Abstract
PURPOSE:
The aim of this study is to describe the prevalence and clinical profile of pediatric diabetic retinopathy (DR) among Omani diabetic children and adolescents attending a tertiary care hospital.
METHODS:
This retrospective cross-sectional study involved the record review of 216 diabetic children attending the diabetes retina clinic of the National Diabetes and Endocrine Centre in the period between June 2015 and November 2018. The retinal evaluation was conducted using direct ophthalmological examination and fundus photography. DR was graded using the Early Treatment DR Study criteria. The statistical analysis was performed using SPSS, version 20.
RESULTS:
The prevalence of DR among the study sample was 3.7% (95% confidence interval: 1.2–6.2). Mild and moderate nonproliferative diabetic retinopathy was seen in 2.8% (6) and 0.9% (2) of patients, respectively. Diabetic maculopathy was observed in 2 (0.9%) cases. Diabetic ketoacidosis was seen in 33.8% of children at presentation. DR was significantly associated with age (P = 0.01), duration (P < 0.001), Type 1 diabetes mellitus (P = 0.01), dyslipidemia (P = 0.005), microalbuminuria (P = 0.001), glycated hemoglobin (P = 0.003), total cholesterol (P = 0.001), high-density lipoproteins (P = 0.001), low-density lipoproteins (P < 0.001), and albumin/creatinine ratio (P < 0.001).
CONCLUSIONS:
This is the first study describing DR among the pediatric age group in Oman. This study reveals a relatively low prevalence of DR and maculopathy among the pediatric diabetic population. However, novel strategies are to be adopted at primary levels to achieve timely screening of diabetic children to enhance the early detection of DR.
Keywords: Diabetes mellitus, diabetic maculopathy, diabetic retinopathy, Oman, pediatric
Introduction
Diabetic retinopathy (DR) is a microvascular complication of diabetes mellitus (DM) and usually one of the first to manifest. Across the years, the world is witnessing a tremendous rise in the prevalence of Type 1 diabetes mellitus (T1DM) and T2DM among children and adolescents.[1,2] Nearly one-half of all new DM cases among this specific group are of T2DM, which is attributed to the concurrent increase in childhood obesity.[3] Thus, more children and young adults are at risk of developing DR and other vision-threatening complications of DM. Puberty might trigger the expression of DR, along with other microvascular diabetic complications, thereby making the detection of retinopathy important in this age group. Almost all Type 1 diabetic patients and two-thirds of Type 2 will eventually develop DR over a period of time.[4] Among Type 1 DM patients, almost 25% develop DR after 5 years, 60% after 10 years, and 80% after 15 years.[5] Around 18% of patients after 15 years of diabetes diagnosis develop proliferative diabetic retinopathy (PDR) with no difference between Type 1 and Type 2.[5]
The Diabetes Control and Complications Trial along with other epidemiological studies in pediatric diabetic patients have emphasized the importance of glycemic control in delaying or preventing the development of DR. In addition to long-term metabolic control, other important risk factors for developing DR include diabetes duration, hypertension, hyperlipidemia, serum levels of advanced glycation end-products, evidence for early-stage atherosclerosis, increased caliber of retinal blood vessels, and several genetic factors.
Several medical organizations worldwide suggest more or less similar retinopathy screening guidelines, recommending annual screening after 3–5 years of the initial diagnosis of diabetes.[4,6] The International Society for Pediatric and Adolescent Diabetes suggests that annual screening for retinopathy for patients aged 11 years should be conducted after 2 years of diagnosis, and for patients aged 9 years after 5 years.[6,7] For this purpose, digital fundus photography can serve as an important screening tool as it has been described to be superior to clinical ophthalmoscopy in the recent literature.[8] Additional advantages of this screening tool are its availability, reproducibility, safety, and cost-effectiveness.[9]
Considerable variation is shown in the prevalence of pediatric DR in the literature, ranging from 0% to 28%.[10,11,12,13,14] However, Oman is one of the ten countries in the world with the highest prevalence of DM, till date, no local studies available addressing DR prevalence among Omani children.[5] In this regard, this is the first attempt to explore this area locally, highlighting the novelty of this research. Such a study will give an insight on the current burden of the disease which might influence screening guidelines for DR in pediatric patients in Oman.
