Abstract
Atherosclerosis is a leading cause of death worldwide. The adverse side effects of currently available drugs urge to find more effective and safe remedial agents. Alternative candidates from natural resources are of great consequence in the emerging of new drugs. Pandanus tectorius (Pandanaceae) was traditionally used in Ayurvedic medicine to cure certain diseases. Thus, the current study conducted to elucidate the potency of P. tectorius fruit as antiatherosclerosis and antihypercholesterolemia agents through the regulation of high density lipoprotein (HDL) receptor (scavenger receptor [SR]-B1) gene expression and 3-hydroxy-3-methylglutaryl coenzyme A reductase reductase (HMGCR) in vitro, respectively. The P. tectorius fruit was noncytotoxic against the HepG2 cell line confirmed by 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide assay. The P. tectorius fruit successfully upregulates the SR-B1 gene expression and downregulate the HMGCR. Moreover, an in vivo study showed that P. tectorius has good activity on the upregulation of HDL and subsequently downregulation of total cholesterol level. Moreover, P. tectorius fruit did not show any increase in toxicity biomarkers serum glutamic oxaloacetic transaminase and serum glutamate pyruvate transaminase in vivo. These results found that P. tectorius fruits have potency as the preventive agent for hypercholesterolemia and atherosclerosis via SR-B1 and HMGCR mechanisms of action.
Key words: 3-hydroxy-3-methylglutaryl coenzyme A reductase, antiatherosclerosis, antihypercholesterolemia, Pandanus tectorius, scavenger receptor-B1
INTRODUCTION
Cardiovascular diseases (CVDs) as myocardial infarction, acute coronary syndrome, and stroke occur due to the formation of plaques in the arteries.[1,2] Hypercholesterolemia, a crucial significant factor for the development of CVDs along with atherosclerosis and heart stroke.[3,4] Various genetic factors such as hypertension, insulin resistance, obesity, and environmental factors as smoking, fat-enriched diet, poor lifestyle, luck exercise considered primary risk factors involved in the expansion of atherosclerosis.[5,6]
Scavenger receptor BI (SR-B1) is a multiligand receptor glycoprotein. SR-B1 receptor mediates uptake of HDL-derived cholesteryl esters into cells and surrounding tissue.[7,8,9] Statins widely used in the prevention of cardiovascular disease and lowing of LDL (low-density lipoproteins). The main aim of the drug is to regulate cholesterol levels via inhibiting -hydroxy-3-methylglutaryl coenzyme A reductase reductase (HMGCR) activity (as a competitive inhibitor). HMGCR considers having a vital role in the rate-limiting step involved in the biosynthesis of cholesterol.[7] However, it involves organ damage (liver injury) and muscle toxicity.[10,11,12] Over the past few decades, herbal medicines have captivated much attention for atherosclerosis due to their potential of multiple targeting steps involved in disease pathogenesis with reduce side effects. Previous studies have reported the Pandanus tectorius to possess cholesterol-reducing activity,[13] and hypolipidemic activity.[14] The caffeoylquinic acid isolated from P. tectorius possessed an antiglycemic and antilipidemic effect.[15,16] Therefore, the current study focus on the P. tectorius fruit as an anti-atherosclerosis agent via regulating the SR-B1 gene expression and HMGCR activity.
MATERIALS AND METHODS
The HepG2 cells obtained from the American Type Culture Collection (Rockville, MD, USA). The plasmid midi kit purchased from Qiagen 100 protocol, cat no (Valencia, CA, USA) all the reagents purchased from Sigma Aldrich. While, the rats (male Spraque Dawley) purchased from Takrif Bistari Enterprise, Kuala Lumpur, Malaysia.
Sampling location
The P. tecorius (fruit) collected from Batu Rakit, Kuala Terengganu, Malaysia (TER0618002). The sample deposited at the Institute of Marine Biotechnology, Universiti Malaysia Terengganu.
Extraction and solvent fractionation of Pandanus tectorius fruits
The P. tectorius (fruit) extraction done by using cold maceration method using methanol to yield methanol extract first (PMK). Then, the PMK was used to produce the PHK (Haxane) and PEK (Ethyl acetate) extract by solvent-solvent partitioning method.
