Abstract
Thanks to their promising properties, essential oils (EOs) have strong potential to remedy several problems such as microorganisms acquired resistance to antimicrobial agents and chemical antioxidants toxicity. Firstly, this work was conducted to determine chemical composition, antioxydant activity, then antibacterial, antifungal, antimycobacterial properties of Rosmarinus officinalis EO. Secondly, EOs combined antimicrobial effect with carvacrol was assessed. Chemical EO analysis was performed using Gas chromatography/mass spectrometer. 1,1-diphenyl-2-picrylhydrazyl test was used to evaluate in vitro antioxidant rosemary oil effect. The antimicrobial activity against seven bacteria, two fungi, and two mycobacterial strains was screened using the broth microdilution method. Thereafter, the checkerboard essay was used to evaluate the antibacterial effect of this EO and Carvacrol. Chemical EO analysis revealed 1,8-cineole (33.88%), camphor (14.66%), and α-pinene (12.76%) as main constituents. The obtained IC50 value (2.77 mg/mL) showed rosemary EO's radical scavenging power. Moreover, all tested microorganisms showed an important sensitivity to this EO (MIC values: 0.007%–1% (v/v)). Furthermore, results highlighted synergistic and partial synergistic interaction for tested products. The studied EO has both antimicrobial and antioxidant potentials. Combined application showed a remarkable synergistic antibacterial potentiation that can be used as an alternative in pharmaceutical and food processing sectors.
Key words: Antimicrobial activity, antioxidant, carvacrol, essential oil, Rosmarinus officinalis, radical scavenging, synergistic
INTRODUCTION
Plants have been used for centuries as disease remedies thanks to their valuable therapeutic components, notably their secondary metabolites which have many biological activities.
Control of food oxidation and pathogenic bacteria is mainly realized by chemical products with great risks to human health.[1]
Currently, microorganism's resistance to antimicrobial agents has led researchers to exploit plant world, particularly biologically active essential oils (EOs) of aromatic and medicinal plants, as an alternative to control food spoilage and harmful pathogens.[2] Furthermore, a combination of different EOs constitutes a new alternative to potentiate their antimicrobial and antioxidant effects for discovering new drugs and reducing their organoleptic impact in food.
Moroccan flora constituted by nearly 3913 plants species,[3] contains an unlimited number of plants having therapeutic properties. Rosmarinus officinalis (Lamiaceae) is a Mediterranean aromatic and medicinal plant, used in cosmetics, folk medicine, and food flavor, thanks to its numerous bioactives components.[4] It also has many other beneficial effects such as its anticancer,[5] antimicrobial, antioxidant,[6] anti-inflammatory,[7] and insecticidal activities.[8]
The present study investigates chemical composition of Moroccan R. officinalis EO, its antioxidant activity, its antimicrobial activity against microorganisms causing food spoilage and pathogenicity and finally the antimicrobial effect of binary combination with carvacrol against Bacillus subtilis.
SUBJECTS AND METHODS
Plant materials
Rosemary aerial parts (leaves and stems) were harvested in June 2015, at Er-Rich (32° 16′ 22″ N, 4° 30′ 26″ W) (Morocco).
Essential oil extraction
The harvested parts were hydrodistillated for 3 h in a Clevenger-type apparatus. The obtained EO was kept, in the dark, at 4°C until further use.
Chemical analysis
The EO composition was determined using a Hewlett-Packard (HP 6890) gas chromatograph (GC) coupled to a Polaris Q ion trap mass spectrometer (GC/MS), according to Chraibi et al.[9] Hence, the temperature was maintained 5 min at 50°C then increased to 200°C at 4°C/min. The chromatography carrier gas was N2 (1.8 mL/min). The mode used was Split (at 72.1 mL/min flow, and 1/50 ratio). The temperature was 250°C for both injector and detector, and 48 min was the final hold time. A “HP Chem Station” computer system was used to follow the evolution of these analyses. 1 μl of diluted samples (1/20 in methanol) was injected manually.
Antiradical activity assay
The R. officinalis EO's antioxidant activity was evaluated using the reduction test of 1,1-diphenyl- 2-picrylhydrazyl (DPPH).[9] Rosemary EO dilutions were prepared in methanol. Then 3 ml of DPPH dissolved in methanol (0.004% w/v) was mixed with 3 ml of each prepared dilution, to get 0.5, 1, 2 and 5 mg/mL final concentrations. After homogenizing, mixtures were vortexed and kept in the dark for 30 min. The optical density was measured at 517 nm, against Butylated hydroxytoluene (BHT) as a positive control.
