Abstract
Insulin-like growth factors (IGFs), specifically IGF1 and IGF2, promote glucose metabolism, with their availability regulated by IGF-binding proteins (IGFBPs). We hypothesized that IGF1 and IGF2 levels, or their bioavailability, are reduced during type 1 diabetes development. Total serum IGF1, IGF2, and IGFBP1–7 levels were measured in an age-matched, cross-sectional cohort at varying stages of progression to type 1 diabetes. IGF1 and IGF2 levels were significantly lower in autoantibody (AAb)+ compared with AAb− relatives of subjects with type 1 diabetes. Most high-affinity IGFBPs were unchanged in individuals with pre–type 1 diabetes, suggesting that total IGF levels may reflect bioactivity. We also measured serum IGFs from a cohort of fasted subjects with type 1 diabetes. IGF1 levels significantly decreased with disease duration, in parallel with declining β-cell function. Additionally, plasma IGF levels were assessed in an AAb+ cohort monthly for a year. IGF1 and IGF2 showed longitudinal stability in single AAb+ subjects, but IGF1 levels decreased over time in subjects with multiple AAb and those who progressed to type 1 diabetes, particularly postdiagnosis. In sum, IGFs are dysregulated both before and after the clinical diagnosis of type 1 diabetes and may serve as novel biomarkers to improve disease prediction.
Introduction
Public health care screening for presymptomatic type 1 diabetes does not yet exist, but efforts are underway to evaluate feasibility of population-based genetic testing and screening for disease-related autoantibodies (AAb) (1–3). A recent consensus article presented a two-step definition of pre–type 1 diabetes: the first being seroconversion to two or more AAb against β-cell antigens (stage 1), with stage 2 occurring when multiple AAb+ subjects demonstrate dysglycemia following an oral glucose tolerance test (OGTT) (4). The duration of progression to overt hyperglycemia is highly variable, ranging from weeks to decades beyond seroconversion (5), and OGTTs are time-consuming and require multiple venous blood sample collections. This exemplifies the need for minimally invasive biomarkers reflective of metabolic dysregulation to complement AAb screening in predicting type 1 diabetes prior to clinical manifestation (stage 3).
Type 1 diabetes is thought to result from deficient immunoregulation together with impaired β-cell viability and/or function (6). The insulin-like growth factor (IGF) axis and particularly IGF1 and IGF2 are candidates for correcting these deficiencies (7). IGFs are hormones produced primarily by the liver that induce cellular proliferation via the widely expressed IGF1 receptor (8). IGF1 and IGF2 are highly homologous to insulin in structure (9,10) and can mediate similar metabolic effects but are unable to compensate for the loss of insulin production in type 1 diabetes. IGF production is temporally regulated such that IGF2 is thought to primarily be important for embryonic and fetal development, whereas IGF1 is vital for postnatal growth (8). Possibly impacting type 1 diabetes pathogenesis, IGFs have been shown to promote the regulation of T cell–mediated inflammation (11–13). IGFs are also known to support endocrine (14,15) and exocrine pancreatic growth (16,17), which, if lacking, might contribute to the profound deficiency of total pancreas mass in subjects with type 1 diabetes (18,19). However, it remains unclear whether insufficient levels or bioavailability of IGF-family ligands may directly promote the pathogenesis of type 1 diabetes and/or serve as reliable biomarkers of disease staging. Additionally, the regulation of IGF levels by insulin (20) begs the question of whether defects in IGF levels may be primary or secondary to the loss of insulin production known to occur before (21) and after (22) clinical diagnosis.
A recent report described suppressed levels of IGF1 in plasma as children seroconvert to AAb positivity (23); yet, current literature lacks information on the circulating levels of IGF2 during the progression to type 1 diabetes. Additionally, circulating IGFs are generally bound to IGF-binding proteins (IGFBPs) that modulate IGF activity by blocking binding to their cognate receptors (24). A longitudinal study suggested that IGFBP3, which is the most prevalent IGFBP in circulation (25), increases prior to seroconversion to at least one type 1 diabetes–related AAb (23), potentially modulating IGF bioavailability. Three out of seven IGFBPs, which are expressed at differing levels and have varying affinities for IGF1 and IGF2 (25), are increased in serum of subjects with established type 1 diabetes (26). However, the majority of these proteins have not been assessed in pre–type 1 diabetes. Herein, we test the hypothesis that IGF1 and IGF2 levels or bioavailability, as inversely related to IGFBPs, are altered during progression to type 1 diabetes.
Research Design and Methods
Subject Enrollment
All procedures were approved by institutional review boards at each institution and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from participants (or their legal guardian in the case of minors) prior to enrollment.
Cross-sectional
Subjects were recruited from clinics at the University of Florida (UF); Nemours Children’s Hospital (Orlando, FL); and Emory University. Peripheral blood samples were collected from nonfasted subjects by venipuncture in serum separator vacutainer tubes (BD Biosciences) and rested overnight prior to processing to minimize variance due to transport times. Sera were stored in the UF Diabetes Institute (UFDI) biorepository at −20°C prior to batch processing. Subjects were selected from the biorepository by age, sex, ethnicity, and BMI matching to subjects positive for at least one AAb against GAD65 (GADA), IA-2 (IA-2A), or zinc transporter 8 (ZnT8A).
Subjects with type 1 diabetes were recruited by the Benaroya Research Institute (BRI) under the BRIDge Study of Type 1 Diabetes institutional review board protocol. Peripheral blood samples were collected from fasted or nonfasted subjects in serum separator vacutainer tubes (BD Biosciences), processed within 24 h, and stored at −80°C until batch processing. Detailed subject demographic information is presented in Table 1.
Table 1.
