Abstract
Background:
The United States Food and Drug Administration (FDA) has licensed three HPV (Human papilloma virus) vaccines. The centers for disease control and prevention (CDC) and advisory committee on immunization practices (ACIP) recommends routine HPV vaccination at age 11 or 12 years. This study aimed to summarize and characterize adverse events following HPV vaccination reported to VAERS database from July 2006 to May 2017.
Methods:
A systematic data mining was performed in the VAERS database for reports associated with HPV vaccine. Clinically relevant Vaccine Event Combinations (VEC) were identified in the VAERS database following HPV vaccination. A VEC was considered for analysis only if a minimum of hundred reports were present in database for the given Adverse Event (AE). The data mining algorithm used in this study was reporting odds ratio. A value of ROR-1.96SE >1 was considered as positive signal.
Results:
VAERS received 49444 reports after receipt of HPV vaccine during the study period. Out of 49444, 2307 unique reactions were identified. A total of 177 death reports and 3526 non death serious reactions were reported to VAERS. ROR showed positive signals for abdominal pain, syncope, dizziness, convulsion, abortion spontaneous, alopecia, amenorrhea, anogenital warts, cervical dysplasia, anaemia, dyskinesia, migrane, blood pressure decreased, fall, head injury, loss of consciousness, pallor, presyncope, seizures.
Conclusion:
The present analysis did not identify any new/unexpected safety concern and was consistent with the safety data from prelicensure trials. Further epidemiological studies are required to systematically validate the data provided by VAERS.
Keywords: Adverse event, human papillomavirus vaccine, signal detection, Vaccine Adverse Event Reporting System database, vaccine safety
INTRODUCTION
Vaccines have historically been the most effective means to fight and eradicate infectious diseases; limitations to their effectiveness, nevertheless, exist.[1] The risk/benefit ratio is one of the highest concerns of any medical intervention. It is difficult or almost impossible to determine all the adverse effects of a vaccine in prelicensure clinical trials due to restricted sample size, reduced follow-up, appraisal of surrogate markers, exclusion of testing in special population, and participants with multiple comorbidities and on polypharmacy.[2,3] The requisite for postmarketing surveillance is a direct result of these limitations.
Human papillomaviruses (HPVs) are an enormous and diverse group of viruses. They are DNA virus that infects epithelial tissues and are classified into low- and high-risk types based on their ability to promote malignant transformation. The viral genes, i.e., E6 and E7 bind to tumor suppressor gene and retinoblastoma tumor suppressor protein, respectively, and inactivate them.[4] An epidemiology study suggests that 75% of all sexually active men and women will develop HPV infection at least once in their lifetime with utmost prevalence existing from adolescence to the age of 29 years.[5] According to the WHO (updated 2016), two HPV types, i.e. HPV-16 and HPV-18 cause 70% of cervical cancers and precancerous cervical lesions.[6] There is high variation in the incubation period for the development of clinical symptoms following HPV infection.[7]
HPV infections and HPV-associated cancers can effectively be prevented by HPV vaccines. The Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) recommends routine HPV vaccination at the age of 11 or 12 years. HPV vaccines are licensed for use in females and males aged 9–26 years.[8]
The most common adverse events (AEs) observed in prelicensure clinical trials were as follows: local reactions (pain, redness, swelling, and pruritus at injection site), pyrexia, nausea, dizziness, diarrhea, vomiting, fatigue, upper respiratory tract infections, oropharyngeal pain, myalgia, and headache.[9,10,11]
There were few postlicensure studies conducted previously to assess the adverse effects following HPV vaccine.[12,13,14,15,16] However, those studies were limited to a specific adverse effect or a constrained study population. Furthermore, there were few case reports documented after HPV vaccination.[17,18,19,20] To our best knowledge, there was only one study done to assess the postlicensure safety surveillance of quadrivalent human papillomavirus recombinant vaccine.[21] However, this study was carried out in the year 2008, after which many new AEs would have unfolded. The aforementioned study covered reports only from June 1, 2006, to December 31, 2008. Extended use of HPV vaccine and a limited number of safety surveillance studies result in an ambiguous situation of HPV vaccine safety.
