Abstract
Human but not mouse islets transplanted into immunodeficient NSG mice effectively accumulate lipid droplets (LDs). Because chronic lipid exposure is associated with islet β-cell dysfunction, we investigated LD accumulation in the intact human and mouse pancreas over a range of ages and states of diabetes. Very few LDs were found in normal human juvenile pancreatic acinar and islet cells, with numbers subsequently increasing throughout adulthood. While accumulation appeared evenly distributed in postjuvenile acinar and islet cells in donors without diabetes, LDs were enriched in islet α- and β-cells from donors with type 2 diabetes (T2D). LDs were also found in the islet β-like cells produced from human embryonic cell–derived β-cell clusters. In contrast, LD accumulation was nearly undetectable in the adult rodent pancreas, even in hyperglycemic and hyperlipidemic models or 1.5-year-old mice. Taken together, there appear to be significant differences in pancreas islet cell lipid handling between species, and the human juvenile and adult cell populations. Moreover, our results suggest that LD enrichment could be impactful to T2D islet cell function.
Introduction
Type 2 diabetes (T2D) is a group of metabolic diseases characterized by chronically elevated blood glucose and lipid levels (1). The inability to maintain euglycemia ultimately results from progressive dysfunction and/or loss of pancreatic islet β- and α-cells, with β-cell–secreted insulin mediating glucose uptake in peripheral tissues and α-cell–secreted glucagon raising blood glucose levels. How chronic hyperglycemia and elevated free fatty acid levels cause islet cell dysfunction and even cell death has been studied principally in rodent models because of limited access to human pancreas samples (2–4). Importantly, the recent development of collaborative human tissue repositories and accessible databases, including the Network of Pancreatic Organ Donors with Diabetes (nPOD), the Genotype Tissue Expression Project, and the Human Islet Research Network, has enabled investigations on normal and diseased pancreatic donor samples.
Notable differences have been revealed between rodent and human islets, including variations in innervation (5), vascularization (5–7), cell type composition (8), hormone secretion (8), islet-enriched transcription factor distribution (9), mitogenic stimuli (10), and stress response signaling (11). Another key difference described recently was that human islets, but not mouse islets, transplanted into immunodeficient NSG mice effectively accumulated lipid droplets (LDs). Moreover, while high-fat diet (HFD)-induced glucolipotoxic conditions led to the expected compensatory changes in transplanted mouse islet β-cell secretion and proliferation, human islets had minimal responses (10). In aggregate, these results suggested not only that islet lipid handling was regulated in a species-specific manner but also that it was associated with α- and β-cell function.
LDs are cellular organelles that regulate the storage and hydrolysis of neutral lipids and serve as a reservoir for cholesterol, acyl-glycerols, and phospholipids used in membrane formation and maintenance (12). On the outer surface of LDs, a number of proteins can be found that are involved in the regulation of lipid metabolism and structural stabilization. The best characterized LD surface proteins are in the perilipin family, which is composed of five members (PLIN1–5) (13). While the PLINs are produced in all cell types, their expression levels and patterns are tissue and developmental stage dependent. For example, PLIN2 and PLIN3 expression predominates in mouse liver, muscle cells (14), and pancreatic islets (15), whereas PLIN1 is significantly upregulated as preadipocytes mature into adipocytes (16). Perilipin protein function has been studied primarily in rodents, and, for example, PLIN2 (17,18) or PLIN3 (19) null mice are protected from hepatic steatosis and have improved glucose tolerance and insulin sensitivity. Furthermore, islet β-cell deletion of PLIN2 improves cellular autophagic flux and reduces endoplasmic reticulum stress (20). While these results indicate that LD accumulation is detrimental to mouse islet β-cells, the effect of LDs on human islet cells is not known.
Here we demonstrate that LDs accumulate in human acinar and islet cells in an age-dependent manner. Specifically, electron microscopy (EM) and fluorescent microscopy analysis showed that LDs begin to accumulate after ∼11 years of age in the postjuvenile period and then increase progressively over time. Transplantation experiments in NSG mice further suggest that there are fundamental molecular differences in the ability of adult human islet cells to accumulate LDs in relation to juvenile islet cells. LDs were also found in the islet-like β-cells produced in the embryonic cell–derived β-cell clusters (eBCs) derived from human embryonic stem (ES) cells by insulin+ cell sorting and reaggregation. Furthermore, LDs were enriched in T2D islet α- and β-cells relative to acinar cells. In contrast, LDs were almost undetectable in the aged, hyperlipidemic, obese, or hyperglycemic rodent pancreatic acinar or islet cells. We discuss the significance that LDs may have to islet cell activity of humans without diabetes and with T2D.
Research Design and Methods
Human, Mouse, and Rat Pancreas Samples
Pancreata and islets from normal and T2D human donors were obtained through a partnership with the International Institute for Advancement of Medicine, National Disease Research Interchange, Integrated Islet Distribution Program, and nPOD (Supplementary Tables 1–3). Juvenile donors were defined as <11 years of age. The fixation and preparation of cryosectioned human pancreas were performed as described previously (21). LD analysis was conducted on the previously described juvenile and adult human islets transplanted under the kidney capsule for 6 weeks in 12-week-old NSG mice fed normal chow (22). The handpicked islets from the normal 47-year-old donor (Supplementary Table 1) were analyzed for LDs by BODIPY 493/503 staining pre- and posttransplantation into 12-week-old NSG mice fed normal chow. The transplants were harvested 6 weeks later as described previously (22). The percentage of LD+ cells relative to islet hormone+ cells within a 0.1 mm2 area was determined before and after transplantation, with at least six distinct areas within the transplant analyzed. Rodent pancreata were dissected and fixed in 4% paraformaldehyde in PBS for 4 h, followed by Tissue-Plus O.C.T. Compound (Fisher Scientific, Waltham, MA) embedding and cryosectioning at 6-μm thickness. Rodent pancreata was obtained from the following animals: 10-week-old normal Sprague-Dawley (SD) rats, 10-week-old Zucker diabetic fatty (ZDF) rats, 8-week-old C57BL6 mice fed a low-fat diet (i.e., 10% calories from fat [5.6% w/w fat]; Research Diet, New Brunswick, NJ) or HFD (60% calories from fat [35.0% w/w fat]; Research Diet) for 8 weeks, 10-week-old db/db mice, 10-week-old db/+ mice, 8-week-old low-density lipoprotein receptor (LDLR) null mice (23) fed a normal chow (i.e., 13% calories from fat [5.6% w/w fat]; Research Diet) or Western diet (i.e., 40% calories from fat [20.0% w/w fat] and 0.15% added cholesterol; Research Diet) for 8 weeks, and 1.5-year-old C57BL6 mice. The body weight, fed blood glucose levels and β-cell mass for these rodents just prior to sacrifice are listed in Supplementary Fig. 1.