Demographic information for UFDI and BRI cross-sectional cohorts
| UFDI | BRI established | ||||||
|---|---|---|---|---|---|---|---|
| AAb− control subjects | AAb− relatives | 1AAb+ | 2–3 AAb+ | Recent onset | Established | ||
| Total subjects, n | 69 | 55 | 25 | 27 | 61 | 68 | 50 |
| Sex, n (%) | |||||||
| Male | 49 (71) | 28 (51) | 11 (44) | 18 (67) | 34 (56) | 36 (53) | 28 (56) |
| Female | 20 (29) | 27 (49) | 14 (56) | 9 (33) | 27 (44) | 32 (47) | 22 (44) |
| Age (years) | 12.6 ± 3.4 | 12.0 ± 3.4 | 10.4 ± 4.5 | 13.1 ± 3.9 | 12.1 ± 3.5 | 11.6 ± 3.6 | 22.6 ± 13.6 |
| Height (m)* | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.3 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.1 |
| Weight (kg)* | 48.3 ± 24.2 | 49.8 ± 24.0 | 41.3 ± 19.3 | 55.4 ± 19.3 | 49.1 ± 20.6 | 50.8 ± 22.7 | 76.1 ± 13.5 |
| BMI (kg/m2)* | 21.0 ± 7.8 | 21.7 ± 7.1 | 20.4 ± 4.8 | 21.2 ± 3.9 | 21.7 ± 5.1 | 22.5 ± 5.9 | 24.3 ± 4.8 |
| BMI percentile (%)*† | 64.1 ± 27.3 | 66.1 ± 29.2 | 75.7 ± 22.1 | 65.5 ± 24.3 | 73.5 ± 24.3 | 76.3 ± 21.0 | 73.2 ± 21.7 |
| Ethnicity, n (%) | |||||||
| Caucasian | 37 (54) | 37 (67) | 16 (64) | 21 (78) | 43 (70) | 37 (54) | 44 (88) |
| African-Am | 16 (23) | 9 (16) | 0 (0) | 2 (7) | 7 (11) | 11 (16) | 0 (0) |
| Hispanic | 8 (12) | 7 (13) | 4 (16) | 4 (15) | 8 (13) | 14 (21) | 1 (2) |
| Asian/Pac-Isl | 5 (7) | 0 (0) | 2 (8) | 0 (0) | 1 (2) | 1 (2) | 3 (6) |
| Native-Am | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| Other | 3 (4) | 2 (4) | 3 (12) | 0 (0) | 2 (3) | 3 (4) | 2 (4) |
| Disease duration (years) | N/A | N/A | N/A | N/A | 0.08 ± 0.06 | 4.35 ± 3.72 | 5.1 ± 10.7 |
| Metabolic status, n (%) | |||||||
| Fasted | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 38 (76) |
| Random | 69 (100) | 55 (100) | 25 (100) | 27 (100) | 61 (100) | 68 (100) | 12 (24) |
Data are means ± SD unless otherwise indicated. UFDI cross-sectional cohorts include AAb− control subjects; AAb− first-degree relatives of a subject with type 1 diabetes; subjects with one, two, or three AAb; subjects with recent-onset type 1 diabetes diagnosed within the past 3 months; and subjects with established type 1 diabetes with disease for ≥3 months. Demographic information for BRI cross-sectional cohort of subjects with established type 1 diabetes also shown. AAb+ subjects included both relatives of subjects with type 1 diabetes and unrelated subjects. African-Am, African American; N/A, not applicable; Native-Am, Native American; Pac-Isl, Pacific Islander.
*Provision of height and weight was voluntary; thus, these data are available for some but not all study subjects.
†Approximately one-half of the BRI cohort are adults and are excluded from BMI percentile for children and teenagers.
Longitudinal
Subjects at risk for type 1 diabetes were recruited and enrolled from 2013 to 2014 in the Type 1 Diabetes Longitudinal Biomarker Trial (T1DBIT) (ClinicalTrials.gov, clinical trial reg. no. NCT01846312 [manuscript in preparation]) by the Pacific Northwest Research Institute and Novo Nordisk Research Center Seattle, Inc. Subjects were followed for up to 18 months postenrollment. Individuals included in this study were positive for at least one islet AAb at the time of enrollment (GADA, IA-2A, or insulin [IAA]), aged ≥ 4 and <40 years, and nonpregnant and not breast-feeding; had no chronic disorders; and used no medication that might impact immune status or progression to type 1 diabetes. Subject demographic information is included in Table 2. Samples were shipped to the clinical site at ambient temperature, processed within 24 h of draw, and stored at −80°C.
Table 2.
Demographic information for longitudinal cohort from Pacific Northwest Research Institute and Novo Nordisk Research Center (T1DBIT)
| T1DBIT | |||
|---|---|---|---|
| 1AAb+ at enrollment | 2–5 AAb+ at enrollment | Developed T1D during study | |
| Total subjects, n | 14 | 20 | 6 |
| Number of time points per subject, mean (range) | 13 (12–13) | 13 (11–13) | 13 (12–13) |
| Time between draws (months) | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.4 |
| Sex, n (%) | |||
| Male | 1 (7) | 12 (60) | 5 (83) |
| Female | 13 (93) | 8 (40) | 1 (17) |
| Age at enrollment (years) | 17.0 ± 8.2 | 14.2 ± 6.5 | 15.0 ± 2.8 |
| Height at enrollment (m)* | 1.6 ± 0.1 | 1.6 ± 0.2 | 1.7 ± 0.1 |
| Weight at enrollment (kg)* | 59.0 ± 18.0 | 53.9 ± 22.4 | 58.3 ± 16.9 |
| BMI at enrollment (kg/m2)* | 23.3 ± 5.9 | 20.7 ± 4.1 | 20.7 ± 3.7 |
| BMI percentile for children and teens at enrollment (%)*† | 68.4 ± 22.3 | 61.1 ± 23.8 | 52.8 ± 32.3 |
| Change in BMI percentile over study*† | r = 0.00; P = 0.97 | r = −0.08; P = 0.25 | r = −0.02; P = 0.86 |
| Ethnicity, n (%) | |||
| Caucasian | 10 (72) | 15 (75) | 4 (67) |
| Hispanic | 1 (7) | 3 (15) | 0 (0) |
| Asian/Pac-Isl | 1 (7) | 0 (0) | 0 (0) |
| Other | 2 (14) | 2 (10) | 2 (33) |
| Disease duration at enrollment (years) | N/A | N/A | N/A |
| Metabolic status, n (%) | |||
| Fasted | 0 (0) | 0 (0) | 0 (0) |
| Random | 14 (100) | 20 (100) | 6 (100) |
Data are means ± SD unless otherwise indicated. Cohort includes subjects 1AAb+ at enrollment, subjects 2–5 AAb+ at enrollment, and subjects who developed type 1 diabetes during the study. Subjects were not selected based on family history. Participants underwent clinic or in-home visits with blood draw and questionnaire collection every 4–6 weeks for a total of 13 samples spanning 12–18 months. Participants who developed T1D during the course of sampling continued on the same frequent sampling schedule at least until completion of their 13th sample visit. At each study visit, nonfasted subjects completed a questionnaire to initiate or update a list of their medical conditions and current medications, if any. Change in BMI reported as Spearman correlation results. N/A, not applicable. Pac-Isl, Pacific Islander.
*Provision of height and weight was voluntary; thus, these data are available for some but not all study subjects.
**Two subjects from 1AAb+ at enrollment and one subject from 2–5 AAb+ at enrollment groups are adults and therefore are excluded from BMI percentile for children and teenagers.
AAb Measurement
For UFDI samples, GADA, IA-2A, and ZnT8A were measured from serum via an Islet Autoantibody Standardization Program (IASP)-validated enzyme-linked immunosorbent assay (ELISA) as previously described (27). For T1DBIT samples, IAA, GADA, IA-2A, IA-2βA, and ZnT8A were measured from serum via radioimmunoassay with positivity defined as the 97.5th percentile of control sera as previously described (28). After enrollment into T1DBIT, seroconversion was defined as at least two consecutive time points with AAb positivity.
Glucose Metabolism
For BRI subjects, glucose, C-peptide, and HbA1c from a mixed-meal tolerance test visit were tested at the University of Washington’s Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA). Serum C-peptide was measured by two-site immunoenzymometric assay on a Tosoh II 600 autoanalyzer, and area under the curve (AUC) C-peptide calculated as previously described (29). HbA1c was measured either at or within 3 months of the time of draw.
