Abstract
BACKGROUND:
Postpartum period and recurrent abortion are stressful conditions that affect women's mental health. Stress and depression lead to the release of stress biomarkers that may be dangerous for the mother and fetus. The aim of this study was to determine stress in the after recurrent pregnancy loss (RPL) and normal vaginal delivery (NVD) in the north of Iran.
MATERIALS AND METHODS:
This case–control study was done on forty women with NVD and forty women with RPL. Stress was measured through measuring serum cortisol, Perceived Stress Scale-14 (PSS-14), and the revised version of the Symptom Checklist-90 (SCL-90-R). Data were analyzed using the Statistical Package for the Social Sciences (SPSS) 22.0 software. Chi-square test, independent-samples t-test, Mann–Whitney U-test, and Pearson correlation were used to analyze the data.
RESULTS:
Findings showed that nonpregnant healthy women had significantly higher cortisol level than RPL women (mean ± standard deviation [SD]: 155.80 ± 84.97 ng/ml and 126.02 ± 50.44 ng/ml, P < 0.011), respectively. Furthermore, they had higher PSS-14 and SCL-90 scores than PRL women (mean ± SD: 25.87 ± 7.48 and 25.5 ± 9.19, P = 0.745, and mean ± SD: 1.27±0.63 and 1.20 ± 0.53, P = 0.624), respectively.
CONCLUSIONS:
High levels of cortisol reflect the acute stress caused by the care of the baby in women. Therefore, social support for the pregnant woman by the health-care team is an essential factor for reducing postpartum depression.
Keywords: Cortisol, postpartum, Perceived Stress Scale-14, recurrent pregnancy loss, Symptom Checklist-90-R, stress
Introduction
Infertility and pregnancy loss are two main problems for women in the reproductive age.[1,2] RPL has increased to 58% over the past decade (from 53 to 74/100,000 fertile women).[3] Recurrent abortion refers to the loss of two or more pregnancies before 20 weeks of gestation.[4] The etiology of RPL is often unknown. Some logical etiologic reasons include immunologic disorders, antiphospholipid antibody syndrome, chromosomal disorders, thrombophilia, infections, endocrine abnormalities, and environmental factors.[5]
The loss of a desirable pregnancy is a considerable negative life occurrence, and this usual problem may cause notable physical and psychological distress.[6] Pregnancy loss is related to anxiety and distress, especially in women who experience RPL.[7] Anxiety symptoms start immediately after abortion and continue until nearly 4–6 months later.[8] In addition, while waiting for the next pregnancy, there is usually a high level of uncertainty and anxiety, which reduces the person's ability to tackle problems.[9] Pregnancy loss and infertility are considered as numerous difficulties which it is necessary for patients to receive the best services in diagnosis, treatment, and psychological support.[10,11] On the other hand, the postpartum period is a serious point for women.[12] The stressful life causes pathological stimulus and psychological stress resulting in continuous activity of the sympathetic nervous system and hypothalamic–pituitary–adrenal (HPA) axis.[13] Stress may cause the release stress-related biomarkers and therewith affect confirmation of pregnancy.[5] Pregnancy loss may cause women to be concerned about the success of the next pregnancy.[10] Women, who anything social supports, have experienced recurrent abortion are at a higher risk of exhibiting psychological morbidity or symptoms after a pregnancy loss or infertility.[14]
Cortisol is generally applied as a stress marker since its production from the adrenal cortex tends to increment looking for psychologic, immunologic, and energetic challenges.[15] Whereas some past researches reported stress to be related to RPL,[16] other studies did not.[17] The present study was done to compare the stress in women with RPL and women with NVD in the north of Iran.
Materials and Methods
Subjects
This case–control study was conducted from May 2015 to August 2016 in Babol, Iran. In this study, women with NVD and without any history of abortion, who were referred to primary health-care centers, were selected as a case group. They were forty healthy, nonpregnant women with at least one living child that did not have a history of infertility, previous abortion, preterm deliveries, or stillbirths. The control group was considered as a group that was probably high in stress. We wanted to know that the amount of stress after delivery is higher or after abortion. Thus, the control group included forty women with RPL that were referred to the Research Center for Infertility of Babol University of Medical Sciences. Recurrent loss was defined as having had two or more consecutive abortions in the first trimester of pregnancy. The inclusion criteria for patients with recurrent abortion included having experienced at least two consecutive idiopathic abortions of a desired pregnancy. The exclusion criteria for patients with recurrent abortion included history of polycystic ovary syndrome; abnormal gynecological status; anatomy and karyotype; abnormal levels of the antiphospholipid antibody, antinuclear antibody, anticardiolipin antibody, antithrombin III, lupus anticoagulant, homocysteine, protein S, protein C, factor V Leiden, antithyroid peroxidase (anti-TPO), thyroid hormones, and prolactin; and abnormal spermogram and karyotype of the sexual partner. The case and control groups were evaluated from 3 months to 1 year after childbirth or abortion, respectively.