Immunofluorescence Analysis and LD Quantification
The cryosections of human and rodent pancreas were made permeable by 0.5% Triton treatment for 10 min followed by blocking with 0.5% BSA in PBS for 30 min. The following primary antibodies were applied overnight at 4°C: insulin (guinea pig; 1:500; Dako, Santa Clara, CA), glucagon (mouse; 1:400; Sigma-Aldrich, St. Louis, MO), somatostatin (goat, 1:400; Santa Cruz Biotechnology, Santa Cruz, CA), PLIN2/ADRP (rabbit; 1:400; Abcam, Cambridge, MA), and PLIN3/Tip47 (rabbit; 1:400; Abcam). Species-matched antibodies conjugated with the Cy2, Cy3, or Cy5 fluorophores were used for secondary detection (1:1,000; Jackson ImmunoResearch, West Grove, PA). Nile Red (1 μg/mL; Sigma-Aldrich) and BODIPY 493/503 (5 μmol/L; Thermo Fisher Scientific) were used interchangeably to detect neutral lipid-enriched LDs by incubating at room temperature for 30 min following secondary antibody treatment. Information on the human donor samples used for LD analysis is provided in Supplementary Tables 1 and 2. DAPI was applied for 5 min before the slides were mounted with the ProLong Gold Antifade reagent (Invitrogen, Carlsbad, CA). Images were acquired on a Zeiss Axio Imager M2 wide-field microscope with Apotome or a Zeiss LSM 880 confocal laser-scanning microscope. Quantification of the LD area as a percentage of the whole acinar or islet cell area was performed using ImageJ and MetaMorph software. At least five distinct areas of a donor transplant were quantified. The islet area was defined by the insulin+, glucagon+, and somatostatin+ staining signals.
Islet Isolation and Real-Time Quantitative RT-PCR
Total RNA from human (Supplementary Table 3) and 8-week-old male C57BL6 mouse islets was isolated using an RNAqueous RNA Isolation Kit (Ambion, Austin, TX) following the manufacturer’s protocol. RNA bioanalysis and quality control were performed in the Vanderbilt Functional Genomics Shared Resource core laboratory, with quantitative RT-PCR analysis only performed when the 28S/18S rRNA ratio was larger than 1.5 and the RNA integrity number greater than 8. TaqMan primer-probe and reagents (Applied Biosystems, Foster City, CA) were used, with GAPDH and β-actin (ACTB) serving as internal controls. Relative mRNA changes were calculated by the comparative ΔCt method using the Applied Biosystems StepOnePlus System.
Preparation of eBCs from Human ES Cells and eBC Transplantation Into NSG Mice
Human eBCs were produced from the INSGFP/W ES cell reporter line using previously published procedures (24). Briefly, a variety of established protocols recapitulate stages of embryonic development that include the formation of gut tube (stages 1–3), pancreatic foregut (stages 4–5), and pancreatic progenitors (stages 6–7) in vitro. This method involves sorting of the immature INS-GFP–producing cells from day 20 and reaggregating and culturing these eBC cells for 4–8 days (days 24–28). The steps to obtain eBCs induce many mature islet β-cell phenotypes upon the cell clusters that are not manifested in vitro with most other procedures (24). The transplantation of 4,000 eBCs (4 × 106 cells) under the kidney capsule of 10- to 20-week-old NSG mice and subsequent isolation of the kidneys bearing the 3- and 9-month-old transplants were described previously (24). The analyzed in vitro day 20, in vitro eBCs, and transplanted eBCs represent a combination of older (24) and newly generated samples. LD levels were analyzed by BODIPY as well as by immunofluorescent staining, i.e., insulin (guinea pig, 1:500; Dako), synaptophysin (mouse, 1:400; LSBio, Seattle, WA), PLIN2/ADRP (rabbit, 1:400; Abcam), and PLIN3/Tip47 (mouse, 1:400; Santa Cruz Biotechnology). LD quantitation was performed over in vitro cell clusters or a 0.1-mm2 area of the in vivo transplants image, with at least three areas analyzed per transplant.
Electron Microscopy
The LD content of human islet β-cells was analyzed by transmission EM (25). Human pancreas was fixed in 2.5% glutaraldehyde prepared in 0.1 mol/L cacodylate buffer. Samples were subsequently imaged on the Philips/FEI Tecnai T12 microscope at various magnifications. LD numbers in human β-cells were determined by manual counting.
Statistics
Data were presented as the mean ± SEM. Results were examined for significance by the unpaired Student t test or one-way ANOVA with Tukey-Kramer post hoc analysis. GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) was used for data analysis. A P value <0.05 was considered significant.
Study Approval
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University and UCSF. Mice were housed and cared for according to the Vanderbilt and UCSF Departments of Animal Care and IACUC/Office of Animal Welfare Assurance Standards and Guidelines. The Vanderbilt University Institutional Review Board declared that studies on deidentified human pancreatic specimens do not qualify as human subject research.
Data and Resource Availability
All data generated or analyzed during this study are included in the published article (and its Supplementary Data). Further detailed information is available from the corresponding author upon reasonable request.