The present study aimed to assess the prevalence and associated clinical characteristics of DR among an Omani pediatric population who attended the diabetes retina clinic of the National Diabetes and Endocrine Centre (NDEC) during 2015–2018, using both; direct ophthalmological examination and digital photography.
Methods
This is a cross-sectional hospital-based study carried out at NDEC which is a tertiary care center accepting pediatric diabetic patients from all over Oman. The current study involved record review of all Omani diabetic children of both genders aged <18 years, who attended the diabetes retina clinic of the NDEC during the period from June 2015 to November 2018. All data were collected from the NDEC health information system. All referred patients from pediatric and combined adolescent diabetes clinics of NDEC underwent thorough ocular evaluation including the assessment of DR at the diabetes retina clinic of the NDEC by an ophthalmologist.
The vision of each eye was recorded separately using Snellen distant vision chart at 6 m and classified according to the International Council of Ophthalmology visual standards. The anterior segment was evaluated with a biomicroscope, and the intraocular pressure (IOP) was measured by either applanation or indentation tonometer. The retinal examination using + 90 D Volk lens and pan-retinal indirect ophthalmoscope, digital photography, and grading of DR category was carried out by the same ophthalmologist after pupil dilatation. We used three-dimensional Optical Coherence Tomography (OCT)-2000 and Type IA TRC-50DX retinal camera to take two digital images per eye (macular view and disc view) along with measurement of average macular thickness and volume. The Early Treatment Diabetic Retinopathy Study grading system was used to define and classify DR.[15] The presence or absence of diabetic maculopathy was detected by the use of OCT; however, no further grading of maculopathy was done for such patients in the current study. Vision-threatening diabetic retinopathy (VTDR) was defined as the presence of severe nonproliferative diabetic retinopathy (NPDR), PDR, or diabetic macular edema (DME). The DR status was labeled according to the worse eye.
Other details including age, gender, weight, height, body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP), duration of diabetes, type of diabetes, associated systemic comorbidities (hypertension and dyslipidemia), microvascular complications, including peripheral neuropathy and nephropathy, presence of diabetic ketoacidosis (DKA) at the time of diagnosis, fasting blood sugar (FBS) and random blood sugar (RBS), lipid profile, including total cholesterol levels, high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), glycated hemoglobin (HbA1c), and albumin/creatinine ratio (ACR) were considered in the study.
The data were analyzed using IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY: IBM Corp). Descriptive measures were expressed as percentages and/or means with standard deviations. Associations were tested using univariate tests including Chi-square and independent t-test according to the nature of variables.
This study was approved by the Research Ethics Committee of the Center of Studies and Research of the Ministry of Health, Oman.
Results
The study sample included 216 Omani pediatric patients with 54.2% of girls. Age ranged from 3 to 17 years with a mean of 12.8 ± 2.8 years. Majority of the patients were Type 1 diabetics, accounting for 94.9%. The duration of diabetes ranged from 1 to 17 years with a mean duration of 4.5 ± 3.3 years. The mean BMI of the study sample was 22.7 ± 5.6 kg/m2, with ranging from 14 to 40.6 kg/m2. Detailed characteristics are shown in Table 1.
Table 1.