This study aimed to summarize and characterize AEs following HPV vaccination reported to the Vaccine AE Reporting System (VAERS) database from July 2006 to May 2017.
METHODS
Vaccine Adverse Event Reporting System
VAERS is a nationwide passive reporting system which was established in 1990 by the US Food and Drug Administration (US-FDA) and the CDC to detect possible safety problems in US-licensed vaccines. The main objectives of VAERS – a spontaneous reporting system – are to detect new, unusual, or rare vaccine AEs, assess the safety of newly licensed vaccines, identify potential patient risk factors for particular types of AEs, monitor increases in known AEs, determine and address possible reporting clusters, and provide a national safety monitoring system that extends to the entire general population.[22]
Vaccine manufacturers, health-care providers, and vaccine recipients (or their parents/guardians) can report the AEs to VAERS. It collects the data on patient information (name, allergies, age at vaccination, and concurrent illness), reporter information, facility information, vaccine information (vaccine, manufacturer, lot number, and route), and any other additional information (if applicable). A central facility that is managed by a private contractor under the direction of CDC and FDA receives all the VAERS reports where report processing, the Medical Dictionary for Regulatory Activities coding in which each report is assigned to one or more preferred terms, data entry, and quality control, is done.[23] The reports which are received are categorized as either serious or nonserious based on the definition in the Code of Federal Regulations, 21CFR312.32.[24] A reaction is considered serious if it results in any one or more of the following:
“Death, a life-threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, or a congenital anomaly/birth defect.”
In addition, the definition specifies that important medical events that do not come under the above definition may also be classified as serious if they “jeopardize the patient or subject and may require medical or surgical intervention to prevent one of the outcomes listed in this definition.”
We comprehended all the reports from July 2006 to May 2017 received to VAERS database for individuals who were vaccinated with human papillomavirus vaccine.
Disproportionality analysis
The data mining algorithm used in this study was reporting odds ratio (ROR). A value of ROR-1.96SE >1 (standard error [SE]) was considered as a threshold value, above which it will be considered as a positive signal.[25] The ROR is the ratio of the odds of reporting of one specific event versus all other events for a given drug compared to the reporting odds for all other drugs present in the database. The higher the value, the stronger the disproportion appears to be.
To consider a vaccine event combination (VEC) to be clinically relevant in this study, a minimum of total hundred reports must be present in the database for an AE. This resulted in 57 clinically relevant VECs by omitting 2250 VECs as those had reports below 100.
RESULTS
A total of 49,444 VECs were reported from July 2006 to May 2017 in the VAERS database. Of these reports, HPV vaccine was administered to 373 (1.26%) people aged between 0 and 8 years, 28,268 (96.11%) aged between 9–26 years, and 771 (2.62%) of age >27 years [Table 1]. We identified vaccination error reports for the age group of 0–8 years. The most common error group was the wrong vaccine administered (70.33%) followed by inappropriate schedule (20.09%) and storage and dispensing errors (9.56%) [Figure 1].
Table 1.
Age distribution of human papillomavirus vaccine recipient, 2006-2017
| 0-8 years | 9-26 years | >26 years | |
|---|---|---|---|
| 2006 | 3 | 284 | 21 |
| 2007 | 20 | 4342 | 133 |
| 2008 | 20 | 4934 | 125 |
| 2009 | 14 | 2816 | 66 |
| 2010 | 25 | 2284 | 49 |
| 2011 | 10 | 2050 | 37 |
| 2012 | 36 | 1883 | 59 |
| 2013 | 42 | 2089 | 78 |
| 2014 | 52 | 2210 | 73 |
| 2015 | 51 | 2294 | 62 |
| 2016 | 49 | 2573 | 59 |
| 2017 | 51 | 1976 | 34 |
Figure 1.