In vitro study
Cytotoxicity study
Cytotoxicity performed by 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT) assay.[17,18] The HepG2 cells seeded at a concentration of 2 × 104 cells/well on 96-well plates. After the treatment, 20 μL fresh MTT solution was added, and the plate incubated at 37°C, 5% CO2. The plate read by ELISA reader at a wavelength of 570 nm.
The effect of extracts on the transcriptional activity of scavenger receptor-B1
Extraction of the reporter plasmid DNA and Lipofectin™ transfection and luciferase activity
Two recombinant plasmids pGL-3 SR-B1 and Mammalian co-reporter vector of Renilla luciferase purified by using plasmid midi kit, Qiagen 100 protocol, cat no 12145 (Valencia, CA, USA). Transient transfection carried out using Lipofectin™ (Invitrogen), and luciferase activity were measured using the Dual-Luciferase™ (Promega).[19]
Inhibitory effect of Pandanus tectorius fruit on 3-hydroxy-3-methylglutaryl coenzyme A reductase reductase enzyme
The inhibition study of HMGCR enzyme performed according to the method provided (Sigma Aldrich, CS1090, St. Louis, USA).
In vivo study
Ethics statement
The Research Ethics approved the experimental design protocol and guidelines in this study.
The Research Ethics includes experimental design, protocol and guidelines in this study approved by Committee (JKEP), Universiti Malaysia Terengganu, with the reference number UMT/JKEPHT/2018/18. 31.
Animal and treatments
A forty male of Sprague dawley rats, healthy with regular activity. The 6–8 weeks old with approximately body weight 160–200 g. All of the Rats divided into four groups (Group A, B, C, and D) randomly, where there are ten rats in each group. Group A: As a negative control (feed by standard food only); Group B: As a positive control (feed by high cholesterol diet food); Whereas Group C and D are treatment groups that were feed by high cholesterol diet food and actives fraction of P. tectorius fruit PEK/PMK (500 g/kg BW), respectively. After the treatment periods, the rats conducted for recovery periods for more than 2 weeks. The blood sample of rats withdrawn to analysis their HDL, total cholesterol, serum glutamic oxaloacetic transaminase (SGOT), and serum glutamine-pyruvate transaminase (SGPT) levels. Cholesterol diet food in this current study made by 2% of cholesterol, 0.1% of propylthiouracil (PTU), 10% of cooking oil, and then mixed with the standard food until 100%.[19]
Measuring total cholesterol level and enzymes activity
The total cholesterol levels analyzed by cholesterol oxidase phenol 4-aminoantipyrine peroxidase (CHOD-PAP) Method. The HDL levels were analyzed using manufacturer precipitant method of HDL cholesterol (Randox, Crumlin Co., Antrim, UK). The enzyme activity measured by using SGPT and SGOT reagents (1000 μL). The activities read by spectrophotometer at 365 nm wavelength.
Statistical analysis
The experiment conducted in triplicates (mean ± standard deviation). The analysis of variance was applied, followed by the Tukey test using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). The significant results considered as P ≤ 0.05 among groups.
RESULTS AND DISCUSSION
Cytotoxicity study and scavenger receptor-B1 gene expression assay
The cytotoxicity analysis shows that PHK, PEK, [Figure 1] and PMK of P. tectorius fruits extract noncytotoxic (half maximal inhibitory concentration [IC50] <30 μg/mL). As a criterion, the IC50 value below 30 μg/mL considers as cytotoxic.[17] Moreover, the role of P. tectorius fruit in the regulation of the regulator of receptor protein SR-B1 also determine. The PEK and PMK exhibited a maximum increase at the concentration of 6.25 μg/mL; however, PHK produced the dose-dependent increase in SR-B1 gene expression, where the maximum exhibition noticed at 50 μg/mL [Figure 2]. Furthermore, PEK produced a higher level of expression (56.56%) comparable to PMK (45.70%). According to Zanoni et al.[20], SR-B1 as an HDL receptor can protect against atherosclerosis and CDV diseases. SR-B1 plays a significant part in cholesterol homeostasis, lipoprotein metabolism, and particularly atherosclerosis.[21,22] SR-B1 is a factor responsible for regulating cholesterol via reverse cholesterol transport.[20,23]
Figure 1.