Each experiment was repeated three times. The antiradical reactivity was determined as follows:
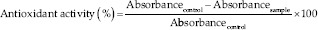
Antimicrobial activity
Minimum inhibitory concentration (MIC) values were established by the microdilution assay, in a 96-well microplate.[10] The emulsifier used (0.15% (w/v) bacteriological agar) was mixed with Mueller Hinton Broth (MHB). EO and carvacrol were serially diluted in the agar supplemented broth to reach concentrations from 4% to 0.003% (v/v). Then, 50 μL of bacterial (106 CFU/mL) was deposed. Finally, bacterial growth was revealed by turning resazurin from purple to pink.
Likewise, MIC's values for Candida albicans and Candida tropicalis were determined according to the protocol described by CLSI.[11] The EO dilution was prepared in YPG broth containing 0.15% agar (w/v). Then, the fungal inoculum (50 μL of 103 CFU/mL) was added to each well of the microplate, which was incubated, for 48 h, at 30°C.
For mycobacterial strains, a similar protocol was followed using Sauton 0.15% agar as culture medium and incubating during 48 and 72 h, respectively, for Mycobacterium smegmatis and Mycobacterium aurum. Experiments were repeated three times.
Fractional inhibitory concentration
The combined antibacterial effect of R. officinalis EO and carvacrol was evaluated using the checkerboard method.[12] The tested concentrations, ranged from 0.125% to 0.0039% and 0.125% to 0.0019% (v/v) for R. officinalis EO and carvacrol, respectively, and were prepared in MHB supplemented with 0.15% w/v agar. Along the microplate x-axis, 50 μL of each concentration of the studied EO were added to the corresponding well from the 1st to the 11th one. Each carvacrol concentration (50 μL) was added into each well following the y-axis. The 12th well was regarded as growth control. Then, all the microplate wells received bacterial inoculum at a final concentration of 106 CFU/mL. Thereafter, microplate was sealed and incubated for 18–20 h, at 37°C. Then, a second incubation was conducted for 2 h, at 37°C, after adding 10 μL of resazurin to each well. Experiments were conducted in triplicate.
The FIC index values were determined as follows:
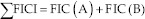
Where:
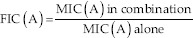
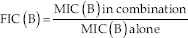
A: R. officinalis EO
B: Carvacrol
The Σ FICI values interpretation enabled to conclude a synergistic interaction for FICI ≤0.5; a partial synergy for 0.5<FIC<0.75; additive interaction for: 0.76<FIC<1.0; indifferent (non-interactive) when 1.0<FIC<4.0; and antagonistic interaction for FIC>4.0.
RESULTS
Chemical composition
Twenty-five constituents representing 98.14% of the total composition of R. officinalis EO were identified. Major compounds were 1,8-cineole, camphor, α-pinene [Table 1].
Table 1.
Chemical composition of Rosmarinus officinalis essential oil
| Kovats index | Constituents | Percentage |
|---|---|---|
| 939 | α-pinene | 12.76 |
| 953 | Camphene | 2.47 |
| 976 | β-pinene | 7.17 |
| 990 | Myrcene | 4.54 |
| 1033 | 1.8- cineole | 33.88 |
| 1143 | Camphor | 14.66 |
| 1162 | β-trans-terpineol | 3.46 |
| 1194 | Myrtenol | 1.23 |
| 1282 | α-terpineol | 2.28 |
| 1418 | β-Caryophyllene | 3.8 |
| 1493 | Ledene | 3.92 |
| 1581 | Caryophyllene oxide | 2.75 |
| Minor components (>1%) | 4.33 | |
| Total (%) | 98.14 | |
Antioxidant activity
The antiradical activity evaluation of rosemary EO showed a scavenging effect (IC50= 2.77 mg/mL) and a linear correlation between the EO concentration and the antioxidant activity (R2 = 0.99) [Figure 1]. However, BHT exhibited a greater radical scavenging activity (IC50= 6.83 μg/mL).
Figure 1.