Characteristics of the study sample
| Characteristics (n) | Percentage or mean±SD |
|---|---|
| Gender | |
| Male (99) | 45.8 |
| Female (117) | 54.2 |
| Age (216) | 12.8±2.8 |
| Duration (216) | 4.5±3.3 |
| Weight (kg) (215) | 57.5±25.7 |
| Height (cm) (216) | 150.9±24.6 |
| BMI (kg/m2) (178) | 22.7±5.6 |
| DM | |
| Type 1 (194) | 94.9 |
| Type 2 (17) | 7.9 |
| Secondary DM (2) | 0.9 |
| MODY (2) | 0.9 |
| Worfram’s syndrome (1) | 0.5 |
| Comorbidities | |
| No comorbidities (205) | 94.9 |
| DLP (7) | 3.2 |
| HTN (2) | 0.9 |
| Multiple (2) | 0.9 |
| Complications | |
| No complications (200) | 92.6 |
| Microvascular (other than retinopathy) (16) | 7.4 |
| DKA at presentation | |
| No (143) | 66.2 |
| Yes (73) | 33.8 |
| HbA1c (214) | 10.0±2.3 |
| RBS mmol/l (26) | 13.5±6.7 |
| ACR mg/mmol (192) | 8.1±47.5 |
| Lipid profile (153) | |
| Total cholesterol | 4.7±1.0 |
| HDL mmol/l | 1.4±0.3 |
| LDL mmol/l | 2.9±0.9 |
| TG mmol/l | 1.4±0.8 |
| First DR screening | |
| Yes (113) | 52.3 |
| No (103) | 47.7 |
| BCVA worse eye | |
| Normal (193) | 90.2 |
| Abnormal (21) | 9.8 |
| IOP (mmHg) | |
| Normal (59) | 85.5 |
| High IOP (10) | 14.5 |
| OCT RE (28) | |
| Average thickness (μm) | 265.7±11.6 |
| Average volume (mm3) | 7.5±0.3 |
| Retinal treatment | |
| No treatment (216) | 100 |
SD: Standard deviations, BMI: Body mass index, DM: Diabetes mellitus, MODY: Maturity-onset diabetes of the young, DLP: Dyslipidemia, HTN: Hypertension, DKA: Diabetic ketoacidosis, HbA1c: Glycated hemoglobin, RBS: Random blood sugar, ACR: Albumin/creatinine ratio, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, TG: Triglycerides, DR: Diabetic retinopathy, BCVA: Best-corrected visual acuity, IOP: Intraocular pressure, OCT: Optical coherence tomography
Dyslipidemia was present in 3.2% of cases. Microvascular complications other than retinopathy were seen in 7.4% of cases. DKA was seen in 33.8% of children at presentation. The mean SBP and DBP were 117.6 ± 12.4 and 73.8 ± 9.2 mm of Hg, respectively. The mean total cholesterol level was 4.7 ± 1.0 mmol/l, and mean LDL was 2.9 ± 0.9 mmol/l in the studied sample. The HbA1c ranged between 5.1% and 19% with mean HbA1c 10.0 ± 2.3, whereas mean FBS was 9.9 ± 4.4 mmol/l.
The prevalence of DR among the study sample was 3.7% (95% confidence interval: 1.2–6.2) [Figure 1]. Out of the total eight cases of DR, mild and moderate NPDR was seen in 2.8% and 0.9% of patients, respectively [Figures 1 and 2]. Diabetic maculopathy (in addition to the retinopathy changes) was observed in two patients only accounting for 0.9% of the sample [Figure 3]. Among the study sample, 52.3% of patients were screened for the first time for the presence of DR at our center.
Figure 1.
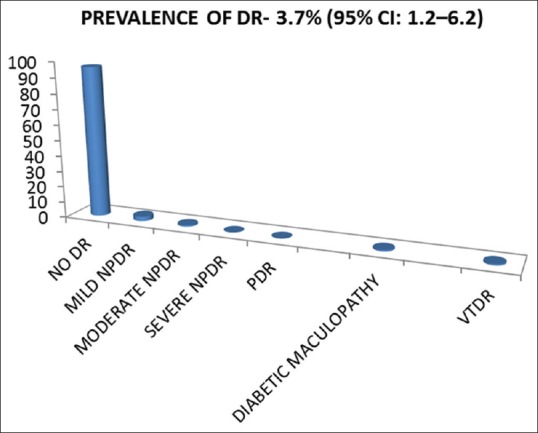
The prevalence of diabetic retinopathy, diabetic maculopathy, and vision-threatening diabetic retinopathy
Figure 2.
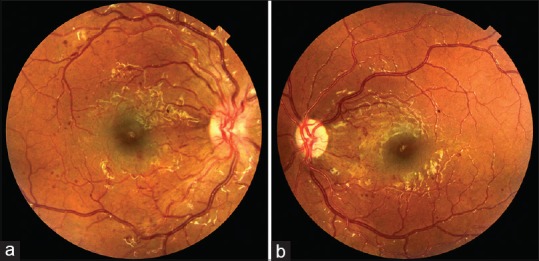
Color fundus photograph of (a) right eye showing nonproliferative diabetic retinopathy (b) left eye showing nonproliferative diabetic retinopathy
Figure 3.