For T1DBIT subjects, plasma C-peptide was measured by two-site immunoenzymometric assay performed on a Tosoh II 600 autoanalyzer. HbA1c was measured using a commercial high-performance liquid chromatography–based assay at each draw by a Clinical Laboratory Improvement Amendments–certified clinical laboratory. Subjects were considered to have prediabetes with HbA1c >5.7% and diagnosed with type 1 diabetes at HbA1c >6.4% (30).
IGF1 and IGF2 Quantification
Total IGF1 and IGF2 were measured under blinded conditions in duplicate from human sera (UFDI, BRI) or plasma (T1DBIT) via commercially available ELISAs (ALPCO), according to the manufacturer’s instructions. This assay provides more complete recovery of IGFs compared with traditional acid-ethanol extraction methods (31). Briefly, samples were pretreated with an acidic buffer to dissociate IGFs from IGFBPs, followed by sample neutralization immediately prior to plating such that the high-affinity interaction between IGF and capture antibody occurs prior to IGF-IGFBP reassociation. Data were collected with a SpectraMax M5 plate reader (Molecular Devices) and analyzed with SoftMax Pro, version 4.8. Data were excluded if the coefficient of variation (CV) between duplicates was >25%.
The mean intra-assay CV for duplicates from an initial subset of samples showed that IGF1 (5.3% [n = 36]) and IGF2 (3.5% [n = 32]) measurements were reproducible and precise. Additionally, IGF1 and IGF2 concentrations were measured across seven plates using manufacturer-provided control sera to assess interassay reproducibility in our hands. Interassay CVs from low or high concentration control sera were 11.6% or 2.4%, respectively, for IGF1 and 13.9% or 9.4% for IGF2.
IGFBP Quantification
IGFBP1–7 levels were measured under blinded conditions using a Luminex assay on a MILLIPLEX platform (Millipore EMD), according to the manufacturer’s instructions. Data were analyzed with MILLIPLEX Analyst, version 5.1.0.0. For reported analytes, values outside of the standard curve were assigned the value of the limit of detection (LOD) in the five-parameter logistic regression, as determined by Milliplex Analyst software. Although a variety of methods may be used to impute values outside of the LOD, selection of “fill in” values provides a relatively unbiased estimate of data variance when <30% of data are outside such limits (32), as seen in our data set.
Statistical Analysis
BMI percentiles were calculated according to subject age and sex with available height and weight data using a publicly available SAS program based on growth charts from the Centers for Disease Control and Prevention (33). Normalized IGF1 and IGF2 percentiles were determined via curve fitting of published reference ranges (Supplementary Tables 1 and 2) binned for age and sex (34).
Analyses were performed using GraphPad Prism software, version 7.0. Data are presented as means ± SD unless otherwise specified. Cross-sectional IGF and IGFBP levels across cohorts are shown as violin plots with median and quartiles overlaid. Multiplicity-adjusted P values <0.05 were considered significant.
Data and Resource Availability
The data sets presented are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Total IGF Levels Decrease in Serum of AAb+ Subjects at Risk for Type 1 Diabetes
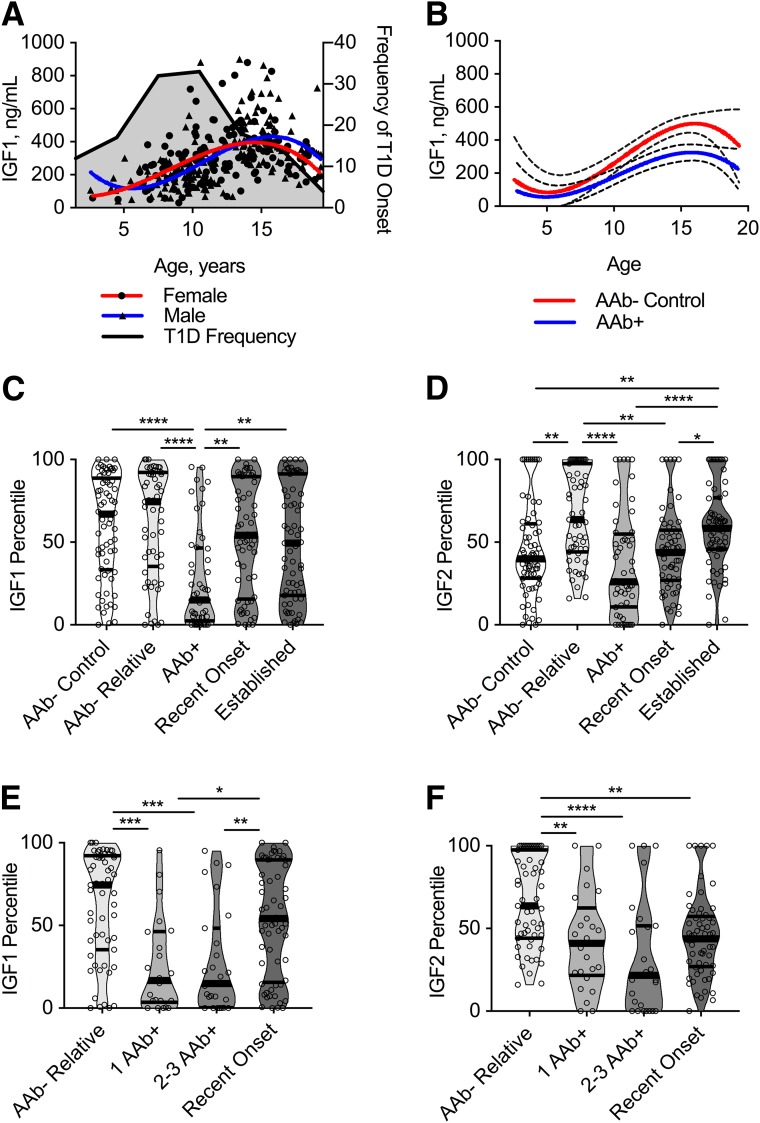
We investigated the IGF axis in a cross-sectional cohort of N = 305 pediatric and adolescent subjects with or at varying degrees of risk for clinical diagnosis of type 1 diabetes. There were no significant differences in any of the continuous or categorical demographics between clinical groups (Table 1). Given that some analytes in the IGF axis are directly modulated by growth hormone (GH) (35), which peaks during puberty, we carefully selected samples from age-matched subjects (Table 1). IGF1 levels have been shown to peak around puberty (36), as replicated in our cohort (Fig. 1A), but IGF2 levels are not regulated by GH (8).
Figure 1.
