Abstract
Drymaria cordata (Caryophyllaceae), commonly known as Abhijalo in Sikkimese-Tibetan, is a creeping herb grown in tropical and subtropical regions of the world. It is used by ethnic groups of Sikkim, North-East India, for the treatment of various diseases including diabetes. This study aimed to investigate the antidiabetic effect of methanol extract from D. cordata leaf (DCME) in streptozotocin (STZ) and nicotinamide (NA)-induced type 2 diabetes in rats. Diabetic Wistar albino rats were treated with DCME at 200 mg/kg and 400 mg/kg orally for 28 days. Metformin (150 mg/kg b.w.) was used as a reference drug. Fasting blood glucose (FBG) level; serum biochemical parameters; and liver, kidney, and antioxidant parameters were estimated, and pancreatic histopathology was performed after 28 days of treatment. Administration of DCME to STZ-NA-induced diabetic rats at 200 mg/kg and 400 mg/kg orally for 28 days exhibited statistically significant (P < 0.05) and dose-dependent reduction of FBG, glycosylated hemoglobin, serum lipid, and hepatorenal antioxidative parameters in DCME-treated groups when compared to those of diabetic controls. Histopathological studies of pancreas in DCME-treated rats showed improvement in β-cell density compared to diabetic group. The results demonstrate the significant antidiabetic potential of D. cordata leaf in albino rats plausibly by reducing oxidative stress and serum lipids levels, justifying the folkloric use of this plant in the treatment of diabetes.
Key words: Antidiabetic activity, Drymaria cordata, streptozotocin–nicotinamide, type 2 diabetes
INTRODUCTION
Diabetes mellitus is a metabolic disorder characterized by the elevation of blood sugar level (hyperglycemia), hyperlipidemia or dyslipidemia, and glycosuria, generally due to insulin resistance or insulin deficiency. There are several etiological factors involved in the development of type 2 diabetes (noninsulin-dependent diabetes mellitus), namely, genetic factor, age, diet, obesity, and lifestyle. Since the last couple of decades, it was observed that type 2 diabetes has become a global health problem. As estimated by the World Health Organization, more than 176 million people are suffering from this disease globally.[1] In the Western countries, diabetes mostly develops with advancement in age, but in Asian countries, it affects mostly young to middle-aged population. Various associated macrovascular (cerebrovascular and cardiovascular diseases) and microvascular complications (neuropathy, retinopathy, and nephropathy) develop during diabetes. It has been suggested that enhanced generation of free radicals, oxidative stress, and deficiency of endogenous antioxidants are the cardinal issues in the development of diabetic complications.[2]
Studies have shown that diabetes elicits redox imbalance of endogenous antioxidant system of our body by overproduction of free radicals (reactive oxygen and nitrogenous species) that are responsible for various cellular and subcellular detrimental changes, thus aggravating the condition. Recent studies suggest that implication of antioxidants in diet or as medicine is an alternative tool for the management of diabetic complications. The plant kingdom offers an untapped source of antioxidant agents which require thorough exploitation.[3,4]
Drymaria cordata (Linn.) Willd. and Schult. (Caryophyllaceae), commonly known as tropical chickweed in English, is a creeping herb that grows in dense patches in moist shady places and also in dry, sun-exposed tropical and subtropical regions of the world. Its stems are green and slender, leaves are opposite and cordate with short petiole, and flowers are small and green.[5] Usage of this plant in agriculture and traditional medicine has been reported in African, Asian, and American countries.[6] In Cameroon, the plant is used in the treatment of peptic ulcer, headaches, nephritis, and female infertility.[5] In Nigerian folk medicine, D. cordata is used to treat sleeping disorders, convulsions, and febrile conditions in children.[7] In Meghalaya, North-East India, this herb is used to cure cough and acute cold attack, sinusitis, burn, snake bite, burns, and skin diseases.[8] In Sikkim, North-East India, this plant is commonly known as Abhijalo in Sikkimese-Tibetan. The local community people of Sikkim use this plant for the treatment of various ailments such as pneumonia, infant fever, sinusitis, nose blockade, headache, and fever.[9] The Nepali tribal practitioners of Sikkim use this whole herb as a folk medicine for the treatment of diabetes.[10]
D. cordata has previously been reported to possess antitussive,[8] antibacterial,[11] anti-inflammatory,[7] anxiolytic,[12] analgesic,[13] and cytotoxic activities.[14] Despite having the above-mentioned ethnomedicinal use in diabetes, till now, there is no study investigating its antidiabetic property. Hence, an experimental attempt has been made to assess the possible antidiabetic effect of D. Cordata leaf against streptozotocin (STZ) and nicotinamide (NA)-induced type-2 diabetes in rats to validate the traditional use of this plant in the management of diabetes.