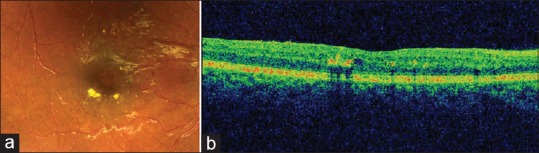
(a) Color fundus photograph showing small exudates and microaneurysms and few small haemorrhages (b) corresponding optical coherence tomography shows retinal thickening and exudates
A vision of 6/7.5 or better in the eye with worse vision was recorded in 90.2% of patients, whereas 9.8% of patients had visual acuity <6/7.5 among whom only one (0.05%) had severe visual impairment related to amblyopia. High IOP was recorded in 14.5% of patients. None of the patients in this study required any ocular treatment for DR. OCT was conducted in 28 patients with the right eye taken as standard and showed an average macular thickness and average macular volume of 265.7 ± 11.6 μm and 7.5 ± 0.3 mm3, respectively.
As illustrated in Table 2, girls showed a slightly higher rate (4.3%) of DR than boys (3%), but the difference was not statistically significant (P = 0.73). The prevalence of DR among Type 1 diabetic children was 3.6% and was found to be significantly higher when compared to children with Type 2 diabetes and other forms of diabetes (P = 0.01). One of the two patients with secondary diabetes also had DR. Longer duration of diabetes was associated with significantly higher prevalence of DR compared to shorter duration (P < 0.001). The mean age among DR group was significantly higher compared to the DR-free group (P = 0.01). It was also observed that the occurrence of DR was higher among patients who were screened before elsewhere compared to those patients, in whom DR screening was conducted for the first time at the center (P = 0.03). The prevalence of DR was generally higher in patients with dyslipidemia (P = 0.005). In addition, the presence of microvascular complications was associated with a higher occurrence of DR (P = 0.001).
Table 2.
Associations between clinical and laboratory characteristics and diabetic retinopathy
| Diabetic retinopathy, n (% or mean with SD) | P | ||
|---|---|---|---|
| Absent | Any DR | ||
| Gender | |||
| Male | 96 (97) | 3 (3) | 0.73 |
| Female | 112 (95.7) | 5 (4.3) | |
| Age | 208 (12.7±2.7) | 8 (15.1±1.8) | 0.01 |
| Type of DM | |||
| Type 1 | 187 (96.4) | 7 (3.6) | 0.01 |
| Type 2 | 17 (100) | 0 (0) | |
| Secondary DM | 1 (50) | 1 (50) | |
| MODY (2) | 2 (100) | 0 (0) | |
| Worfram’s syndrome (1) | 1 (100) | 0 (0) | |
| First DR screening | |||
| Yes | 112 (99) | 1 (0.9) | 0.03 |
| No | 96 (93.2) | 7 (6.8) | |
| Duration of DM | 208 (4.3±3.0) | 8 (8.9±5.4) | <0.001 |
| Comorbidities | |||
| No comorbidities | 199 (97.1) | 6 (2.9) | 0.005 |
| Dyslipidemia | 5 (71.4) | 2 (28.6) | |
| HTN | 2 (100) | 0 (0) | |
| Multiple | 2 (100) | 0 (0) | |
| Complications | |||
| No DM complications | 195 (97.5) | 5 (2.5) | 0.001 |
| Micro vascular complications | 13 (81.2) | 3 (18.8) | |
| DKA at presentation | |||
| No | 138 (96.5) | 5 (3.5) | 0.82 |
| Yes | 70 (95.9) | 3 (4.1) | |
| BMI (kg/m2) | 170 (22.8±5.7) | 8 (20.6±3.3) | 0.27 |
| BCVA worse eye | |||
| Normal | 186 (96.4) | 7 (3.6) | 0.80 |
| Abnormal | 20 (95.2) | 1 (4.8) | |
| OCT RE | |||
| Average thickness (μm) | 23 (265.3±12.0) | 5 (267.5±10.8) | 0.71 |
| Average volume (mm3) | 23 (7.5±0.3) | 5 (7.6±0.3) | 0.77 |
| HbA1c | 206 (10.0±2.3) | 8 (12.4±2.1) | 0.003 |
| ACR (mg/mmol) | 184 (5.2±28.2) | 8 (74.9±188.0) | <0.001 |
| Lipid profile | |||
| Total cholesterol | 146 (4.6±1.0) | 7 (6.0±1.4) | 0.001 |
| HDL (mmol/l) | 146 (1.4±0.3) | 7 (1.8±0.3) | 0.001 |
| LDL (mmol/l) | 146 (2.8±0.8) | 7 (4.2±1.3) | <0.001 |
| TG (mmol/l) | 146 (1.1±0.8) | 7 (1.1±0.5) | 0.89 |
DR: Diabetic retinopathy, DM: Diabetes mellitus, MODY: Maturity-onset diabetes of the young, HTN: Hypertension, DKA: Diabetic ketoacidosis, BMI: Body mass index, BCVA: Best-corrected visual acuity, OCT: Optical coherence tomography, HbA1c: Glycated hemoglobin, ACR: Albumin/ creatinine ratio, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, TG: Triglycerides, RE: Right eye
There was a significant difference in the mean HbA1c between the DR-free and DR-groups (P = 0.003). In general, the difference in the mean total cholesterol (P = 0.001), HDL (P = 0.001), and LDL (P < 0.001) levels between DR-free and DR group were statistically significant. The association between ACR and DR was also found to be statistically significant (P < 0.001).
