Abstract
Background
Prayer is amongst the oldest and most widespread interventions used with the intention of alleviating illness and promoting good health. Given the significance of this response to illness for a large proportion of the world's population, there has been considerable interest in recent years in measuring the efficacy of intercessory prayer for the alleviation of ill health in a scientifically rigorous fashion. The question of whether this may contribute towards proving or disproving the existence of God is a philosophical question lying outside the scope of this review of the effects of prayer. This revised version of the review has been prepared in response to feedback and to reflect new methods in the conduct and presentation of Cochrane reviews.
Objectives
To review the effects of intercessory prayer as an additional intervention for people with health problems already receiving routine health care.
Search methods
We systematically searched ten relevant databases including MEDLINE and EMBASE (June 2007).
Selection criteria
We included any randomised trial comparing personal, focused, committed and organised intercessory prayer with those interceding holding some belief that they are praying to God or a god versus any other intervention. This prayer could be offered on behalf of anyone with health problems.
Data collection and analysis
We extracted data independently and analysed it on an intention to treat basis, where possible. We calculated, for binary data, the fixed‐effect relative risk (RR), their 95% confidence intervals (CI).
Main results
Ten studies are included in this review (7646 patients). For the comparison of intercessory prayer plus standard care versus standard care alone, overall there was no clear effect of intercessory prayer on death (5 RCTs, n=3389, random‐effects RR 1.00 CI 0.74 to1.36). For general clinical state there was also no significant difference between groups (5 RCTs, n=2705, RR intermediate or bad outcome 0.98 CI 0.86 to 1.11). Four studies found no effect for re‐admission to Coronary Care Unit (4 RCTs, n=2644, RR 1.00 CI 0.77 to 1.30).Two other trials found intercessory prayer had no effect on re‐hospitalisation (2 RCTs, n=1155, RR 0.93 CI 0.71 to 1.22).
Authors' conclusions
These findings are equivocal and, although some of the results of individual studies suggest a positive effect of intercessory prayer, the majority do not and the evidence does not support a recommendation either in favour or against the use of intercessory prayer. We are not convinced that further trials of this intervention should be undertaken and would prefer to see any resources available for such a trial used to investigate other questions in health care.
Plain language summary
Intercessory Prayer for the alleviation of ill health
Intercessory prayer is one of the oldest and most common interventions used with the intention of alleviating illness and promoting good health. It is practised by many faiths and involves a person or group setting time aside to petition God (or a god) on behalf of another who is in some kind of need. This review examines whether there is a difference in outcome for people who are prayed for by name whilst ill, or recovering from an illness or operation, and those who are not. Both groups of people still received their usual treatment for their illness. Ten trials were found which randomised a total of 7646 people. The majority of these compared prayer (for someone to become well) plus treatment as usual with treatment as usual without prayer. One trial had two prayer groups, comparing participants who knew they were being prayed for with those who did not. Another trial prayed retroactively, randomising people a month to 6 years after they were admitted to hospital. Each trial had people with different illnesses. These included leukaemia, heart problems, blood infection, alcohol abuse and psychological or rheumatic disease. In one trial people were judged to be at high or low risk of death and placed in relevant groups.
Overall, there was no significant difference in recovery from illness or death between those prayed for and those not prayed for. In the trials that measured post‐operative or other complications, indeterminate and bad outcomes, or readmission to hospital, no significant differences between groups were also found. Specific complications (cardiac arrest, major surgery before discharge, need for a monitoring catheter in the heart) were significantly more likely to occur among those in the group not receiving prayer. Finally, when comparing those who knew about being prayed for with those who did not, there were fewer post‐operative complications in those who had no knowledge of being prayed for.
The authors conclude that due to various limitations in the trials included in this review (such as unclear randomising procedures and the reporting of many different outcomes and illnesses) it is only possible to state that intercessory prayer is neither significantly beneficial nor harmful for those who are sick. Further studies which are better designed and reported would be necessary to draw firmer conclusions.
Summary of findings
Summary of findings for the main comparison. Summary of findings 1. INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE.
| INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE for various illnesses | ||||||
|
Patient or population: patients with various illnesses Settings: in hospital Intervention: INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE | |||||
| Death by end of trial | Medium risk population | RR 0.72 (0.38 to 1.38) | 3389 (5) | ⊕⊕⊝⊝ low1,2 | ||
| 96 per 1000 | 69 per 1000 (36 to 132) | |||||
| Clinical state: 1. Improved/not improved: intermediate or bad outcome | Medium risk population | RR 0.98 (0.86 to 1.11) | 2705 (5) | ⊕⊕⊝⊝ low1,2 | ||
| 269 per 1000 | 264 per 1000 (231 to 299) | |||||
| Clinical state: 2. Significant complications (readmission to CCU) | Medium risk population | RR 1 (0.77 to 1.3) | 2644 (4) | ⊕⊕⊕⊝ moderate1 | ||
| 84 per 1000 | 84 per 1000 (65 to 109) | |||||
| Leaving the study early | Medium risk population | RR 0.75 (0.43 to 1.31) | 3446 (6) | ⊕⊕⊕⊝ moderate1 | ||
| 2 per 1000 | 2 per 1000 (1 to 3) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described
2 Considerable heterogeneity
Summary of findings 2. Summary of findings 2. INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE.
| INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE for blood stream infections | ||||||
|
Patient or population: patients with blood stream infections Settings: in hospital Intervention: INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE | |||||
| Death by end of trial | Medium risk population | RR 0.93 (0.84 to 1.03) | 3393 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 302 per 1000 | 281 per 1000 (254 to 311) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described
2 Very rare type of study
Summary of findings 3. Summary of findings 3. AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE.
| AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE for scheduled to receive non‐emergency CABG | ||||||
|
Patient or population: patients with scheduled to receive non‐emergency CABG Settings: in hospital Intervention: AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE | |||||
| Death by end of trial | Medium risk population | RR 0.92 (0.44 to 1.95) | 1198 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 24 per 1000 | 22 per 1000 (11 to 47) | |||||
| Clinical state: 1. Improved/not improved: intermediate or bad outcome | Medium risk population | RR 1.06 (0.79 to 1.4) | 1198 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 134 per 1000 | 142 per 1000 (106 to 188) | |||||
| Clinical state: 2. Significant complications (readmission to CCU) | Medium risk population | RR 0.91 (0.64 to 1.29) | 1198 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 99 per 1000 | 90 per 1000 (63 to 128) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described
2 Very rare type of study
Summary of findings 4. Summary of findings 4. AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER.
| AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER for people who are ill | ||||||
|
Patient or population: patients with scheduled to receive non‐emergency CABG Settings: in hospital Intervention: AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER | |||||
| Death by end of trial | Medium risk population | RR 0.82 (0.4 to 1.68) | 1205 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 27 per 1000 | 22 per 1000 (11 to 45) | |||||
| Clinical state: 1. Improved/not improved: intermediate or bad outcome | Medium risk population | RR 0.78 (0.6 to 1.02) | 1205 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 181 per 1000 | 141 per 1000 (109 to 185) | |||||
| Clinical state: 2. Significant complications (readmission to CCU) | Medium risk population | RR 0.95 (0.67 to 1.36) | 1205 (1) | ⊕⊕⊕⊝ moderate1,2 | ||
| 94 per 1000 | 89 per 1000 (63 to 128) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation not well described
2 Very rare type of study
Background
Description of the intervention
Prayer is amongst the oldest and most widespread interventions used with the intention of alleviating illness and promoting good health (McCaffrey 2004; Barnes 2004). Recent years have seen considerable interest in the beneficial effects of religious belief and communal religious involvement on health outcomes (Koenig 2000). Research has been done to investigate the effect for the patient of the complex matter of belonging to a religious tradition and undertaking its distinctive practices. One aspect of this is offering and receiving intercessory prayers for the sick. In this study we consider effect for the patient of intercessory prayer being offered on their behalf, separated from the question of his or her religious affiliation.
Prayer, defined as the "solemn request or thanksgiving to God or object of worship" (OED 1989), is an ancient and widely used intervention. There are many different forms of intercessory prayer; it is found in highly developed belief systems and is also practised sporadically by individuals in times of need, relatively free from formal involvement in organised religion. Indeed, one plausible derivation of the word ‘God’ and its Indo‐European cognates is from a root meaning "the one who is called upon" (OED 1989). Prayer has relation to other spiritual disciplines, including meditation and thanksgiving. This review focuses on intercessory prayer which, for the purposes of this study, involves a person or group setting time aside to petition God (or a god) on behalf of another person who is in some kind of need. Intercessory prayer is organised, regular, and committed, and those who practise it will hold some committed belief that they are praying to God (or a god).
How the intervention might work
The mechanism(s) by which prayer might work is unknown and hypotheses about this will depend to a large extent on religious beliefs. This review seeks to answer the question of effect not mechanism and it does not seek to answer the question of whether any effects of prayer confirm or refute the existence of God. In determining the direction of any effect, it is important to note that a religious believer may suggest that the nature of divine intervention could be subtle ‐ more subtle, indeed, than is likely to be revealed by the results of a randomised trial. Significance could be attached, for instance, to the question of whether a person has a ‘good death’ (approached with courage and having achieved a sense of peace) or a ‘bad death’, even though the ‘clinical outcome’ may be measured and recorded as the same. We nonetheless take the stand that claims for intercessory prayer for the sick which go beyond such subtleties can be subject to empirical testing and, potentially, proof and so, whilst not wishing to belittle such distinctions (as, for instance, between a ‘good’ and a ‘bad’ death), we will test the starker claims that are made for prayer which are of a measurable, directly clinical nature.
Why it is important to do this review
As with all systematic reviews, this review is necessary to bring together the relevant research evidence, to present that evidence and to seek to resolve uncertainties about the effects of intercessory prayer. We note that the results of this review will be of interest to those who are involved with the ‘debate about God’ ‐ both religious believers and atheists ‐ but these results cannot directly stand as ‘proof’ or ‘disproof’ of the existence of God. The extent and manner to which God’s existence can be determined by reference to events in the world is one of the most significant, and ancient, questions in theology‐philosophy, and is contested. (For a recent survey see Denys Turner (Turner 2004)). One strand of discussion, for instance, concentrates on the existence of the world rather than any given state of affairs within it. In the words of Ludwig Wittgenstein, ‘It is not how things are in the world that is mystical, but that it exists' (Wittgenstein 1974). We do not, therefore, seek to pose or answer any questions about the existence of God with this reviews. There are several challenges when assessing the results of randomised trials of prayer. There are potential problems with trial methodology. For example, ‘contamination’. The ‘control group’ of patients who are not prayed for within the trial may, nonetheless, be the subject of prayers offered by others. For instance, a sizeable number of people ‐ particularly those within religious orders and comparable fraternities ‐ are devoted to the practice of praying for all who are in need. Nonetheless, those who pray for the sick do so out of a conviction that their contribution makes a difference. They do not refrain from praying out of the consideration that someone, somewhere else, may also be praying. This conviction and its consequent practices are sufficiently deeply engrained as to make such studies worthwhile, since this background level of prayer should be evenly distributed to the two intervention groups through the process of random allocation.
A second consideration is the question of whether it makes any sense to speak of a ‘blind’ trial if the action (or not) of the intervention is determined by a putative divine agent. Most of the world’s religious traditions, from within which the prayer under consideration here would be offered, understand God to be omniscient, that is, all‐knowing. Therefore there could be no concealment of allocation nor concealment of the group to which a person has been allocated before God, who might choose to influence the patient outcomes because of or instead of the allocation. However, these are theological questions, and this review proceeds on scientific principles in that it is a widely held belief that intercessory prayer is beneficial for those who are unwell because God directs the outcome of those for whom prayers are offered differently from those for whom it is not. As noted above, we are not seeking to assess whether God is or is not the agent of action for prayer but, by using the same study designs used to test other interventions in healthcare we will assess the effects of the intervention. For this reason we also exclude from consideration such theological considerations as the injunction "Do not put the Lord your God to the test" (Deuteronomy 6:16) or questions as to whether God generally veils his presence from observation: in the words of the philosopher GF Hegel, "God does not offer himself for observation" (Hegel 2008).
Objectives
1. To evaluate the effects of intercessory prayer as an intervention for those with health problems.
2. If possible, to undertake sensitivity analyses to assess the specific efficacy of prayer for (i) people suffering from life threatening conditions and (ii) people suffering from less serious health problems.