MATERIALS AND METHODS
Plant material
The plant D. cordata (L.) Willd. and Schult. (Family: Caryophyllaceae) was collected from Bermiok, South Sikkim, India, during the month of July 2018. The plant specimen was identified by David Lalsama Baite, Scientist-in-Charge, Botanical Survey of India (BSI), Sikkim Himalayan Regional Center (SHRC), Gangtok, Sikkim - 737 103, India, and the voucher specimen had been deposited and accessioned at the Herbarium of BSI, SHRC, Gangtok, India, with acronym BSHC (Accession No. 0239).
Preparation of extract
Fresh leaves of D. cordata were thoroughly washed and cleaned with tap water. Then, the leaves were dried under shade, ground mechanically, and kept in an airtight container. Powdered plant material (300 g) was extracted successively with petroleum ether (60°C–80°C), chloroform, and methanol in a Soxhlet extractor. The methanol extract was concentrated in vacuo to obtain the dry extract (methanol extract from D. cordata [DCME], 11.06% w/w) which was stored at 4°C for use in the study.
Experimental animals
Adult male Wistar albino rats (150–200 g) of mature normoglycemic, with fasting blood glucose (FBG) levels of 85–90 mg/dL, and Swiss albino mice (20–25 g) used in this study were obtained from the registered breeder – Saha Enterprise, Kolkata (Reg. no. 1828/PO/BT/S/15/CPCSEA). The animals were maintained under standard environmental conditions and had free access to standard diet and water ad libitum. The rats were housed at an ambient temperature of 25°C with 12 h light/dark cycle. All the animal experimental procedures described were reviewed and approved by the University's Animal Ethical Committee (Ref. No. AEC/PHARM/1502/09/2015), Jadavpur University, Kolkata 700 032, West Bengal, India.
Chemicals
Chemicals used in this study were obtained from various sources as bovine serum albumin, (Sigma-Aldrich, St. Louis, Missouri, USA), STZ (Hi-media, Mumbai, Maharashtra, India) NA (Hi-media), metformin hydrochloride (USV Pvt. Ltd., Mumbai, Maharashtra India), and alpha glucosidase (SISCO Research Laboratory, Mumbai, Maharashtra, India). Trichloroacetic acid, thiobarbituric acid (TBA), DTNB, reduced glutathione (GSH), NADH, and alpha amylase were obtained from Hi-media. All chemicals and solvents were of analytical grade from India.
Preliminary phytochemical studies
Preliminary phytochemical study was performed on DCME for qualitative identification of various phytoconstituents.[15]
Acute toxicity study
Acute oral toxicity study of DCME was performed on Swiss albino mice according to the Organization for Economic Co-operation and Development guidelines 425.[16]
In-vitro antidiabetic assays
Alpha amylase inhibition assay
This assay was performed according to the standard method with minor modifications. Different concentrations (10, 20, 40, 60, 80, and 100 μg/ml) of standard (acarbose) and DCME were prepared in prelabeled test tubes. 50-μl phosphate buffer (100 mM, pH: 6.8) and 10-μl α-amylase (2 U/ml) were added in each test tube and incubated at 37°C for 30 min. Then, the 20 μl of 1% soluble starch (100 mM phosphate buffer pH: 6.8) was added as a substrate and incubated further at 37°C for 30 min; 100 μl of the DNS color reagent was then added and boiled for 10 min. The absorbance of the resulting mixture was measured at 540 nm using a spectrophotometer (Chemline-1320, Chemline India Ltd., New Delhi, India). Without test (DCME) or standard, the reaction mixture was set up in parallel as control, and each experiment was performed in triplicates. The results were expressed as percentage inhibition, which was calculated using the following formula.[17]
Inhibitory activity (%) = (1 − As/Ac) ×100
Where
As is the absorbance in the presence of test substance and Ac is that of control.
Alpha-glucosidase inhibition assay
The α-glucosidase inhibitory activity of DCME was carried out according to the standard method with slight modifications. Varying concentrations (10, 20, 40, 60, 80, and 100 μg/ml) of standard (acarbose) and DCME were prepared in prelabeled test tubes. Then, 50-μl phosphate buffer (100 mM, pH: 6.8) and 10-μl alpha-glucosidase (1 U/ml) were added in each test tube and preincubated at 37°C for 15 min. Then, 20-μl p-nitrophenyl-α-glucopyranoside (P-NPG, 5 mM) was added as a substrate and incubated further at 37°C for 20 min. The reaction was stopped by adding 50-μl Na2 CO3 (0.1 M). The absorbance of the released p-nitrophenol was measured at 405 nm by using spectrophotometer (Chemline-1320). Without standard or test (DCME), the reaction mixture was set up in parallel as a control, and each experiment was performed in triplicates. The results were expressed as percentage inhibition, which was calculated using the following formula.[17]
Inhibitory activity (%) = (1 − As/Ac) ×100
Where As is the absorbance in the presence of test substance and Ac is the absorbance of control.