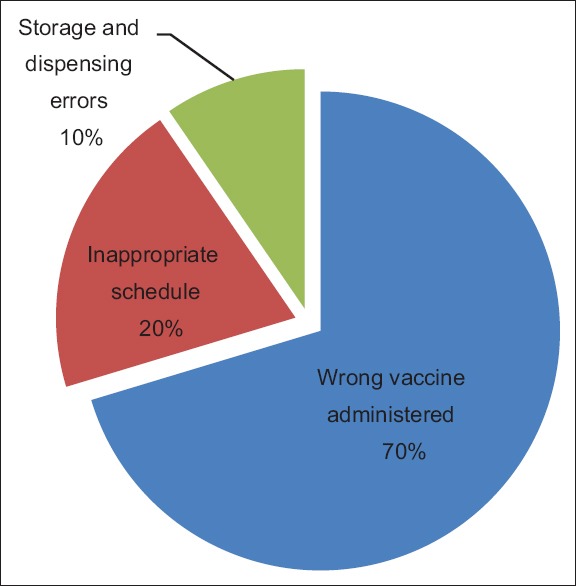
Vaccination error reports for human papillomavirus vaccine for the age group of 0–8 years, Vaccine Adverse Event Reporting System, 2006–2017
Out of 49,444 reports, 2307 unique reactions were identified by removing duplicates. ROR showed positive signals for abdominal pain, syncope, dizziness, convulsion, spontaneous abortion, alopecia, amenorrhea, anogenital warts, cervical dysplasia, anemia, dyskinesia, migraine, fall, head injury, loss of consciousness, pallor, presyncope, and seizures.
Serious reports
Serious reactions of 57 VECs were looked through in the VAERS database following HPV administration from July 2006 to May 2017 [Table 2]. A total of 177 death reports were identified. There was zero death reported in the year 2006 which increased to 39 in the year 2016. There were ten deaths reported in 2017. The nondeath serious reactions include life-threatening, hospitalized, prolonged hospitalization, and disability. There were 3526 nondeath serious reactions reported to VAERS [Table 3].
Table 2.
Number of serious events reported to vaccine adverse event reporting system after human papillomavirus vaccine
| Medical condition* | Number of serious reports |
|---|---|
| Blood and lymphatic system disorders | |
| Anemia | 22 |
| Lymphadenopathy | 6 |
| Cardiac disorders | |
| Cyanosis | 8 |
| Chest pain | 22 |
| Gastrointestinal disorders | |
| Abdominal pain | 202 |
| Abdominal discomfort | 19 |
| Diarrhea | 11 |
| Vomiting | 45 |
| Nausea | 48 |
| Musculoskeletal and connective tissue disorders | |
| Arthralgia | 87 |
| Myalgia | 31 |
| Back pain | 34 |
| Nervous system disorders | |
| Dyskinesia | 4 |
| Abasia | 77 |
| Tremor | 15 |
| Syncope | 42 |
| Dizziness | 71 |
| Convulsion | 85 |
| Amnesia | 21 |
| Migraine | 17 |
| Headache | 93 |
| Guillain Barre Syndrome | 26 |
| Paraesthesia | 26 |
| Seizure | 21 |
| Presyncope | 4 |
| Burning sensation | 4 |
| Investigation | |
| Blood pressure decreased | 9 |
| Vascular disorders | |
| Pallor | 12 |
| Infections and infestations | |
| Cellulitis | 7 |
| Skin and subcutaneous tissue disorders | |
| Hyperhidrosis | 9 |
| Urticaria | 12 |
| Rash | 35 |
| Blister | 3 |
| Erythema | 11 |
| Pruritis | 9 |
| Alopecia | 19 |
| Cold sweat | 4 |
| Psychiatric disorders | |
| Anxiety | 24 |
| Reproductive system and breast disorders | |
| Amenorrhea | 17 |
| Cervical dysplasia | 1 |
| Pregnancy, puerperium and perinatal conditions | |
| Spontaneous abortion | 18 |
| Respiratory, thoracic, and mediastinal disorders | |
| Dyspnea | 37 |
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | |
| Anogenital warts | 1 |
| General disorders and administration site conditions | |
| Hyposthenia | 48 |
| Pyrexia | 52 |
| Asthenia | 73 |
| Malaise | 28 |
| Chills | 5 |
| Condition aggravated | 28 |
| Fatigue | 49 |
| Edema peripheral | 11 |
| Immediate postinjection reaction | 11 |
| Swelling | 33 |
| Injury, poisoning and procedural complications | |
| Fall | 17 |
| Head injury | 6 |
*MedDRA SOC. SOC=System organ class
Table 3.