Results
LD Accumulation in Human Pancreatic Islets Is Dependent On Donor Age
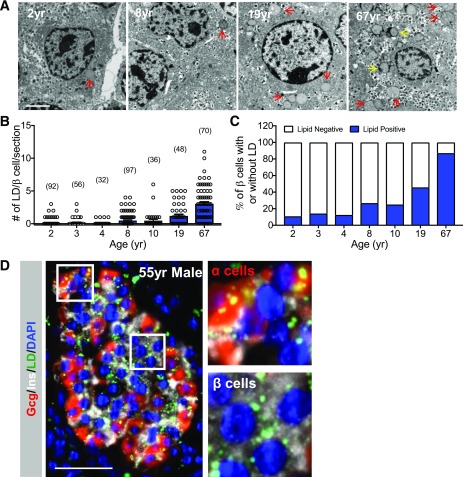
Earlier work established that adult human islets transplanted into immunodeficient NSG mice have many more LDs in their β-cells than transplanted adult mouse islets. Furthermore, these human islets were much less responsive to metabolic HFD-induced stress signals than mouse islets (10). To examine how lipids are normally stored in human islet β-cells, we used EM and the BODIPY or Nile Red lipophilic dyes to detect pancreatic LDs in healthy human pancreas donor samples ranging in age from 0.8 to 67 years. EM analysis revealed that there were relatively few LDs in juvenile (i.e., less than ∼11 years after birth) donor β-cells compared with adults (Fig. 1A–C). Staining with the lipophilic dyes showed that the LDs were detected in both adult islet α- and β-cells (Fig. 1D).
Figure 1.
LD accumulation in human islet β-cells increases with age. A: Representative EM images of healthy human islet β-cells from a 2-, 8-, 19-, and 67-year-old donor, which illustrates that very few LDs (red arrows) are found in juvenile donors compared with adult. The yellow arrows depict lipofuscins. Scale bar = 2 μm. yr, years. B: The number of lipid granules (including lipofuscins) per donor β-cell, with the quantity of β-cells counted in parenthesis. C: Percentage of β-cells containing lipid granules in human donors of different ages. D: Representative immunofluorescent image showing LD accumulation in healthy adult islet α- and β-cells from a 55-year-old (55yr) donor. Staining was performed to detect insulin (Ins) (white), glucagon (Gcg) (red), LDs (BODIPY [green]), and nuclei (DAPI [blue]). Scale bar = 50 μm. Right: magnified view of LD build-up in α- and β-cells within the marked area in the left panel. Error bars indicate SEM.
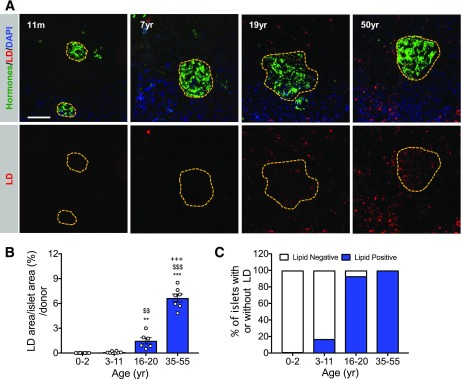
Examination of the LD content in the intact human pancreas with lipophilic dyes revealed an age-dependent accumulation pattern within pancreatic acinar and islet cells similar to that found by EM imaging of islet β-cells. Thus, LDs were almost undetectable within the pancreas until the postjuvenile period, but they were much more prevalent in acinar and islet cells in the 16- to 20- and 35- to 55-year-old donors (Fig. 2 and Supplementary Fig. 2).
Figure 2.
Relatively few LDs are present in the juvenile human pancreas. A: Pancreatic LDs are difficult to detect by Nile Red neutral lipid staining in juvenile (11 months old [11m] to 7 years old [7yr]) in comparison with teenage (19 years old) and adult (50 years old) human donors. The islet hormone area is depicted in green and LDs (Nile red) are depicted in red and nuclei in blue (DAPI). Scale bar = 50 μm. B: Percent of the islet LD area in the various age-groups (n = 6–8 donors/group). yr, years. C: The percent of LD containing islets in the donor age-groups. All error bars indicate SEM. **P < 0.01 and ***P < 0.001 vs. 0- to 2-year-old group; $$P < 0.01 and $$$P < 0.001 vs. 3- to 11-year-old group; +++P < 0.001 vs. 16- to 20-year-old group.
Age-Dependent LD Accumulation in Adult Human Islets Transplanted in NSG Mice
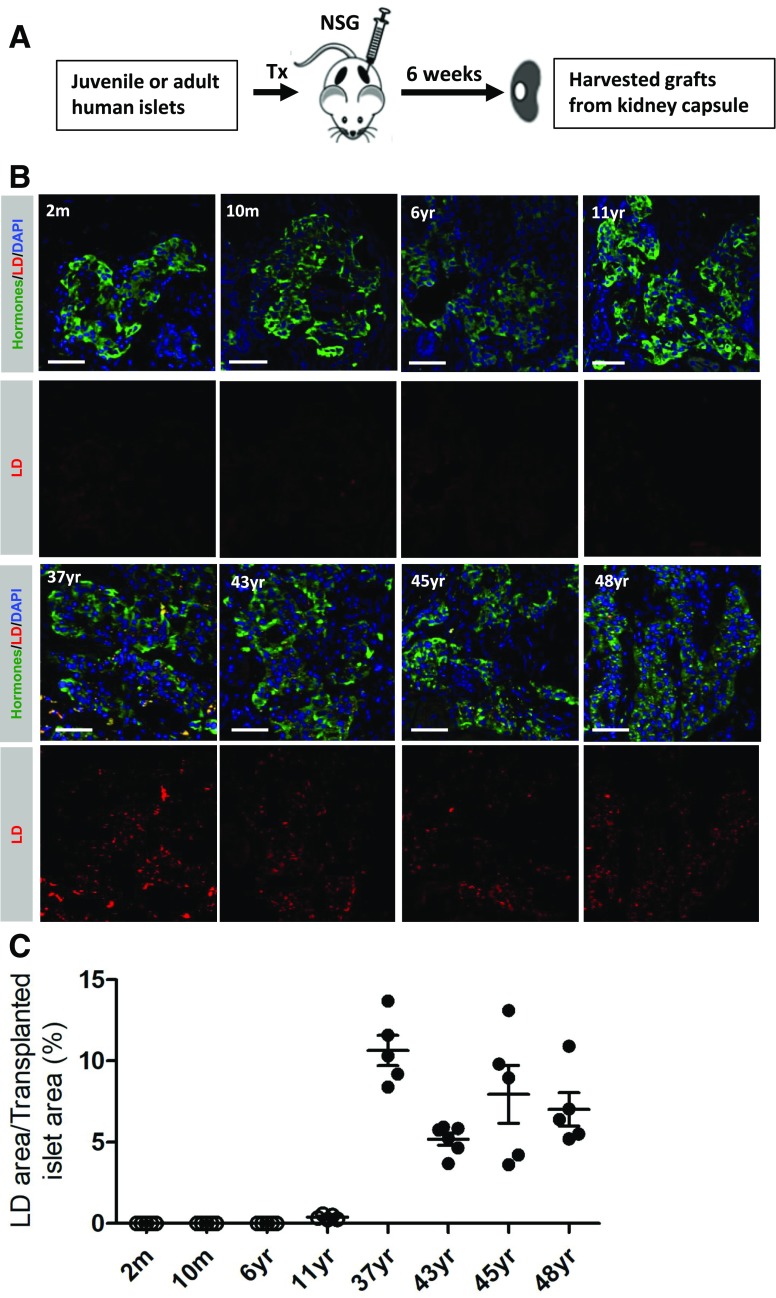
A number of molecular differences have been described in juvenile and adult human and rodent islet β-cells that influence β-cell function and mass (10,22). For example, islet β-cell proliferation decreased in an age-dependent manner in these samples, with juveniles having a much higher rate of replication (22). Consequently, we analyzed preexisting juvenile (2 months to 11 years old) and adult (37–48 years old) (Supplementary Table 4) human islet transplants (22) for the presence of LDs to determine if accumulation reflects molecular and/or environmental/dietary exposure differences between the adult and juvenile islet cell populations.