On the other hand, there was no significant association of DR with gender, obesity level, DKA, SBP, DBP, FBS, RBS, TGs, best-corrected visual acuity, IOP, and OCT parameters.
Discussion
Detecting DR early in its course is important for timely multidisciplinary interventions to reverse or delay long-term poor visual outcomes. This is the first study in Oman to explore the burden of pediatric DR among young diabetic population.
The prevalence of pediatric DR in the current study is lower than that reported in other countries including Ethiopia (4.7%), Tanzania (22.2%), Australia (24%), and France (4.5%).[12,13,14,16] However, it is higher compared to a study from Iceland (0%).[10] This disparity in the reported prevalence may be genuine to some extent due to the inherent genetic predisposition for the expression of DR in different populations. However, it can also be attributed to the quality of DM care and health infrastructure in different countries as well as study characteristics, methodology, and sample size used. In this regard, it was noted that the present study used a larger sample size than that of Tanzania (99) and Ethiopia (86); however, it was smaller than that used in the France study (504). The children in this study were comparable to those in Tanzania in terms of disease duration and mean age; however, the reason for the much low prevalence of DR in this study compared to the former can be explained by the unfavorable social and economic environment faced by diabetic children in Tanzania. However, other studies showed a higher mean age and marginally longer duration of DM. Although the patients depicted a high mean-HbA1c in this study compared to the aforementioned literature except for the French study, the low prevalence can be explained by the accessibility of modern diabetic care to the general population in Oman. The use of 2-fields fundus photography instead of 7-fields fundus photography in this study might be one of the reasons of the low prevalence of DR compared to the Australian study, considering comparable health infrastructure in these countries. However, the 2-fields color fundus photography with the sensitivity and specificity of 96% and 89%, respectively, is one of the most common modes of detecting DR used in many studies, favoring patient compliance with screening.[17] At this point, it is also noteworthy that a low DR prevalence is usually observed in community-based population studies as noted in France, but in contrast to that, this study revealed a low prevalence, despite being a hospital-based study, which can again be attributed to optimum sample size and good standards of DM care. However, there are exceptions to that as shown in the Australian study, which depicted a high prevalence in community-based study.
Proliferative DR was not reported in any of our patients, which is similar to the findings in other studies.[16,18] None of the children with DR in the current study received any kind of treatment for DR, such as focal or pan-retinal laser photocoagulation or intravitreal injection of anti-vascular endothelial growth factor agents. This observation supports those mentioned in the literature indicating that DR requiring laser treatment rarely occurs in the pediatric age group.[7,12,19]
The present study reveals a low prevalence of diabetic maculopathy and VTDR. There are very limited literature available depicting diabetic maculopathy in children. A study from Ethiopia observed a high prevalence of maculopathy (2.3%) in children which can be attributed to smaller sample size, higher mean age, and longer duration of disease in that study.[16] Another study from Brazil also depicts a very high prevalence of 9.4% in Type 1 DM patients; however, their study group was less comparable to the present study group considering sample size, mean age, and the duration of DM.[20] This variation can also be due to differences in DM care standards and methodologies applied in former studies. In addition, the differences in definition of diabetic maculopathy can be another explanation for this variation as there is difference in clinical definition of clinically significant macular edema and OCT-detected DME. It is also noteworthy that the diagnosis of DME itself is challenging as there is no consensus on OCT-based severity classification for DME.