3. In addition, we compared the outcomes of well 'blinded' and poorly 'blinded' studies in order to investigate the extent to which knowing that one is being prayed for influences the primary outcome of recovery.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. Where a trial was described as "double blind" but it was only implied that the study was randomised, if the participants' demographic details in each group were similar, we included it. We excluded quasi‐randomised studies, in which treatment allocation was not concealed, such as those allocating by using alternate days of the week.
Types of participants
We included any person with a physical or mental health problem irrespective of age, gender, or race.
Types of interventions
1. Intercessory prayer: routine care (see below) plus personal, focused, committed, and organised intercessory prayer on behalf of another.
2. Routine care: the relevant medical and non‐medical care normally given to people diagnosed with their particular illness in the setting in which the trial was done.
Types of outcome measures
We grouped outcomes into those measured in the short term (up to six weeks), medium term (six weeks to six months) and long term (six months and more).
Primary outcomes
1. Death ‐ any cause
2. Clinical state ‐ No important change in clinical state (as defined by individual studies)
3. Service outcomes ‐ Hospitalisation
4. Quality of life ‐ No clinically important change in quality of life
5. Satisfaction with treatment ‐ Leaving the studies early
Secondary outcomes
1. Death 1.1 Suicide 1.2 Due to illness 1.3 Natural
2. Clinical state 2.1 Course of illness (as defined by individual studies) 2.2 Complications (as defined by individual studies) 2.3 Medication use (as defined by individual studies) 2.4 Average endpoint scores in clinical state (as defined by individual studies)
3. Service outcomes 3.1 Number of days in hospital 3.2 Number of days to discharge 3.3 Re‐admission
4. Quality of life 4.1 Average endpoint quality of life score 4.2 Average change in quality of life scores 4.3 No clinically important change in specific aspects of quality of life 4.4 Average endpoint specific aspects of quality of life 4.5 Average change in specific aspects of quality of life
5. Satisfaction with treatment 5.1 Recipient of care not satisfied with treatment 5.2 Recipient of care average satisfaction score 5.3 Recipient of care average change in satisfaction scores 5.4 Carer not satisfied with treatment 5.5 Carer average satisfaction score 5.6 Carer average change in satisfaction scores
6. Mental state 6.1 No clinically important change in general mental state 6.2 Not any change in general mental state 6.3 Average endpoint general mental state score 6.4 Average change in general mental state scores 6.5 No clinically important change in specific symptoms 6.6 Not any change in specific symptoms 6.7 Average endpoint specific symptom score 6.8 Average change in specific symptom scores
7. Behaviour 7.1 No clinically important change in general behaviour 7.2 Average endpoint general behaviour score 7.3 Average change in general behaviour scores 7.4 No clinically important change in specific aspects of behaviour 7.5 Average endpoint specific aspects of behaviour 7.6 Average change in specific aspects of behaviour
8. Adverse effects 8.1 Clinically important general adverse effects 8.2 Average endpoint general adverse effect score 8.3 Average change in general adverse effect scores 8.4 Clinically important specific adverse effects 8.5 Average endpoint specific adverse effects 8.6 Average change in specific adverse effects
Search methods for identification of studies
Electronic searches
For this update we searched the following electronic databases:
a. AMED, CINAHL, EMBASE and MEDLINE on Ovid (June 2007) were searched using Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with:
((pray* or god or faith* or religio or spiritual*) in ti, ab) or ((spirituality or religion) in sh)
b. ATLA Religion Database on EBSCO Host (June 2007) was searched using the phrase:
pray* and trial*
c. Web Sites We searched Clinicaltrials.gov on National Institute for Health using the phrase
pray or prayer or god or religion or religious
Searching other resources
We checked all references in the articles selected for further relevant trials.
Searches undertaken for previous versions of this review are included in Appendix 1.
Data collection and analysis
The methods described below differ from those in earlier versions of this review (Roberts 2000, Roberts 2007). The methods in this 2009 version have been brought up to date and are in keeping with the new format of Cochrane reviews and recent methodological developments. These changes have not materially effected how we have or will manage data, but we have included the 'Methods' section from the previous review for those who are interested (Appendix 2).
Selection of studies
Material downloaded from electronic sources included details of author, institution, or journal of publication. The principal review author (LR) inspected all reports. These were then re‐inspected independently by a second author (IA) in order to ensure reliable selection. We resolved any disagreement by discussion, and where there was still doubt, we obtained the full article for further inspection. When we had obtained the full articles, LR and IA decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and added these trials to the list of those awaiting classification.
Data extraction and management
1. Extraction
Two authors (LR and IA) independently extracted data from included studies. Again, any disagreements were discussed, decisions documented and, if necessary, authors of studies were contacted for clarification. With remaining problems Clive Adams (Co‐ordinating Editor of the Cochrane Schizophrenia Group) helped clarify issues and those final decisions were documented.
2. Management
Data were extracted onto standard, simple forms.
3. Scale‐derived data
We included continuous data from rating scales only if the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000) and the instrument is either a self‐report or completed by an independent rater or relative (not by the therapist).
Assessment of risk of bias in included studies
Again working independently, two authors (LR and IA) assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). This tool encourages consideration of how the randomisation sequence was generated, how allocation was concealed, the integrity of blinding at outcome measurement, the completeness of outcome data, selective reporting and other biases. We would have excluded any studies where sequence generation was at high risk of bias or where allocation was clearly not concealed. If disputes arose as to the correct category for a trial this was resolved through discussion, and guidance from Clive Adams. Where possible, we extracted (and report here) information on the religious beliefs of the authors reporting the included studies because of the possibility that this is related to the risk of bias.
Measures of treatment effect
We adopted p=0.05 as the conventional level of statistical significance but are especially cautious where results were only slightly below this, and we report 95% confidence intervals in preference to p‐values.
1. Binary data
For binary outcomes we calculated a standard estimation of the fixed‐effect risk ratio (RR) and its 95% confidence interval (CI). For statistically significant results we calculated the number needed to treat/harm statistic (NNT/H), and its 95% CI using Visual Rx (http://www.nntonline.net/) taking account of the event rate in the control group.
2. Continuous data
2.1 Summary statistic
For continuous outcomes we estimated a fixed‐effect weighted mean difference (WMD) between groups. We did not calculate effect size measures.
2.2 Endpoint versus change data
We preferred to use scale endpoint data, which typically cannot have negative values and is easier to interpret from a clinical point of view. Change data are often not ordinal and are problematic to interpret. If endpoint data were unavailable, we used change data.
2.3 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: (a) standard deviations and means are reported in the paper or obtainable from the authors; (b) when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996)); (c) if a scale starts from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above will be modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale which includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. Skewed data from studies of less than 200 participants were entered in additional tables rather than into the data analysis. Skewed data pose less of a problem when looking at means if the sample size is large and these were entered into syntheses.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This increases the risk of type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in an included study, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients for their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of an included study, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, we will only use data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, the additional treatment arms were presented in comparisons. Where the additional treatment arms were not relevant, these data were not reproduced.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, the findings of a trial must lose credibility (Xia 2007 ‐ direct link). We are forced to make a judgment where this is for the very short‐term trials likely to be included in this review. We decided that if more than 40% of data be unaccounted for at 8 weeks we would not reproduce these data or use them within analyses.
2. Binary
If attrition for a binary outcome is between 0 and 40% and outcomes of these people are described, we included these data as reported. Where these data were not clearly described, for the primary outcome we assumed the worst for each person who was lost, and for adverse effects we assumed rates similar to those among patients who did continue to have their data recorded.
3. Continuous
If attrition for a continuous outcome is between 0 and 40% and completer‐only data were reported, we have reproduced these.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies without any comparison to judge clinical heterogeneity.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I‐squared statistic
This provided an estimate of the percentage of inconsistency thought to be due to chance. I‐squared estimate greater than or equal to 50% was interpreted as evidence of high levels of heterogeneity (Higgins 2002).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1995). These are described in section 10.1 of the Cochrane Handbook (Higgins 2009). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were ten or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
Where possible we employed a fixed‐effect model for analyses. We understand that there is no closed argument for preference for use of fixed or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random‐effects does put added weight onto the smaller of the studies ‐ those trials that are most vulnerable to bias. For this reason we favour using fixed‐effect models employing random‐effects only when investigating heterogeneity.
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for prayer.
Subgroup analysis and investigation of heterogeneity
If data are clearly statistically heterogeneous we first checked that data were correctly extracted and entered and that we had made no unit of analysis errors. If the high levels of heterogeneity remained we did not undertake a meta‐analysis at this point for if there is considerable variation in results, and particularly if there is inconsistency in the direction of effect, it may be misleading to quote an average value for the intervention effect. Instead we would have explored possible sources of heterogeneity. We do not pre‐specify any characteristics of studies that may be associated with heterogeneity except those relating to the quality of trial method. If no clear association could be shown by sorting studies by quality of methods a random‐effects meta‐analysis was performed. Should another characteristic of the studies be highlighted by the investigation of heterogeneity, perhaps some clinical heterogeneity not hitherto predicted but plausible causes of heterogeneity, these post‐hoc reasons will be discussed and the data analysed and presented. However, should the heterogeneity be substantially unaffected by use of random‐effects meta‐analysis and no other reasons for the heterogeneity be clear, the results of the individual trials would be presented without a meta‐analysis.
Sensitivity analysis
If necessary, we analysed the impact of including studies with high attrition rates in a sensitivity analysis. We aimed to include trials in a sensitivity analysis if they are described as 'double‐blind' but only implied randomisation had taken place (but no such trials have been included in the 2009 update). If we found no substantive differences within primary outcome when these high attrition and 'implied randomisation' studies were added to the overall results, we included them in the final analysis. However, if there was a substantive difference, we excluded them and only included clearly randomised trials and those with attrition below 40%.
Results
Description of studies
Please also see 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
The original electronic search identified 196 citations and four included studies were identified from these. More recent searches identified six new excluded studies and six new included studies, taking the total number of included studies to ten. Three of the new included studies (Benson 2006, Krucoff 2001 and Walker 1997) were ongoing studies in the original review.
Included studies
The number of included studies now stands at ten with six new studies, Aviles 2001, Benson 2006, Krucoff 2001, Leibovici 2001 and Walker 1997. In all but one of the studies prayer was undertaken after the onset of ill‐health, concurrent with routine treatment, however, in one study, Leibovici 2001, the prayers were ‘retroactive’, that is, they were undertaken after the clinical outcomes were recorded.
1. Duration
Studies ranged from short term with follow‐up for the 'remainder of the admission' (Byrd 1988, Leibovici 2001) to long term with a follow‐up of 15 months (Collipp 1969). Most of the studies, however, were of mid‐term duration. Aviles 2001, Joyce 1964, Krucoff 2001 and Walker 1997 had follow up of six months. Harris 1999 states that participants were the focus of prayer for 28 days but does not comment on the duration of follow‐up. Benson 2006 was also a short trial with prayer for only 14 days, starting the night before coronary artery bypass surgery (CABG) and outcomes were measured through the 30 days after surgery.
2. Participants
A total of ten studies which randomised 7646 people are included in this review. Seven of the ten included studies focused on people who were 'acutely ill' with life‐threatening conditions: children with leukaemia (Collipp 1969), those admitted to a coronary care unit (Aviles 2001, Benson 2006, Byrd 1988, Harris 1999 and Krucoff 2001) and people with a blood stream infection (Leibovici 2001). The participants in Joyce 1964 were ill with psychological or rheumatic disease and in Walker 1997 the participants were being treated for alcohol abuse. Collipp 1969 was the only trial not to include adults. The mean age of participants in this trial was around seven years. All other studies randomised people over the age of 18 years.
3. Setting
Participants were mixture of inpatients and outpatients. All received prayer from outside their medical surroundings.
4. Study size
Study size varied from small (Collipp 1969 n=18, Joyce 1964 n=48) to very large (Leibovici 2001 n=3393, Benson 2006 n=1804, Harris 1999 n=1013).
5. Interventions
5.1 Intercessory Prayer
Patients in the intercessory prayer groups received relevant routine care plus daily intercessory prayer. The types of intercessory prayer varied slightly but all prayers were given with the intent that these intercessions would aid recovery of the patient.