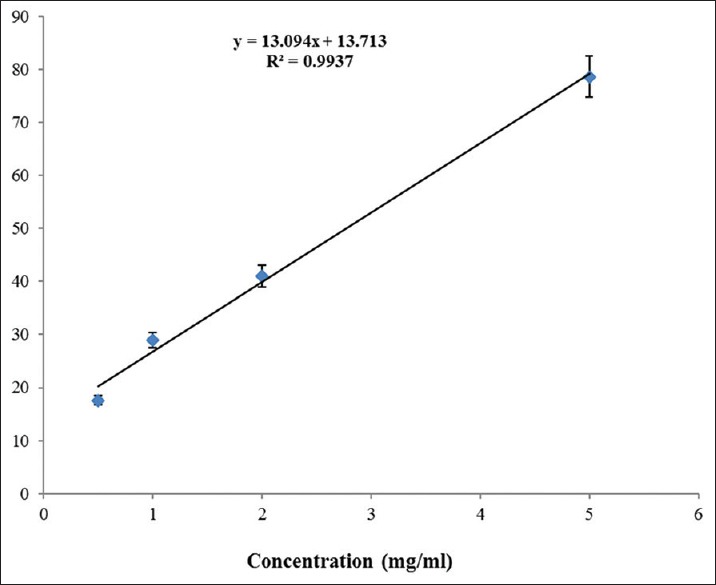
Antioxidant activity of Rosmarinus officinalis essential oil
Antimicrobial activity
A strong antimicrobial power was found against all tested Gram-positive bacteria, especially Saphyloccoccus aureus, Micrococcus luteus, and Bacillus cereus (respective MIC values of 0.007%, 0.015%, and 0.031% (v/v)). In addition, 0.062% (v/v) was sufficient to inhibit B. subtilis growth. The tested EO was more efficient against Escherichia coli and Salmonella enterica; while Pseudomonas aeruginosa was the most resistant concerning Gram-negative bacteria [Table 2].
Table 2.
Minimum inhibitory concentrations of Rosmarinus officinalis essential oil against tested strains
| Strains | Concentrations % |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.062 | 0.031 | 0.015 | 0.007 | 0.003 | |
| Bacillus subtilis | − | − | − | − | − | − | − | + | + | + | + |
| Bacillus cereus | − | − | − | − | − | − | − | − | + | + | + |
| Micrococcus luteus | − | − | − | − | − | − | − | − | − | + | + |
| Escherichia coli | − | − | − | − | − | − | + | + | + | + | + |
| Salmonella enterica | − | − | − | − | − | + | + | + | + | + | + |
| Pseudomonas aeruginosa | − | − | − | + | + | + | + | + | + | + | + |
| Candida albicans | − | − | − | − | − | − | + | + | + | + | + |
| Candida tropicalis | − | − | − | − | − | − | − | + | + | + | + |
| Mycobacterium aurum | − | − | − | − | − | − | + | + | + | + | + |
| Mycobacterium smegmatis | − | − | − | − | − | + | + | + | + | + | + |
−: Not growth, +: Growth
The fungal strains were also sensitive to R. officinalis EO, with respective MIC values of 0.062% and 0.125% (v/v) for C. tropicalis and C. albicans [Table 2]. R. officinalis EO has also showed an important activity against mycobacterial strains (MIC: 0.125% and 0.25% (v/v)) [Table 2].
Carvacrol was active on all tested strains, with MIC values ranging from 0.015%, for S. aureus and M. luteus to 1% (v/v) for P. aeruginosa.
The results of antibacterial combined effect against B. subtilis are presented in Table 3.
Table 3.
Fractional inhibitory concentration, fractional inhibitory concentration index, and outcome of interactions between Rosmarinus officinalis essential oil and carvacrol against Bacillus subtilis
| Components | MIC % (v/v) |
FIC% (v/v) | FICI | Outcome | |
|---|---|---|---|---|---|
| Alone | In combination | ||||
| EO | 0. 0625 | 0.031 | 0.496 | 0.527 | Partial synergy |
| Carvacrol | 0.125 | 0.0039 | 0.0312 | ||
| EO | 0. 0625 | 0.015 | 0.24 | 0.36 | Synergistic |
| Carvacrol | 0.125 | 0.015 | 0.12 | ||
| EO | 0. 0625 | 0.007 | 0.112 | 0.36 | Synergistic |
| Carvacrol | 0.125 | 0.031 | 0.248 | ||
| EO | 0. 0625 | 0.0019 | 0.030 | 0.526 | Partial synergy |
| Carvacrol | 0.125 | 0.062 | 0.496 | ||
FIC: Fractional inhibitory concentration, MIC: Minimum inhibitory concentration, EO: Essential oil, FICI: Fractional inhibitory concentration index
The checkerboard assay generated four combinations. FIC index calculated values were comprised between 0.36 and 0.527, giving two interaction types between the studied EO and carvacrol.