Total IGF1 and IGF2 levels are significantly decreased in serum of AAb+ subjects at high risk for type 1 diabetes onset. Subjects from UF cross-sectional cohort. A: IGF1 levels correlate with age for males (Spearman correlation: P < 0.0001, r = 0.60) and females (P < 0.0001, r = 0.38), with an earlier peak in females compared with males. Ages at onset of type 1 diabetes in this cohort (includes subjects with recent onset and established disease) were binned based on frequency and are overlaid in gray. B: Best fit curves were significantly different for AAb− control vs. AAb+ subjects (P = 0.0002, extra sum of squares F test). Data are shown as mean (solid line) with 95% CI (dashed lines). C: Violin plots showing IGF1 levels normalized for age and sex are decreased in AAb+ subjects compared with AAb− control subjects, AAb− relatives, and subjects with recent-onset and established type 1 diabetes. D: IGF2 levels normalized for age and sex decreased in AAb+ subjects and subjects with recent-onset disease compared with AAb− relatives and those with established type 1 diabetes. E and F: Upon stratification of the AAb+ group by number of AAb, decreases in IGF1 (E) and IGF2 (F) percentiles remain significant for those with any number of AAb. Kruskal-Wallis with Dunn multiple comparisons test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The peak age of type 1 diabetes onset in the UFDI cohort was ∼10–12 years (Table 1 and Fig. 1A). This may be a critical time period for the maintenance of immune tolerance to β-cell antigens, as puberty represents a dynamic period of growth and development that includes metabolic stress (37). Although IGF1 levels peaked around puberty in all groups (Fig. 1A), AAb+ subjects showed significantly lower IGF1 levels for their age compared with AAb− control subjects (Fig. 1B). Analyzing IGF levels normalized for age and sex (Fig. 1C and D and Supplementary Table 3) revealed that total serum IGF1 and IGF2 levels were significantly lower in AAb+ subjects compared with AAb− relatives, and both analytes appeared to recover following type 1 diabetes diagnosis, although with different kinetics. Intriguingly, this observation of lower IGF1 and IGF2 levels not only applied to high-risk subjects with multiple AAb (≥2AAb+) but was also noted in subjects with only a single type 1 diabetes–related AAb (1AAb+) (Fig. 1E and F and Supplementary Table 3), which occurs early in the disease process and prior to detectable loss of β-cell mass or function (considered pre–stage 1 disease) (4). These findings remained statistically significant in analysis of raw (Supplementary Fig. 1 and Supplementary Table 4) and age-normalized IGF1 and IGF2 data (Fig. 1C–F and Supplementary Table 3). The majority of individuals showed IGF1 and IGF2 levels within normal published reference ranges (34). However, those <5th percentile, which is traditionally diagnostic for idiopathic GH deficiency (38), were enriched in 1AAb+ and ≥2AAb+ subjects compared with all other groups (Fig. 1E and F and Supplementary Tables 3 and 5), despite a lack of consensus on whether growth is impaired in pre–type 1 diabetes (39).
For determination of IGF bioavailability, IGFBP levels were measured in the same cohort. We found that circulating IGFBP1 levels were significantly higher in AAb+ versus control subjects. In addition, this increase remained statistically significant in subjects with established type 1 diabetes (Supplementary Fig. 2A and Supplementary Table 6). IGFBP1 levels are inversely associated with insulin levels (40); thus, high IGFBP1 may reflect the initiation of metabolic dysregulation in AAb+ subjects. The major component of IGFBP in serum, IGFBP3, was detected at comparable levels across all groups examined (Supplementary Fig. 2B and Supplementary Table 6). IGFBP2, IGFBP4, and IGFBP5 were not above the assay LOD in the majority of subjects in this study. IGFBP6 and IGFBP7 levels were decreased in subjects with recent-onset type 1 diabetes compared with AAb− control subjects or relatives, respectively (Supplementary Fig. 2C and D and Supplementary Table 6), while IGFBP7 levels were also significantly lower in AAb+ subjects compared with AAb− relatives (Supplementary Fig. 2D and Supplementary Table 6). Importantly, in contrast to IGF1 and IGF2, there is not a published reference range for this IGFBP laboratory assay. Upon stratification of the cohort by number of AAb, IGFBPs with high affinity for IGFs (i.e., IGFBP1, IGFBP3, and IGFBP6) were not significantly different between AAb−, 1AAb+, and ≥2AAb+ subjects (Supplementary Fig. 2E–G and Supplementary Table 6). In contrast, the low-affinity IGFBP7 showed significantly lower concentrations in ≥2AAb+ subjects and subjects with recent-onset type 1 diabetes compared with AAb− subjects (Supplementary Fig. 2H and Supplementary Table 6). Together, these data suggest that most high-affinity IGFBPs do not appear to be modulated in pre–type 1 diabetes and that IGFBP measurements do not improve the capacity of IGF1 and IGF2 levels to distinguish type 1 diabetes stages (Supplementary Table 7).
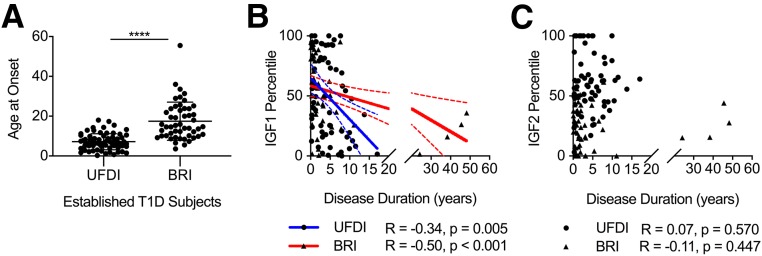
IGF1 Decreases With Increased Duration of Type 1 Diabetes
Since IGF1 and IGF2 percentiles were significantly decreased during progression to disease, we next asked whether IGF modulation was associated with type 1 diabetes duration. We measured serum IGF levels from a cross-sectional BRI cohort with established type 1 diabetes (N = 50), comprised primarily of fasted subjects (Table 1), compared with UFDI subjects with established type 1 diabetes (N = 68), reported above. Of note, however, disease onset occurred at a significantly older age for the BRI versus the UFDI cohort (Fig. 2A). IGF1 percentiles appeared to drop with longer type 1 diabetes duration in both the primarily pediatric UFDI cohort and the BRI cohort comprised of children and adults (Fig. 2B). Interestingly, the slope of IGF1 loss appeared to be steeper in the younger-onset UFDI cohort than in the older-onset BRI cohort (Fig. 2B), mirroring the known faster loss of C-peptide in younger-onset subjects (41). IGF2 percentiles, on the other hand, were not associated with disease duration in either cohort (Fig. 2C).
Figure 2.
IGF1 decreases with increased duration of type 1 diabetes. A: Disease onset for the BRI cohort was at significantly older age than for the UFDI cohort with established type 1 diabetes (Mann-Whitney U test). B: IGF1 percentile for age and sex decreases with increasing disease duration in cross-sectional established type 1 diabetes cohorts from UFDI and BRI. C: IGF2 percentile is not associated with disease duration in either cohort. Data are overlaid with best fit lines (solid) and 95% CI (dashed lines). Spearman correlation: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The observation of reduced levels of IGF1 with increasing type 1 diabetes duration suggested a potential association between IGF1 levels and remaining β-cell function. In subjects from the BRI cohort, IGF1 showed a trend toward correlation with mixed-meal tolerance test–stimulated C-peptide AUC (Supplementary Fig. 3A), although HbA1c (Supplementary Fig. 3B) and fasting blood glucose levels were not associated with IGF1 (Supplementary Fig. 3C). As expected, C-peptide AUC significantly decreased with disease duration (Supplementary Fig. 3D). Therefore, we cannot rule out the possibility that the trending association between IGF1 and C-peptide may be driven by other factors related to disease duration. These data supported the notion that IGF1 levels may be associated with residual β-cell function and fueled our investigation of whether IGFs were modulated longitudinally during the development of type 1 diabetes.