5.1.1 Religious background of those interceding
The religious background of the original researchers is likely to have affected their selection of interceders and was mentioned in some studies. Joyce 1964 was undertaken by two researchers, one of whom started with the belief that prayer 'worked' and the other that it did not. The author of Collipp 1969 recruited "...friends of ours in Washington..." to undertake the experimental intervention and concluded the article with the statement "every physician has prescribed this remedy [prayer] and nearly every physician has seen it succeed". Harris 1999 did not comment on the religious feelings of its authors.
All intercessors had religious belief but their background and level of religious activity varied. Byrd 1988 accepted people as intercessors if they were "'born‐again' Christians with an active Christian life as manifested by daily devotional prayer and active Christian fellowship with a local church.” In Collipp 1969 intercessors were "friends of ours in Washington who [...] agreed to organize a prayer group." Joyce 1964 stipulated two required conditions which needed to be fulfilled: (a) a willingness to accept up to six participant names and (b) residence more than 30 miles from the London Hospital. In Harris 1999 intercessors did not have to belong to any particular denomination, but needed to agree with the statements: "I believe in God. I believe that He is personal and is concerned with individual lives. I further believe that He is responsive to prayers for healing made on behalf of the sick". They also all reported at least weekly church attendance and daily prayer habits before the trial. The volunteers in Walker 1997 were initially recruited from the 'Albuquerque Faith Initiative', a community organisation designed to educate religious professionals and laity about substance abuse. They needed to report at least five years experience of intercessory prayer and believe that at least one prayer had been actively answered.
The remaining five studies were unclear about the level of religious activity previously undertaken by its intercessors. Aviles 2001 recruited from local religious groups and community interest meetings but stated the 'beliefs of intercessors was not quantified'. Krucoff 2001 listed several off‐site prayer groups (United School of Christianity, Buddhist, Roman Catholic, Jewish, Fundamentalist Christian, Baptist and Moravian) but gave no other background information. Benson 2006 listed three Christian groups (St Pauls Monastery, Community of Tersian Carmelties and Silent Unity) but no further details. Leibovici 2001 was also unclear about religious background.
5.1.2 Type of prayer given
Apart from Krucoff 2001, prayer was undertaken daily in all studies. Krucoff 2001 had a Jewish prayer which was placed on the Western Wall for the duration of the trial. One trial, Benson 2006, gave a specific phrase ("for a successful surgery with a quick, healthy recovery and no complications") to be added to the daily prayer of the group.
Most trials used intercessors who prayed within the Judeo‐Christian framework although they had a range of backgrounds: Protestant, Roman Catholic and other interdenominational bodies promoting Christian healing (Quakers and the Guild of Health). Intercessors in Krucoff 2001 belonged to seven different nominations, or faiths (as above).
Some participants were prayed for by groups (Benson 2006, Joyce 1964, Collipp 1969, Krucoff 2001), others by individuals (Joyce 1964, Byrd 1988, Harris 1999, Leibovici 2001 and Walker 1997). Efforts were made in some studies to ensure daily prayer was maintained throughout the trial. Byrd 1988 states that prayer was "under the direction of a co‐ordinator", and in Collipp 1969 intercessors received weekly reminders and participated in frequent discussions about their commitment (despite being unaware that they were participating in a study). Intercessors in Harris 1999 were randomly placed in groups of five members, each with a team leader. Prayer, however, was offered individually, not in groups. None of the intercessors in any trial personally knew the participant for whom they were praying.
In Leibovici 2001 prayer was offered after the period of illness and its outcome. Such retrospective prayer is practised by some people.
5.2 Routine care
Those in the control group received the routine care relevant to their setting, including the medication that would normally be given to people suffering from their particular illness. Joyce 1964 provided "standard, uninterrupted medical care" for all the participant groups (rheumatic and psychological conditions) but no further details are available. Collipp 1969 provided drug therapy for all participants (children with leukaemia). Each child received combinations of between two and five drugs (methotrexate, 6‐mercaptopurine, vincristine, prednisone, daunamycin, bis‐chlorethyl‐nitrosourea, cytosinearabioside, tryptophane mustard and fluorinated progesterone). In Byrd 1988, Harris 1999 and Aviles 2001 all participants were treated on the coronary care unit and the control groups received "usual or standard care". It was assumed, but not stated, in a few studies that all participants received standard medical care. Krucoff 2001 defined standard care by 'the absence of any noetic therapy" and Leibovici 2001stated that there was no "sham intervention".
5.3 Awareness of intervention.
Only one study, Benson 2006, specifically informed some participants that they were receiving intercessory prayer from people other than 'friends and family'. This was to determine the effects of awareness of prayer and in accordance with a pre‐specified analysis plan, we separated this data for additional analysis.
6. Outcomes
6.1 Scales used
The criteria for accepting scale data has changed since the original publication of this review. However, no trial in this review presents usable scale data from a valid scale which would be needed to meet this new criteria (see Data extraction and management). After discussion, therefore, we decided to retain the original data and discuss this below.
a. Clinical State Scale and Clinical Attitude Scale Only Joyce 1964 employed these scales. As neither is referenced we assumed they have not been validated. Data from these scales, however, were graded into dichotomous positive or negative outcomes and as un‐validated clinical opinion for these outcomes is acceptable for this review these too have been included. The Clinical State Scale graded changes in the participants' illness by an examination by a physician, from zero (very poor) to four (very good). We considered data from this scale to be similar to data from the Byrd score and therefore presented them as dichotomous data for Clinical state. The Attitude Scale was also used in a similar way and data from this scale are presented as dichotomous data for Behaviour. Data were categorised into 1. positive scores for (a) stoical; (b) positive and cooperative attitude; (zero for 'non‐committal' attitude); and 2. negative scores for (a) apprehensive; and (b) critical and complaining.
b. Byrd Score (Byrd 1988) This was developed by Byrd 1988 to assess clinical state. Clinical outcomes were categorised into 'good', 'intermediate' and 'bad'. It is not clear if those undertaking the categorisation were blind to the data at the time. The scoring system was based on the presence or absence of complications, for example a patient would be categorised 'good clinical state' if they had no or only one relatively unserious complication/s such as mild unstable angina, supraventricular tachyarrhythmia or mild congestive heart failure without pulmonary oedema. A patient would be categorised as 'bad' if they suffered from complications such as extension of initial infarction, cerebrovascular accident or death. A full list of categorisation of complications is given in the paper. Harris 1999 replicated this study and although they developed their own scoring system (MAIHU‐ CCU score, see below) they also used the Byrd score for clinical state to compare data. After discussion, we have accepted this use of the Byrd Score as a form of peer review and used data from the Byrd score.
c. Mid American Heart Institute‐Cardiac Care Unit scoring system: MAHI‐CCU score (Harris 1999) Harris 1999 created their own scoring system to categorise clinical state into good, intermediate or bad. The scoring system is a 'continuous variable that attempts to describe outcomes from excellent to catastrophic'. Points are given if a patient suffers from a complication and the severity of complications are graded, for example, if, after one day a patient developed unstable angina (one point) was treated with antiangian agents (one point) and then suffered a cardiac arrest (five points) their weighted MAHI‐CCU score would be seven. Although the MAHI‐CCU has not been peer reviewed, because this review was originally prepared before the Cochrane Schizophrenia Group's change in its guidance on the use of data from scales, we decided to leave the data from the MAHI‐CCU scoring system in this review but are aware it may be prone to bias because it is a scoring system created by the authors of the paper (Marshall 2000)
d. Major Cardiovascular End Points (MACE) (Krucoff 2001) Krucoff 2001 used MACE to assess the clinical state of their participants. Again, complications such as death, myocardial infarction congestive heart failure or bypass surgery were used as markers. We felt these were similar enough to the categories used by the above studies to categorise participants in Krucoff 2001 with MACE as 'intermediate or bad' clinical state. Benson 2006 also used 'major events' (defined by the New York State Cardiac Surgery Reporting System) as an outcome, and again, we felt this data could be used in the same way.
6.2 Choosing 'significant complication'. In Byrd 1988 those complications with statistically significant findings are re‐highlighted in the text of the paper and it is these that are quoted elsewhere (AIDS daily summary). We asked an independent collaborator (Dr Evandro da Silva Freire Coutinho), blind to these data, to choose one 'complication' for presentation in the analysis. He chose 'readmission to Coronary Care Unit (CCU)'. As newly included studies also present data for complications we have kept this outcome as a 'primary significant complication' but also report data for other complications arising after treatment.
Excluded studies
There are now 15 excluded studies in this review. Six trials have been added since the original version of this review (Abbot 2000, Conti 1999, Green 1993, Harrison 1999a, O'Mathuna 1999 and Toth 1999). Three of these (Abbot 2000, O'Mathuna 1999 and Toth 1999) were not trials but reviews. The other three studies were randomised trials not using intercessory prayer as one of the interventions. We excluded Galton 1883, as it was a retrospective study, although cited by Byrd 1988, Collipp 1969 and Joyce 1964. Two studies examined the effect of providing a religious solution to a hypothetical personal problem (Lilliston 1981, Lilliston 1982). A further three trials investigated the effect of specifically "non‐contact therapeutic touch" for dermal healing (Wirth 1994a), the results of non‐traditional prayers on physiological measures (Wirth 1994b), and "distance healing by volunteers trained in LeShan's meditation techniques" (Greyson 1997). As a result of the update of February 2000, Sicher 1998 was excluded as the intervention was 'distance healing'. This technique may have included an element of prayer but did not specifically involve personal, focused, committed and organised intercessory prayer on behalf of another alone. Sicher 1998 is the published result of 'Targ 1993', which, in previous versions of this review, was listed as an 'Ongoing Study'.
We had previously included Cha 2001, but this has been changed to an excluded study in the 2009 update, after we learnt of the controversy surrounding it and the removal of the study from the website of the Journal of Reproductive Medicine.
Awaiting classification
No further publications of Larson 1997 and Choi 1997 have been found despite considerable searching, so we decided to remove these from 'awaiting classification' (previously, ‘awaiting assessment’) and have now excluded them. If further publications come to light we will assess them.
Ongoing studies
In the 2009 update, three studies that were previously in this section (Benson 2006, Krucoff 2001, Walker 1997) have been included. We have not identified any currently ongoing studies.
Risk of bias in included studies
Allocation
All included studies were stated to be randomised. Joyce 1964 stated that allocation was decided by the spin of a coin, Collipp 1969 by randomly selecting names, and Byrd 1988 by a computer generated list. In none of these studies was it stated that those in charge of allocation were blind as to what a 'heads or tails' of the spinning coin meant, to how the computer generated list was to be used or where the next name out of the bag was to go.
Harris 1999 had a rather unexpected method of randomisation. All new admissions to the CCU were identified daily in the chaplain's office, and these new patients were "randomly" assigned to either group by the chaplain's secretary based on the last digit of the medical record number; even numbers assigned to the prayer group, odd to 'usual care'. After some discussion we came to the conclusion that this method of allocation was adequate. Nevertheless, for every outcome that included data from Harris 1999 we undertook an analyses to investigate the sensitivity of the finding to removal of these data. The six studies added to the update were also all stated to be randomised and all described how this was achieved. Aviles 2001, and Walker 1997 used computer programmes. Krucoff 2001and Benson 2006 allocated participants by on‐site envelopes and Leibovici 2001 used a random number generator.
Blinding
Participants in Joyce 1964 were not aware of their participation in a trial and the rater was unaware of the group to which each patient had been allocated. Byrd 1988 obtained written consent from all participants but neither they nor those rating outcomes knew of their group allocation. In Collipp 1969 neither the children with leukaemia nor their parents knew of their inclusion in a trial and, in addition, all physicians were blinded. Even those praying were unaware that they were taking part in a study. Harris 1999 did not obtain written consent, so all participants may have been blind as we have found no report of them having given verbal consent. CCU staff, data collectors and statisticians involved in the studies were also blind to allocation. Five of the six new studies were double blind and described how this was achieved. In each case “double blind” was used to indicate that both the patient and the people responsible for their health care did not know whether the patient was in the prayer or the control group. Of note, Aviles 2001 stated that participants, care‐givers and interviewers were blind to allocation. Finally, Leibovici 2001 did not obtain consent from participants to maintain blindness ‐ the intervention took place after the outcome. The study was double blind; those praying did not know the outcome for any of the patients. One study, Benson 2006, specifically told one group of participants they were receiving intercessory prayer while the other groups were uncertain of their allocation. This was to assess the effects of awareness of intercessory prayer on recovery. All carers and researchers were unaware of each patient's allocation in this trial.