Death and nondeath serious reactions reported to vaccine adverse event reporting system after human papillomavirus vaccination from 2006-2017
| Death | Life-threatening | Hospitalized | Prolonged hospitalization | Disability | |
|---|---|---|---|---|---|
| 2006 | 0 | 5 | 13 | 0 | 6 |
| 2007 | 12 | 78 | 179 | 47 | 111 |
| 2008 | 19 | 71 | 298 | 81 | 155 |
| 2009 | 14 | 93 | 259 | 40 | 158 |
| 2010 | 14 | 54 | 143 | 14 | 87 |
| 2011 | 11 | 38 | 98 | 17 | 49 |
| 2012 | 6 | 35 | 129 | 13 | 41 |
| 2013 | 19 | 41 | 96 | 14 | 49 |
| 2014 | 16 | 45 | 105 | 17 | 83 |
| 2015 | 17 | 38 | 96 | 11 | 83 |
| 2016 | 39 | 49 | 120 | 14 | 87 |
| 2017 | 10 | 44 | 116 | 24 | 82 |
After a thorough review of all the serious reports, we found that abdominal pain (202 reports) and headache (93 reports) were the two most common events reported. The maximum reports were found in the year 2008 (36 reports) and 2009 (37 reports) and then gradually decreased to 13 reports in 2016. While headache is usually regarded as temporary and nonlife-threatening condition, the serious reports labeled in this study were due to hospitalization or disability.
Reporting of nervous system disorders following human papillomavirus vaccination
The nervous system disorders include syncope, dizziness, convulsion, migraine, presyncope, loss of consciousness, seizure, and dyskinesia. We identified 1739 reports of syncope, 2478 reports of dizziness, 763 reports of convulsion, 84 reports of migraine, 101 reports of presyncope, 560 reports of loss of consciousness, 124 reports of seizure, and 164 reports of dyskinesia from 2006 to 2017. The positive signals were obtained for syncope (cumulative ROR-1.96SE: 15.14), dizziness (3.19), convulsion (2.01), migraine (2.38), presyncope (5.40), loss of consciousness (6.79), seizure (2.65), and dyskinesia (2.17) [Table 4].
Table 4.
Human papillomavirus vaccine and reporting of events, cumulative reporting odds ratio value, 2006-2017
| Adverse events | ROR-1.96SE |
|---|---|
| Syncope | 15.149 |
| Dizziness | 3.192 |
| Convulsion | 2.010 |
| Migraine | 2.388 |
| Presyncope | 5.400 |
| Loss of consciousness | 6.791 |
| Seizure | 2.655 |
| Abdominal pain | 2.243 |
| Anemia | 2.191 |
| Alopecia | 6.879 |
| Pallor | 2.043 |
| Spontaneous abortion | 5.509 |
| Amenorrhea | 59.641 |
| Dyskinesia | 2.176 |
| Head injury | 8.238 |
| Anogenital warts | 137.755 |
| Cervical dysplasia | 87.544 |
| Fall | 4.735 |
ROR=Reporting odds ratio
Reporting of gastrointestinal disorders following human papillomavirus vaccination
The gastrointestinal disorder includes abdominal pain. We identified 1136 reports of abdominal pain from 2006 to 2017. The cumulative ROR-1.96 value was 2.24 [Table 4].
