shown in Figure 7. All compounds are able to establish H-bond interactions with Asp78 and the three conserved water molecules W1, W2, and W3 that are known to play a crucial role in binding to the substrate and to some small molecule inhibitors (Figure 7)35. A further H-bond interaction bridged by W1 is established by all compounds with the backbone nitrogen of Gly82. The hydrophobic moieties of the three molecules are accommodated inside the hydrophobic cavity lined by Met83, Leu92 and Phe123. According to the predicted binding mode, Glb15 and Glb23 accept an additional H-bond from the side chain of Arg97 (Figure 7(B,C)).
Figure 7.
Predicted binding modes of Glb08 (A), Glb15 (B), and Glb23 (C) in LbHsp90N homology model. Glb08, Glb15 and Glb23 are shown in sticks, cyan carbon atoms. LbHsp90 is displayed as grey cartoon with active site residues as sticks, grey carbon atoms (only residues involved in H-bond with the molecules are labelled for clarity). Hydrogen bonds are shown as yellow dashed lines and water molecules as red spheres. Nitrogen atoms are Coloured blue, oxygen red and sulphur yellow. Hydrogen atoms are omitted.
Discussion
Hsp90 is a well-established target for the treatment of cancer, and is becoming an attractive target also for the therapy of NTD caused by a wide variety of protozoan parasites. In fact, Hsp90 is essential for survival and proliferation of protozoan parasites during their intracellular mammalian stage. Since the ATPase activity executed in the N-terminal domain of Hsp90 is critical for chaperone functions, small molecules able to prevent ATP hydrolysis are expected to inhibit Hsp90, leading to client protein degradation and cell death37. In this work, with the ultimate goal to identify hit compounds able to interfere with the replication of L. braziliensis, we established a multidisciplinary approach to target LbHsp90N. A structure-based virtual screening combining similarity search with molecular docking simulations was used to screen a commercial library of compounds, and to identify a shortlist of candidate inhibitors of LbHsp90N that were tested in vitro. First, DSF and cell viability assays were employed to test the potential of the compounds to bind recombinant LbHsp90, and to impair the survival of L. braziliensis promastigotes, respectively. In DSF, both the full length LbHsp90 and its N-terminal domain LbHsp90N were used to monitor whether the compounds might bind within the N-terminal end of the protein endowed with ATP hydrolysis activity.
Among the test set, we found that compounds Glb02, Glb08, Glb11, Glb16, Glb25 and Glb27 induced a change of Tm in at least one LbHsp90 construct, indicating a direct interaction with the target. While some compounds increased protein stability (positive ΔTm values), others determined a reduction in the thermal stability (negative ΔTm values) that could be explained by ligand-dependent conformational alterations of the flexible and modular LbHsp9038.
Cell viability assay confirmed that above compounds are endowed with leishmanicidal activity, in addition to Glb14, Glb15, Glb17 and Glb23 that were not highlighted by DSF. In contrast, Glb02 and Glb11 did not show leishmanicidal activity against the promastigote form of the parasite. In order to provide deeper insights into the interaction between Glb compounds and LbHsp90, Glb08, Glb16, Glb17, Glb23, Glb25 and Glb27 were also tested by means of tryptophan fluorescence quenching assay. Specifically, ligand interaction with the tryptophan residue located in the ATP binding site of Hsp90 N-terminal domain, which is highly conserved in Hsp90 from multiple species31, was monitored.
Notably, all tested compounds proved to bind in the N-terminal region of LbHsp90. However, these molecules proved to bind also hHsp90, generally with similar KDapp as in the LbHsp90. Whether this evidence impacts negatively on the selectivity profile of hit compounds disclosed in this work, this should not be a surprising outcome because of the very high degree of sequence and structural similarity between human and L. braziliensis Hsp90.
ATPase activity is essential for the function of Hsp90. The hydrolysis of ATP requires a series of conformational changes, starting from the apo state, passing through the closed ATP bonded state to culminate in ATP hydrolysis and release of ADP and Pi, with Hsp90 returning to its original conformation39–41. This suggests that compounds able to mimic or to compete with ATP might behave as inhibitors of Hsp90. By monitoring the compounds effect on Hsp90 ATPase activity, we found that Glb08, Glb15, Gl23, Glb25 and Glb27 did not exhibit extensive inhibition against hHsp90, while Glb08, Glb15 and Glb23 were able to inhibit around 90% of ATPase activity in LbHsp90. IC50 determination revealed that these molecules inhibit LbHsp90 ATPase activity to a similar extent of the reference inhibitor GA.