Transplantation of adult human islets under the kidney capsule of NSG mice for 6 weeks resulted in both an approximately twofold greater number of cells containing LDs and a trend toward an increase of the LD to islet area compared with the pretransplanted state (Supplementary Fig. 3A). However, the LD accumulation pattern was as observed with the intact healthy human pancreas, with LDs almost exclusively found in transplanted adult and not juvenile islets (Fig. 3). These data suggest that the age-dependent increase in LD numbers in the human pancreas is mainly determined by molecular differences between the juvenile and adult islet cells, which is also supported by gene expression analysis of these human α- and β-cells populations (10,22).
Figure 3.
Intrinsic human islet factors determine the LD levels. A: Workflow schematic of the healthy juvenile and adult islet transplantation studies. Islets were transplanted under the kidney capsule for 6 weeks. B: Representative images of juvenile and adult islets showing that LD number is much higher in adult transplanted islets. The islet hormone area is depicted in green and LDs are depicted (Nile red) in red and nuclei (DAPI) in blue. Scale bar = 50 μm. C: Quantification of the percent of LD+ cells in the transplanted hormone+ cell area ± SEM. m, months old; Tx, transplant; yr, years old.
LDs Accumulate in Human eBCs Produced In Vitro and After Transplantation in NSG Mice
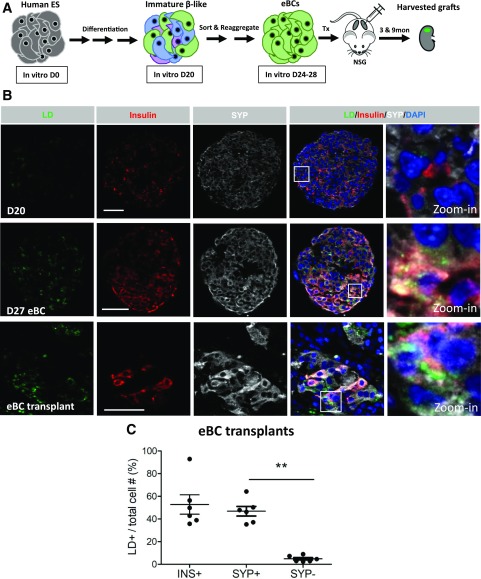
Much effort has been made in recent years to develop human β-like cells from ES cells for therapeutic and research purposes (26). A notable advancement in these protocols was the recent observation that reaggregation of immature day 20 β-like cells into enriched β-cell clusters (i.e., eBCs) induced molecular and functional islet-like properties (24). For example, clustering of the β-cells enhanced metabolic maturation by driving mitochondrial oxidative respiration, which is essential for the improved glucose-stimulated insulin secretion properties displayed by eBCs in relation to the day 20 cells. In addition, RNA-seq analysis revealed that in vitro derived eBC β-cells closely resemble adult islet cells, express similar levels of many cell regulators (PDX1, NKX6.1, NKX2.2, PAX6, and MAFB), and produce the machinery important to insulin synthesis and secretion (24). However, they produce low levels of certain adult β-cell markers (e.g., MAFA, UCN3, and G6PC2).
We next analyzed if LDs accumulated in human ES cell–derived β-like cells and, if so, how this was related to the differing islet cell properties associated with in vitro derived versus transplanted eBCs (Fig. 4). BODIPY+ LDs were detected in the in vitro derived eBCs harboring functional β-cells, but not in the immature and dysfunctional day 20 β-like cells (Fig. 4B). LDs were also readily detected in the more mature adult-like eBCs produced upon transplantation under the kidney capsule of NSG mice for 3 or 9 months, with the synaptophysin staining highlighting the endocrine cell population and insulin highlighting the β-cells (Fig. 4B). Hence, LDs were found in the eBC transplants within ∼55% of insulin+ cells, 45% of synaptophysin+ cells, and <10% of synaptophysin− cells (Fig. 4C). Transplanted eBC β-cells also produce increased levels of the adult markers that were deficient in the in vitro clusters (24). Like what was observed in adult human islets, LDs were found in a larger fraction of transplanted eBC insulin+ cells than pretransplanted, in vitro derived eBCs (Supplementary Fig. 3B). Collectively, our results strongly suggest that adult (and likely postjuvenile) human islet β-cells have a greater ability to accumulate LDs than juvenile human islets.
Figure 4.
LDs detected in human ES cell–derived eBCs. A: A simplified schematic representing human ES cell differentiation into eBCs and the transplantation of eBCs into NSG mice. The eBCs are produced by FACS isolating the insulin+ synaptophysin+ cells from day 20 (D20), cell reaggregation, and then culturing for 4–8 days. eBCs improve their secretory properties upon transplantation (24). 3 & 9mon, 3 and 9 months. B: Representative images taken of day 20 spheres, day 27 eBCs, and a 9-month eBC graft immunostained with BODIPY (green), insulin (red), SYP (white), and DAPI (blue). LDs are enriched in insulin+ eBC cells but are also detectible in some insulin− cells. The white square illustrates the zoomed-in area. Scale bar = 50 μm. C: LD distribution within the eBC transplant insulin+, synaptophysin+, or synaptophysin− cell populations. n = 6. Error bars indicate SEM. **P < 0.01. INS, insulin; SYP, synaptophysin; Tx, transplant.