In-vivo antidiabetic activity
Experimental induction of diabetes
Before diabetes induction, the rats were fasted for 18 h without withdrawing drinking water. STZ was dissolved in 0.1 M citrate buffer (pH: 4.5) just before administration and NA was administered (110 mg/kg, b.w., i.p.) 15 min before administration of STZ. Then, STZ was administered (45 mg/kg, b.w., i.p.). Diabetic status was confirmed by estimating the FBG level. Animals having FBG level ≥250 mg/dl were selected for further antidiabetic study.[18]
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) was carried out in overnight fasted normal glycemic (85–90 mg/dl) rats. The rats were divided into three groups (n = 6): Group I served as normal control and received distilled water (5 mL/kg b.w., p.o.), and Groups II and III received DCME at doses of 200 and 400 mg/kg b.w., p.o., respectively. After these treatments, all the groups received glucose (2 g/kg b.w.) orally. Blood was withdrawn from the tail vein just prior to and 30, 60, and 120 min after oral glucose administration.[19] The blood glucose levels were measured using a single-touch glucometer (Contour TS, Bayer Healthcare AG, Leverkusen, Germany).
Experimental design
The experimental protocol was designed for 28 days. The rats were divided into five groups, with six animals in each group (n = 6).
Normal control group: The rats received normal saline (0.5 ml/kg, p.o.) for 28 days
Diabetic control group: Diabetic group consisted of rats treated with STZ (45 mg/kg, b.w., i.p.) and NA (110 mg/kg, b.w., i.p.)
Extract-treated group (DCME 200 mg/kg): The diabetic rats were treated with DCME (200 mg/kg b.w.), orally for 28 days
Extract-treated group (DCME 400 mg/kg): DCME (400 mg/kg b.w.) was administered orally for 28 days
Metformin-treated group: The diabetic rats were treated with metformin (150 mg/kg, p.o.) for 28 days.
Estimation of glycosylated hemoglobin level
Glycosylated hemoglobin (HbA1c) level was determined from whole blood sample by using commercially available assay kits (Coral Clinical System, Tulip diagnostics Pvt. Ltd., India).
Estimation of serum biochemical parameters
After 28 days of treatment, i.e., at the 29th day, all the rats were kept in fasting condition for 12 h and anesthetized with ether. Then, the anesthetized rats were sacrificed by cervical decapitation method. Blood was collected from cardiac puncture. Serum was separated by centrifugation at 3000 rpm for 10 min. The collected serum was analyzed for various biochemical parameters such as serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), serum alkaline phosphatase (SALP), and total protein by commercially available assay kits (Arkray Helathcare Pvt. Ltd., Mumbai, Maharashtra, India).
Evaluation of serum lipid profile
Serum lipids such as total cholesterol, high-density lipoprotein (HDL)–cholesterol, and triglyceride in STZ–NA-induced diabetic rats were determined by using commercially available kits (Span Diagnostics Ltd., Surat, Gujarat, India).
Estimation of tissue antioxidant parameters
Livers and kidneys collected from the sacrificed animals were homogenized separately in 10 ml of phosphate buffer (20 mM, pH: 7.4) and centrifuged at 12,000 rpm for 30 min at 4°C. The supernatants were collected, and lipid peroxidation (LPO) (malondialdehyde [MDA]), GSH, and superoxide dismutase (SOD) were estimated by using commercially available reagent kits (Erba Diagnostics, Mumbai, Maharashtra, India).
Histopathological studies
Histopathological studies were carried out with the parts of pancreas which were isolated from the sacrificed rats. The tissues were washed with normal saline and immediately fixed in 10% formalin for 24 h, dehydrated with alcohol, embedded in paraffin, and then cut into 4–5-μm-thick sections and stained with hematoxylin-eosin dye for photomicroscopic observations done at ×40.
Statistical analysis
All the data were expressed as mean ± standard error of mean. The results were analyzed for statistical significance by one-way analysis of variance followed by post hoc Dunnett's test using Graph pad prism 5.0 software (Graph Pad Software, San Diego USA). P< 0.05 was considered statistically significant.
RESULTS
Preliminary phytochemical studies
This revealed the presence of triterpenoids, steroids, alkaloids, and flavonoids in DCME.
Acute toxicity study
No toxicity was observed after the administration of DCME up to 2000 mg/kg b.w. in mice. Two doses of DCME 200 mg/kg b.w. and 400 mg/kg b.w. were selected for the present study.
In-vitro alpha amylase and alpha-glucosidase inhibitory activity
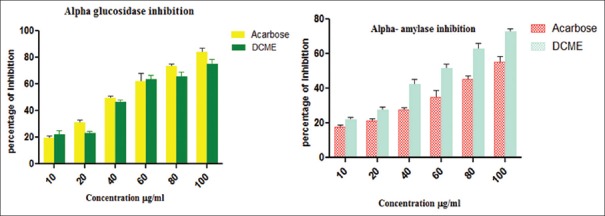
This in-vitro study revealed the presence of DCME alpha amylase and alpha-glucosidase inhibitory activity, and the corresponding IC50 values were found to be 85.32 ± 5.11 μg/ml and 59.66 ± 7.21 μg/ml, respectively [Figure 1].
Figure 1.