We have not been able to extract data fully for the Risk of Bas table in this update (see Figure 1) because of misplaced papers and the need to reach the deadline for publication. We have not extracted information on incomplete outcome data, selective reporting and other potential sources of bias from Collipp 1969, Joyce 1964 or Walker 1997. We will amend this for a forthcoming issue of The Cochrane Library.
1.
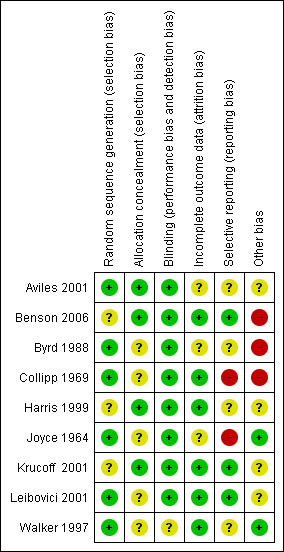
Review authors' judgements for each quality item
Incomplete outcome data
Analysis in Joyce 1964 was by sequential, paired analysis and this led to two of the 19 original pairs being eliminated because one of each pair was found not to satisfy the criteria for admission to the study. Also one member of a third pair failed to attend despite repeated requests and data from that pair were lost. Aviles 2001, Benson 2006, Byrd 1988, Harris 1999, Krucoff 2001 and Leibovici 2001 all had a design where loss of data was difficult and full follow‐up was possible.
Selective reporting
The only evidence we found of a failure to report selected results was that Aviles 2001 stated that they were going to report on quality of life and we could not see where they had done this. The opposite ‐ where a few primary outcomes were pre‐stated but many secondary outcomes were then presented ‐ was more prevalent (Benson 2006, Byrd 1988, Harris 1999). Byrd 1988 especially highlighted the statistically significant effects from the secondary outcomes over the equivocal results of the primary outcomes. This caused us some difficulties (please Description of studies).
Other potential sources of bias
The Benson 2006 and Byrd 1988 studies were undertaken by researchers with a prior belief in the positive effects of prayer. The beliefs of the researchers in the other trials are not clear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
As outlined above (Allocation) we did have some concerns as to whether Harris 1999 should be included. We included and excluded Harris 1999 from all outcomes for which that study reported data. In every case it tightened confidence intervals but made no substantive difference in the findings with a few exceptions. When synthesised with other data it tended to be conservative in its findings and make the findings less favourable for the intervention of intercessory prayer. The first exceptions are where all data for a given outcome are from the Harris 1999 study (anaemia/transfusion, atrial fibrillation, cardiopulmonary arrest, catheterization, implanted cardiac defibrillator, interventional coronary procedure, intra‐aortic balloon pumpSwan‐Ganz catheter). The second group of exceptions are where exclusion of Harris 1999 does result in the finding changing from a null finding to one that becomes statistically significant in favour of prayer (congestive heart failure, diuretics, intubation/ventilation, major surgery before discharge, pneumonia).
We had not anticipated a study of prayer in which people prayed for those who had already had an outcome (albeit one unbeknownst to those intervening). In the last update we added this study to the others but, after conversations with the ombusmen of the Cochrane Collaboration and the Editor in Chief, we have moved it to a seperate comparison.
1. INTERCESSORY PRAYER (CONTEMPORANEOUS) versus ROUTINE CARE
1.1 Death
Five studies present data on death (Aviles 2001, Benson 2006, Collipp 1969, Byrd 1988, Harris 1999). Overall there was no clear effect of intercessory prayer on death (5 RCTs, n=3389, random‐effects RR 1.00 CI 0.74 to 1.36). Aviles 2001 separated data into high‐risk patients and low‐risk patients. This study identified no effect of intercessory prayer on people with low risk of death (1 RCT, n=315, RR 0.57 CI 0.23 to 1.39) or high risk (1 RCT, n=445, RR 1.03 CI 0.59 to 1.81).
1.2 Clinical state
1.2.1 Intermediate or bad outcome
Five studies present data for general clinical state (Joyce 1964, Byrd 1988, Benson 2006, Krucoff 2001, Harris 1999). Overall there was no significant difference in clinical state between intervention groups (5 RCTs, n=2705, RR 0.98 CI 0.86 to 1.11).
1.3 Significant complications
1.3.1 Re‐admission to Coronary Care Unit (CCU)
Four studies found no significant effect for re‐admission to CCU (4 RCTs, n=2644, RR 1.00 CI 0.77 to 1.30).
1.3.2 Presence of any post operative complications by 30 days
Only Benson 2006 grouped complications into one outcome of 'any complication'. Compared with routine care, people unaware of receiving intercessory prayer were not shown to be more or less likely to have post operative complications than those not receiving prayer (1 RCT, n=1201, RR 1.02 CI 0.92 to 1.14).
1.3.3 Various complications
Other studies presented data for specific complications. A total of 33 complications were listed as outcomes, of these, only three showed any significant effect. The numbers suffering cardiac arrest before the end of trial, numbers needing major surgery and/or Swan‐Ganz catheter were all significantly lower in the prayer group. Aviles 2001, Byrd 1988 and Harris 1999 report data for cardiac arrest ‐ the result is just statistically significant (3 RCTs, n=2174, RR 0.46 CI 0.21 to 0.99, NNT 100 CI 69 to 5377). The combined analysis of Byrd 1988 and Harris 1999 found significantly more people in standard care underwent major surgery during the trial (2 RCTs, n=1383, RR 0.69 CI 0.51 to 0.95, NNT 27 CI 17 to 162). One study, Harris 1999, found fewer people in the prayer group needed a Swan‐Ganz catheter. This result is also marginally statistically significant (1 RCT, n=990, RR 0.80 CI 0.66 to 0.98, NNT 16 CI 9 to 153).
1.4 No change or deterioration in attitude
Joyce 1964 did not detect a significant difference for people receiving intercessory prayer in regard to attitude deterioration or change compared to those receiving routine care for their condition (1 RCT, n=38, RR 0.94 CI 0.73 to 1.21).
1.5 Service use
1.5.1 Rehospitalisation, any reason
The combined analysis of Aviles 2001 and Byrd 1988 shows no significant difference on rehospitalisation (2 RCTs, n=1155, RR 0.93 CI 0.71 to 1.22).
1.5.2 Number of visits to emergency department (specific to cardiac problem)
Harris 1999 found no significant difference between groups in the number of visits to emergency room after discharge (1 RCT, n=1789, RR 1.28 CI 0.73 to 2.24).
1.5.3 Mean number of days in hospital
Two studies presented skewed data for this outcome, results were equivocal (Byrd 1988 and Harris 1999).
1.5.4 Mean number of days in CCU
Skewed data from Byrd 1988 found no significant difference for this outcome.
1.6 Leaving the study early
We found no significant difference between groups for numbers of people leaving a study early (6 RCTs, n=3446, RR 0.75 CI 0.43 to 1.31).
2. INTERCESSORY PRAYER (RETROSPECTIVE) versus ROUTINE CARE
2.1 Death
There is one relevant study (Leibovici 2001). Overall there was no clear effect of 'retroactive' intercessory prayer on death (n=3393, RR 0.93 CI 0.84 to 1.03).
2.2 Leaving the study early
As this study was retroactive and record keeping was, by all accounts complete, no one could leave the study in the conventional sense.
3. AWARENESS OF INTERCESSORY PRAYER versus ROUTINE CARE
Benson 2006 was the only trial to assess the effect of 'awareness of prayer'.
3.1 Death
No significant difference was found for death (1 RCT, n=1198, RR 0.92 to 1.40)
3.2 Clinical state
3.2.1 Intermediate or bad outcome
Again, there was no significant difference on clinical state between people aware they were being prayed for and people not being prayed for by the end of the trial (1 RCT, n=1198, RR 0.91 CI 0.64 to 1.29).
3.3 Significant complications
3.3.1 Re‐admission to CCU
There was no significant difference between groups for this outcome (1 RCT, n=1198, RR 1.04 CI 1.04 to 1.28).
3.3.2 Presence of any post operative complication by 30 days
An effect for this outcome, with those not receiving prayer having fewer post operative complications than those aware they were receiving prayer (1 RCT, n=1198, RR 1.15 CI 1.04 to 1.28, NNT 14 CI 8 to 50).
3.4 Leaving the study early
No significant difference between groups was found for leaving the study early (1 RCT, n=1198, RR 2.98 CI 0.60 to 14.71).
4. AWARENESS OF PRAYER versus UNCERTAINTY OF PRAYER
4.1 Death
Benson 2006 found no difference in death between groups (1 RCT, n=1205, RR 0.82 CI 0.40 to 1.68).
4.2 Clinical state
4.2.1 Intermediate or bad outcome
Although fewer people aware of receiving prayer had a 'bad or intermediate' clinical state by the end of the trial, the result is not statistically significant (1 RCT, n=1205, RR 0.78 CI 0.60 to 1.02).
4.3 Significant complications
4.3.1 Re‐admission to CCU
No significant difference between groups was found for re‐admission to CCU (1 RCT, n=1205, RR 0.95 CI 0.67 to 1.36).
4.3.2 Presence of any post operative complication by 30 days
Benson 2006 found a difference between groups, favouring those uncertain of receiving prayer ‐ but it was marginally statistically significant (1 RCT, n=1205, RR 1.12 CI 1.01 to 1.24, NNT 17 CI 9 to 201).
4.4 Leaving the study early
Very few people left the study early and the result is non significant with a wide confidence interval (1 RCT, n=1205, RR 3.01 CI 0.61 to 14.88).
Discussion
Summary of main results
1. INTERCESSORY PRAYER versus ROUTINE CARE
1.1 Death
Overall, the trial data show no more or no fewer deaths in the intercessory prayer group. There is no clear effect of this type of prayer, within these very unusual randomised trials. People of faith may speculate as to why this is so. There could be many plausible reasons when considered from this perspective. Those of no theological belief will also be able to use these data to support their hypotheses.
1.2 Clinical state
1.2.1 Intermediate/poor outcome
Five studies (n=2705) presented data relating to intermediate or poor outcome (Benson 2006, Byrd 1988, Harris 1999, Joyce 1964, Krucoff 2001). Results were equivocal and heterogeneous (I2 =78%). Harris 1999 carried most weight in this meta‐analysis, and although this was a trial replicating Byrd 1988, it found prayer to be less effective. Harris 1999 suggests this could be due to "important differences between the two study designs" (more stringent blinding, lack of informed consent possibly changing participant group, amount of participant information given to intercessors). It is also important to note that in Byrd 1988 the point at which decisions were made relating to the definitions of 'good', 'intermediate' and 'poor' was not stipulated. It is not clear whether these important decisions were made before or after seeing the data and whether those doing the analysis were blind to group allocation. Therefore, bias may have influenced the result of the rather positive Byrd 1988. It is also interesting that Benson 2006, another large trial, also found less favourable results for prayer than the smaller trials. The outcomes of these two larger trials were different to the results of other prayer trials. Participants in Benson 2006 received prayer for only 14 days and as the original authors noted, this may not be enough time for prayer to be effective. It may also be that the study measured an outcome which is not effected by prayer or that prayer has no effect at all.
Other problems with interpreting clinical state results are the use of non‐published scales. In the protocol for this review we stated that only published scales would be reported in an attempt to avoid the presentation of invalid data. However, we were limited in that all data for this outcome came from scales for which the validation status is not clear. The Byrd score used by Byrd 1988 and Harris 1999 was created by Byrd 1988 and replicated by Harris 1999. We felt this was a form of 'peer review' and included these data but realise it could be prone to bias and more valid scale data are needed to confirm this result. The Clinical State Scale, as used in Joyce 1964, is also not referenced and it is unclear if it is a valid measure of health or can be used with any degree of reliability. Data on this outcome are so positive that it may be widely quoted. Even if this scale is indeed a valid measure of health, such a result from a small trial (n=38) must be viewed with caution.
1.3 Significant complications
The presence of post operative complications was a main outcome in several trials. Byrd 1988 and Harris 1999 both presented long lists of possible complications. As noted above, we asked a colleague to choose a generic 'significant' complication blind to the data. He chose 'Readmission to Coronary Care Unit' and results from four studies (n=2644) found no overall difference in these readmissions.