Data collection
Five milliliters of peripheral blood samples from all women from a large antecubital vein was taken into ethylenediaminetetraacetic acid (EDTA)-coated tubes during the follicular phases of the menstrual cycle at 8 AM. Serum samples were separated from EDTA by centrifugation. After centrifugation, the serum was collected and placed in cryotubes and stored at −80°C until use. The level of cortisol in blood serum samples was ascertained by a competitive enzyme immunoassay (IBL, Hamburg, Germany) according to the manufacturer's instructions in the Cellular and Molecular Research Center, Babol University of Medical Sciences, Iran. Each participant completed the Cohen's global measure of perceived stress questionnaire. PSS-14 contains 14 items to evaluate the public perceived stress in the last month. This scale evaluates risk factors for behavioral disorders and presents stressful relationships. Scoring system is on the Likert scale: never = 0, almost never = 1, only occasionally = 2, often = 3, and almost = 4. Questions 5–10 and 13 are inversely scored (from never = 4 to almost = 0). The lowest point is 0 and the highest is 56, which shows high perceived stress.[18]
The range of PSS scores was divided into classified quartiles. The upper two and lower two quartiles were combined (28 being the usable cutoff value for the upper bound) and were marked as stressed and not stressed, respectively. This cutoff value was selected on a study from Pakistan.[19]
The SCL-90-R evaluates the following nine symptoms: somatization, sensitivity, obsessive–compulsive disorder, aggression, phobic anxiety, paranoid, depression, anxiety, and psychotic tendency. This checklist comprises ninety questions with five response options (0 = not at all, 1 = a little bit, 2 = moderately, 3 = quite a bit, and 4 = extremely), and it is widely used in the field of psychiatry. The total score is evaluated as the Global Severity Index (GSI). A mean above 1 in GSI is abnormal and score below 1 is normal.[20]
Ethical considerations
The Ethics Committee of Babol University of Medical Sciences approved the study (ID: MUBABOL.REC.2015.42). The participants signed a written informed consent form prior to the participation in the study, in keeping with the recommendations of the Declaration of Helsinki.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) 22.0 software. The different sociodemographic characteristics, PSS-14, SCL-90-R, and cortisol levels between the case and control groups were determined using Chi-square test, independent samples t-test, and Mann–Whitney U-test, respectively. Pearson correlation was used to identify the significant relationship of PSS-14, SCL-90-R, and cortisol with demographic and reproductive variables. P < 0.05 was considered statistically significant.
Results
The sociodemographic characteristics of the case and control groups are presented in Table 1. The comparison of the two groups for serum cortisol levels showed that women with NVD had significantly higher cortisol concentrations than RPL women (mean ± standard deviation [SD]: 155.80 ± 84.97 ng/ml and 126.02 ± 50.44 ng/ml, P < 0.011, Mann–Whitney test), respectively, as shown in Figure 1. Furthermore, the comparison of the two groups for scores of the PSS-14 and SCL-90-R scales showed that women with NVD had higher score than RPL women (mean ± SD: 25.87 ± 7.48 and 25.5 ± 9.19, P = 0.745, and mean ± SD: 1.27±0.63 and 1.20 ± 0.53, P = 0.624), respectively [Table 2]. Pearson correlation showed no significant correlation of the cortisol levels, SCL-90-R and PSS-14 with age, BMI, occupation, satisfaction with income as well as PSS-14 and SCL-90-R with cortisol levels in the two groups, whereas PSS-14 and SCL-90-R were statistically significant in the two groups (P < 0.0001).
Table 1.
Sociodemographic characteristics in normal vaginal delivery and recurrent pregnancy loss groups
| Characteristics | NVD* (n=40), n (%) | RPL** (n=40), n (%) | P† |
|---|---|---|---|
| Age (year), mean±SD*** | 27.32±4.85 | 29.07±5.13 | 0.121 |
| BMI**** (kg/m2) | 26.79±5.40 | 26.06±3.49 | 0.479 |
| Gravidity (mean±SD) | 1.45±0.50 | 2.80±1.06 | 0.0001 |
| Occupation | |||
| Employed | 5 (12.5) | 4 (10) | 0.723 |
| Homemaker | 35 (87.5) | 36 (90) | |
| Husband occupation | |||
| Employee | 12 (30) | 5 (12.5) | 0.0001 |
| Worker | 21 (52.5) | 6 (15) | |
| Self-employed | 7 (17.5) | 29 (72.5) | |
| Satisfaction with income | |||
| High | 16 (40) | 9 (22.5) | 0.064 |
| Middle | 22 (55) | 31 (77.5) | |
| Low | 2 (5) | 0 |
†The data were assessed using Chi.square and t.tests. *NVD=Normal vaginal delivery, **RPL=Recurrent pregnancy loss, ***SD=Standard deviation, ****BMI=Body mass index
Figure 1.