In-vitro alpha amylase and alpha glucosidase activities of methanol extract from Drymaria cordata
Oral glucose tolerance test
The OGTT was performed on normal glycemic rats (85–90 mg/dl). After glucose administration to the normal rats, blood glucose levels increased in the first 30 min and gradually decreased in 60 min and became normal in 120 min, which has been shown in Table 1.
Table 1.
Effect of Drymaria cordata methanolic extract on oral glucose tolerance test
| Groups | 0 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|
| Normal control | 87.83±3.9 | 129.7±2.9 | 112.7±1.5 | 108.0±2.08 |
| DCME 200 mg/kg | 89.33±1.2 | 121.0±1.5* | 118.3±1.8* | 101.7±1.4* |
| DCME 400 mg/kg | 82±1.5 | 119.3±1.2* | 109.3±2.4* | 94.6±2.02* |
| Metformin 150 mg/kg | 80.5±2.5 | 105±2.30* | 95.2±3.20* | 86±1.80* |
*Values significantly differ from normal control where P<0.05. DCME: Drymaria cordata methanolic extract
Fasting blood glucose and body weight
It was found that FBG level was statistically significantly (P < 0.05) elevated in STZ–NA-induced diabetic rats as compared to the normal control group. On administration of DCME in diabetic rats at the doses of 200 and 400 mg/kg for 28 days, statistically significant (P < 0.05) reduction of FBG level toward normal was found as compared to the diabetic control group [Table 2]. The final body weights were statistically significantly (P < 0.05) decreased in the diabetic control group as compared to the normal control group. Administration of DCME at the doses of 200 and 400 mg/kg statistically significantly (P < 0.05) improved the body weight when compared to the diabetic control group, which has been shown in Table 3.
Table 2.
Effect of Drymaria cordata methanolic extract on fasting blood glucose (mg/dl)
| Groups | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| Normal control | 77±2.23 | 80.33±1.80 | 81.66±3.41 | 80.66±2.50 | 76±1.92 |
| STZ (110 mg/kg) + NA (45 mg/kg) | 414.33±3.52a* | 493.66±2.19a* | 520±1.15a* | 525.67±1.80a* | 546.65±3.40a* |
| STZ + DCME 200 mg/kg | 428.63±2.13 | 393.33±1.75b* | 344.41±2.04b* | 278.34±1.19b* | 212.20±1.32b* |
| STZ + DCME 400 mg/kg | 414.33±2.10 | 308.34±1.45b* | 221.33±1.46b* | 176.24±2.11b* | 104.66±3.40b* |
| Diabetic + metformin 150 mg/kg | 425.31±3.33 | 254.22±2.10b* | 118.66±1.26b* | 84.42±1.14b* | 79.13±2.15b* |
Each values are expressed as mean±SEM (n=6), a*Diabetic control group versus normal control group (P<0.05), b*All treated group versus diabetic control group on corresponding day (P<0.05), *Values significantly differ from each other where P<0.05. SEM: Standard error of mean, DCME: Drymaria cordata methanolic extract, STZ: Streptozotocin, NA: Nicotinamide
Table 3.
Effect of Drymaria cordata methanolic extract on body weight (g)
| Groups | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| Normal control | 175±2.11 | 177±0.76 | 174±0.89 | 175±1.28 | 176±0.87 |
| Diabetic control | 171±1.79a | 165±0.88a* | 135±0.89a* | 138±0.87a* | 132±0.98a* |
| Diabetic + DCME 200 mg/kg | 165±1.28b | 158±0.89b* | 148±1.33b* | 146±0.89b* | 142±1.22b* |
| Diabetic + DCME 400 mg/kg | 170±2.21b | 158±1.11b* | 149±1.22b* | 152±0.89b* | 155±0.88b* |
| Diabetic + metformin 150 mg/kg | 171±0.88b | 161±0.98b* | 163±0.88b* | 163±1.01b* | 165±0.97b* |
Each value is expressed as mean±SEM (n=6). a*Diabetic control group versus normal control group (P<0.05), b*All treated groups versus diabetic control group on corresponding day (P<0.05), *Values significantly differ from each other where P<0.05. SEM: Standard error of mean, DCME: Drymaria cordata methanolic extract
Glycosylated hemoglobin
HbA1c level was found decreasing in DCME-treated rats statistically significantly (P < 0.05) when compared to diabetic rats [Figure 2].
Figure 2.