Analysis of data from the list of specific complications found three out of 33 outcomes were statistically significant, all favouring prayer. However, the two studies presenting these data (Byrd 1988, Harris 1999) presented results for such a long list of complications that statistical analysis was likely to highlight some as 'statistically significantly' improved by the use of prayer by chance alone (Bender 2008). The authors of Byrd 1988 do state how multiple analysis of variables can lead to spurious 'significant' results but go on to re‐report these in the text of the paper. It is these results that are then selectively quoted in other papers, leading to reporting bias (Anonymous 1995). Benson 2006 did not present results for individual specific complications but summated data into 'presence of any complication' and found no effect.
1.4 No change or deterioration in attitude
Joyce 1964 presented data from an unknown 'Attitude Scale' and the same arguments apply to this as to the Clinical State Scale (see above). Results are equivocal. Even if the Attitude Scale is a valid measure of attitude, the trial was too small to detect a difference unless it was very large.
1.5 Service use
No effect was found for any service use outcomes. There were no significant differences between groups for rehospitalisation (n=1155) or visits to emergency departments (n=1789).
1.6 Leaving the study early
Six studies (n=3446) found no overall difference in the numbers leaving studies early. However, it might be better to regard this outcome as “loss to follow up” rather than leaving the study early because, in most trials, the patients were not aware that they were being studied.
2. INTERCESSORY PRAYER (RETROSPECTIVE) versus ROUTINE CARE
2.1 Death
The purpose of this review is not to consider the putative means by which prayer may or may not be effective, but only to consider the results of well‐constructed trials. Leibovici 2001 attempted to test the effects of such prayer and did not find any clear effect (n=3393, RR 0.93 CI 0.84 to 1.03). Retrospective prayer may be considered theologically controversial, but we are not concerned with theology. Our aim is to review the empirical evidence for the efficacy of prayer as a treatment for ill‐health rather than to consider questions of metaphysics. We judge ourselves bound to analyse the results of any trial that fits our original criteria (including our initial definition of prayer) and which is methodologically well constructed. Having set our protocol we are convinced that it would be unscientific to modify it to exclude a study that fits our criteria for inclusion, for all it approaches this from an unusual angle.
2.2 Leaving the study early
We understand that record keeping was complete so no one could leave the study in the conventional sense.
3. AWARENESS OF INTERCESSORY PRAYER versus ROUTINE CARE
Benson 2006 found no significant effect for 'awareness of prayer' (where the participants were told and gave consent for intercessory prayer on their behalf, for their recovery from illness) for death, clinical state or leaving the study early. There was however, a significant effect favouring standard care for 'presence of post operative complications' (1 RCT, n=1198, RR 1.15 CI 1.04 to 1.28, NNH 14 CI 8 to 50). This result is from a single trial and needs replication if it is to be accepted. One suggestion, made by the original authors, as to why people not receiving prayer should have fewer postoperative complications than those who knew they were being prayed for is the limited time prayer was offered to those in the prayer group. This result, which is just statistically significant could also be due the play of chance when there is truly no difference between the groups or be a real, toxic effect of the knowledge that one is being prayed for.
4. AWARENESS OF INTERCESSORY PRAYER versus UNCERTAINTY OF PRAYER
Here the intervention 'awareness of prayer' is as above and those in the 'uncertainty of prayer' group were told they may or may not be the focus of intercessory prayer, by either an individual or group praying for their recovery from illness.
The single trial that investigated this, Benson 2006, found no significant differences between groups for any outcomes, except for presence of post operative complications (1 RCT, n=1205, RR 1.12 CI 1.01 to 1.24, NNH 17 CI 9 to 201). As above, this suggests that awareness of a person or group praying for you may have moderate untoward effects, but this finding needs replication if it is to be accepted.
Overall completeness and applicability of evidence
There is considerable interest relating to this widely used health care intervention and studies relevant to prayer and distance healing continue to be carried out, although we know of no ongoing studies directly relevant to this review. There are, however, a number of studies, at least as extensive in number as those included in this review, which deal with interventions of a spiritual nature not focused specifically on the supplication of God or a god. These include such practices as ‘distance healing’. We restricted our review to ‘intercessory prayer’, as widely understood and were limited by a lack of usable data within the included trials.
A further issue is that the healthcare conditions of the trial participants were diverse, although the majority did suffer from coronary disease (Aviles 2001, Benson 2006, Byrd 1988, Harris 1999, Krucoff 2001). This could be a limitation or a strength, but all of the conditions were serious and life‐threatening and we expect that the beneficial, harmful or non‐existent effects of prayer should be similar across different health problems.
Quality of the evidence
We have not yet fully investigated the quality of the evidence arising from the trials in this review but we do not consider it to be radically different from what is common for randomised trials of, for example, drug interventions or psychological therapies. In some ways, the original researchers for these prayer trials have been more clear about their potential for bias and their prior beliefs than would be expected in a trial of a drug run by the pharmaceutical industry or of a psychological therapy run by a psychotherapist. Overall, the reporting of the included studies could have been improved and it is likely that all studies fall into a category of having at least a moderate risk of bias. For example, sequence generation, concealment, blinding were not well described in any of the studies and this poor description of these methodological parameters has been shown to be associated with an over estimate of effect (Jüni 2001). Having said that, the overall estimates were non‐significant so it is not clear how increasing the methodological quality of the evidence would have changed the result.
Potential biases in the review process
Searching for these unusual studies is not easy. There is no clear place where they would be published or indexed. Several databases have been used but it is likely that we have missed some eligible trials. These are likely to be those studies that are smaller and more difficult to publish ‐ and, perhaps less positive for prayer (Egger 1995).
We have excluded Cha 2001 from this version of the review because of the comments received feedback and knowledge gained by our own investigations. We may have been incorrect in doing this and still do not have proof that the study was bogus but the behaviour of the principal investigator raises concerns about its legitimacy which makes it safer to exclude it at this time.
We have also received comments suggesting that publication in the 2001 Christmas issue of the BMJ should preclude a study from inclusion because its methods or findings would be "jest" (Feedback). We found no evidence that this was true. Leibovici 2001 is an unusual and original design ‐ but it appears to have used a rigorous design, and, although a challenging study, it was conducted carefully and is presented respectfully. We do not agree that it should be regarded as a "jest" and excluded on those grounds but, as with all Cochrane reviews, we present its results explicitly and others may wish to remove it before conducting their own meta‐analysis using the RevMan 5 data file which is available alongside this review. We do acknowledge that this study will pose philosophical problems for some readers. We aim not to involve ourselves in such philosophical discussions but to include all studies that tested empirical claims in a rigorous, empirical way.
Agreements and disagreements with other studies or reviews
This version of the review broadly concurs with findings from those that preceded it (Roberts 2000, Roberts 2007).
Authors' conclusions
Implications for practice.
1. For people receiving health care
The studies that have been done, reported and included in this review do not show a clear effect of intercessory prayer. However, because this review highlighted no clear effects does not mean that intercessory prayer does not work. The limitations in trial design and reporting are enough to hide a real beneficial effect and we found no data to contraindicate the use of prayer for seriously ill people.
2. For those intervening with prayer
As we state near the beginning of this review, the trials included in this review cannot prove or refute the existence or actions of God. We have sought to use empirical methods to investigate the effectiveness of intercessory prayer for those who are sick and, mostly, this review suggests no real effect of prayer on health outcomes for the patients being prayed for.
3. For managers or policymakers
In the light of the best available data, there are no grounds to change current practices in relation to the provision, or not, of prayer or the associated facilities.
Implications for research.
1. General
Future studies, if there should be any, should follow CONSORT guidance on reporting and best practice on their methodological conduct. This review would have more data and greater confidence in its results should this guidance have been followed by the trials that we have included.
2. Specific ‐ should there be more trials?
The evidence presented so far is interesting enough to support further study. However, if resources were available for such a trial, we would probably use them elsewhere. There are many other treatments that are in urgent need of evaluation and that are likely to be more suited to investigation in a randomised trial. Should someone else have resources for a randomised trial of intercessory prayer, we have suggested a design based on the best of the trials we have seen already (Table 5).
1. Suggested design for future trial.
| Methods | Allocation: centralised sequence generation with table of random numbers or computer generated code, stratified by severity of illness, sequence concealed till interventions assigned. Blinding: those recruiting and assigning participants, those administering intervention, those assessing outcomes, all blind to allocated group. Duration: minimum of 26 weeks. |
| Participants | Diagnosis: Any person with a physical or mental health problem. N=300.* Age: adults. Sex: men and women. Setting: anywhere. |
| Interventions | 1. Intercessory prayer: standard care (see below) plus personal, focused, committed and organised intercessory prayer on behalf of another. N=150. 2. Standard care: the relevant medical and non‐medical care normally given to people diagnosed with their particular illness. N=150. |
| Outcomes | Key problem prayed for resolved**. Quality of life: functioning. Service outcomes: healthy days**, days in hospital. Satisfaction with care: patients / carers. Adverse effects: including mortality. Economic data. |
| Notes | * Size of study to detect a 10% difference in improvement with 80% certainty. ** Primary outcome. *** If scales are used to measure outcome then there should be binary cut off points, defined before study starts, of clinically important improvement. |
Feedback
Jørgensen, Hrobjartsson and Gøtzsche, 16 April 2008
Summary
This review is riddled by serious flaws such as lack of critical appraisal of the included trials and findings, lack of a necessary discussion of the relevant sources of bias, and undue interference of theological reasoning. We list the most important problems: 1) The largest included study was published in the Christmas issue of the BMJ (1, which is characterized by articles written in jest. This was also the case for the study in question (2). It carries 75% of the weight in one of the main meta‐analyses of the review where the authors report a statistically significant effect on death, relative risk 0.88, 95% confidence interval 0.80 to 0.97. However, nowhere did the authors of the Cochrane review mention that this study evaluated the effect of prayer taking place 4‐10 years after the patients had either left the hospital alive or had died from their bloodstream infection. Thus, the study randomised dead patients and then studied whether they were dead or alive. The authors argued that we cannot assume "...that God is limited by a linear time". 2) One of their methodological reservations are also encountered within alternative medicine where the true benefit of an intervention often seems to escape what is measurable in a scientific setting: “If understanding of God is as limited as the Holy Literature suggests (1 Corinthians 13:12), the consequences of divine intervention may be considerably more subtle than could be measured in the crude results of a trial”, and “It could be that any effect of prayer are due to elements beyond present scientific understanding”. If these are real concerns for the authors, they should not have undertaken the review at all and their reservations also disagree with the stated premises for the review, that only non‐divine effects were to be assessed. This theological reservation is no more applicable to a scientific investigation of prayer than to trials of any other medical intervention, as a God may also intervene in these cases. 3) The theological reasoning leads the authors to untenable statements, e.g. “A caring God may not wish to prolong suffering, so death therefore might be a positive outcome of prayer”. This is a perfect immunization of the hypothesis that will make any trial of prayer meaningless. If people survive, it is good for them, and if they die, it is also good for them. 4) The authors found one study that reported an increased risk of surgical complications due to prayer, but only if the patients are aware that people pray for their improved health (relative risk 1.15, 95% confidence interval 1.04 to 1.28). Instead of discussing the plausibility of this finding, and the finding that knowledge of the intervention did not affect the other positive outcomes in this review, e.g. increased chance of successful in‐vitro fertilisation, the authors conclude that people intervening with prayer should be “cautious about informing the recipient” when it comes to surgery and that managers and policymakers may wish to exercise some caution about “praying at the bedside of those who are about to have a surgical operation”. 5) When discussing the effect of prayer on what the authors call “clinical state”, they attempt to explain the lack of effect as being because the participants only received prayer for 14 days, which may not have been enough for prayer to be effective. The authors do not mention the far more plausible interpretation that the lack of effect of prayer is because prayer has no effect. This review does not live up to the scientific standards one can reasonably expect of a Cochrane review. The review as currently published should be withdrawn from the Cochrane Library, not least because it suggests that all scientific studies are meaningless, as we will never know whether one or more gods intervened in our carefully planned experiments.
References
Reply
The Editorial Base and the Comments Editor of the Cochrane Schizophrenia Group do wish to apologise to those sending the comment on the ‘Intercessory prayer for the alleviation of ill health’ review which appeared in Issue 1 of 2009. This comment was erroneously attributed to “Peter C. Gøtzsche ‐ Director, Nordic Cochrane Center at Rigshospitalet in Copenhagen, Denmark” and should read, as it does now, “Karsten Juhl Jørgensen, Asbjørn Hrobjartsson, Peter C. Gøtzsche”.
We regret that the feedback finds problems with this review, even to the extent in one case of saying that the review is 'riddled with serious flaws'. We have made every effort to make such corrections as we think were justified and thank those commenting for highlighting them. We have addressed them in the same order as appears above.