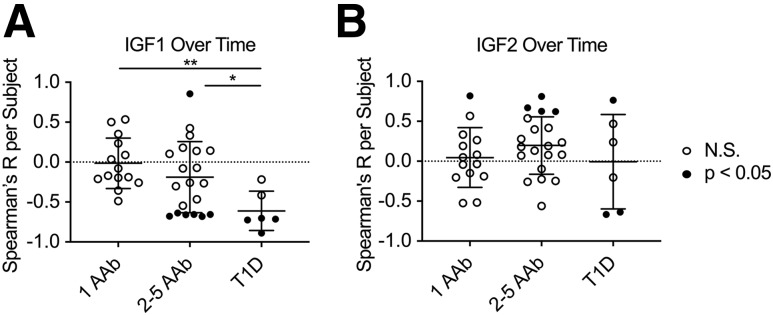
IGF Levels Show Longitudinal Stability in Single AAb+ Subjects
We next assessed the longitudinal stability of IGF1 and IGF2 from up to 13 time points in the T1DBIT cohort of N = 40 pediatric and young adult subjects at risk for type 1 diabetes (Table 2). Importantly, the groups were well age and BMI matched, and subject BMI did not significantly deviate during the study (Table 2). We hypothesized that IGF modulation may mirror the loss of insulin preceding diagnosis and that continued loss of endogenous insulin postonset may also be associated with a loss of IGF1, in particular. For subjects who entered the study with 1AAb+ and did not progress to disease, IGF1 and IGF2 levels were relatively stable within the individual but variable between subjects (Table 3 and Supplementary Figs. 4 and 5), as one would expect given the puberty-adjacent age range of this cohort (Table 2). There were no significant differences in intrasubject CVs for IGF1 or IGF2 in comparison of those 1AAb+ at enrollment, those ≥2AAb+ at enrollment, and those who developed type 1 diabetes during the study (Table 3).
Table 3.
Within- and between-subject CVs (%) for raw IGF1 and IGF2 measurements
| 1AAb+ at enrollment | 2–5 AAb+ at enrollment | Developed T1D during study | ||||
|---|---|---|---|---|---|---|
| Intrasubject | Intersubject | Intrasubject | Intersubject | Intrasubject | Intersubject | |
| IGF1 | 16.3 ± 4.4 | 38.1 ± 4.7 | 18.6 ± 7.1 | 45.5 ± 4.3 | 13.7 ± 4.3 | 22.6 ± 5.3 |
| IGF2 | 11.0 ± 3.9 | 24.6 ± 3.6 | 13.4 ± 4.9 | 23.4 ± 5.0 | 14.3 ± 5.6 | 14.6 ± 4.3 |
Measurements in subjects 1AAb+ at enrollment, with 2–5 AAb+ at enrollment, or who developed type 1 diabetes during the study. CVs were calculated from 11–13 time points per subject.
Longitudinal IGF1 Levels Decrease Over Time in Multiple AAb+ Subjects
For assessment of grouped IGF trajectories, IGF levels were normalized by their baseline values per subject to minimize the effects of age and sex. Subjects with 1AAb+ at time of enrollment did not show a significant change in IGF1 or IGF2 levels over the course of the study (Fig. 3 and Supplementary Figs. 4 and 5). In contrast, a subset of subjects with ≥2AAb+ showed a significant decline in IGF1 levels over time (Fig. 3A and Supplementary Fig. 6). Indeed, all subjects with longitudinally decreasing IGF1 levels were positive for three or more AAb, although many subjects with three or more AAb had stable IGF1 levels (Table 4). With regard to AAb specificities, all of the subjects with decreasing IGF1 levels possessed IA-2A and ZnT8A (Table 4), which have previously been suggested to serve as an age-independent means of identifying those progressing quickly to disease onset (42). One ≥2AAb+ subject did show a potentially puberty-related increase in IGF1 levels over time (Fig. 3A and Supplementary Fig. 6); however, we wish to highlight that this subject lost IAA reactivity during the study (Table 4) and, therefore, may have a lower risk of developing disease (43). Additionally, a subset of ≥2AAb+ subjects showed increased IGF2 levels over time (Fig. 3B and Supplementary Fig. 7), although it is important to note that a similar trajectory was observed in one of the 1AAb+ subjects (Fig. 3B and Supplementary Fig. 5). In those who developed type 1 diabetes during the study, IGF1 levels decreased significantly over time (Fig. 3A and Supplementary Fig. 8), and this decrease was seen immediately prior to diagnosis, with most continuing to slowly decrease (Supplementary Fig. 8). For IGF2, there was more variability in individual levels and no consistency in trend over time (Fig. 3B and Supplementary Fig. 9).
Figure 3.
Longitudinal IGF1 levels were stable in subjects with 1AAb+ at enrollment and decreased over time in those with multiple AAb+ at enrollment and subjects who progressed to type 1 diabetes. Subjects from longitudinal T1DBIT cohort. A: IGF1 does not show any correlation with time of longitudinal follow-up in 1AAb+ subjects, while a subset of those with multiple AAb+ and who developed type 1 diabetes show significantly decreasing IGF1 over time. B: IGF2 does not show any correlation with time of longitudinal follow-up in 1AAb+ subjects, while a subset of those with multiple AAb+ show significantly increasing IGF2 levels and those who developed type 1 diabetes show variable IGF2 levels over time. ●, subjects with significant slopes; ○, subjects with nonsignificant slopes in IGF over the course of the study. N.S., not significant. Kruskal-Wallis with Dunn multiple comparisons test: *P < 0.05, **P < 0.01.
Table 4.