Combinations of (1/2 MIC R. officinalis + 1/32 MIC carvacrol) and (1/32 MIC R. officinalis + 1/2 MIC carvacrol) showed a partial synergistic effect against B. subtilis with respective FIC indexes of 0.527 and 0.526. Moreover, combinations of (1/4 MIC R. officinalis + 1/8 MIC carvacrol) and (1/8 MIC R. officinalis + 1/4 MIC carvacrol) exhibited an inhibitory activity (FIC index = 0.36), indicating a synergistic interaction [Table 3].
DISCUSSION
Rosemary EO composition was similar to those previously found for EOs of rosemary from Korea,[13] Morocco,[14] and Tunisia,[8] especially for the main compounds. While, it differs from that found for Italian rosemary EO (Verbenone, 21.8%; camphor 14.6%).[15] Even if BHT showed a stronger antioxidant effect (IC50 = 6.83 μg/mL) compared to rosemary EO (2.77 mg/mL), several studies have proved BHT to be cytotoxic due to its potential carcinogenic action.[16] Moreover, a large variation in the antioxidant activity of R. officinalis EO has been reported by several authors;[8,17] and explained by their hydroxylated derivatives concentrations.[18]
This activity could be attributed to EO's molecular structure, because antioxidant activity was reported to be related to hydroxyl groups substitution in the phenolic aromatic rings, contributing to their hydrogen donating ability and to oxygenated monoterpenes and mixture of mono and sesquiterpene hydrocarbons.[4,19] In addition according to Bajalan,[6] the antioxidant activity is positively correlated with EOs major compounds such as β-pinene or 1,8-cineole. In contrast, the minor compounds can interact directly to create a mixture of biological activities.[20]
Several studies have previously reported the antimicrobial activity of R. officinalis EO.[6,14]
These antimicrobial properties could be explained by the EO's chemical composition, very abundant in monoterpenoids compounds possessing high antimicrobial activity. According to Bakkali et al.,[21] biological activities of EOs are mostly linked to their major compounds. In fact, first and second main compounds (1,8-cineole and camphor) are known for their antimicrobial properties.[22,23] α-pinene also have a strong potential of antimicrobial activity,[24] thanks to oxygen function's presence which increase antimicrobial properties of terpenoids. In addition, a major compound does not possess the antimicrobial effect alone, but minor compounds can also act on microbes.
Thus, the synergistic interaction between different EO constituents could be responsible for increasing their antimicrobial power.[25]
Regarding FIC index, all combinations gave an interesting antibacterial effect which is synergistic and higher than the effect of the EO or the carvacrol alone toward B. subtilis. This synergy is of major economic interest because the antimicrobial effect has been potentiated using a combination of the two products at extremely low concentrations. In fact synergism among carvacrol and hydrocarbons monoterpenes such as α-pinene, myrcene, and camphene representing 19.75% of the total composition of R. officinalis EO was demonstrated by Ultee research team.[26] In addition, these hydrocarbons interact with the cell membrane facilitating carvacrol penetration.[27]
Finally, being the first main compound of R. officinalis EO with 33.88%, 1,8 cineol was found to interact synergistically with carvacrol toward multiplication and survival of spoilage and pathogenic microorganisms.[28]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rim KT. Reproductive toxic chemicals at work and efforts to protect workers' health: A literature review. Saf Health Work. 2017;8:143–50. doi: 10.1016/j.shaw.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calo JR, Crandall PG, O'Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems – A review. Food Control. 2015;54:111–9. [Google Scholar]
- 3.Fennane M, IBN Tattou M. Statistics and comments on the current inventory of the vascular flora of Morocco. Bull Inst Sci. 2012;34:1–9. [Google Scholar]
- 4.Ojeda-Sana AM, van Baren CM, Elechosa MA, Juárez MA, Moreno S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013;31:189–95. [Google Scholar]
- 5.González-Vallinas M, Reglero G, Ramírez de Molina A. Rosemary (Rosmarinus officinalis L.) extract as a potential complementary agent in anticancer therapy. Nutr Cancer. 2015;67:1221–9. doi: 10.1080/01635581.2015.1082110. [DOI] [PubMed] [Google Scholar]
- 6.Bajalan I, Rouzbahani R, Pirbalouti AG, Maggi F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of R. officinalis. Ind Crops Prod. 2017;107:305–11. [Google Scholar]