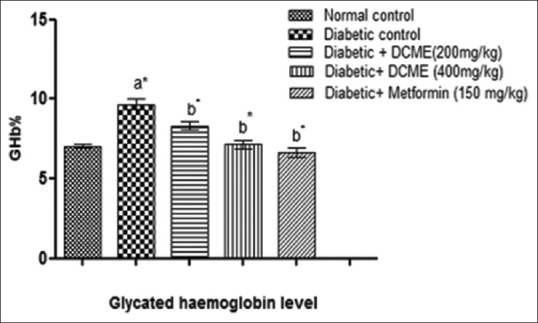
Effect of methanol extract from Drymaria cordata on glycosylated hemoglobin level. Each bar represents mean ± standard error of mean (n = 6). a*Diabetic control group versus normal control group, b*All treated groups versus diabetic control group, where all bars are statistically significant from each other. P<0.05
Serum biochemical parameters
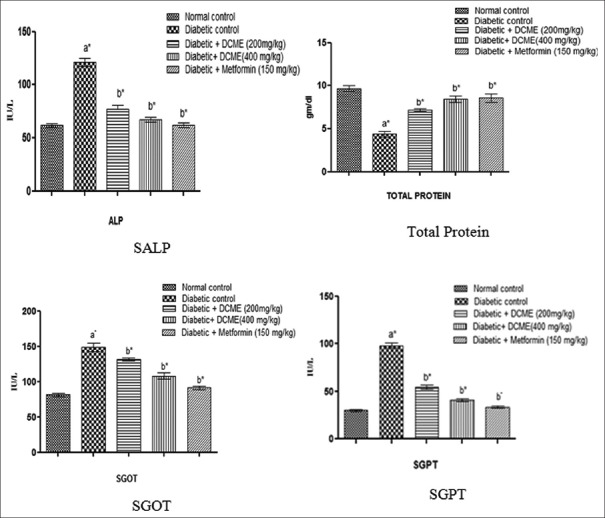
Serum biochemical parameters such as SALP, SGOT, SGPT showed statistically significant (P < 0.05) reduction in DCME-treated group when compared to the STZ–NA control group. Content of total protein increased in the treated group when compared to the STZ–NA control [Figure 3].
Figure 3.
Effect of methanol extract from Drymaria cordata on serum biochemical parameters. Each bar represents mean ± standard error of mean (n = 6). a*Diabetic control group versus normal control group, b*All treated groups versus diabetic control group, where all bars are statistically significant from each other. P <0.05
Serum lipid profile
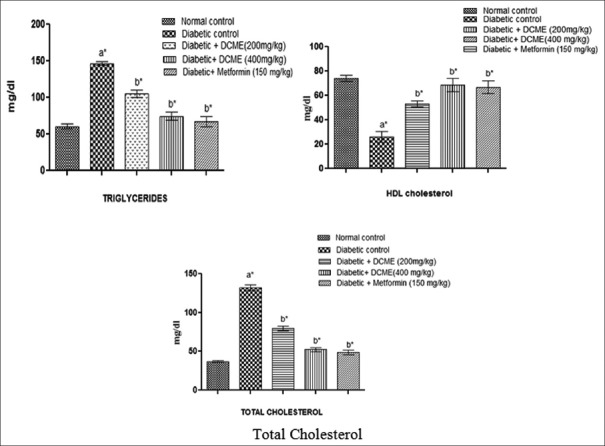
Serum lipid profile such as triglyceride and total cholesterol level was statistically significantly (P < 0.05) higher in diabetic rats compared to normal rats, and HDL level statistically significantly (P < 0.05) decreased in diabetic rats compared to normal rats. After administration of DCME at the doses of 200 mg/kg and 400 mg/kg b.w., triglyceride and total cholesterol level statistically significantly (P < 0.05) reduced and HDL level statistically significantly (P < 0.05) increased, when compared to the diabetic control group [Figure 4].
Figure 4.
Effect of methanol extract from Drymaria cordata on serum lipid profile. Each bar represents mean ± standard error of mean (n = 6). a*Diabetic control group versus normal control group, b*All treated groups versus diabetic control group, where all bars are statistically significant from each other.P <0.05
Tissue antioxidant parameters
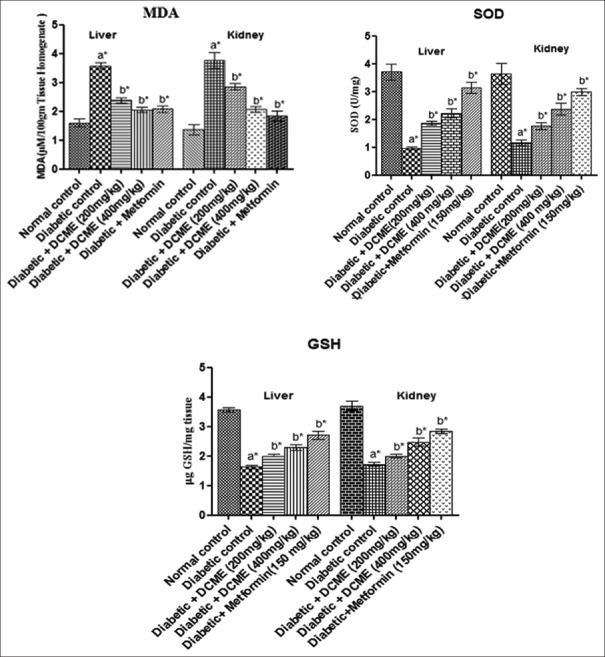
Hepatic and renal MDA levels statistically significantly (P < 0.05) reduced, and SOD and GSH levels statistically significantly (P < 0.05) elevated and MDA level decreased statistically significantly (P < 0.05) in DCME-treated rats compared to diabetic rats [Figure 5].