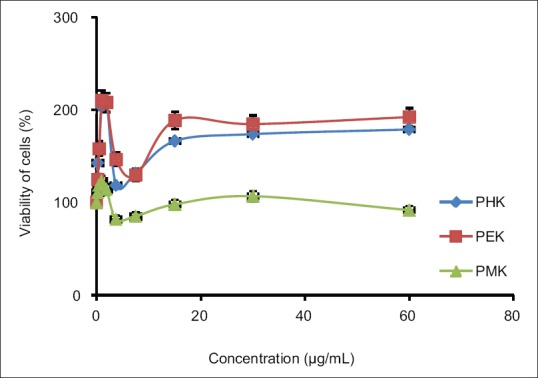
Cytotoxicity property PHK, PEK and PMK of Pandanus tectorius fruit against HepG2 cell lines. PHK (Pandanus Hexane Extract), PEK (Pandanus Ethyl acetate Extract) and PMK (Pandanus Mathanol Extract)
Figure 2.
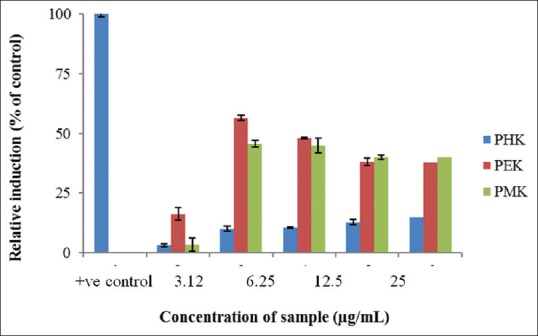
The activity value of scavenger receptor-B1 promoter that given PHK, PEK, PMK and positive control (rosiglitazone)
A reductase 3-hydroxy-3-methylglutaryl coenzyme inhibition activity
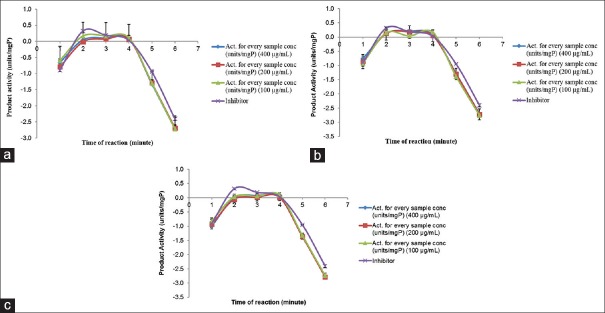
To further investigate the cholesterol regulatory effects of P. tectorius fruit, HMGCR activity assay performed. Figure 3a–c shows that extracts (PHK, PEK, and PMK) inhibited the HMGCR enzyme activity at 400, 200, and 100 mg/mL. PEK achieved the highest activity (0.21 units/mgP or 65.63% activity) comparable to the Pravastatin (+ve control), followed by PHK and PMK. The activity of P. tectorius extracts (PHK, PEK, and PMK) correlated to our previous study, which showed the presence of the phenolic and flavonoids constituents in extracts.[17]
Figure 3.
(a-c) The effect of PHK on 3-hydroxy-3-methylglutaryl coenzyme A reductase reductase activity at concentration of 400, 200, and 100 μg/mL compare to the inhibitor (Pravastatin)
In vivo study
Bodyweight and cholesterol levels
The high cholesterol diet food intake de-regulate the HMGCR contributes to fat stored and weight gain of rats.[24,25] The decrease in the total cholesterol level in Groups C and D are seen after 2 weeks of treatment of the P. tectorius fruits, with 67% and 65% compared to the cholesterol Group (B), respectively (significantly different at P < 0.05) [Figures 4 and 5]. The reduction in total cholesterol levels in Groups B, C, D was comparative to Group A which shows that PEK and PMK might consider as preventive agents for hypercholesterolemia and atherosclerosis. Similarly, the increase in HDL level [Figure 6] might be due to the presence of secondary metabolites in P. tectorius fruit. The β– carotene presence represented by the deep orange-red color of the fully ripe fruit. The previous studies showed that the P. tectorius (fruit) contains secondary metabolites such as (vanillin, chrysin, trans-ethyl caffeate, naringenin, caffeoylquinic acid, and tangeretin).[14,26] The HDL particles lower cholesterol levels and reducing the risk of atherosclerosis by it binding to hepatic SR-B1.[27,28] The sterol regulatory element-binding proteins (SREBPs) belong to a class of transcription factors (membrane-bound) that play a fundamental role in the regulation of cholesterol and fatty acids biosynthesis.[29] Li Y et al., 2013 studied that the activation of SREBPs and the lipogenic process may trigger lipid accumulation and deposition in an atherosclerotic aorta.[30] Thus, the sample, which has an increasing the SR-B1 gene expression, could also have a capability in inhibiting HMGCR activity. These two mechanisms of action could be correlated to the capability of P. tectorius fruits to reduce total cholesterol levels and prevent atherosclerosis.