Trajectory of IGF1 and IGF2 in T1DBIT subjects and analysis of AAb positivity
| Subject identification number | Age (years) | Sex | IGF1 | IGF2 | Number of AAb | GADA | IA-2A | IA-2βA | ZnT8A | IAA |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 14 | F | Stable | Stable | 1 | N | Y | N | N | N |
| 37 | 6 | F | Stable | Stable | 1 | N | N | N | N | Y→N |
| 13 | 10 | F | Stable | Stable | 1 | N | N | N | N | Y |
| 12 | 13 | F | Stable | Stable | 1 | N | N | N | N | Y |
| 25 | 14 | F | Stable | Stable | 1 | Y | N | N | N | N→Y |
| 19 | 14 | F | Stable | Stable | 1 | N | N | N | N | Y |
| 32 | 14 | M | Stable | Stable | 1 | N | N | N | N | Y |
| 34 | 16 | F | Stable | Stable | 1 | Y | N | N | N | N→Y→N |
| 27 | 16 | F | Stable | Stable | 1 | N | N | N | N | Y |
| 5 | 17 | F | Stable | Stable | 1 | N | N | N | N | Y→N |
| 29 | 18 | F | Stable | Stable | 1 | Y | N | N | N | N |
| 30 | 18 | F | Stable | Stable | 1 | N | N | N | N | Y |
| 22 | 33 | F | Stable | Up‡ | 1 | N | N | N | N | Y |
| 38 | 37 | F | Stable | Stable | 1 | N | N | N | N | Y |
| 36 | 12 | M | Stable | Stable | 2 | Y | N | N | N | Y |
| 26 | 18 | M | Stable | Stable | 2 | Y | N | N | N | Y |
| 3 | 8 | M | Stable | Stable | 2 | Y | N | N | Y→N | N |
| 33 | 6 | F | Stable | Stable | 2 | N | N | N→Y | Y→N | Y |
| 35 | 17 | F | Stable | Stable | 2 | Y | N | N | Y | N→Y→N→Y |
| 8 | 4 | M | Stable | Stable | 3 | Y | Y | Y | N | N→Y→N |
| 17 | 15 | F | Stable | Up‡ | 3 | Y | N | N | Y | Y→N |
| 31 | 13 | M | Stable | Stable | 4 | Y | Y | Y | Y | N |
| 2 | 15 | F | Stable | Stable | 4 | Y | Y | Y | Y | N |
| 4 | 18 | M | Stable | Up‡ | 4 | Y | Y | Y | Y | N |
| 11 | 35 | F | Stable | Stable | 4 | Y | Y | Y | Y | N |
| 18 | 17 | M | Stable | Stable | 4 | Y | Y | Y | Y | N→Y→N |
| 40 | 5 | M | Stable | Stable | 5 | Y | Y | Y | Y | Y |
| 23 | 14 | F | Down† | Up‡ | 3 | N→Y→N→Y | Y | Y | Y | N |
| 20 | 14 | M | Down† | Stable | 3 | N | Y | Y | Y | N→Y |
| 39 | 14 | F | Down† | Stable | 4 | Y | Y | Y | Y | N |
| 15 | 18 | F | Down† | Stable | 4 | Y | Y | Y | Y | N |
| 16 | 15 | M | Down† | Up‡ | 5 | Y | Y | Y | Y | Y |
| 10 | 17 | M | Down† | Stable | 5 | Y→N→Y | Y | Y | Y | Y |
| 9 | 11 | M | Up‡ | Stable | 3 | Y | N | N | Y | Y→N |
| 1* | 10 | M | Stable | Stable | 5 | Y | Y | Y | Y | Y |
| 28* | 17 | M | Stable | Stable | 4 | Y | Y | Y | Y | N→Y |
| 7* | 14 | M | Down† | Up‡ | 5 | Y | Y | Y | Y | Y→N→Y |
| 21* | 15 | M | Down† | Down† | 3 | N→Y | Y | Y | Y | N→Y |
| 14* | 17 | M | Down† | Down† | 4 | Y | Y | Y | Y | N→Y |
| 24* | 17 | F | Down† | Stable | 4 | Y | Y | Y | Y | N |
F, female; M, male; N, no; Y, yes. IGF trajectory designated as follows:
†levels significantly decreased or
‡significantly increased during the study, as determined by Spearman correlation of IGF level vs. time.
*These subjects developed type 1 diabetes during the study; therefore, IAA seroconversion may be a consequence of exogenous insulin therapy.
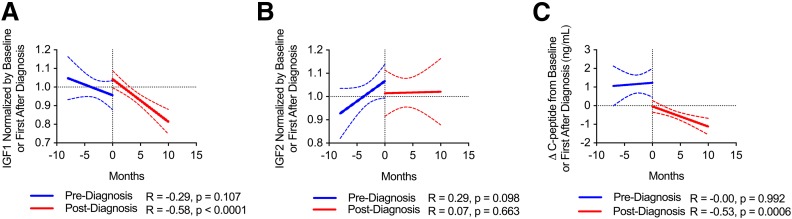
Longitudinal IGF1 Levels Decrease Over Time Following Type 1 Diabetes Diagnosis
For those subjects who developed type 1 diabetes within the time frame of the T1DBIT study, we compared the trajectory of IGFs pre- and postdiagnosis within the same individual. Here, IGFs were normalized by either baseline values prediagnosis or values for the sample collected nearest to diagnosis, respectively. IGF1 was shown to remain relatively stable prediagnosis but to significantly decrease postdiagnosis (Fig. 4A and Supplementary Fig. 8). On the other hand, the pre- and postdiagnosis trajectories for IGF2 were not significantly different (Fig. 4B and Supplementary Fig. 9). We also observed that longitudinal C-peptide levels significantly decreased postdiagnosis (Fig. 4C), suggesting that IGF1 and C-peptide may be simultaneously modulated.
Figure 4.
Subjects who progressed to type 1 diabetes showed reduction in IGF1 postdiagnosis. Subjects from longitudinal T1DBIT cohort. Grouped IGF trajectory data for subjects progressing to type 1 diabetes during the study reveal that IGF1 remains stable prediagnosis and decreases with time postdiagnosis in the same subjects (A) and that IGF2 levels were not significantly different in comparison of pre- and postdiagnosis trajectories (B). C: Random C-peptide levels are stable prediagnosis and decrease with time postdiagnosis. Data were normalized by baseline levels before or immediately after diagnosis. Best fit lines (solid) shown with 95% CI (dashed). Spearman correlation.
Discussion
The current dogma surrounding the pathogenesis of type 1 diabetes suggests that disease occurs from inciting autoimmunity leading to a fundamental metabolic defect. However, the metabolic and growth factor derangements that occur prior to overt hyperglycemia remain incompletely characterized during the natural history of the disease. Therefore, we sought to further characterize the IGF axis in both cross-sectional and longitudinal cohorts representing the various stages of type 1 diabetes. Herein, we report that IGF1 and IGF2 defects exist prior to type 1 diabetes onset. In agreement with our results, Peet et al. (23) found IGF1 levels to be significantly lower in AAb+ versus AAb− subjects, but in this previous study, the difference was only apparent at 12 months of age. IGFBP3 concentrations were not significantly different when comparing AAb+ against AAb− individuals at all ages (23), again in agreement with our cross-sectional data. To our knowledge, our results are the first to report that total IGF2 levels were also significantly decreased in AAb+ compared with AAb− relatives, analogous to the observations with IGF1. The majority of high-affinity IGFBPs, however, were not significantly altered in pre–type 1 diabetes, suggesting that total IGF levels may accurately reflect IGF bioavailability.