Reporting of blood and lymphatic system disorders following human papillomavirus vaccination
Anemia was the only blood and lymphatic system disorder associated with this study. We identified 73 reports of anemia from 2006 to 2017. The cumulative ROR-1.96 was found to be 2.19 [Table 4].
Reporting of skin and subcutaneous tissue disorders following human papillomavirus vaccination
Alopecia was the only condition associated with skin and subcutaneous tissue disorders. We identified 251 reports of alopecia from 2006 to 2017. The cumulative ROR-1.96 value was 6.87 [Table 4].
Reporting of vascular disorders following human papillomavirus vaccination
There was one condition associated with vascular disorders, namely pallor. We identified 197 reports of pallor from 2006 to 2017. The cumulative ROR-1.96 value was 2.04 [Table 4].
Reporting of reproductive system and breast disorders following human papillomavirus vaccination
The reproductive system and breast disorders include amenorrhea and cervical dysplasia. We identified 140 reports of amenorrhea and 110 reports of cervical dysplasia from 2006 to 2017. The cumulative ROR-1.96 value for amenorrhea and cervical dysplasia was found to be 59.64 and 87.54, respectively [Table 4].
Reporting of pregnancy, puerperium, and perinatal conditions following human papillomavirus vaccination
There was one condition associated with pregnancy, puerperium, and perinatal conditions, namely spontaneous abortion. We identified 263 reports of spontaneous abortion from 2006 to 2017. The cumulative ROR-1.96 value was 5.05 [Table 4].
Reporting of injury, poisoning, and procedural complications following human papillomavirus vaccination
The injury, poisoning, and procedural complications include head injury and fall. We identified 144 reports of head injury and 374 reports of fall from 2006 to 2017. The cumulative ROR-1.96 value was 8.23 for head injury and 4.73 for fall [Table 4].
Reporting of neoplasms' benign, malignant, and unspecified conditions following human papillomavirus vaccination
The neoplasms' benign, malignant, and unspecified conditions include anogenital warts. We identified 131 reports of anogenital warts from 2006 to 2017. The cumulative ROR-1.96 value was 137.75 [Table 4].
DISCUSSION
We completed a comprehensive appraisal of AEs reported to VAERS following HPV vaccination from July 2006 to May 2017. As expected, the age groups of 9–26 years accounted for 96.22% of total HPV vaccine reports, the group for which HPV vaccine is routinely recommended. Three hundred and seventy-three reports were received after the administration of the vaccine to people aged between 0 and 8 years, and most of these reports were “incorrect vaccine administered,” “inappropriate age,” “given by mistake,” “accidentally vaccinated,” and “injection by error.” A study conducted by Hibbs et al. showed that human papillomavirus and rotavirus vaccine were most frequently involved in vaccination error reports.[26]
Syncope is the most common AE documented after vaccination which can result in hospitalization for medical evaluation or because of injury.[15] Our study identified positive signal and a total of 1739 reports of syncope following HPV vaccine administration out of which 42 were recognized as serious reports. Furthermore, there were 374 reports for fall and 144 reports for a head injury which might have resulted due to syncope. Vaccine providers must follow the ACIP recommendation to strongly consider observing patients for 15 min after vaccination to prevent syncope-related injuries.[27] However, there are no sufficient data to study how well the vaccine providers followed these guidelines.
There are no adequate and well-controlled studies in pregnant women, and animal reproduction studies are not always predictive of human responses; therefore, HPV vaccines should be used with caution during pregnancy. Studies on animals showed no vaccine-related fetal malformations or other evidence of teratogenesis; however, clinical studies in humans observed few cases of congenital anomaly in pregnancies following HPV vaccine administration.[9,10,11] In this study, data mining analysis identified positive signal for “spontaneous abortion” and hence may require close monitoring when the vaccine is given to a pregnant woman. Further studies are required to assess the safety of HPV vaccine in pregnancy.