1. Comments made about the Christmas issue of the BMJ and the Leibovici 2001 study in particular are not fully accurate. Several articles in the late December issues of the BMJ are written with humour and some in pure spoof. Most are not. They may be written with humour and have an odd perspective, but are, nevertheless, interesting and well thought out research. The Leibovici 2001 was not in jest. It is a rather serious paper, intended as a challenge (direct link to comments).
2. Our brief theological discussions were meant to be illustrative of some of the wider academic background to these questions and were not intended to relate to the studies themselves. We note that this distinction could be taken further and have attempted in this revision to make a clearer distinction between philosophical or theological background and the entirely empirical matter under discussion.
3. We do not feel that it would benefit the purposes of this review to argue the point brought to the fore by the commentators. We have tried to state the evidence ‐ about outcomes such as death ‐ and let the readers draw their own conclusions. Perhaps we have been unsuccessful in that ‐ and for this we would wish to apologise. We are, however, aware that readers will differ as to how they perceive the data, and the phrase highlighted by the comment illustrates this. The comment suggests that some paths of logic may make trials of prayer meaningless. Whether we agree or disagree is not important. These trials have been undertaken and, we still feel, merit thoughtful review.
4. We accept these points and have tried to reword the relevant passages.
5. The commentators are right in highlighting this shortcoming and we have addressed it in the text. We are unsure upon what the commentators base their claim of plausibility and suspect that such a sweeping claim is based in faith.
We disagree that this review does not live up to the scientific standards one can reasonably expect of a Cochrane review. It is a complex review, and like many others has been and remains imperfect. With successive revisions we endeavour to improve it.
We disagree that this review should be withdrawn: prayer is a very widely used intervention in response to ill‐health, the studies are judged on their merits and our analysis is sound.
We regret that we have caused some commentators to suppose that we were suggesting that "all scientific studies are meaningless". We are also a little perplexed by this response. We strongly believe otherwise.
Similarly, we are sorry that some commentators feel that this review merits censoring on the grounds that they perceive it to be suggesting "all scientific studies are meaningless, as we will never know whether one or more gods intervened in our carefully planned experiments". We have proceeded on the basis that empirical claims are made for prayer and that these can be empirically tested.
Contributors
Contributors of feedback: Karsten Juhl Jørgensen, Asbjørn Hrobjartsson and Peter C. Gøtzsche. All authors: The Nordic Cochrane Centre, Rigshospitalet Dept. 3343, Copenhagen, Denmark.
Contributors of response: Leanne Roberts and Andrew Davison.
Jackson, 24 March 2009
Summary
I corresponded with Leanne Roberts (author) in 2007 by email. This feedback's an encapsulation of the main points of that email exchange. The IVF study cited has been debunked and reference to it ought to be withdrawn. There are several excellent papers explaining why intercessory prayer cannot be studied using a RCT, rendering meta‐analyses of RCTs meaningless. (Chibnall 2001 is one of the best but there are many others ‐ email me for a more complete list). Please can this review be overhauled, emphasising that RCTs of prayer are meaningless and that scientific study of prayer ought to be limited to its social effects ideally using qualitative methodologies? There's a lot of pseudoscience being done in this area. Thanks.
Reply
Dr Jackson makes two separate points. The first concerns the Cha study (Cha 2001). We are in agreement with him. Where there is justified dispute as to methodology or scholarly integrity we will exclude studies from the review. Consequently, this study was removed from the last substantial update in Issue 2, 2009 of the Cochrane Library.
Dr Jackson's second point is more wide‐ranging. We agree with him in opposing surveys that are undertaken for the purpose of proving or disproving a prior commitment. He suggests that other reviewers in this field have undertaken to ‘ignore papers, or cherry‐pick perceived weak points’. If this is so then we join with him in his disapproval. This is, however, a serious accusation. Dr Jackson’s willingness to classify authors in this field widely as either ‘pro‐IP’ (intercessory prayer) or as ‘anti‐IP’ is, in our opinion, too extensive a charge. For our part, in identifying and analysing studies for this review we have sought to use scrupulously objective standards. We therefore see ourselves for the purpose of the review as neither ‘pro‐’ nor ‘anti‐IP’.
Dr Jackson urges us to include ‘an argument against further IP studies being performed’ in the future. It seems that there are two possible reasons for taking such a position. First, that the scientific results show IP to have been disqualified or confirmed with a very high degree of certainty. The results are by no means conclusive enough for us to urge such a course on this basis. A second reason for stopping studies of IP would be that it is inherently flawed to use randomised controlled trials (RCTs) to investigate this subject. This point deserves more detailed consideration.
Arguments for or against the applicability of RCTs for IP rest upon prior, conceptual commitments of a theological nature. We take any discussion of the ‘god’ under consideration to be ‘theological’. One has to have a sense of the entity one is talking about, and its relationship to the world, regardless of whether one believes in its existence or not.
Some of those who have written on divine agency conceive of God[1] and intercessory prayer in a way that raises serious questions about the legitimacy of RCTs in these studies. Others, however, have understood God and the mechanics of intercessory prayer in a way that makes sense of studying IP in this way. For instance, RCTs might well investigate the action of God understood as a relatively limited being among beings. There might be problems if we understand God as beyond time and space and as omniscient.
It is not possible to rule upon the validity of RCTs for IP without taking a particular line on these questions. We cannot agree with Dr Jackson that studies of this kind should be ruled out, simply because this cannot be recommended without making assumptions of a philosophical and theological nature, whether theistic or atheistic. Such assumptions might provide valid personal motivation in the case of the involvement of the individual scientist. They cannot be a reason for a journal to rule out a meta‐analysis.
Laying aside theological‐metaphysical questions for the sake of our review, we are simply left with a set of empirical studies that can be judged on their merits as RCTs, and subjected to careful meta‐analysis. As it happens, the theological‐philosophical convictions of at least one of the authors involved in our review disposes them against the use of RCTs for investigating IP. He or she holds that this must be put to one side and wishes to examine the empirical evidence as empirical evidence.
In conclusion, we do not think that one could rule out further RCTs for IP on a priori grounds separate from ‘theological’ assumptions as to the properties of the God in which one does or does not believe. One could not urge for these studies to be discontinued on a priori grounds without bringing theological ideology into science. IP may be studied by empirical means until such time the empirical findings themselves suggest an end to the studies.
[1] In this discussion we refer to ‘God’; we are, however, deliberately agnostic, and this could as easily be taken to refer to ‘gods’.
Contributors
Contributors of feedback: Chris Jackson, anaesthetist. Contributors of response: Leanne Roberts, Steve Hall and Andrew Davison.
Jørgensen, Hòbjartsson and Gøtzsche, 6 January 2011
Summary
In the updated Cochrane review of intercessory prayer (1), the authors have chosen not to exclude a study,Leibovici 2001(2) that we have shown to be unfit for inclusion (3).
In the updated review, the authors noted that: ‘We have also received comments suggesting that publication in the 2001 Christmas issue of the BMJ should preclude a study from inclusion because its methods or findings would be "jest". We found no evidence that this was true.’
This seems to be part misquotation, part neglect. We have not suggested that publication in the Christmas issue of BMJ in general precludes inclusion of studies in Cochrane reviews (3). However, we do think it is problematic to include a study written in jest if there is evidence in favour of this. That was the case for the Leibovici 2001 study. We provided the evidence in our first response to the Cochrane review in a reference to a rapid response in the BMJ. Leibovici clearly described his trial as a ‘non‐study’ and emphasised that his aim was not to test intercessory prayer, but to illustrate the limitations of which interventions can be sensibly tested in randomised trials:
‘() if the pre‐trial probability is infinitesimally low, the results of the trial will not really change it, and the trial should not be performed. This, to my mind, turns the article into a non‐study, although the details provided in the publication (randomisation done only once, statement of a wish, analysis, etc) are correct. The article has nothing to do with religion. I believe that prayer is a real comfort and help to a believer. I do not believe it should be tested in controlled trials.’ (4)
The review authors should therefore have had easy access to evidence that our assertion of the Leibovici 2001 study is correct.
We recently wrote to authors of articles published in the BMJ Christmas issue to clarify the intentions behind them and investigate if they were given undue weight when they were subsequently quoted (5). The authors, including Leibovici, were given two options and could characterise their study as either:
A) a serious study (conducted to answer a scientific question, and could have been published in another scientific journal), or B) a spoof study (written specifically for the BMJ Christmas issue with no attempt of addressing the proposed scientific question, but with an implicit ironic or tongue‐in‐cheek objective).”
The answer from Leibovici was unmistakable: ‘Certainly B ‐ although the details of the 'study' that was done (basically dividing an old database into 2 random parts and comparing them) were described correctly in the article.’ Not all authors of papers in the BMJ Christmas issue chose option B, however.
We suggest a prompt amendment to the review with an exclusion of the Leibovici study.
1. Roberts L, Ahmed I, Hall S, Davison A. Intercessory prayer for the alleviation of ill health. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD000368. DOI: 10.1002/14651858.CD000368.pub3.
2. Leibovici L. Effects of remote, retroactive intercessory prayer on outcomes in patients with bloodstream infection: randomised controlled trial. BMJ 2001;323:1450‐1.
3. Gøtzsche PC, Jørgensen KJ, Hróbjartsson A. Serious flaws? Feedback, Cochrane Library, April 18 2008.
4. Leibovici L. Author's reply. BMJ 2002;324:1037.
5. Felding UA, Jørgensen KJ, Hróbjartsson A. [A new source of scientific error: SILLY‐bias]. Ugeskr Laeger 2009; 171: 3784‐9.
Reply
We note the further comment from our colleagues in Denmark, who continue to argue against the inclusion of Leibovici 2001. The objectives of the original trialists when they complete a study must not influence its inclusion in a review for which the methods of the study make it eligible. To ensure that Cochrane reviews are rigorous they are preceded by a protocol – as was the case with this particular review. This protocol was open to comment and criticism. We received no comments on the inclusion criteria. It is clearly not part of the reviewers' remit to judge the motivation of those conducting eligible trials. We are satisfied that we have applied our protocol as objectively and rigorously as possible. We are keen that all studies meeting the clearly stated inclusion criteria should be reported (even if later stated to have been "written in jest"), rather than being kept hidden and perpetuating publication bias.
Contributors
Contributors of feedback: Karsten Juhl Jørgensen, Asbjørn Hrobjartsson and Peter C. Gøtzsche. All authors: The Nordic Cochrane Centre, Rigshospitalet Dept. 3343, Copenhagen, Denmark.
Contributor of reply: Leanne Roberts.
Sormani, Rada 2013
Summary
Readers (Maria Sormani and Gabriel Rada) noticed data for the outcome of death entered in review were different to data in the published paper Aviles 2001. The number of deaths in the Aviles 2001 paper was 31 for those in the standard care group, in the review it was entered as 80. Maria and Gabriel contacted editorial base via email in June 2013.
Reply
Authors of the review checked the data and the data had been entered incorrectly. Outcome data for death were amended but this correction did not change the overall result or conclusions for this outcome. The correction, did however, remove heterogeneity from this result.
Authors have corrected text of the review to reflect the above change.
Contributors
Maria Pia Sormani and Gabriel Rada ‐ noticed error in data.