Pancreatic LDs Are Barely Detectible in Rodent Models of Aging or Metabolic Syndrome
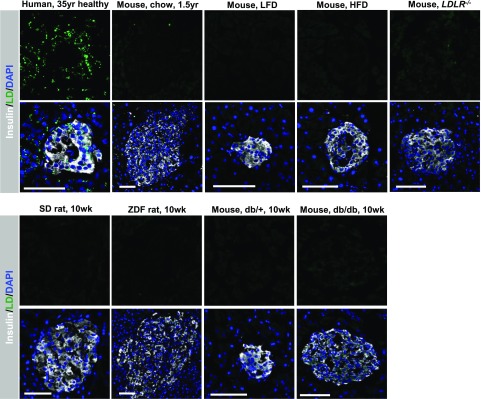
LDs were rarely found in mouse islets transplanted into NSG mice (10). Likewise, LDs were almost undetectable in the intact rodent pancreas when compared with (for example) a 35-year-old human donor (Fig. 5 and Supplementary Fig. 4). Thus, pancreatic LDs were very difficult to find in relatively young or aged 1.5-year-old mice, as well as hyperlipidemic mice fed an HFD, dyslipidemic LDLR−/− mice fed a fat- and cholesterol-rich Western diet, hyperlipidemic ZDF rats, or hyperglycemic db/db mice. In addition, few (if any) LDs were found in hypertriglyceridemic apolipoprotein C3 transgenic mouse islets (27). These data indicate that there is also a fundamental difference between the rodent and adult human pancreas in LD formation and/or degradation.
Figure 5.
Pancreas LDs are rarely found in the aging mouse or in rodent models of hyperlipidemia and hyperglycemia. BODIPY+ (green) LDs are rarely detected in the pancreas of 1.5-year-old (1.5yr) C57BL6 mice, C57BL6 mice fed a low-fat diet (LFD) or HFD, and LDLR−/− mice (top) or SD rats, ZDF rats, control db/+ mice, and diabetic db/db mice (bottom). BODIPY staining of the 35-year-old human donor is shown for comparison (top left). Scale bar = 50 μm. The physiological conditions of the rodents prior to islet isolation are provided in Supplementary Fig. 1. 10wk, 10 weeks old.
PLIN2 and PLIN3 Transcript Levels Do Not Change With Age or Diabetic State
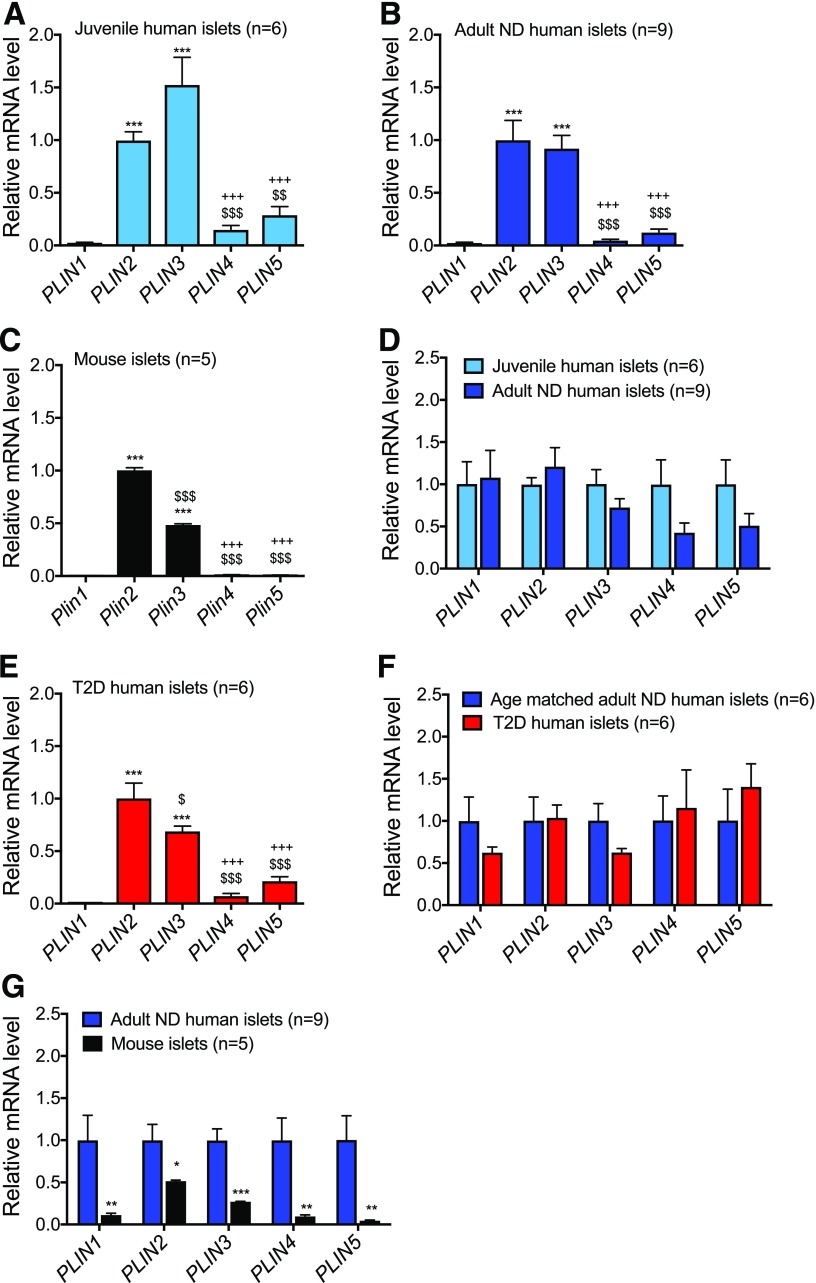
Perilipin proteins are integral to LD structure and degradation and cellular lipid homeostasis (28). PLIN2 and PLIN3 are the major perilipin family members in adult human islets (15). Here we compared perilipin mRNA levels between mouse and human islets, juvenile and adult human islets, and human islets from donors with and without T2D. As anticipated, PLIN2 and PLIN3 were the predominant perilipin gene products of mouse as well as healthy juvenile and adult human islets (Fig. 6A–C). There was no significant difference in PLIN1, PLIN2, PLIN3, PLIN4, or PLIN5 mRNA expression between the different aged human islet populations (Fig. 6D), despite the increase in LD content in adult islets (Figs. 1 and 2). There was also no apparent change in perilipin gene expression within age-matched nondiabetic and T2D islets (Fig. 6E and F).