Poor glycemic control observed in the majority of our study participants is similar to those reported in other African countries.[13,16] Higher HbA1c levels among patients with DR than among those without DR is consistent with previous reports.[7,13,16,19,21] The current study supports the well-known fact that poor glycemic control indicated by higher HbA1c levels is associated with increased occurrence of DR.[7,13,19,21]
In this study, Type 1 DM children were found to have significantly higher risk of developing DR than those of Type 2 DM. This is consistent with many similar studies conducted worldwide.[19,21] For example, Lee et al. observed the risk of DR among Type 1 DM patients to be three times the risk among Type 2 DM patients in a recent meta-analysis.[21] Similar findings were also reported by Wang et al. in another study from US.[19]
This study supports the association of age, duration of diabetes, comorbidities including dyslipidemia and hypertension, and microalbuminuria with the development of DR among children, as observed in previous studies.[7,12,19,21,22] In addition, laboratory factors including ACR, lipid profile (total cholesterol levels, HDL, LDL) were also found to be hazardous for the development of pediatric DR.[7,22]
The present study does not reveal any statistically significant gender inclination in young diabetics, though it showed insignificant higher rate of DR among girls which is in agreement with previous studies.[22]
The current study indicated that less than half of the studied population had previous retinopathy screening at primary referral centers. About one-fourth and one-fifth of the children with the duration of more than 5 and 10 years, respectively, were screened for the first time at our institute. Keeping in view, the good quality of DM care at primary and secondary levels in Oman, this emphasizes the need to explore the reasons behind the poor adherence to the guidelines related to timely screening.
Although the prevalence of DKA reported in this survey was high compared to international literature (25%–30%), it is still much less in comparison to the prevalence found in Congo Brazzaville and Tanzania.[13,23,24] This disparity can be attributed to the variation in health-care system and frequency of diabetes. However, another local study reported more or less similar prevalence of DKA (31%) among Omani children.[25]
The use of ophthalmologic imaging along with clinical ophthalmoscopy has made this work more authentic in validating our results. In addition, the entire study participants were assessed at a single center with standard uniform methods throughout the study period with the same retina specialist involved in the entire process of the evaluation of patients beginning from clinical examination, digital photography with grading, and documentation of DR category. Optimal sample size has reinforced present work in terms of validity. On the other hand, this study was conducted in a tertiary national referral center, and hence may not reflect the primary care setting. However, our results are still valid for tertiary care settings. In addition, since most Type 1 diabetic patients are referred to this center and they come from different regions across the country, the results can be extrapolated to pediatric diabetics of Oman to some extent.
Conclusions
This is the first study addressing DR among the pediatric age group in Oman. The present study reveals a relatively low prevalence of DR among the pediatric diabetic population. This study also emphasizes the importance of age, duration of diabetes, and HbA1c as risk factors among Omani children. Strict glycemic control remains the key to decrease the development and progression of DR in young diabetics. In consideration of the rising burden of both T1DM and T2DM among children, cost-effective and timely screening of these children is the demand of the present time without causing any undue onus on the patients and health-care system. Novel strategies are to be developed which might use a combination of fundus photography and telemedicine at primary health-care setting to enhance the early detection of DR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Green A, Patterson CC Eurodiab Tiger Study Group Europe and Diabetes. Trends in the incidence of childhood-onset diabetes in Europe 1989-1998. Diabetologia. 2001;44(Suppl 3):B3–8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 2.Day C. The rising tide of type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1:37–43. [Google Scholar]
- 3.D’Adamo E, Caprio S. Type 2 diabetes in youth: Epidemiology and pathophysiology. Diabetes Care. 2011;34(Suppl 2):S161–5. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, et al. American diabetes association. Diabetic retinopathy. Diabetes Care. 2002;25(Suppl 1):S90–3. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Ophthalmology – Diabetic Retinopathy – Middle East. 2016. Oct, [Last accessed on 2019 Aug 05]. Available from: https://www.aao.org/topic-detail/diabetic-retinopathy-middle-east .
- 6.Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence. 2011. [Last accessed on 2015 Apr 09]. Available from: https://www.idf.org/sites/default/files/Diabetes-in-Childhood-and-AdolescenceGuidelines.pdf .