Cervical dysplasia is a precancerous condition that has the potential to advance in progressive stages to cervical cancer. However, not all cases progress and some of the mild lesions resolve spontaneously.[28] HPV vaccines are given to prevent cervical dysplasia or cervical intraepithelial neoplasia lesions,[29] but in this study, we identified 110 reports of cervical dysplasia following HPV vaccination. In most of the reports, the Pap test result showed atypical squamous cells of undetermined significance, and patients were at high risk for cervical cancer. Given this potential association, cervical dysplasia should be closely monitored. Furthermore, there were reports of papillomaviral infections, the primary rationale for which this vaccine is indicated. Most of the healthy population after vaccination with HPV got infected with papilloma viral infection, and the source of infection is uncertain. The results for this event could not be computed due to mathematical limitations since there were zero reports of papillomaviral infection due to other vaccines.
Guillain–Barre syndrome (GBS) is a nervous system disorder which is the most frequent cause of acute flaccid paralysis worldwide.[30] Ojha et al. conducted a study to analyze the postmarketing surveillance data following HPV4 vaccination which suggests that GBS is not frequently reported AE.[31] The data mining analysis in our study did not identify any disproportionate reporting for GBS following HPV vaccination which has been consistent with the above study. However, the extent of underreporting to VAERS should be considered.
Signals detected through analysis of VAERS data almost always require confirmation through a controlled study and should be used in conjunction with medical judgment since there are many limitations. The foremost limitation of VAERS is its inability to establish a causal relationship between the AE and the vaccine. The positive signal for any of the AEs does not mean that a causal relationship can be established. It requires extensive studies and pharmacological evidence to prove the same.[32] Once proved, any of the action can be taken depending on the seriousness of the reaction, namely revising label or revising formulary or drug recall. Other drawbacks include underreporting, lack of denominator data which are imperative for calculating report rates, the absence of control group, lack of verification of reported diagnosis, insufficient clinical information recorded, and bias in the reporting.[33,34] Follow-up studies are necessary to confirm suspected vaccine associations identified by VAERS reports. Although VAERS shares immanent limitations of all passive surveillance systems, it has a horizon of scope and can provide important signals that may require further evaluation.
CONCLUSION
Vaccination with HPV has a great potential to decrease the global morbidity and mortality of HPV-associated disorders. Nevertheless, adverse effects following HPV vaccination should be carefully monitored. The present analysis did not identify any new/unexpected safety concern and was consistent with the safety data from prelicensure trials. Health-care providers should be cautious about the plausibility to encounter the AEs associated with HPV vaccine observed in postmarketing surveillance. Further epidemiological studies are required to systematically validate the data provided by VAERS. Our findings may be useful when discussing the risks and benefits of HPV vaccination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Grammatikos AP, Mantadakis E, Falagas ME. Meta-analyses on pediatric infections and vaccines. Infect Dis Clin North Am. 2009;23:431–57. doi: 10.1016/j.idc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad SR. Adverse drug event monitoring at the food and drug administration. J Gen Intern Med. 2003;18:57–60. doi: 10.1046/j.1525-1497.2003.20130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy DL, Goldman SA, Lillie RB. Pharmacoepidemiology. 3rd ed. John Wiley & Sons, Ltd; 2000. Spontaneous reporting in the United States; pp. 149–74. [Google Scholar]
- 4.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: Novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 5.papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Human Papillomavirus (HPV) and Cervical Cancer. World Health Organization. 2016 [Google Scholar]