Figure 4.
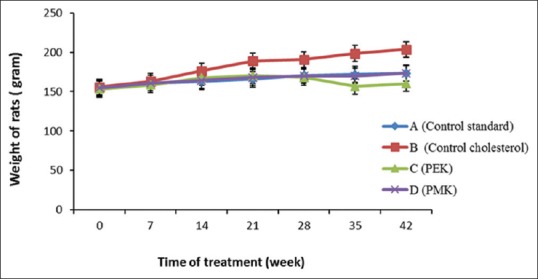
Rats body weight during 42 days of treatment that taken each week
Figure 5.
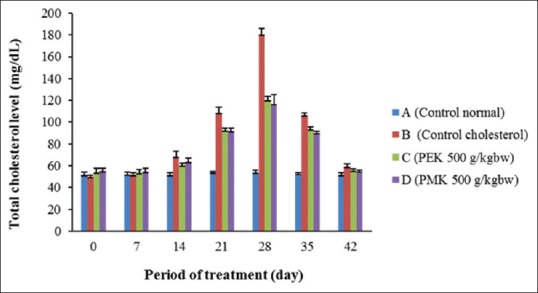
The reduction of total cholesterol levels during treatment on groups of standard food (A); control cholesterol food (B); PEK 500 g/kg BW (C); and PMK 500 g/kg BW (D)
Figure 6.
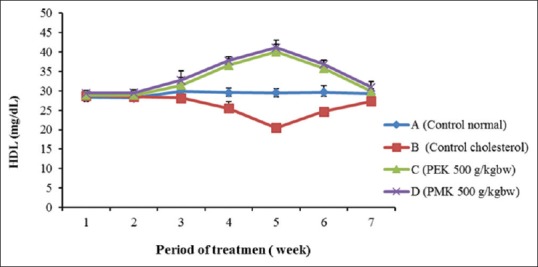
The HDL cholesterol level during 42-day treatment
Furthermore, the SGOT and SGPT levels were determined to measure the toxicity of P. tectorius fruit on the liver. The influence of P. tectorius (PEK and PMK) in Group C and D exhibited SGOT activities ranging from 132.303 to 134.653 U/l (Unit per liter) and from 134.140 to 135.380 U/I, respectively comparable to toxic value 242.10 ± 36.40 U/I (Jeong et al., 2005).[31] Similarly, Groups C and D exhibited SGPT activity is less with range 64.872–66.462 U/I as compare to previous reported toxic ranges 232.65 ± 17.38 U/I–283,30 ± 30.10 U/I range [Table 1].[32] Hence, the administration of PEK and PMK of P. tectorius fruits at a dose of 500 mg/kg BW can be categorized as nontoxic and selected as a safe therapeutic dose [Table 2].
Table 1.
The serum glutamic oxaloacetic transaminase activities of rat plasma during treatment on each group
| Group | Activity of SGOT (U/I) for every 7 days during treatment |
||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
| A | 134.23±1.04 | 134.51±1.28 | 134.25±1.41 | 134.19±0.95 | 134.25±0.86 | 134.19±0.888 | 134.02±1.15 |
| B | 134.32±1.14 | 137.14±0.93* | 137.35±1.19* | 138.53±1.41* | 137.93±1.22* | 137.78±1.22* | 137.91±1.17* |
| C | 134.65±0.68 | 133.59±0.96 | 132.30±1.55 | 132.32±1.13 | 132.42±1.99 | 132.54±2.07 | 132.70±1.07 |
| D | 134.63±0.58 | 134.14±0.75 | 134.40±0.84 | 134.93±0.62 | 135.13±0.77 | 135.38±0.41 | 135.29±0.87 |
Group A as control of standard food, Group B as control of cholesterol food, Group C as PEK (Pandanus Ethyl acetate Extract), and Group D as PMK (Pandanus Methanol Extract). *P<0.05 significantly different in comparison among normal, cholesterol, and treatment group; each value is presented as mean±SD (n=10). SGOT: Serum glutamic oxaloacetic transaminase, SD: Standard deviation
Table 2.