- 7.Sultan MB, Starita C, Huang K. Epidemiology, risk factors and management of paediatric diabetic retinopathy. Br J Ophthalmol. 2012;96:312–7. doi: 10.1136/bjophthalmol-2011-300169. [DOI] [PubMed] [Google Scholar]
- 8.Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: A comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol. 2002;134:204–13. doi: 10.1016/s0002-9394(02)01522-2. [DOI] [PubMed] [Google Scholar]
- 9.Pajunp€a€a H. The costs of photographic screening for diabetic retinopathy and the quality of life and mortality of visually disabled patients. Acta Univ Ouluensis Med. 1999;522:355–3221. [Google Scholar]
- 10.Kristinsson JK, Gudmundsson JR, Stefánsson E, Jónasson F, Gíslason I, Thórsson AV. Screening for diabetic retinopathy. Initiation and frequency. Acta Ophthalmol Scand. 1995;73:525–8. doi: 10.1111/j.1600-0420.1995.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 11.Gallego PH, Wiltshire E, Donaghue KC. Identifying children at particular risk of long-term diabetes complications. Pediatr Diabetes. 2007;8(Suppl 6):40–8. doi: 10.1111/j.1399-5448.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 12.Massin P, Erginay A, Mercat-Caudal I, Vol S, Robert N, Reach G, et al. Prevalence of diabetic retinopathy in children and adolescents with type-1 diabetes attending summer camps in france. Diabetes Metab. 2007;33:284–9. doi: 10.1016/j.diabet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Majaliwa ES, Munubhi E, Ramaiya K, Mpembeni R, Sanyiwa A, Mohn A, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National hospital in Dar ES Salaam, Tanzania. Diabetes Care. 2007;30:2187–92. doi: 10.2337/dc07-0594. [DOI] [PubMed] [Google Scholar]
- 14.Donaghue KC, Craig ME, Chan AK, Fairchild JM, Cusumano JM, Verge CF, et al. Prevalence of diabetes complications 6 years after diagnosis in an incident cohort of childhood diabetes. Diabet Med. 2005;22:711–8. doi: 10.1111/j.1464-5491.2005.01527.x. [DOI] [PubMed] [Google Scholar]
- 15.Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 16.Shibeshi MS, Fantahun B, Kebede T, Tilahun B. Pediatric diabetic retinopathy: Experience of a tertiary hospital in Ethiopia. BMC Res Notes. 2016;9:116. doi: 10.1186/s13104-016-1941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson JA, Strachan FM, Hipwell JH, Goatman KA, McHardy KC, Forrester JV, et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med. 2003;20:528–34. doi: 10.1046/j.1464-5491.2003.00969.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 19.Wang SY, Andrews CA, Herman WH, Gardner TW, Stein JD. Incidence and risk factors for developing diabetic retinopathy among youths with type 1 or type 2 diabetes throughout the United States. Ophthalmology. 2017;124:424–30. doi: 10.1016/j.ophtha.2016.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteves JF, Kramer CK, Azevedo MJ, Stolz AP, Roggia MF, Larangeira A, et al. Prevalence of diabetic retinopathy in patients with type 1 diabetes mellitus. Rev Assoc Med Bras (1992) 2009;55:268–73. doi: 10.1590/s0104-42302009000300017. [DOI] [PubMed] [Google Scholar]
- 21.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hautala N, Hannula V, Palosaari T, Ebeling T, Falck A. Prevalence of diabetic retinopathy in young adults with type 1 diabetes since childhood: The Oulu cohort study of diabetic retinopathy. Acta Ophthalmol. 2014;92:749–52. doi: 10.1111/aos.12426. [DOI] [PubMed] [Google Scholar]
- 23.Soltész G. IDDM in Hungarian children: Population-based clinical characteristic and their possible implication for diabetic health care. Hungarian Childhood Diabetes Epidemiology Study Group. Padiatr Padol. 1992;27:63–6. [PubMed] [Google Scholar]
- 24.Yudkin JS. Insulin for the world's poorest countries. Lancet. 2000;355:919–21. doi: 10.1016/S0140-6736(99)09225-9. [DOI] [PubMed] [Google Scholar]
- 25.Al-Yaarubi S, Ullah I, Sharef SW, Al Shidhani A, Al Hanai S, Al Kalbani R, et al. Demographic and clinical characteristics of type 1 diabetes mellitus in omani children -single center experience. Oman Med J. 2014;29:119–22. doi: 10.5001/omj.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