- 7.Rahbardar MG, Amin B, Mehri S, Mirnajafi-Zadeh SJ, Hosseinzadeh H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis and rosmarinic acid in a rat model of neuropathic pain. Biomed Pharmacother. 2017;86:441–9. doi: 10.1016/j.biopha.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Badreddine BS, Olfa E, Samir D, Hnia C, Lahbib BJ. Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae) Asian Pac J Trop Med. 2015;8:98–103. doi: 10.1016/S1995-7645(14)60298-4. [DOI] [PubMed] [Google Scholar]
- 9.Chraibi M, Fikri-Benbrahim K, Ou-Yahyia D, Balouiri M, Farah A. Radical scavenging and disinfectant effect of essential oil from Moroccan Mentha pulegium. Int J Pharm Pharm Res. 2016;8:116–9. [Google Scholar]
- 10.Sadiki M, Elabed A, Elaabedy A, Elabed A, Farah A, Iraqui M, et al. Characterization and antibacterial activity of the essential oil from Thymus vulgaris cultivated in morocco (Taounate) against ten bacteria. World J Pharm Res. 2015;4:314–25. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. 2nd ed. NCCLS Document M27-A2. 22(15) Clinical and Laboratory Standards Institute. 2002 [Google Scholar]
- 12.Sadiki M, Balouiri M, Barkai H, Maataoui H, Koraichi S, Elabed S. Synergistic antibacterial effect of M. communis and T. vulgaris essential oils fractional inhibitory concentration index. Int J Pharm Pharm Sci. 2014;1:121–4. [Google Scholar]
- 13.Yang SA, Jeon SK, Lee EJ, Shim CH, Lee IS. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat Prod Res. 2010;24:140–51. doi: 10.1080/14786410802496598. [DOI] [PubMed] [Google Scholar]
- 14.Fadil M, Fikri-Benbrahim K, Rachiq S, Ihssane B, Lebrazi S, Chraibi M, et al. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur J Pharm Biopharm. 2018;126:211–20. doi: 10.1016/j.ejpb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, et al. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–32. [Google Scholar]
- 16.Taghvaei M, Jafari SM. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol. 2015;52:1272–82. doi: 10.1007/s13197-013-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raškovic A, Milanovic I, Pavlovic N, Cebovic T, Vukmirovic S, Mikov M. Antioxidant activity of rosemary (R. officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Alter Med. 2014;14:225–34. doi: 10.1186/1472-6882-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beretta G, Artali R, Facino RM, Gelmini F. An analytical and theoretical approach for the profiling of the antioxidant activity of essential oils: The case of Rosmarinus officinalis L. J Pharm Biomed Anal. 2011;55:1255–64. doi: 10.1016/j.jpba.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Yen WJ, Wang BS, Chang LW, Duh PD. Antioxidant properties of roasted coffee residues. J Agric Food Chem. 2005;53:2658–63. doi: 10.1021/jf0402429. [DOI] [PubMed] [Google Scholar]
- 20.Zaibet W. Maire, and their application as antimicrobial agents in Low Density Polyethylene (LDPE). D. Thesis. Setif, Algeria: Faculty of Technology, Ferhat ABBAS University; 2016. Chemical composition and biological activity of essentiel oils of Daucus aureus (Desf) and Reutera lutea (Desf.) [Google Scholar]
- 21.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – A review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 22.Hendry ER, Worthington T, Conway BR, Lambert PA. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J Antimicrob Chemother. 2009;64:1219–25. doi: 10.1093/jac/dkp362. [DOI] [PubMed] [Google Scholar]
- 23.Hadian M, Rajaei A, Mohsenifar A, Tabatabaei M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against S. typhimurium during refrigerated storage. Lwt Food Sci Technol. 2017;84:394–401. [Google Scholar]
- 24.Leite AM, Lima ED, Souza EL, Diniz MD, Trajano VN, Medeiros IA. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev Bras Ciênc Farm. 2007;43:121–6. [Google Scholar]
- 25.Benelli G, Pavela R, Iannarelli R, Petrelli R, Cappellacci L, Cianfaglione K, et al. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: Larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind Crops Prod. 2017;96:186–95. [Google Scholar]
- 26.Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000;63:620–4. doi: 10.4315/0362-028x-63.5.620. [DOI] [PubMed] [Google Scholar]
- 27.De Azeredo GA, Stamford TL, Nunes PC, Neto NJ, De Oliveira ME, DeSouza EL. Combined application of essential oils from Origanum vulgare and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Res Inter. 2011;44:1541–48. [Google Scholar]
- 28.de Sousa JP, de Azerêdo GA, de Araújo Torres R, da Silva Vasconcelos MA, da Conceição ML, de Souza EL. Synergies of carvacrol and 1,8-cineole to inhibit bacteria associated with minimally processed vegetables. Int J Food Microbiol. 2012;154:145–51. doi: 10.1016/j.ijfoodmicro.2011.12.026. [DOI] [PubMed] [Google Scholar]