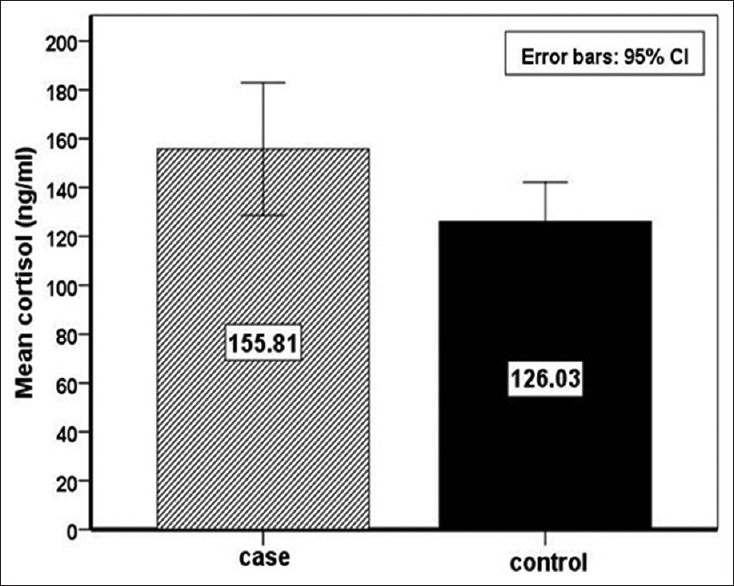
Serum cortisol levels in case and control groups
Table 2.
Comparison of psychological stress in normal vaginal delivery and recurrent pregnancy loss groups
| Psychological scores† | NVD* (n=40), n (%) | RPL** (n=40), n (%) | P† |
|---|---|---|---|
| PSS-14*** | |||
| <28, n (%) | 11 (27.5) | 17 (42.5) | 0.160 |
| >28, n (%) | 29 (72.5) | 23 (57.5) | |
| Total, mean±SD | 25.87±7.48 | 25.35±6.88 | 0.745 |
| SCL-90**** | |||
| <1, n (%) | 14 (35) | 15 (37.5) | 0.816 |
| >1, n (%) | 26 (65) | 25 (62.5) | |
| Total, mean±SD | 1.27±0.63 | 1.20±0.53 | 0.624 |
†The data were assessed using Chi.square and t. test. *NVD =Normal vaginal delivery, **RPL =Recurrent pregnancy loss, *** PSS.14=Perceived Stress Scale.14, ****SCL.90=Symptom Checklist.90, SD=Standard deviation
Discussion
RPL is one of the common complications in women of childbearing age that the psychological and immune factors are a wide ratio of many causes.[6,21] In our study, the results showed that the stress level in the women with NVD was higher compared with unexplained RPL. The most studies in this area emphasize the stress after abortion or after childbirth alone or in the women with abortion and pregnant women, and we did not find studies which have been made in the 1st year after abortion or childbirth.
In one study, the PSS score in spontaneous abortion was higher than pregnant controls.[17] The results of one study indicated that patients with RPL had significantly higher scores on the PSS than fertile controls. In this study, stress was introduced as a risk factor of RPL.[5] Furthermore, the results of another study showed that patients with RPL had more psychological distress on the SCL-90 scale.[20] We should note that most studies in this area emphasize the stress after abortion or after childbirth alone or in the women with abortion and pregnant women, and we did not find studies which have been made in the 1st year after abortion or childbirth.