It is worth noting that any attempts to characterise the complex between LbHsp90N and selected Glb compounds by X-ray crystallography failed. Atomistic details of the interaction of Glb08, Glb15 and Glb23 to LbHsp90N were provided by molecular modelling. These compounds establish interactions with LbHsp90 Asp78 and Gly82, while non-polar moieties are accommodated inside the hydrophobic cavity lined by Met83, Leu92 and Phe123. Beyond this, Glb15 and Glb23 also performed an additional interaction through Arg97. To evaluate the coherence of these predicted binding modes, they were compared with X-ray crystallographic structures available for LmHsp90N. The complexes of LmHsp90 with ADP (PDB-ID 3U6721) with 17-DMAP-geldanamycin (PDB-ID 3Q5J), and with 17-AEP-geldanamycin (PDB-ID 3Q5L) showed that the interactions predicted for our molecules are also relevant for substrates and inhibitors binding to LmHsp90N42.
Although the characterisation of the mechanism of action of these compounds would require additional efforts, taken together our results highlighted Glb08, Glb15, and Glb23 as confirmed inhibitors of LbHsp90 that are worth of further development. These molecules might serve as source of inspiration in the design of further generation of LbHsp90 small molecule inhibitors with improved potency, selectivity, and drug-like features.
Conclusions
In this work, we used a multidisciplinary approach boosted by computational screening to identify a number of small molecules able to impair the viability of L. braziliensis promastigotes, to bind LbHsp90, or to inhibit ATPase activity exerted by LbHsp90N. Particularly, our molecules bear a guanidine-like pharmacophore that – to the best of our knowledge – is not included in any known Hsp90 inhibitors targeting the N-terminal domain reported to date. These molecules could be used as a starting point to design further generations of LbHsp90 inhibitors with improved activity, drug-like properties, and selectivity.
Supplementary Material
Acknowledgements
The authors wish to thank the OpenEye Free Academic Licencing Programme for providing a free academic licence for molecular modelling and chemoinformatics software.
We acknowledge Prof. Dr. Reinaldo Alves de Brito and Dr. Felipe Rafael Torres (Department of Genetics and Evolution, Federal University of São Carlos) by the access to the equipment and technical support during DSF experiments.
Funding Statement
FAPESP [2011/23110–0; 2012/50161–8; 2013/10712–8, 2013/18009–4, 2014/07206–6, FAPESP: 2017/26131–5 and 2017/07335–9], CNPq [471415/2013–8 and 303129/2015–8] and CAPES.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Nii-Trebi NI. Emerging and neglected infectious diseases: insights, advances, and challenges. Biomed Res Int 2017;2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcantara LM, Ferreira TCS, Gadelha FR, Miguel DC.. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. Int J Parasitol Drugs Drug Resist 2018;8:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012;7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varikuti S, Jha BK, Volpedo G, et al. Host-directed drug therapies for neglected tropical diseases caused by protozoan parasites. Front Microbiol 2018;9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hombach A, Clos J.. No stress – Hsp90 and signal transduction in Leishmania. Parasitology 2014;141:1156–66. [DOI] [PubMed] [Google Scholar]
- 6.Graefe SE, Wiesgigl M, Gaworski I, et al. Inhibition of HSP90 in Trypanosoma cruzi induces a stress response but no stage differentiation. Eukaryot Cell 2002;1:936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seraphim TV, Ramos CHI, Borges JC, The interaction networks of Hsp70 and Hsp90 in the Plasmodium and Leishmania parasites. In: Houry W, ed. The molecular chaperones interaction networks in protein folding and degradation. Interactomics and systems biology. 1. New York: Springer; 2014:445–81. [Google Scholar]
- 8.Wandinger SK, Richter K, Buchner J.. The Hsp90 chaperone machinery. J Biol Chem 2008;283:18473–7. [DOI] [PubMed] [Google Scholar]
- 9.Sahasrabudhe P, Rohrberg J, Biebl MM, et al. The plasticity of the Hsp90 Co-chaperone system. Mol Cell 2017;67:947–61 e5. [DOI] [PubMed] [Google Scholar]
- 10.Schopf FH, Biebl MM, Buchner J.. The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 2017;18:345–60. [DOI] [PubMed] [Google Scholar]
- 11.Pallavi R, Roy N, Nageshan RK, et al. Heat shock protein 90 as a drug target against protozoan infections: biochemical characterization of HSP90 from Plasmodium falciparum and Trypanosoma evansi and evaluation of its inhibitor as a candidate drug. J Biol Chem 2010;285:37964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soti C, Nagy E, Giricz Z, et al. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol 2005;146:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers MV, Workman P.. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett 2007;581:3758–69. [DOI] [PubMed] [Google Scholar]