- 7.Moscicki AB. Impact of HPV infection in adolescent populations. J Adolesc Health. 2005;37:S3–9. doi: 10.1016/j.jadohealth.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Petrosky E, Bocchini JA, Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. GARDASIL® Quadrivalent Package Inserts. Food and Drug Administration. 2006 [Google Scholar]
- 10.Food and Drug Administration. CERVARIX Bivalent Package Inserts. Food and Drug Administration; 2009 [Google Scholar]
- 11.Food and Drug Administration. GARDASIL® 9 – Package Insert. Food and Drug Administration. 2014 [Google Scholar]
- 12.Brotherton JM, Gold MS, Kemp AS, McIntyre PB, Burgess MA, Campbell-Lloyd S, et al. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ. 2008;179:525–33. doi: 10.1503/cmaj.080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang LW, Crawford N, Tang ML, Buttery J, Royle J, Gold M, et al. Hypersensitivity reactions to human papillomavirus vaccine in Australian schoolgirls: Retrospective cohort study. BMJ. 2008;337:a2642. doi: 10.1136/bmj.a2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomljenovic L, Shaw C. Death after quadrivalent human papillomavirus (HPV) vaccination: causal or coincidental. Pharm Regul Aff S. 2012;12:2. [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Syncope after vaccination – United states, January 2005-July 2007. MMWR Morb Mortal Wkly Rep. 2008;57:457–60. [PubMed] [Google Scholar]
- 16.Geier DA, Geier MR. Quadrivalent human papillomavirus vaccine and autoimmune adverse events: A case-control assessment of the vaccine adverse event reporting system (VAERS) database. Immunol Res. 2017;65:46–54. doi: 10.1007/s12026-016-8815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildemann B, Jarius S, Hartmann M, Regula JU, Hametner C. Acute disseminated encephalomyelitis following vaccination against human papillomavirus. Neurology. 2009;72:2132–3. doi: 10.1212/WNL.0b013e3181aa53bb. [DOI] [PubMed] [Google Scholar]
- 18.Debeer P, De Munter P, Bruyninckx F, Devlieger R. Brachial plexus neuritis following HPV vaccination. Vaccine. 2008;26:4417–9. doi: 10.1016/j.vaccine.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 19.Rossi M, Bettini C, Pagano C. Bilateral papilledema following human papillomavirus vaccination. J Med Cases. 2011;2:222–4. [Google Scholar]
- 20.Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papillomavirus vaccination. Pharmacotherapy. 2008;28:1194–7. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 21.Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 22.VAERS [Internet] Vaccine Adverse Event Reporting System (VAERS) [Cited 2017 Oct 08]. Available from: https://vaers.hhs.gov/about.html .
- 23.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS) Vaccine. 2015;33:4398–405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Code of Federal Regulations. 21CFR312.32 Postmarketing Reporting of Adverse Experiences. 2017 [Google Scholar]
- 25.Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS). Data Mining Applications in Engineering and Medicine. InTech. 2012 [Google Scholar]
- 26.Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro TT. Vaccination errors reported to the vaccine adverse event reporting system, (VAERS) United States, 2000-2013. Vaccine. 2015;33:3171–8. doi: 10.1016/j.vaccine.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC, et al. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) MMWR Recomm Rep. 2002;51:1–35. [PubMed] [Google Scholar]
- 28.Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 29.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 30.Yuki N, Hartung HP. Guillain-barré syndrome. N Engl J Med. 2012;366:2294–304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 31.Ojha RP, Jackson BE, Tota JE, Offutt-Powell TN, Singh KP, Bae S, et al. Guillain-barre syndrome following quadrivalent human papillomavirus vaccination among vaccine-eligible individuals in the united states. Hum Vaccin Immunother. 2014;10:232–7. doi: 10.4161/hv.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subeesh V, Singh H, Maheswari E, Beulah E. Novel adverse events of vortioxetine: A disproportionality analysis in USFDA adverse event reporting system database. Asian J Psychiatr. 2017;30:152–6. doi: 10.1016/j.ajp.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, et al. The vaccine adverse event reporting system (VAERS) Vaccine. 1994;12:542–50. doi: 10.1016/0264-410x(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 34.Banks D, Woo EJ, Burwen DR, Perucci P, Braun MM, Ball R, et al. Comparing data mining methods on the VAERS database. Pharmacoepidemiol Drug Saf. 2005;14:601–9. doi: 10.1002/pds.1107. [DOI] [PubMed] [Google Scholar]