The serum glutamate pyruvate transaminase activities of rat plasma during treatment on each group
| Group | Activity of SGPT (U/I) for every 7 days during treatment |
||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
| A | 64.46±1.20 | 63.95±1.61 | 64.73±1.23 | 64.96±1.18 | 64.85±1.12 | 65.15±0.94 | 64.34±0.57 |
| B | 64.66±0.68 | 67.32±1.66* | 66.95±2.98* | 71.83±0.41* | 70.99±4.13* | 71.17±1.78* | 71.39±2.47* |
| C | 64.87±0.86 | 65.98±0.76 | 66.46±0.94 | 64.95±1.31 | 65.04±1.28 | 64.87±0.89 | 64.87±0.96 |
| D | 65.21±0.69 | 65.70±0.97 | 65.74±0.94 | 65.83±0.95 | 65.52±0.90 | 65.10±0.83 | 65.69±1.19 |
Group A as control of standard food, Group B as control of cholesterol food, Group C as PEK, and Group D as PMK. *P<0.05 significantly different in comparisn among normal, cholesterol, and treatment group; each value is presented as mean±SD (n =10). SGPT: Serum glutamic pyruvate transaminase, SD: Standard deviation
CONCLUSIONS
All samples from P. tectorius fruits extracts showed good activity on increasing of SR-B1 gene expression and inhibiting HMGCR activity in the in vitro study, and also reducing total cholesterol levels in the in vivo study. Besides, the cytotoxicity assay has shown that P. tectorius fruit was proven to have noncytotoxic activity on HepG2 cells. The SGOT and SGPT levels remained constant after treatment with PEK, and PMK reflected the nontoxic activity of P. tectorius fruits. Thus, the study proved that P. tectorius (fruits) as an excellent alternative source of natural phytochemicals for anti-hypercholesterolemia and anti-atherosclerosis potential agents via a regulatory effect on SR-B1 and HMGCR activities.
Financial support and sponsorship
Ministry of Higher Education (MOHE) – Malaysia: Fundamental Research Grant Scheme (FRGS) Fasa I/2017 (Vote No 59477) and Fasa I/2014 (Vote No 59346).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, et al. The yin and yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman duff memorial lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–42. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 2.Keaney JF., Jr Atherosclerosis: From lesion formation to plaque activation and endothelial dysfunction. Mol Aspects Med. 2000;21:99–166. doi: 10.1016/s0098-2997(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 3.Wald NJ, Law MR. Serum cholesterol and ischaemic heart disease. Atherosclerosis. 1995;118(Suppl):S1–5. [PubMed] [Google Scholar]
- 4.Krieger M. The “best” of cholesterols, the “worst” of cholesterols: A tale of two receptors. Proc Natl Acad Sci U S A. 1998;95:4077–80. doi: 10.1073/pnas.95.8.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, et al. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis. 2009;202:263–71. doi: 10.1016/j.atherosclerosis.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: Exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2:160–6. doi: 10.1007/s11883-000-0111-3. [DOI] [PubMed] [Google Scholar]
- 7.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–9. [PubMed] [Google Scholar]
- 8.Brundert M, Ewert A, Heeren J, Behrendt B, Ramakrishnan R, Greten H, et al. Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl ester by the liver in mice. Arterioscler Thromb Vasc Biol. 2005;25:143–8. doi: 10.1161/01.ATV.0000149381.16166.c6. [DOI] [PubMed] [Google Scholar]
- 9.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo A, Tsujita Y, Kuroda M, Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur J Biochem. 1977;77:31–6. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- 11.Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151:43–9. doi: 10.1001/archinte.151.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A. Adverse effects of statins – Mechanisms and consequences. Curr Drug Saf. 2009;4:209–28. doi: 10.2174/157488609789006949. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Park YB, Bae KH, Bok SH, Kwon YK, Lee ES, et al. Cholesterol-lowering activity of naringenin via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and acyl coenzyme A: cholesterol acyltransferase in rats. Ann Nutr Metab. 1999;43:173–80. doi: 10.1159/000012783. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Wu C, Wu H, Sheng L, Su Y, Zhang X, et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS One. 2013;8:e61922. doi: 10.1371/journal.pone.0061922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Zhang X, Zhang X, Luan H, Sun G, Sun X, et al. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J Nutr Biochem. 2014;25:412–9. doi: 10.1016/j.jnutbio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Zhang X, Wu C, Wu H, Guo P, Xu X. Anti-hyperlipidemic caffeoylquinic acids from the fruits of Pandanus tectorius Soland. J Appl Pharm Sci. 2013;3:016–9. [Google Scholar]
- 17.Andriani Y, Tengku-Muhammad TS, Mohamad H, Saidin J, Syamsumir DF, Chew GS, et al. Phaleria macrocarpa Boerl. (Thymelaeaceae) leaves increase SR-BI expression and reduce cholesterol levels in rats fed a high cholesterol diet. Molecules. 2015;20:4410–29. doi: 10.3390/molecules20034410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Andriani Y, Ramli NM, Syamsumir DF, Kassim MN, Jaafar J, Azis AN, et al. Phytochemical analysis antioxidant antibacteria and cytotoxicity activities of keys and cores part of Pandanus tectorius fruits. Arabian J Chem. 2015:3555–64. [Google Scholar]
- 20.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. CHD exome consortium; cardiogram exome consortium; global lipids genetics consortium. Science. 2016;351:1166–71. [Google Scholar]
- 21.Eckhardt ER, Cai L, Sun B, Webb NR, Deneys RW. High density lipoprotein uptake by scavenger receptor SRB1. J Biol Chem. 2004;273:4372–81. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- 22.Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276:25287–93. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM. Scavenger receptor B-1 emerges as anti-atherogenic candidate. Cell Metab. 2016;23:755–7. doi: 10.1016/j.cmet.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Murray K. Harper Biochemistry. New York: Mc Graw Hill Companie; 2002. [Google Scholar]
- 25.Ahmed MS, Olfat MY. Physiological studies on the risk factors responsible for atherosclerotic in rat. Nat Sci. 2010;5:144–55. [Google Scholar]
- 26.Zhang X, Guo P, Sun G, Chen S, Yang M, Fu N, et al. Phenolic compounds and flavonoids from the fruits of Pandanus tectorius Soland. J Med Plants Res. 2012;6:2622–6. [Google Scholar]
- 27.Li M, Diao Y, Liu Y, Huang H, Li Y, Tan P, et al. Chronic moderate alcohol intakes accelerate SR-B1 mediated reverse cholesterol transport. Sci Rep. 2016;6:33032. doi: 10.1038/srep33032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro-González I, Pérez-Sánchez H, Martín-Pozuelo G, García-Alonso J, Periago MJ. The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR:In vivo study and molecular modelling. PLoS One. 2014;9:e83968. doi: 10.1371/journal.pone.0083968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffler U, Hobbie K, Wilson R, Bai R, Rahman A, Malarkey D, et al. Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine. 2009;36:311–25. doi: 10.1007/s12020-009-9224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Xu S, Jiang B, Cohen RA, Zang M. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS One. 2013;8:e67532. doi: 10.1371/journal.pone.0067532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong WI, Jeong DH, Do SH, Kim YK, Park HY, Kwon OD, et al. Mild Hepatic fibrosis in cholesterol and sodium cholate diet-feed rats. J Vet Med Sci. 2005;67:235–42. doi: 10.1292/jvms.67.235. [DOI] [PubMed] [Google Scholar]
- 32.Ravikumar S, Gnanadesigan M. Hepatoprotective and antioxidant activity of a mangrove plant Lumnitzera racemosa. Asian Pac J Trop Biomed. 2011;1:348–52. doi: 10.1016/S2221-1691(11)60078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]