Figure 5.
Effect of methanol extract from Drymaria cordata on tissue (liver and kidney) antioxidant parameters. Each bar represents mean ± standard error of mean (n = 6). a*Diabetic control group versus normal control group, b*All treated groups versus diabetic control group, where all bars are statistically significant from each other. P <0.05
Histopathology of pancreas
Pancreatic histopathological observations revealed that DCME-treated groups exhibited marked pancreatic β-cell protective activity. There was dose-dependent gradual improvement of pancreatic β-cell density in DCME-treated groups as compared to diabetic rats [Figure 6].
Figure 6.
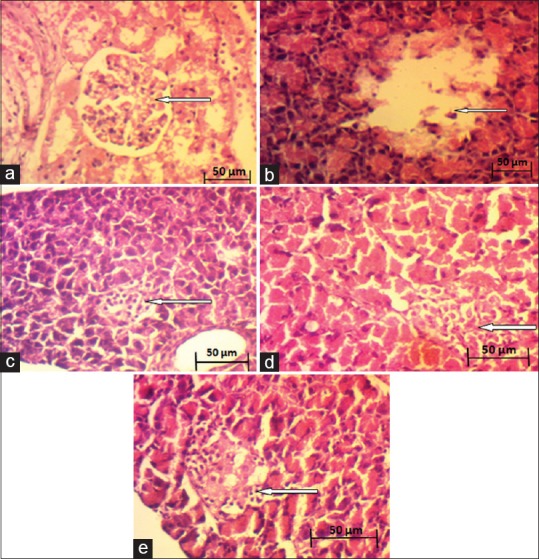
Effect of methanol extract from Drymaria cordata on histopathology of pancreas . (a) Normal control. (b) Diabetic control. (c) DCME (200 mg/kg). (d) DCME (400 mg/kg). (e) Metformin (150 mg/kg)
DISCUSSION
The present study probes the antidiabetic, antihyperlipidemic, and antioxidant activities of methanol extract of D. cordata leaf (DCME) in STZ–NA-induced diabetic rats. D. cordata has various ethnomedicinal uses in North-East India. Along with diet control and exercise, oral hypoglycemics have conventionally been used in the treatment of type 2 diabetes. Although these agents can control hyperglycemia and glycosuria, they increase cardiovascular complications and cannot prevent the associated macrovascular and microvascular complications in the long term.[20] Medicinal plants and natural products thereof historically possessed a paramount impact in drug discovery and served as the pivot of premature medications.[21] This has been made a vista in the pursuit of alternative options from traditional or folk medicine to treat this disease.
From the acute toxicity study, DCME was found to be safe orally at the dose of 2000 mg/kg. Alpha-amylase and alpha-glucosidase are endo-enzymes of the digestive tract which are responsible for the hydrolysis of starch and disaccharides to glucose and breakdown of long-chain carbohydrates. The inhibitors of these enzymes are considered as the potential targets in the management of diabetes mellitus.[22] In-vitro assays showed that DCME have in-vitro alpha-amylase and alpha-glucosidase inhibitory activity, and it can be suggested from the results that DCME can reduce the absorption of monosaccharides which helps control blood sugar level. OGTT was performed to determine the clearance of glucose from blood with time after glucose intake. It is an important parameter to determine diabetic status and insulin resistance.[23] It was found that DCME have significant glucose tolerance effect when compared with normal control group. Administration of DCME at the oral doses of 200 mg/kg and 400 mg/kg b.w. per day to rats for 28 days demonstrated the antidiabetic activity by significantly lowering the FBG level when compared with diabetic control rats.
Concentration of HbA1c in blood is an important diagnostic parameter to determine diabetic condition, indicating the state of glycemia. HbA1c level ≥6.5% is considered as a high-risk state of diabetes, and it is directly proportional to the FBG content. DCME significantly reduced HbA1c level in diabetic rats. In diabetic condition, it is observed that free fatty acids' level in systemic circulation is increased. The circulating free fatty acids have deleterious effect on the endothelial functions by various pathways and mechanisms which include free radical generation and protein kinase C activation, and thus aggravate dyslipidemia.[24] Dyslipidemia implies high plasma level of triglycerides, low-density lipoprotein-cholesterol, cholesterol, and low plasma level of HDL-cholesterol. In diabetic condition, free radicals' (reactive oxygen and nitrogen species) production is increased due to less or no scavenging of these excess free radicals, resulting in increased oxidative stress.[25] Oxidative stress leads to several micro- and macrovascular complications of diabetes (as stated in introduction). It was observed that DCME has significant lipid (triglyceride and total cholesterol)-lowering ability when compared with diabetic control. HDL-cholesterol, which is regarded as good cholesterol, significantly increased in DCME-treated groups.