Figure 6.
Little change in human islet PLIN2 and PLIN3 mRNA levels with respect to age or T2D. Relative PLIN1, 2, 3, 4, and 5 mRNA expression for islets in juvenile humans (age range 14 months–9 years) (A), adult humans (age range 39–68 years) (B), adult male C57BL6 mice (C), juvenile and adult humans (D), adults with T2D (age range 47–61 years) (E), age-matched adults without diabetes (ND) or with T2D (F), and adult humans and C57BL6 mice (G). There are no significant changes in PLIN mRNA expression between either healthy juvenile and adult islets (D) or adult healthy and T2D islets (F). The number of independent human and mouse islet samples analyzed is shown, with the adult ND number reduced for an age-matched comparison with T2D donor islets in F. Data presented relative to PLIN2 in A–C and E and relative to juveniles in D and to adult humans without diabetes in F and G. Error bars indicate SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PLIN1 in A–C and E or vs. adult humans in G; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. PLIN2; +++P < 0.001 vs. PLIN3 in A–C and E.
Islet PLIN2 and 3 gene expression was lower than in mice than in humans (i.e., mouse pLIN2 approximately twofold lower and mouse pLIN3 approximately fourfold lower than human [Fig. 6G]), although this change was much smaller than the differences in the EM (15) or lipophilic dye LD signals between species (Fig. 5 and Supplementary Fig. 4). Moreover, PLIN2 and PLIN3 protein staining levels were very low and diffuse in rodent and juvenile human islets compared with adult humans (Supplementary Fig. 4). In addition, most of the PLIN2 and 3 protein signals colocalized with BODIPY+ in adult human islets (Fig. 7F) and eBCs (Supplementary Fig. 5). These results demonstrate that islet LDs contain the PLIN2 and/or PLIN3 proteins.
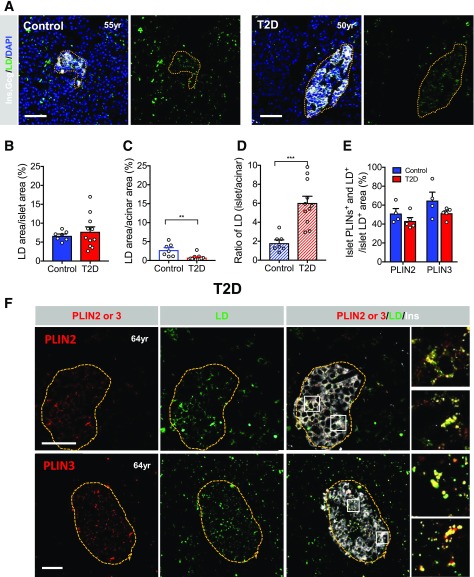
Figure 7.
LDs are enriched in T2D islets. A: Representative images contrasting the uniform BODIPY pattern in pancreas islet and acinar cells of a healthy donor to the enriched pattern of a T2D donor islet. Yellow dotted lines outline the islet area and DAPI (blue) shows the pancreas cell nuclei. B: Quantification of the percent LD area in islets. C: Quantification of the percent LD area in acinar cells. D: Quantification of the ratio of percent LD islet to acinar cell area. E: Quantitation of the islet PLIN2 or PLIN3. F: Islet PLIN2 or PLIN3 (red) colocalization with BODIPY+ (LD [green]) cells in the T2D pancreas. The yellow dotted lines outline the islet insulin+ (Ins) (white) area. Magnified insets are provided to more clearly show PLIN2/3 and LD colocalization. Scale bar = 50 μm. Error bars indicate SEM. **P < 0.01, ***P < 0.001. yr, years old.
LDs Were Enriched in Human T2D Islets
Strikingly, BODIPY staining revealed that LD density was visibly higher in T2D than in healthy islets when compared with the evenly distributed signal between the endocrine and exocrine compartments of healthy control donors (Fig. 7A). The approximately threefold enrichment within T2D islets was mainly attributed to lower LD numbers in acinar cells (Fig. 7B–D). The PLIN2 or PLIN3 proteins coassociated with 50–70% of the islet BODIPY+ signals in healthy and T2D islets (Fig. 7E and F). Collectively, our results support the possibility that there is a functional consequence of LD accumulation in the human pancreas.
Discussion
A number of modifiable factors have been linked to islet β-cell deterioration in T2D including chronic hyperglycemia and elevated levels of free fatty acids like palmitate (29). In this study, we observed age-related accumulation of LDs in human pancreatic islet and exocrine cells. The increase in LDs was readily detectable by EM and neutral lipid staining in islet α- and β-cells during the postjuvenile period, with accumulation continuing throughout adulthood. The majority of human islet neutral lipid staining was also shown to colocalize with PLIN2 and PLIN3, LD identity proteins. Moreover, our human islet transplantation results strongly indicate that molecular factors, and not environmental, impact the ability of adult human islet cells to accumulate LDs in comparison with juvenile human islets. To our knowledge, such a distinct temporal pattern of LD build-up has not been described in major lipid storage/utilization tissues like skeletal muscle, heart, or liver. Moreover, α- and β-cells from human T2D islets were found to have a higher LD content when compared with surrounding acinar cells. These observations are distinct from earlier studies showing age-dependent accumulation of an uncharacterized lipid storage vesicle entity in human β-cells (30), which was subsequently described as lipofuscin (31), an age-dependent electron-dense, autofluorescent material produced from indigestible lysosomal protein aggregates in postmitotic cells also found in our analysis. Notably, lipofuscins are not enriched in T2D islet cells (31). In addition, LDs were detected in the functional islet β-cells derived from human ES cells. In contrast, LDs were not readily detectable in the rodent pancreas under normal, aging, or pathophysiological conditions. These results raise the possibility that LD accumulation is functionally consequential to human pancreatic acinar and islet cells.