As we observed for all three cohorts in this study, others have previously reported that IGF1 significantly decreases post–diagnosis of type 1 diabetes (44,45). IGF1 levels have also been positively correlated with C-peptide as a measure of residual β-cell function (46). Confirmation of the association between IGF1 levels and disease duration/metabolism provided strong support for evaluating longitudinal IGF trajectories in subjects with pre–type 1 diabetes. We noted that IGF1 and IGF2 levels show longitudinal stability in the T1DBIT cohort, supporting the concept of tracking these analytes as potential prognostic biomarkers. In fact, our intraperson CVs for IGF1 in 1AAb+ subjects were remarkably similar to those previously reported in healthy adults (47). This report is the first to demonstrate that IGF1 decreased over time in ≥2AAb+ subjects but did not change over time in 1AAb+ subjects, suggesting that this change was not related to age or puberty—and, importantly, identifying a new potential explanation for the differing rates of progression among 1AAb+ versus ≥2AAb+ subjects. We found that the subjects with decreasing IGF1 were positive for IA-2A and ZnT8A, AAb that tend to appear closer to clinical diagnosis as a consequence of antigenic spreading (48). These data, in combination with the association between IGF1 and C-peptide changes, suggest that IGF1 and insulin may be lost simultaneously, with these analytes potentially synergizing to dysregulate glucose metabolism prior to clinical diagnosis.
In terms of potential study limitations, the UF and T1DBIT samples were drawn from nonfasted subjects, limiting our ability to address the association with glucose levels in these cohorts. We also found that ≥2AAb+ subjects trended toward increasing IGF2 levels over time. However, the levels were variable in the cohort and increasing levels were also seen in a 1AAb+ subject, which brings this finding under question as potentially an age-related increase rather than strictly a consequence of disease pathogenesis. Clearly, this issue needs to be addressed in future efforts, possibly within a larger study. An additional and potentially important caveat to note is that AAb were measured using different techniques for the T1DBIT and UFDI cohorts. Specifically, for the T1DBIT cohort, AAb were measured via radioimmunoassay, but the UFDI cohort used ELISA. The IASP has yet to validate an ELISA with adequate sensitivity and specificity for IAA (49); therefore, IAA were not measured in the cross-sectional UFDI subjects. Since IAA and GADA are often the first AAb to appear in the natural history of T1D (50), we concede that it is possible that some proportion of the UFDI 1AAb+ subjects may actually possess multiple AAb. This discrepancy may explain why we saw low IGF1 levels cross-sectionally in 1AAb+ and ≥2AAb+ UFDI subjects but only noted decreasing IGF1 levels in multiple AAb+ subjects from the T1DBIT cohort.
While the trajectories of IGF2 were not significantly different pre- and postdiagnosis for the collective T1DBIT cohort, IGF1 was shown to decrease over time postdiagnosis compared with prediagnosis in the same individual. Despite the small sample size for the subjects progressing to type 1 diabetes, using the same subject as a comparison within a short time frame, before and after diagnosis, provides more confidence that the effect is not driven solely by covariates like age and puberty. Our longitudinal findings mirror those of a recently published study that reported IGF1 decreased by 2 years postdiagnosis; however, this study did not have prediabetes time points for comparison (45). This study noted a temporary increase in IGF1 immediately after type 1 diabetes onset, which they ascribe to the impact of exogenous insulin delivery (45). While we did see this rebound in the cross-sectional UFDI cohort, the T1DBIT cohort does not appear to show this effect. It is important to note that in the cited study, the average HbA1c at diagnosis was 10.5% (45). Recent-onset UFDI subjects were identified from presentation in the clinic, so the average HbA1c upon diagnosis was likely higher than in our longitudinal cohort wherein HbA1c was measured monthly, resulting in prompt diagnoses at HbA1c values close to 6.5%. Therefore, some endogenous insulin, potentially acting directly on the liver, may have remained in the T1DBIT cohort with initially lower exogenous insulin dosages (51), explaining the discrepancy in IGF1 rebound postdiagnosis in all of the cohorts described.
The results presented herein suggest that decreased total IGF levels may occur both before and after the clinical diagnosis of type 1 diabetes (stage 3 disease). Aberrant modulation of IGFs prior to disease onset could complement existing disease staging efforts in combination with AAb surveillance and glycemic monitoring. Longitudinal studies of longer duration in fasted subjects are needed to assess whether decreasing IGF1 levels in multiple AAb+ subjects would improve disease prediction in place of or in combination with OGTT. These data additionally support the further investigation of IGF modulation as potentially contributing to type 1 diabetes pathogenesis, with IGFs representing a novel therapeutic target to possibly inhibit autoimmunity or preserve pancreatic health (7) in AAb+ subjects.
Supplementary Material
Article Information
Acknowledgments. The authors thank Joshua Peterson and Kieran McGrail (UF), Jordan Klaiman and Rachel Hartley (BRI), and Rachel Hervey (Pacific Northwest Research Institute) for technical assistance with sample processing, biorepository management, and procurement of demographic data. Special thanks are extended to all study subjects and their families for generously participating. The authors thank the clinical staff at UF, Nemours Children’s Hospital, Emory University, BRI (specifically, Jenna Snavely and others conducting the BRIDge Study of Type 1 Diabetes), Pacific Northwest Research Institute, and Novo Nordisk Research Center for sample acquisition.
Funding. Project support was provided by grants from the National Institutes of Health (F31 DK117548 to M.R.S., T32 DK108736 to M.A.A., Clinical and Translational Science Award URL1TR001427 to D.A.S., P01 AI42288 to M.A.A. and T.M.B., and R01 DK106191 to T.M.B.), the National Institute of Diabetes and Digestive and Kidney Diseases–supported Human Islet Research Network (RRID:SCR_014393, hirnetwork.org [UC4 DK104216-01 to D.A.S.]), the Children’s Miracle Network (to M.R.S. and M.J.H.), and JDRF (3-SRA-2016-209-Q-R to C.S.).
Duality of Interest. J.D.W. and M.v.H. are paid employees of Novo Nordisk. W.A.H. has received research support from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.R.S. researched and analyzed the data in all figures and tables and wrote the manuscript. C.H.W. conceived the study and reviewed and edited the manuscript. S.M.G. researched the data in Fig. 1A–C and reviewed and edited the manuscript. A.L.P. contributed to discussion and reviewed and edited the manuscript. R.B. advised on statistical analysis and reviewed and edited the manuscript. A.M. recruited subjects. M.J.H., D.A.S., J.D.W., M.v.H., W.A.H., and C.S. designed the respective cohorts and recruited subjects, contributed to discussion, and reviewed and edited the manuscript. M.A.A. and T.M.B. conceived the study and reviewed and edited the manuscript. T.M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017 and the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at https://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0942/-/DC1.