Leanne Roberts ‐ contact person and lead author of review.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2014 | Feedback has been incorporated | Feedback incorporated, error in data corrected. Overall conclusions not changed. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 5 October 2011 | Amended | Contact details updated. |
| 16 February 2011 | Feedback has been incorporated | New feedback received and author's response incorporated |
| 16 February 2010 | Amended | This version of the review contains an amendment agreed upon during a conference call with the Cochrane Collaboration's Editor in Chief, two representatives from Cochrane Ombudsman, the Cochrane Schizophrenia Group's Comments Editor, the Director of the UK Cochrane Centre and the Co‐ordinating Editor of this group. A new comparison has been added (2. INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE) and some text included which is relevant to this new comparison. |
| 1 February 2010 | Amended | Plain language summary added |
| 1 December 2009 | Amended | One study moved into separate comparison (number 2) |
| 12 August 2009 | Feedback has been incorporated | Authors response to feedback incorporated. |
| 24 March 2009 | Feedback has been incorporated | New feedback received ‐ awaiting authors response. |
| 18 February 2009 | Amended | The Editorial Base and the Comments Editor of the Cochrane Schizophrenia Group do wish to apologise to those sending the comment on the ‘Intercessory prayer for the alleviation of ill health’ review which appeared in Issue 1 of 2009. This comment was erroneously attributed to “Peter C. Gøtzsche ‐ Director, Nordic Cochrane Center at Rigshospitalet in Copenhagen, Denmark” and should read, as it does now, “Karsten Juhl Jørgensen, Asbjørn Hrobjartsson and Peter C. Gøtzsche”. |
| 18 February 2009 | New citation required and conclusions have changed | New trials included, one trial removed, analysis redone, text substantially amended. |
| 16 December 2008 | New search has been performed | Addressed criticism ‐ removed one study (Cha 2001). |
| 30 June 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Iain Chalmers and Sarah Lewington for advice during the initial production of this review, Karen Bailie for her help with information relating to the treatment of children with leukaemia, Catherine Sargent for her involvement in the preparation of this review and Evandro da Silva Freire Coutinho for choosing a relevant 'complication' for us to use for the Byrd 1988 study. We would also like to thank Claire Irving for her help with the 2005, 2007 and 2009 updates by undertaking study selection, data extraction, and rewriting of the review into the current format, and Nancy Owens for her assistance with the 2009 revision. We would also like to thank the editorial base of the Cochrane Schizophrenia Group for their editorial support.
Appendices
Appendix 1. Search strategy for the first and second versions of the review
1. The first version was Roberts 2000.
We identified relevant randomised trials by searching the following electronic databases:
a. ATLA Religion Database Silver platter Inspires 4.0 (1949 ‐ May 1997), using the phrase: random*
b. Biological Abstracts on Silver platter Inspires 4.0 (January 1985 to September 1999), using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab))]
c. CINAHL on Silverplatter WinSPIRS 4.0 (January 1982 to October 1999) using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab) or explode "PRAYER"/ all topical subheadings / all age subheadings or explode "RELIGION‐AND‐PSYCHOLOGY"/ all topical subheadings / all age subheadings or explode "MENTAL‐HEALING"/ all topical subheadings / all age subheadings)]
d. Cochrane Schizophrenia Group's Register (December 1999), using the phrase: (pray* or god or spiritual or faith)
e. CCTR of The Cochrane Library (Issue 4, 1999), using the Cochrane Schizophrenia Group's phrase for schizophrenia (see Group search strategy) combined with: [AND (pray*:ti or god:ti or faith*:ti or spiritual*:ti or religi*:ti or pray*:ab or god:ab or faith*:ab or spiritual*:ab or religi*:ab)]
f. EMBASE on Silverplatter WinSPIRS 4.0 (January 1980 to October 1999), using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab))]
g. MEDLINE on Silverplatter WinSPIRS 4.0 (January 1966 to December 1999), using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab) or "MENTAL‐HEALING"/ all subheadings
h. PsycLIT on Silverplatter WinSPIRS 4.0 (January 1887 to December 1999), using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab) or explode "RELIGIOUS‐PRACTICES" or explode "SPIRITUALITY"
i.Sociofile Silverplatter WinSPIRS 4.0 1/1974 ‐ 12/1996, using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab) or explode "prayer" or explode "faith‐healing" or explode "spiritual"))]
j. Sociological Abstracts Silverplatter WinSPIRS 4.0 (1963 to September 1999), using the Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with: [AND ((pray* in ti) or (pray* in ab) or (god in ti) or (god in ab) or (spiritual in ti) or (spiritual in ab) or (faith in ti) or (faith in ab) or faith‐healing in de)]
2. The second version was Roberts 2007
For the 2005‐6 update ‐ we identified relevant randomised trials by searching the following electronic databases:
a. AMED, CINAHL, EMBASE and MEDLINE on Ovid (2004 ‐ November 2005) was searched using Cochrane Schizophrenia Group's phrase for randomised controlled trials (see Group search strategy) combined with:
((pray* or god or faith* or religio or spiritual*) in ti, ab) or ((spirituality or religion) in sh)
b. ATLA Religion Database on EBSCO Host (2004 ‐ November 2005) was searched using the phrase:
pray* and trial*
c. BIOSIS Previews on CityplaceEdina (2003 ‐ November 2005) was searched using the phrase:
(randomi* or trial* or blind*)) and (pray* or god or religio* or spiritual* or faith*)
d. CENTRAL (The Cochrane Library 2005, Issue 4) was searched on using the phrase:
pray* or god or spiritual* or faith* or religio* (limited to 2003 ‐ 2005)
e. Cochrane Schizophrenia Group Trials Register (November 2005) was searched using the phrase:
*pray* or *god* or *spiritual* or *faith* or *religi*
f. ISI Proceedings on Thomson ISI (2003 ‐ November 2005) was searched using the phrase:
(pray* or god or religio*) and randomi*
g. ISI Web of Science on Thomson ISI (1981 ‐ 2004) was searched using the phrase:
(pray* or god or religio*) and randomi*
h. National Research Register (2005, Issue 4) was searched using the phrase:
pray* or god or religio*
i NTIS (1990 ‐ 2005) was searched using the phrase:
prayer or god
j. We searched Sociological Abstracts and ASSIA (Applied Social Sciences Index & Abstracts) on Cambridge Scientific Abstracts (2003 ‐ 2005) using the phrase:
randomi* and (pray* or god* or faith*)
k. Web Sites
We searched Clinicaltrials.gov on National Institute for Health using the phrase
pray or prayer or god or religion or religious
Appendix 2. Methods section of previous version of this review
1. Selection of trials Material downloaded from electronic sources included details of author, institution or journal of publication. The principal reviewer (LR) inspected all reports. These were then re‐inspected by IA in order to ensure reliable selection. We resolved any disagreement by discussion, and where there was still doubt, we obtained the full article for further inspection. Once we had obtained the full articles, LR and IA decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and added these trials to the list of those awaiting assessment. 2. Assessment of methodological quality We assessed the methodological quality of the trials included in this review using the criteria described in the Cochrane Handbook (Higgins 2009) and the Jadad Scale (Jadad 1996). The former is based on the evidence of a strong relationship between allocation concealment and direction of effect (Schulz 1995). The categories are defined below: A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment). For the purpose of the analysis in this review, we included trials if they met the Cochrane Handbook criteria A or B. The Jadad Scale measures a wider range of factors that impact on the quality of a trial. The scales include three items: 1. Was the study described as randomised? 2. Was the study described as double‐blind? 3. Was there a description of withdrawals and drop outs? Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomisation or the blinding/masking procedures described are inadequate. For this review we used a cut‐off of two points on the Jadad scale to check the assessment made by the handbook criteria. However, we did not use the Jadad Scale to exclude trials. 3. Data collection LR independently extracted data from selected trials, while IA separately re‐extracted information from two different samples (10%). When disputes arose we attempted arose resolution by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment. 4. Data synthesis 4.1 Data types We assessed outcomes using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such as 'little change', 'moderate change' or 'much change') or dichotomous (for example, either 'no important changes' or 'important changes' in a person's behaviour) measures. Currently RevMan does not support categorical data so they could not be analysed as such. 4.2 Incomplete data We did not include trial outcomes if more than 40% of people were not reported in the final analysis. 4.3 Dichotomous ‐ yes/no ‐ data We used an 'intention to treat' analysis. On the condition that more than 60% of people completed the study, everyone allocated to the intervention were counted, whether they completed the follow up or not. We assumed that those who dropped out had the negative outcome, with the exception of death. Where possible, we tried to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. If the authors of a study had used a predefined cut‐off point for determining clinical effectiveness this was used by the reviewers where appropriate. Otherwise we generally assumed that if there had been a 50% reduction in a scale‐derived score, this could be considered as a clinically significant response. We calculated the relative risk (RR) and its 95% confidence interval (CI) based on the random‐effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that, RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. We inspected data to see if an analysis using a fixed‐effect model made any substantive difference in outcomes that were not statistically significantly heterogeneous. When the overall results were significant we calculated the number needed to treat (NNT) and the number‐needed‐to‐harm (NNH) as the inverse of the risk difference. 4.4 Continuous data 4.4.1 Normally distributed data: continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards were applied to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors; (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); (c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them. When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), it is difficult to tell whether data are non‐normally distributed (skewed) or not. Skewed data from studies of less than 200 participants would have been entered in additional tables rather than into an analysis. Skewed data poses less of a problem when looking at means if the sample size is large and would have been entered into a synthesis. For change data (endpoint minus baseline), the situation is even more problematic. In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALLSTAT electronic statistics mailing list, we presented change data in MetaView in order to summarise available information. In doing this, we assumed either that data were not skewed or that the analyses could cope with the unknown degree of skew. Without individual patient data it is impossible to test this assumption. Where both change and endpoint data were available for the same outcome category, we presented only endpoint data. We acknowledge that by doing this much of the published change data were excluded, but argue that endpoint data are more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. We have contacted authors of studies reporting only change data for endpoint figures. We reported non‐normally distributed data in the 'other data types' tables. 4.4.2 Rating scales: A wide range of instruments is available to measure mental health outcomes. These instruments vary in quality and many are not valid, or even ad hoc. For outcome instruments some minimum standards have to be set. It has been shown that the use of rating scales which have not been described in a peer‐reviewed journal (Marshall 2000) is associated with bias; therefore we excluded the results of such scales. Furthermore, we stipulated that the instrument should either be a self report or be completed by an independent rater or relative (not the therapist), and that the instrument could be considered a global assessment of an area of functioning. However, as it was expected that therapists would frequently also be the rater, we included such data but commented on the data as 'prone to bias'. Whenever possible we took the opportunity to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. Where continuous data were presented from different scales rating the same effect, we presented both sets of data and inspected the general direction of effect. 4.4.3 Summary statistic For continuous outcomes we estimated a weighted mean difference (WMD) between groups, again based on the random‐effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. 4.5 Cluster trials Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999). Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique. 5. Investigation for heterogeneity Firstly, we undertook consideration of all the included studies within any comparison to judge clinical heterogeneity. Then we visually inspected graphs to investigate the possibility of statistical heterogeneity. This was supplemented using, primarily, the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 75%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency was high, we did not summate data, but presented these separately and investigated reasons for heterogeneity. 6. Addressing publication bias We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. 7. General Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for prayer.
Data and analyses
Comparison 1. INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by end of trial | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 high risk patients | 1 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.59, 1.81] |
| 1.2 low risk patients | 1 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.23, 1.39] |
| 1.3 all patients | 5 | 3389 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.36] |
| 2 Clinical state: 1. Improved/not improved: intermediate or bad outcome | 5 | 2705 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 3 Clinical state: 2. Significant complications (readmission to CCU) | 4 | 2644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 4 Clinical state: 3. Presence of any post operative complications by 30 days | 1 | 1201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 5 Clinical state: 4. Significant complications (various) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 antianginal agents | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.31] |
| 5.2 antiarrhythmics | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.20] |
| 5.3 antibiotics | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.28] |
| 5.4 anemia/transfusion | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 5.5 angina (unstable) | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.57, 1.79] |
| 5.6 arterial pressure monitoring | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.12] |
| 5.7 atrial fibrillation | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.38, 1.64] |
| 5.8 cardiac arrest by end of trial | 3 | 2182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.21, 0.99] |
| 5.9 cardiopulmonary arrest | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.29, 3.05] |
| 5.10 catheterization | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 5.11 central pressure monitoring | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.06] |
| 5.12 congestive heart failure | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.29] |
| 5.13 coronary angiography | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.46, 1.56] |
| 5.14 diuretics | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| 5.15 extension | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.13, 1.51] |
| 5.16 gastrointestinal bleeding | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 5.17 hypotension | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.39, 1.87] |
| 5.18 implanted cardiac defibrillator | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.69, 5.12] |
| 5.19 interventional coronary procedure | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 5.20 intra‐aortic balloon pump | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.33, 1.37] |
| 5.21 intropic agents | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.23] |
| 5.22 intubation/ventilation | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.20] |
| 5.23 major surgery before discharge | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.95] |
| 5.24 pacemaker (permanent) | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.40, 1.45] |
| 5.25 pacemaker (temporary ) | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.57, 2.17] |
| 5.26 pneumonia | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.35] |
| 5.27 post‐PCI ischemia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.48] |
| 5.28 sepsis | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.39, 1.87] |
| 5.29 supraventricular tachyarrhythmia | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.06] |
| 5.30 Swan‐Ganz catheter | 1 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.98] |
| 5.31 third degree heart block | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.43, 7.43] |
| 5.32 vasodilators | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.11] |
| 5.33 ventricular fibrillation/tachycardia | 2 | 1383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.53, 1.51] |
| 6 Clinical state: 5. Mean number of discharge medications (data likely to be skewed) | Other data | No numeric data | ||
| 7 Clinical state: 6. No change or deterioration in attitude | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.21] |
| 8 Service use: 1. Rehospitalisation (any reason) | 2 | 1155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.22] |
| 9 Service use: 2. Number of 'visits to emergency department after discharge (specific to cardiac problem) | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.62] |
| 10 Service use: 3. Mean number of days in hospital (data likely to be skewed) | Other data | No numeric data | ||
| 11 Service use: 4. Mean number of days in CCU (data likely to be skewed) | Other data | No numeric data | ||
| 12 Leaving the study early | 6 | 3446 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.43, 1.31] |
1.1. Analysis.
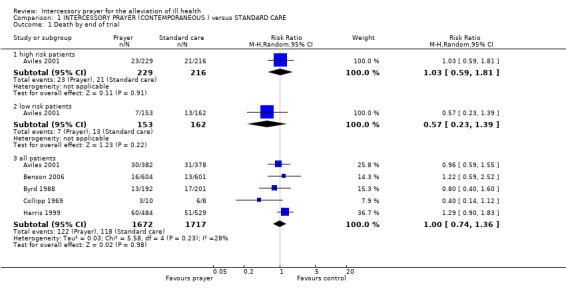
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 1 Death by end of trial.