The ability of human pancreatic endocrine cells to accumulate lipid-storage vesicles in an age-dependent manner was first suggested upon analysis of a 6- and 67-year-old pancreas donor by EM and Oil Red O neutral lipid staining (30). However, the molecular characterization of these lipid-enriched vesicles was incomplete. Here we show that PLIN2 and/or PLIN3 colocalize with much of the lipophilic dye staining in human adult normal and T2D pancreas, demonstrating the prevalence of LDs in this tissue. Notably, we did not observe a significant difference in expression of the mRNAs encoding these proteins between juvenile and adult human islets or between islets from humans without diabetes or with T2D, despite the increase in LD content. While free fatty acids and glucose regulate PLIN2 protein levels in adult human islets (32), the inability to detect LDs in transplanted human juvenile islets in relation to adult demonstrated that nutrient signaling alone could not explain these accumulation differences. In addition, a striking discordance was recently reported between mRNA and protein synthesis in juvenile and adult mouse islets, with network analysis illustrating specific age-dependent changes in the production of proteins important (for example) in fatty acid and lipid metabolism (33). However, the lack of LD build-up in human juvenile islets transplanted into NSG mice implies that these age-associated changes are not simply a matter of environment.
Instead, it is very likely that molecular differences between juvenile and adult human islet cells influence LD accumulation. For example, many unique changes in chromatin landscape, gene expression, β-cell proliferation, and glucose-stimulated insulin secretion have been described between these cell populations, including in key transcription factors (e.g., MAFA, MAFB, SIX2, and SIX3) (34,35). Additionally, many fatty acid synthesis–related gene products (e.g., FASN, SCD1, and ACLY) were significantly higher in human adult β-cells compared with juvenile, while fatty acid transporter gene expression (e.g., FAT and FATP1–5) was not significantly altered (34). Adult mouse islets also have increased levels of enzymes involved in lipid metabolism in comparison with juvenile mice (33). Understanding the basis and significance in how genetic differences in expression of metabolic regulators of fatty acid flux increase LD accumulation in aging human pancreatic islet cells could potentially be of relevance to diabetes diagnosis and treatment. Future efforts should also focus on understanding whether LD accumulation is related to sex, BMI, and differences between α- and β-cells, an effort that will require a large donor population as exemplified upon demonstrating the variability in human islet cell mass (36).
Lysosomal-derived lipid-rich and electron-dense structures lipofuscins or “age-pigments” (37) have also been shown by EM to accumulate in an age-associated manner in isolated human islets obtained from 1- to 81-year-old donors (31). Lipofuscins are clearly visible in the EM image of a 67-year-old donor (i.e., marked by the yellow arrows in Fig. 1A), but in contrast to LDs, they do not serve a dynamic role in the storage and hydrolysis of neutral lipids. Collectively, we propose that our results in the intact pancreas and eBCs suggest that LDs are associated with islet cell function. Furthermore, the presence of LDs in a larger fraction of the transplanted islet cell population further indicates that effectors of in vivo β-cell maturation are impacting this remodeling process.
Reducing PLIN2 levels in the Akita mouse model of type 1 diabetes or the mouse MIN6 β-cell line improved β-cell activity by increasing autophagic flux and reducing ER stress (20). Nevertheless, it is unclear if this observation is of relevance to humans, since LDs were very difficult to detect by EM or with lipophilic dyes in the rodent pancreas. Significantly, sequestering free fatty acids in LDs can have both beneficial and detrimental effects depending on levels and time of exposure by buffering their toxic (e.g., palmitate) or protective (e.g., oleate) effects (38). If so, limitations in LD formation or storage could contribute to α- and β-cell dysfunction in T2D. It is of interest that human islet amyloid deposition, which is associated with β-cell dysfunction in T2D (39,40), was only markedly induced in NSG mice fed an HFD and not simply under hyperglycemic conditions (10). Consequently, it will be of interest to determine if the increased LD accumulation associated with the HFD is linked to amyloid deposition.
Arguably, future efforts should first focus on understanding the functional consequence of LD build-up in adult human islet α- and β-cells under basal as well as pathological conditions. The temporal and T2D-associated pattern of LD accumulation suggests a possible relationship in controlling the activity of islet cells. For example, could LDs influence the properties of the four functionally distinct human islet β-cell populations (41) and/or islet cell dysfunction/dedifferentiation in diabetes (42)? Additionally, the increased cellular distribution of LDs and expression of more adult islet identity markers in transplanted eBCs in relation to the in vitro derived cultures suggests a further maturation of this islet-like β-cell population. This proposal is supported by the distinct molecular and functional properties of these β-cell populations (24). Importantly, genetically engineered human ES cells have been used to examine the impact of a variety of transcription factors on islet cell formation, including islet-enriched PDX1, RFX6, and ARX (43). Consequently, it will be of interest to determine how eBC maturation is impacted in PLIN2 and/or PLIN3 knockout cells.
In summary, we report that LD accumulation is both age and disease dependent in the human pancreas and raises a limitation of using rodent models to study the relationship between LD accumulation and islet function. Future experimentation should involve (for example) human reagents, like the endocrine β-cell lines (44), human ES cells, and primary islets using both targeted and unbiased approaches. Ultimately, understanding the significance of increasing LD accumulation in pancreatic islet α- and β-cells from humans who are aging and who have diabetes could potentially be applied to the development of therapeutic strategies for diabetes.