References
- 1.Raab J, Haupt F, Scholz M, et al.; Fr1da Study Group . Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 2016;6:e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hommel A, Haupt F, Delivani P, et al.; The Freder1k Study Group . Screening for type 1 diabetes risk in newborns: the Freder1k Pilot Study in Saxony. Horm Metab Res 2018;50:44–49 [DOI] [PubMed] [Google Scholar]
- 3.Winkler C, Haupt F, Heigermoser M, et al.; GPPAD Study Group . Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials-GPPAD-02 study design and first results. Pediatr Diabetes 2019;20:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care 2010;33:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro MR, Atkinson MA, Brusko TM. Pleiotropic roles of the insulin-like growth factor axis in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2019;26:188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont J, Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res C Embryo Today 2003;69:257–271 [DOI] [PubMed] [Google Scholar]
- 9.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 1978;253:2769–2776 [PubMed] [Google Scholar]
- 10.Rinderknecht E, Humbel RE. Primary structure of human insulin-like growth factor II. FEBS Lett 1978;89:283–286 [DOI] [PubMed] [Google Scholar]
- 11.Bilbao D, Luciani L, Johannesson B, Piszczek A, Rosenthal N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol Med 2014;6:1423–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang G, Geng XR, Song JP, et al. Insulin-like growth factor 2 enhances regulatory T-cell functions and suppresses food allergy in an experimental model. J Allergy Clin Immunol 2014;133:1702–1708.e5 [DOI] [PubMed] [Google Scholar]
- 13.Giuliani C, Saji M, Bucci I, et al. Transcriptional regulation of major histocompatibility complex class I gene by insulin and IGF-I in FRTL-5 thyroid cells. J Endocrinol 2006;189:605–615 [DOI] [PubMed] [Google Scholar]
- 14.Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes 2002;51:966–976 [DOI] [PubMed] [Google Scholar]
- 15.Modi H, Jacovetti C, Tarussio D, et al. Autocrine action of IGF2 regulates adult β-cell mass and function. Diabetes 2015;64:4148–4157 [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H, Okamura D, Starr ME, Saito H, Evers BM. Age-dependent reduction of the PI3K regulatory subunit p85α suppresses pancreatic acinar cell proliferation. Aging Cell 2012;11:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kido Y, Nakae J, Hribal ML, Xuan S, Efstratiadis A, Accili D. Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J Biol Chem 2002;277:36740–36747 [DOI] [PubMed] [Google Scholar]
- 18.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016;59:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battaglia M, Atkinson MA. The streetlight effect in type 1 diabetes. Diabetes 2015;64:1081–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt RI, Simpson HL, Sönksen PH. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 2003;20:3–15 [DOI] [PubMed] [Google Scholar]
- 21.Evans-Molina C, Sims EK, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group . β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 2018;3:120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenbaum CJ, Beam CA, Boulware D, et al.; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peet A, Hämäläinen AM, Kool P, Ilonen J, Knip M, Tillmann V; DIABIMMUNE Study Group . Circulating IGF1 and IGFBP3 in relation to the development of β-cell autoimmunity in young children. Eur J Endocrinol 2015;173:129–137 [DOI] [PubMed] [Google Scholar]
- 24.Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013;20:R321–R339 [DOI] [PubMed] [Google Scholar]
- 25.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer 2014;14:329–341 [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Purohit S, Sharma S, et al. IGF-binding proteins in type-1 diabetes are more severely altered in the presence of complications. Front Endocrinol (Lausanne) 2016;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasserfall C, Montgomery E, Yu L, et al. Validation of a rapid type 1 diabetes autoantibody screening assay for community-based screening of organ donors to identify subjects at increased risk for the disease. Clin Exp Immunol 2016;185:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo W, LaGasse JM, Zhou Z, et al. A novel high-throughput method for accurate, rapid, and economical measurement of multiple type 1 diabetes autoantibodies. J Immunol Methods 2000;244:91–103 [DOI] [PubMed] [Google Scholar]
- 29.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al.; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care in diabetes--2014. In Clinical Practice Recommendations, 2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 31.Ranke MB. Diagnostics of Endocrine Function in Children and Aolescents. 3rd rev. and extended ed. Basel, Switzerland, Karger, 2003, p. 166–199. [Google Scholar]
- 32.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 2004;112:1691–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190 [PubMed] [Google Scholar]
- 34.Blum W, Schweizer R. Insulin-Like Growth Factors and Their Binding Proteins. Ranke M, Ed. Basel, Switzerland, Karger, 2003, p. 166–199 [Google Scholar]
- 35.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol 2011;7:11–24 [DOI] [PubMed] [Google Scholar]
- 36.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–2450 [DOI] [PubMed] [Google Scholar]
- 37.Bonner-Weir S, Aguayo-Mazzucato C, Weir GC. Dynamic development of the pancreas from birth to adulthood. Ups J Med Sci 2016;121:155–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bussières L, Souberbielle JC, Pinto G, Adan L, Noel M, Brauner R. The use of insulin-like growth factor 1 reference values for the diagnosis of growth hormone deficiency in prepubertal children. Clin Endocrinol (Oxf) 2000;52:735–739 [DOI] [PubMed] [Google Scholar]
- 39.Nambam B, Schatz D. Growth hormone and insulin-like growth factor-I axis in type 1 diabetes. Growth Horm IGF Res 2018;38:49–52 [DOI] [PubMed] [Google Scholar]
- 40.Brismar K, Fernqvist-Forbes E, Wahren J, Hall K. Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metab 1994;79:872–878 [DOI] [PubMed] [Google Scholar]
- 41.Besser REJ, Ludvigsson J, Hindmarsh PC, Cole TJ. Exploring C-peptide loss in type 1 diabetes using growth curve analysis. PLoS One 2018;13:e0199635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorus FK, Balti EV, Vermeulen I, et al.; Belgian Diabetes Registry . Screening for insulinoma antigen 2 and zinc transporter 8 autoantibodies: a cost-effective and age-independent strategy to identify rapid progressors to clinical onset among relatives of type 1 diabetic patients. Clin Exp Immunol 2013;171:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endesfelder D, Hagen M, Winkler C, et al. A novel approach for the analysis of longitudinal profiles reveals delayed progression to type 1 diabetes in a subgroup of multiple-islet-autoantibody-positive children. Diabetologia 2016;59:2172–2180 [DOI] [PubMed] [Google Scholar]
- 44.Palta M, LeCaire TJ, Sadek-Badawi M, Herrera VM, Danielson KK. The trajectory of IGF-1 across age and duration of type 1 diabetes. Diabetes Metab Res Rev 2014;30:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chisalita SI, Ludvigsson J. Insulin-like growth factor-1 at diagnosis and during subsequent years in adolescents with type 1 diabetes. J Diabetes Res 2018;2018:8623560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedman CA, Frystyk J, Lindström T, et al. Residual beta-cell function more than glycemic control determines abnormalities of the insulin-like growth factor system in type 1 diabetes. J Clin Endocrinol Metab 2004;89:6305–6309 [DOI] [PubMed] [Google Scholar]
- 47.Borofsky ND, Vogelman JH, Krajcik RA, Orentreich N. Utility of insulin-like growth factor-1 as a biomarker in epidemiologic studies. Clin Chem 2002;48:2248–2251 [PubMed] [Google Scholar]
- 48.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlosser M, Mueller PW, Törn C, Bonifacio E, Bingley PJ, Laboratories P; Participating Laboratories . Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia 2010;53:2611–2620 [DOI] [PubMed] [Google Scholar]
- 50.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steck AK, Larsson HE, Liu X, et al.; and the TEDDY Study Group . Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes 2017;18:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.