In our study, the results showed that the maternal cortisol serum level in the women with NVD was higher compared with unexplained RPL. Liu et al. reported a high cortisol level in mothers from 9 to 12 months after delivery due to stress.[22] de Rezende et al. stated that the cortisol level in the depressed postpartum women was significantly higher than nondepressed postpartum women and nonpostpartum healthy women in the 6-month postpartum.[23] In contrast to our study, the results of a study showed that cortisol levels decreased from the third trimester to 3-month postpartum.[24] Moreover, in another study, urine and blood cortisol levels were not associated with postpartum depression.[25] In another study, spontaneous abortion correlated to increased maternal cortisol.[26]
In most of the studies we have reviewed, stress and cortisol levels were high in women with recurrent abortion, but there was no comparison with normal fertile women in the 1st year after delivery. It should be noted that higher stress and cortisol in normal fertile women than women with recurrent abortion in our study can be on the maternal acute stress due to baby care, breastfeeding, and home affairs. The results of our study showed that there was no significant correlation between cortisol levels and PSS-14 and SCL-90 with demographic and reproductive characteristics, whereas in one study, an increase in cortisol level was associated with an increase in abortion and delayed fetal growth.[27] In another study, cortisol level was higher in the mothers with secondary schooling than less schooling.[22] In our study, cortisol levels showed no significant correlation with PSS-14 and SCL-90 scores in the two groups. In one study, researchers found no relationship between stress scales or cortisol level and the risk for recurrent abortion.[16] The results of one study showed that the association between maternal self-reported anxiety by SCL-90 scale and salivary cortisol levels during pregnancy is very weak. Furthermore, mothers with higher anxiety showed lower cortisol.[28] Another study reported a lower morning cortisol level in pregnant women with higher anxiety.[29]
On the other hand, in one study, perceived stress during pregnancy was correlated with cortisol supply, especially in the mothers who had high levels of childhood difficulty.[30] Furthermore, in the study of Li et al., there was a low relationship between PSS-14 and cortisol level, and they suggest that stress is a risk factor of PRL.[5] It should be noted that social support for pregnant woman by the health-care team is an essential factor cause reducing the postpartum depression.[10] One of the strengths of our study was the concurrent investigation of cortisol, SCL-90, and PSS-14 scales in the two groups of women with RPL and NVD which have received less attention in other studies. The weakness of our study was that stress of women assessed with self-report questionnaires. In the future, it is suggested that the severity of stress will be assessed with interviewing.
A limitation of our study was failure to evaluate cortisol immediately after abortion due to lack of immediate referral after abortion. Another limitation of this study was the small sample size. It is recommended that further longitudinal studies be conducted with a larger sample size for assessment of stress during 6 months after delivery.
Conclusions
A high level of cortisol reflects the acute stress caused by the care of baby in women. Therefore, women should receive a lot of psychological support in this period. A high level of cortisol reflects the acute stress caused by the care of baby in women. Therefore, women should receive a lot of psychological support in this period.
Financial support and sponsorship
This study was supported by a research grant (9434919) from Babol University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study is the result of a doctoral thesis. The authors would like to thank Babol University of Medical Sciences for all their support and the participants in this study.
References
- 1.Adib Rad H, Basirat Z, Mostafazadeh A, Faramarzi M, Bijani A, Nouri HR, et al. Evaluation of peripheral blood NK cell subsets and cytokines in unexplained recurrent miscarriage. J Chin Med Assoc. 2018;81:1065–70. doi: 10.1016/j.jcma.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Basirat Z, Adib Rad H, Esmailzadeh S, Jorsaraei SG, Hajian-Tilaki K, Pasha H, et al. Comparison of pregnancy rate between fresh embryo transfers and frozen-thawed embryo transfers following ICSI treatment. Int J Reprod Biomed (Yazd) 2016;14:39–46. [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmark Roepke E, Matthiesen L, Rylance R, Christiansen OB. Is the incidence of recurrent pregnancy loss increasing? A retrospective register-based study in Sweden. Acta Obstet Gynecol Scand. 2017;96:1365–72. doi: 10.1111/aogs.13210. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil Steril. 2013;99:63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Newell-Price J, Jones GL, Ledger WL, Li TC. Relationship between psychological stress and recurrent miscarriage. Reprod Biomed Online. 2012;25:180–9. doi: 10.1016/j.rbmo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Toffol E, Koponen P, Partonen T. Miscarriage and mental health: Results of two population-based studies. Psychiatry Res. 2013;205:151–8. doi: 10.1016/j.psychres.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Mevorach-Zussman N, Bolotin A, Shalev H, Bilenko N, Mazor M, Bashiri A, et al. Anxiety and deterioration of quality of life factors associated with recurrent miscarriage in an observational study. J Perinat Med. 2012;40:495–501. doi: 10.1515/jpm-2011-0313. [DOI] [PubMed] [Google Scholar]