Supplementary Material
Article Information
Funding. X.T. is supported by a JDRF Fellowship (DF-2019-738-A-N). Imaging was performed with National Institutes of Health (NIH) support from the Vanderbilt University Medical Center Cell Imaging Shared Resource (National Cancer Institute grant CA-68485, National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] grants DK-20593, DK-58404, and DK-59637, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant HD-15052, and National Eye Institute grant EY08126). This research was performed using resources and/or funding provided by the NIH (F30-DK-118830 [J.T.W.], AA026302 [R.M.C.], K08 AA021424 [R.M.C.], DK-105821 [M.H.], DK-108666 [M.H.], DK-106755 [A.C.P. and R.S.], DK-050203 [R.S.], and DK-090570 [R.S.]), NIDDK-supported Human Islet Research Network (RRID: SCR_014393, DK-104211 [A.C.P.], DK-108120 [A.C.P.], and DK-112232 [A.C.P.]), and the Vanderbilt Diabetes Research and Training Center (DK-20593). G.G.N. is supported by a Kraft Family Fellowship and a JDRF postdoctoral fellowship (1-PNF-2016-320-S-B). A.C.P. is also supported by the JDRF, Leona M. and Harry B. Helmsley Charitable Trust, and U.S. Department of Veterans Affairs (BX 000666). The islet hormone analysis was performed in the Vanderbilt University Medical Center Islet Procurement and Analysis Core (NIDDK grant DK-20593) and Vanderbilt University Neurochemistry Core (grant U54 HD083211). Human pancreatic islets were provided by the NIDDK-funded Integrated Islet Distribution Program at the City of Hope (NIH grant DK-098085). This work was supported by the ADA Foundation (grant 1-17-IBS-140).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.T., C.D., A.C.P., and R.S. designed the initial experiments. X.T., C.D., and A.K. and G.G.N. executed and analyzed the experiments with input from J.T.W., M.H., A.C.P., and R.M.C. The manuscript was principally written by X.T., C.D., and R.S., although X.T., C.D., J.T.W., G.G.N., A.K., R.M.C., M.H., A.C.P., and R.S. reviewed versions. R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
X.T. and C.D. contributed equally.
This article contains Supplementary Data online at https://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0281/-/DC1.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somesh BP, Verma MK, Sadasivuni MK, et al. Chronic glucolipotoxic conditions in pancreatic islets impair insulin secretion due to dysregulated calcium dynamics, glucose responsiveness and mitochondrial activity. BMC Cell Biol 2013;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa H, Carroll RJ, Swift HH, Steiner DF. Long-term elevation of free fatty acids leads to delayed processing of proinsulin and prohormone convertases 2 and 3 in the pancreatic beta-cell line MIN6. Diabetes 1999;48:1395–1401 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011;14:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 7.Dai C, Brissova M, Hang Y, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 2012;55:707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad E, Stein R, Hunter CS. Revealing transcription factors during human pancreatic β cell development. Trends Endocrinol Metab 2014;25:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai C, Kayton NS, Shostak A, et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J Clin Invest 2016;126:1857–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brozzi F, Nardelli TR, Lopes M, et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 2015;58:2307–2316 [DOI] [PubMed] [Google Scholar]
- 12.Walther TC, Farese RV Jr. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 2012;81:687–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Gruia-Gray J, Copeland NG, et al. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome 2001;12:741–749 [DOI] [PubMed] [Google Scholar]
- 14.Kimmel AR, Sztalryd C. The perilipins: major cytosolic lipid droplet–associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu Rev Nutr 2016;36:471–509 [DOI] [PubMed] [Google Scholar]
- 15.Trevino MB, Machida Y, Hallinger DR, et al. Perilipin 5 regulates islet lipid metabolism and insulin secretion in a cAMP-dependent manner: implication of its role in the postprandial insulin secretion. Diabetes 2015;64:1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urs S, Smith C, Campbell B, et al. Gene expression profiling in human preadipocytes and adipocytes by microarray analysis. J Nutr 2004;134:762–770 [DOI] [PubMed] [Google Scholar]
- 17.Carr RM, Peralta G, Yin X, Ahima RS. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One 2014;9:e97118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McManaman JL, Bales ES, Orlicky DJ, et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res 2013;54:1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr RM, Patel RT, Rao V, et al. Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am J Physiol Regul Integr Comp Physiol 2012;302:R996–R1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen E, Tsai TH, Li L, Saha P, Chan L, Chang BHJ. PLIN2 is a key regulator of the unfolded protein response and endoplasmic reticulum stress resolution in pancreatic β cells. Sci Rep 2017;7:40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brissova M, Haliyur R, Saunders D, et al. α cell function and gene expression are compromised in type 1 diabetes. Cell Rep 2018;22:2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai C, Hang Y, Shostak A, et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest 2017;127:3835–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 1993;92:883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair GG, Liu JS, Russ HA, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol 2019;21:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai C, Brissova M, Reinert RB, et al. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 2013;62:4144–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sneddon JB, Tang Q, Stock P, et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 2018;22:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y-Z, Cheng X, Zhang T, et al. Effect of hypertriglyceridemia on beta cell mass and function in ApoC3 transgenic mice. J Biol Chem 2016;291:14695–14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura S, Gan J-W, Brzostowski J, et al. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem 2002;277:32253–32257 [DOI] [PubMed] [Google Scholar]
- 29.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cnop M, Grupping A, Hoorens A, Bouwens L, Pipeleers-Marichal M, Pipeleers D. Endocytosis of low-density lipoprotein by human pancreatic beta cells and uptake in lipid-storing vesicles, which increase with age. Am J Pathol 2000;156:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 2010;53:321–330 [DOI] [PubMed] [Google Scholar]
- 32.Vernier S, Chiu A, Schober J, et al. β-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets 2012;4:379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wortham M, Benthuysen JR, Wallace M, et al. Integrated in vivo quantitative proteomics and nutrient tracing reveals age-related metabolic rewiring of pancreatic β cell function. Cell Rep 2018;25:2904–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arda HE, Li L, Tsai J, et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab 2016;23:909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cyphert HA, Walker EM, Hang Y, et al. Examining how the MAFB transcription factor affects islet β-cell function postnatally. Diabetes 2019;68:337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013;36:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray DA, Woulfe J. Lipofuscin and aging: a matter of toxic waste. Sci SAGE KE 2005;2005:re1. [DOI] [PubMed] [Google Scholar]
- 38.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta 2010;1801:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao P, Marek P, Noor H, et al. Islet amyloid: from fundamental biophysics to mechanisms of cytotoxicity. FEBS Lett 2013;587:1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigihara N, Fukunaka A, Hara A, et al. Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy. J Clin Invest 2014;124:3634–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorrell C, Schug J, Canaday PS, et al. Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016;101:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Li QV, Lee K, et al. Genome editing of lineage determinants in human pluripotent stem cells reveals mechanisms of pancreatic development and diabetes. Cell Stem Cell 2016;18:755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharfmann R, Rachdi L, Ravassard P. Concise review: in search of unlimited sources of functional human pancreatic beta cells. Stem Cells Transl Med 2013;2:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.