- 14.Giannini G, Battistuzzi G.. Exploring in vitro and in vivo Hsp90 inhibitors activity against human protozoan parasites. Bioorg Med Chem Lett 2015;25:462–5. [DOI] [PubMed] [Google Scholar]
- 15.Sidera K, Patsavoudi E.. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov 2014;9:1–20. [PubMed] [Google Scholar]
- 16.Brandau S, Dresel A, Clos J.. High constitutive levels of heat-shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem J 1995;310:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesgigl M, Clos J.. Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani. Mol Biol Cell 2001;12:3307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiesgigl M, Clos J.. The heat shock protein 90 of Leishmania donovani. Med Microbiol Immun 2001;190:27–31. [DOI] [PubMed] [Google Scholar]
- 19.Shonhai A, Maier AG, Przyborski JM, Blatch GL.. Intracellular protozoan parasites of humans: the role of molecular chaperones in development and pathogenesis. Protein Pept Lett 2011;18:143–57. [DOI] [PubMed] [Google Scholar]
- 20.Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007;23:2947–8. [DOI] [PubMed] [Google Scholar]
- 21.Pizarro JC, Hills T, Senisterra G, et al. Exploring the Trypanosoma brucei Hsp83 potential as a target for structure guided drug design. PLoS Negl Trop Dis 2013;7:e2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson MP, Pincus DL, Rapp CS, et al. A hierarchical approach to all-atom protein loop prediction. Proteins 2004;55:351–67. [DOI] [PubMed] [Google Scholar]
- 23.Case DA, Darden TA, Cheatham I, et al. AMBER 12. San Francisco: University of California; 2012. [Google Scholar]
- 24.ROCS 3.2.0.4; OpenEye Scientific Software: Santa Fe, NM. http://www.eyesopen.com.
- 25.Hawkins PC, Skillman AG, Nicholls A.. Comparison of shape-matching and docking as virtual screening tools. J Med Chem 2007;50:74–82. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins PC, Skillman AG, Warren GL, et al. Conformer generation with OMEGA: algorithm and validation using high quality structures from the Protein Databank and Cambridge Structural Database. J Chem Inf Model 2010;50:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGann M. FRED pose prediction and virtual screening accuracy. J Chem Inf Model 2011;51:578–96. [DOI] [PubMed] [Google Scholar]
- 28.Schrödinger Release 2017-4: LigPrep, Schrödinger, LLC, New York, NY, 2017. [Google Scholar]
- 29.Szybki 1.8.0.1 4; OpenEye Scientific Software: Santa Fe, NM. http://www.eyesopen.com.
- 30.QUACPAC 1.6.3.1: OpenEye Scientific Software, Santa Fe, NM. http://www.eyesopen.com.
- 31.Silva KP, Seraphim TV, Borges JC.. Structural and functional studies of Leishmania braziliensis Hsp90. BBA-Proteins Proteom 2013;1834:351–61. [DOI] [PubMed] [Google Scholar]
- 32.Minari K, de Azevedo EC, Kiraly VTR, et al. Thermodynamic analysis of interactions of the Hsp90 with adenosine nucleotides: a comparative perspective. Int J Biol Macromol 2019;130:125–38. [DOI] [PubMed] [Google Scholar]
- 33.Batista FA, Gava LM, Pinheiro GM, et al. From conformation to interaction: techniques to explore the Hsp70/Hsp90 network. Curr Protein Pept Sci 2015;16:735–53. [DOI] [PubMed] [Google Scholar]
- 34.Richter K, Muschler P, Hainzl O, Buchner J.. Coordinated ATP hydrolysis by the Hsp90 dimer. J Biol Chem 2001;276:33689–96. [DOI] [PubMed] [Google Scholar]
- 35.Roe SM, Prodromou C, O'Brien R, et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 1999;42:260–6. [DOI] [PubMed] [Google Scholar]
- 36.Palma LC, Ferreira L, Petersen A, et al. A docking-based structural analysis of geldanamycin-derived inhibitor binding to human or Leishmania Hsp90. Sci Rep-Uk 2019;9:14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat R, Tummalapalli SR, Rotella DP.. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J Med Chem 2014;57:8718–28. [DOI] [PubMed] [Google Scholar]
- 38.Seraphim TV, Silva KP, Dores-Silva PR, et al. Insights on the structural dynamics of Leishmania braziliensis Hsp90 molecular chaperone by small angle X-ray scattering. Int J Biol Macromol 2017;97:503–12. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Soroka J, Buchner J.. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. BBA-Mol Cell Res 2012;1823:624–35. [DOI] [PubMed] [Google Scholar]
- 40.Ali MMU, Roe SM, Vaughan CK, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 2006;440:1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hessling M, Richter K, Buchner J.. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol 2009;16:287–93. [DOI] [PubMed] [Google Scholar]
- 42.Pozzi C, Tassone G, Mangani S.. X-ray crystallography contributions to drug discovery against parasite. Annu Rep Med Chem 2018;51:175–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.