During diabetic condition, endogenous antioxidant mechanism becomes less effective to scavenge overproduced free radicals, which causes several pathophysiological changes in different organs. Liver and kidney are important vital organs of storage, detoxification, metabolism, and excretion of metabolites and xenobiotics, so they are particularly vulnerable to oxidative damage. The reason of liver and kidney tissue destruction is oxidative stress involving the secretion of cytokines, mainly tumor necrosis factor-α, interleukin-1, and interferon-c.[26] Due to hepatotoxicity, liver function enzymes, namely, SGOT, SGPT, and SALP levels become increased. It was observed that in diabetic group, serum hepatic marker levels were significantly higher than that of the normal control group. SGOT, SGPT, and SALP levels significantly reduced toward the normal values after 28 days of treatment with DCME when compared with those of diabetic control.
Free radicals such as superoxide (o2−) and hydroxyl (OH−) cause initiation of tissue LPO. LPO is usually measured by the formation of TBA reactive substance, i.e., MDA.[27] Increased levels of hepatic and renal MDA in diabetic rats indicated the impairment of endogenous antioxidant systems. In DCME-treated groups, MDA level decreased significantly compared to diabetic control which reveals that DCME has protective property over oxidative damage at cellular level.
SOD is an important endogenous enzymatic antioxidant which helps to protect organs from oxidative damage by free radicals. GSH serves as a nonenzymatic endogenous antioxidant system which detoxifies hydrogen peroxide radical with the help of glutathione peroxidase and prevents LPO.[28] In diabetic control group, SOD and GSH were depleted due to oxidative insult and less synthesis of GSH in diabetic condition. Hepatic and renal SOD activities and GSH levels were significantly elevated in DCME-treated rats compared to diabetic control rats, indicating prominent boasting of endogenous antioxidative defense prevalent at tissue level of DCME-treated diabetic rats.
Histopathological examination of pancreas of DCME-treated diabetic rats showed dose-dependent improvement in β-cell density compared to diabetic control group, indicating development at cellular level by DCME treatment.
It was evident from the present study that DCME demonstrated marked antidiabetic activity in albino rats in a dose-dependent manner. Preliminary phytochemical studies indicated the presence of flavonoids and alkaloids in DCME. Flavonoids are well-reviewed putative natural antioxidants.[29,30] Previous researchers reported that the aerial parts of D. cordata contained flavonoids which had antioxidant property.[31] The observed antioxidative effect in vivo may be due to the presence of flavonoids. The results demonstrate significant antidiabetic potential of D. cordata leaf plausibly through abrogation of excess oxidative stress and serum lipid levels, corroborating the traditional use of this plant in folk medicine of Sikkim, North-East India. To the best of our knowledge, this is the first report of antidiabetic evaluation of D. cordata.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to the Department of Pharmaceutical Technology, Faculty of Engineering and Technology, Jadavpur University, Kolkata - 700 032, India, for providing necessary facilities related to the study. The authors are thankful to the Department of Science and Technology (DST), West Bengal, India, for providing financial supports.
REFERENCES
- 1.Jaiswal YS, Tatke PA, Gabhe SY, Vaidya AB. Antidiabetic activity of extracts of Anacardium occidentale linn. Leaves on n-streptozotocin diabetic rats. J Tradit Complement Med. 2017;7:421–7. doi: 10.1016/j.jtcme.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–73. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Dey P, Chandra S, Chatterjee P, Bhattacharya S. Neuropharmacological properties of Mikania scandens (L.) willd. (Asteraceae) J Adv Pharm Technol Res. 2011;2:255–9. doi: 10.4103/2231-4040.90883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya S. Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharm Biol. 2017;55:349–54. doi: 10.1080/13880209.2016.1235207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nono NR, Nzowa KL, Barboni L, Tapondjou AL. Drymaria cordata (Linn.) Willd (Caryophyllaceae): Ethnobotany pharmacology and phytochemistry. Adv Biol Chem. 2014;4:160–7. [Google Scholar]
- 6.Akindele AJ, Ibe IF, Adeyemi OO. Analgesic and antipyretic activities of Drymaria cordata (Linn.) willd (Caryophyllaceae) extract. Afr J Tradit Complement Altern Med. 2012;9:25–35. doi: 10.4314/ajtcam.v9i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeyemi OO, Akindele AJ, Nwaubani N. Anti-inflammatory activity of Drymaria cordata extract. J Nat Rem. 2008;8:93–100. [Google Scholar]