- 8.Geller PA, Kerns D, Klier CM. Anxiety following miscarriage and the subsequent pregnancy: A review of the literature and future directions. J Psychosom Res. 2004;56:35–45. doi: 10.1016/S0022-3999(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 9.Boivin J, Lancastle D. Medical waiting periods: Imminence, emotions and coping. Womens Health (Lond) 2010;6:59–69. doi: 10.2217/whe.09.79. [DOI] [PubMed] [Google Scholar]
- 10.Haghparast E, Faramarzi M, Hassanzadeh R. Psychiatric symptoms and pregnancy distress in subsequent pregnancy after spontaneous abortion history. Pak J Med Sci. 2016;32:1097–101. doi: 10.12669/pjms.325.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasha H, Basirat Z, Esmailzadeh S, Faramarzi M, Adibrad H. Marital intimacy and predictive factors among infertile women in Northern Iran. J Clin Diagn Res. 2017;11:QC13–7. doi: 10.7860/JCDR/2017/24972.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmalian H, Nasiri Amiri F, Kheyrkhah F. Prevalence of pre and postpartum depression symptoms and some related factors. J Babol Univ Med Sci. 2008;10:67–75. [Google Scholar]
- 13.Valsamakis G, Chrousos G, Mastorakos G. Stress, female reproduction and pregnancy.Psychoneuroendocrinology. 2019 Feb 1;100:48–57. doi: 10.1016/j.psyneuen.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Bhat A, Byatt N. Infertility and perinatal loss: When the bough breaks. Curr Psychiatry Rep. 2016;18:31. doi: 10.1007/s11920-016-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–8. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DB, Grisso JA, Joffe MM, Brensinger C, Shaw L, Datner E. Does stress influence early pregnancy loss? Ann Epidemiol. 2003;13:223–9. doi: 10.1016/s1047-2797(02)00419-2. [DOI] [PubMed] [Google Scholar]
- 17.Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod. 1998;13:2296–300. doi: 10.1093/humrep/13.8.2296. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 19.Shah M, Hasan S, Malik S, Sreeramareddy CT. Perceived stress, sources and severity of stress among medical undergraduates in a Pakistani medical school. BMC Med Educ. 2010;10:2. doi: 10.1186/1472-6920-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiura-Ogasawara M, Nakano Y, Ozaki Y, Furukawa TA. Possible improvement of depression after systematic examination and explanation of live birth rates among women with recurrent miscarriage. J Obstet Gynaecol. 2013;33:171–4. doi: 10.3109/01443615.2012.745490. [DOI] [PubMed] [Google Scholar]
- 21.Adib Rad H, Basirat Z, Mostafazadeh A, Faramarzi M, Bijani A, Aghajanpour-Mir SM. The role of HLA-G in recurrent pregnancy loss: A case-control study. Ann Trop Med Public Health. 2018;13:SX738. [Google Scholar]
- 22.Liu CH, Fink G, Brentani H, Brentani A. An assessment of hair cortisol among postpartum Brazilian mothers and infants from a high-risk community in São Paulo: Intra-individual stability and association in mother-infant dyads. Dev Psychobiol. 2017;59:916–26. doi: 10.1002/dev.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Rezende MG, Garcia-Leal C, de Figueiredo FP, Cavalli Rde C, Spanghero MS, Barbieri MA, et al. Altered functioning of the HPA axis in depressed postpartum women. J Affect Disord. 2016;193:249–56. doi: 10.1016/j.jad.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 24.Conde A. Figueiredo B 24-h urinary free cortisol from mid-pregnancy to 3-months postpartum: Gender and parity differences and effects. Psychoneuroendocrinology. 2014;50:264–73. doi: 10.1016/j.psyneuen.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: Systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A. 2006;103:3938–42. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field T, Diego M. Cortisol: The culprit prenatal stress variable. Int J Neurosci. 2008;118:1181. doi: 10.1080/00207450701820944. [DOI] [PubMed] [Google Scholar]
- 28.van den Heuvel MI, van Assen MA, Glover V, Claes S, Van den Bergh BR. Associations between maternal psychological distress and salivary cortisol during pregnancy: A mixed-models approach. Psychoneuroendocrinology. 2018;96:52–60. doi: 10.1016/j.psyneuen.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Simon CD, Adam EK, Holl JL, Wolfe KA, Grobman WA, Borders AE, et al. Prenatal stress and the cortisol awakening response in african-american and caucasian women in the third trimester of pregnancy. Matern Child Health J. 2016;20:2142–9. doi: 10.1007/s10995-016-2060-7. [DOI] [PubMed] [Google Scholar]
- 30.Adib-Rad H, Basirat Z, Faramarzi M, Mostafazadeh A, Bijani A. Psychological distress in women with recurrent spontaneous abortion: A case-control study. Turk J Obstet Gynecol. 2019;16:151–7. doi: 10.4274/tjod.galenos.2019.88899. [DOI] [PMC free article] [PubMed] [Google Scholar]