- 8.Mukherjee PK, Saha K, Bhattacharya S, Giri SN, Pal M, Saha BP. Studies on antitussive activity of Drymaria cordata willd. (Caryophyllaceae) J Ethnopharmacol. 1997;56:77–80. doi: 10.1016/s0378-8741(97)01512-2. [DOI] [PubMed] [Google Scholar]
- 9.Idrisi MS, Badola HK, Singh R. Indigenous knowledge and medicinal use of plants by local communities in Rangit valley, South Sikkim, India. NeBIO. 2010;1:34–5. [Google Scholar]
- 10.Shrestha B, Basnett H, Pal P. Herbal remedies practised by traditional practitioners of Nepali tribe in Sikkim. Univers J Pharm Sci Res. 2015;1:51–3. [Google Scholar]
- 11.Mukherjee PK, Bhattacharya S, Saha K, Giri SN, Pal M, Saha BP. Antibacterial evaluation of Drymaria cordata Willd. (Fam. Caryophyllaceae) extract. Phytother Res. 1997;11:249–250. [Google Scholar]
- 12.Barua CC, Roy JD, Buragohain B, Barua AG, Borah P, Lahkar M. Anxiolytic effect of hydroethanolic extract of Drymaria cordata L willd. Indian J Exp Biol. 2009;47:969–73. [PubMed] [Google Scholar]
- 13.Barua CC, Roy JD, Buragohain B, Barua AG, Borah P, Lahkar M. Analgesic and anti-nociceptive activity of hydroethanolic extract of Drymaria cordata willd. Indian J Pharmacol. 2011;43:121–5. doi: 10.4103/0253-7613.77337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowemimo A, van de Venter M, Baatjies L, Koekemoer T. Cytotoxic activity of selected Nigerian plants. Afr J Tradit Complement Altern Med. 2009;6:526–8. doi: 10.4314/ajtcam.v6i4.57186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatesan S, Sankar V, Sankar AS. Preliminary phytochemical studies on leaves of Drymaria cordata willd. Anc Sci Life. 2003;23:16–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. Paris: Organisation for Economic Co-operation and Development Publishing; 2008. [Google Scholar]
- 17.Telagari M, Hullatti K. In vitroα-amylase and α-glucosidase inhibitory activity of Adiantum caudatum linn. And Celosia argentea linn. Extracts and fractions. Indian J Pharmacol. 2015;47:425–9. doi: 10.4103/0253-7613.161270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review) Acta Physiol Hung. 2014;101:408–20. doi: 10.1556/APhysiol.101.2014.4.2. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Bhattacharya S, Prasanna A, Suresh Kumar RB, Pramanik G, Haldar PK. Preclinical evaluation of antihyperglycemic activity of Clerodendrum infortunatum leaf against streptozotocin-induced diabetic rats. Diabetes Ther. 2011;2:92–100. doi: 10.1007/s13300-010-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty M, Bala A, Bhattacharya S, Haldar PK. Hypoglycemic effect of ethyl acetate fraction of methanol extract from Campylandra aurantiaca rhizome on high-fat diet and low-dose streptozotocin-induced diabetic rats. Pharmacogn Mag. 2018;14:539–45. [Google Scholar]
- 21.Biswas M, Bhattacharya S, Mukhopadhyay R, Haldar PK. Dregea volubilis (L. f.) Benth. (Asclepiadaceae): An appraisal on pharmacognostic, phytochemical and pharmacological studies. Orient Pharm Exp Med. 2018;18:1–8. [Google Scholar]
- 22.Tiwari N, Thakur AK, Kumar V, Dey A, Kumar V. Therapeutic targets for diabetes mellitus: An update. Clin Pharmacol Biopharm. 2014;3:117. [Google Scholar]
- 23.Kumar A, Lingadurai S, Shrivastava TP, Bhattacharya S, Haldar PK. Hypoglycemic activity of Erythrina variegata leaf in streptozotocin-induced diabetic rats. Pharm Biol. 2011;49:577–82. doi: 10.3109/13880209.2010.529615. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg IJ. Clinical review 124: Diabetic dyslipidemia: Causes and consequences. J Clin Endocrinol Metab. 2001;86:965–71. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 25.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboonabi A, Rahmat A, Fauziah O. Effect of pomegranate on histopathology of liver and kidney on generated oxidative stress diabetic induced rats. J Cytol Histol. 2014;6:294. [Google Scholar]
- 27.Biswas M, Kar B, Bhattacharya S, Kumar RB, Ghosh AK, Haldar PK. Antihyperglycemic activity and antioxidant role of Terminalia arjuna leaf in streptozotocin-induced diabetic rats. Pharm Biol. 2011;49:335–40. doi: 10.3109/13880209.2010.516755. [DOI] [PubMed] [Google Scholar]
- 28.Forman HJ. Glutathione – From antioxidant to post-translational modifier. Arch Biochem Biophys. 2016;595:64–7. doi: 10.1016/j.abb.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee P, Chandra S, Dey P, Bhattacharya S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J Adv Pharm Technol Res. 2012;3:136–8. doi: 10.4103/2231-4040.97298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharya S. The role of medicinal plants and natural products in melioration of cadmium toxicity. Orient Pharm Exp Med. 2018;18:177–86. [Google Scholar]
- 31.Nono RN, Nguelefack-Mbuyo EP, Nzowa LK, Ponou BK, Teponno RB, Nguelefack TB, et al. Antioxidant C-glycosylflavones of Drymaria cordata (Linn.) willd. Arch Pharm Res. 2016;39:43–50. doi: 10.1007/s12272-015-0691-7. [DOI] [PubMed] [Google Scholar]