1.2. Analysis.
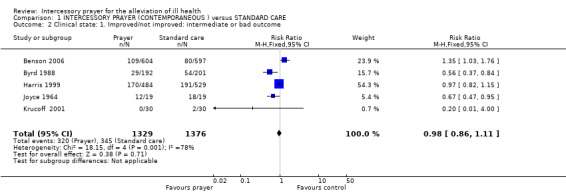
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 2 Clinical state: 1. Improved/not improved: intermediate or bad outcome.
1.3. Analysis.
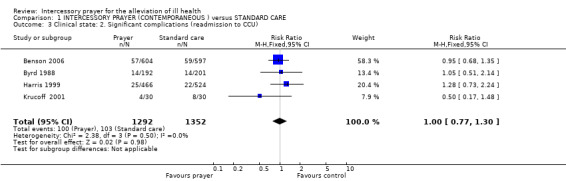
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 3 Clinical state: 2. Significant complications (readmission to CCU).
1.4. Analysis.
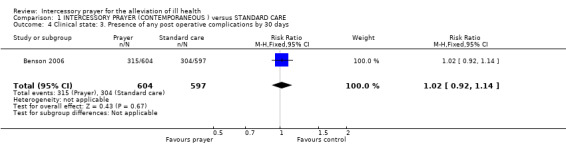
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 4 Clinical state: 3. Presence of any post operative complications by 30 days.
1.5. Analysis.
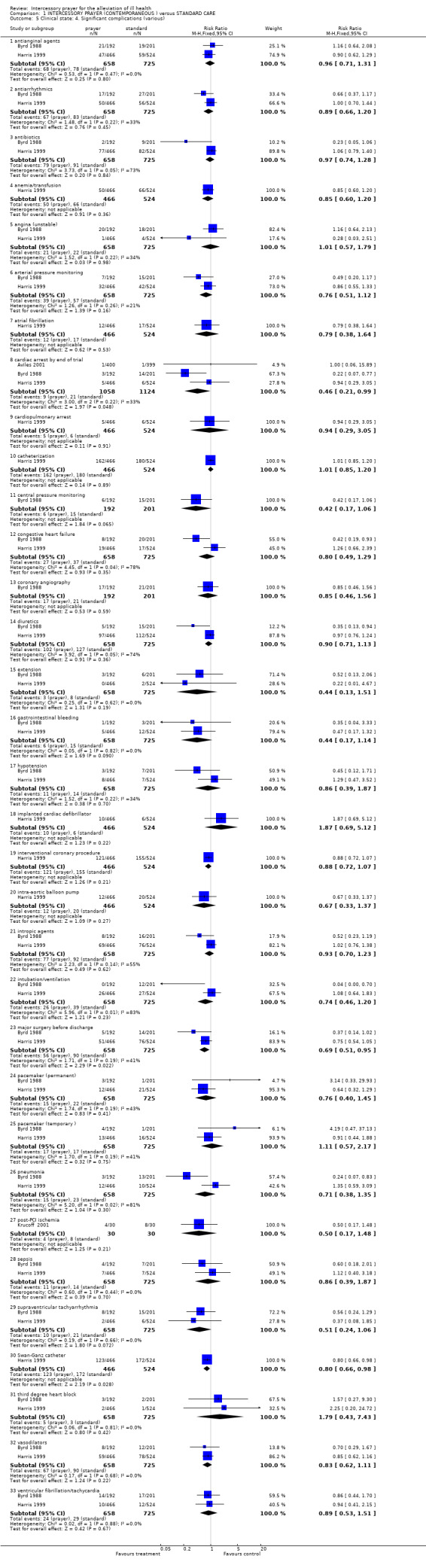
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 5 Clinical state: 4. Significant complications (various).
1.6. Analysis.
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 6 Clinical state: 5. Mean number of discharge medications (data likely to be skewed).
| Clinical state: 5. Mean number of discharge medications (data likely to be skewed) | ||
|---|---|---|
| Study | Prayer | Standard care |
| Byrd 1988 | N = 192 Mean = 3.7 S.D. = 2.2 | N = 201 Mean = 4.0 S.D. = 2.4 |
1.7. Analysis.
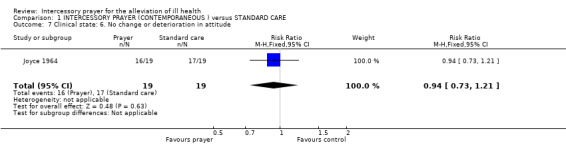
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 7 Clinical state: 6. No change or deterioration in attitude.
1.8. Analysis.
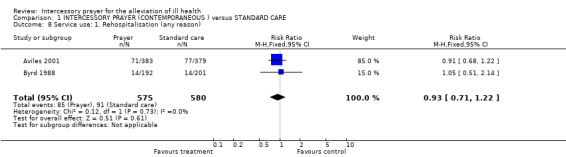
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 8 Service use: 1. Rehospitalisation (any reason).
1.9. Analysis.
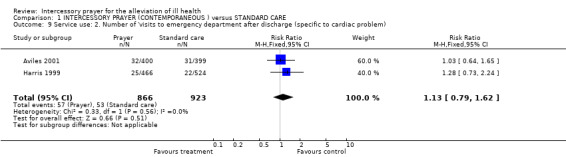
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 9 Service use: 2. Number of 'visits to emergency department after discharge (specific to cardiac problem).
1.10. Analysis.
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 10 Service use: 3. Mean number of days in hospital (data likely to be skewed).
| Service use: 3. Mean number of days in hospital (data likely to be skewed) | ||
|---|---|---|
| Study | Prayer | Standard care |
| Byrd 1988 | N = 192 Mean = 7.6 S.D. = 8.9. | N = 201 Mean = 7.6 SD = 8.7. |
1.11. Analysis.
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 11 Service use: 4. Mean number of days in CCU (data likely to be skewed).
| Service use: 4. Mean number of days in CCU (data likely to be skewed) | ||
|---|---|---|
| Study | Prayer | Standard care |
| Byrd 1988 | N =192 Mean = 2 S.D. = 2.5 | N = 201 Mean = 2.4 S.D. = 4.1 |
1.12. Analysis.
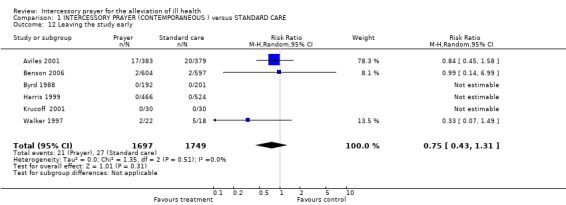
Comparison 1 INTERCESSORY PRAYER (CONTEMPORANEOUS ) versus STANDARD CARE, Outcome 12 Leaving the study early.
Comparison 2. INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by end of trial | 1 | 3393 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 2 Leaving the study early | 1 | 3393 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
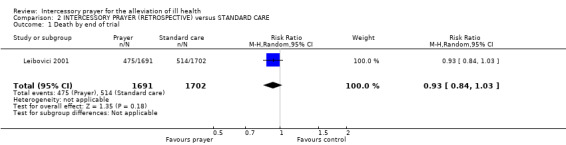
Comparison 2 INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE, Outcome 1 Death by end of trial.
2.2. Analysis.
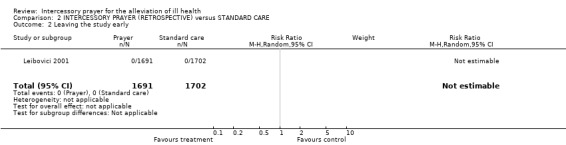
Comparison 2 INTERCESSORY PRAYER (RETROSPECTIVE) versus STANDARD CARE, Outcome 2 Leaving the study early.
Comparison 3. AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by end of trial | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 all patients | 1 | 1198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.44, 1.95] |
| 2 Clinical state: 1. Improved/not improved: intermediate or bad outcome | 1 | 1198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.40] |
| 3 Clinical state: 2. Significant complications (readmission to CCU) | 1 | 1198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.29] |
| 4 Clinical state: 3. Presence of any post operative complications by 30 days | 1 | 1198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.04, 1.28] |
| 5 Leaving the study early | 1 | 1198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.60, 14.71] |
3.1. Analysis.
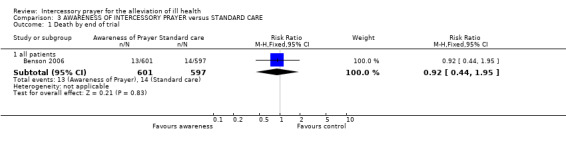
Comparison 3 AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE, Outcome 1 Death by end of trial.
3.2. Analysis.
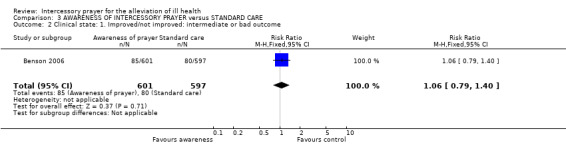
Comparison 3 AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE, Outcome 2 Clinical state: 1. Improved/not improved: intermediate or bad outcome.
3.3. Analysis.
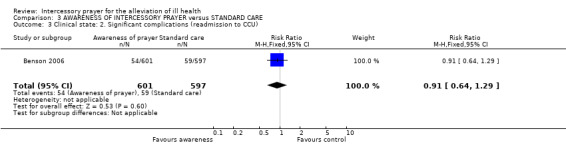
Comparison 3 AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE, Outcome 3 Clinical state: 2. Significant complications (readmission to CCU).
3.4. Analysis.
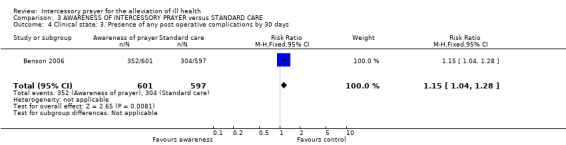
Comparison 3 AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE, Outcome 4 Clinical state: 3. Presence of any post operative complications by 30 days.
3.5. Analysis.
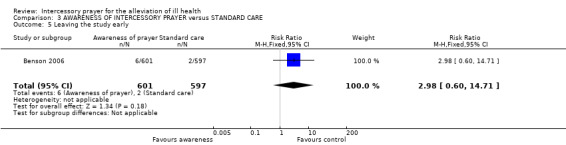
Comparison 3 AWARENESS OF INTERCESSORY PRAYER versus STANDARD CARE, Outcome 5 Leaving the study early.
Comparison 4. AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by end of trial | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 all patients | 1 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.40, 1.68] |
| 2 Clinical state: 1. Improved/not improved: intermediate or bad outcome | 1 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.02] |
| 3 Clinical state: 2. Significant complications (readmission to CCU) | 1 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.36] |
| 4 Clinical state: 3. Presence of any post operative complications by 30 days | 1 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.01, 1.24] |
| 5 Leaving the study early | 1 | 1205 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.61, 14.88] |
4.1. Analysis.
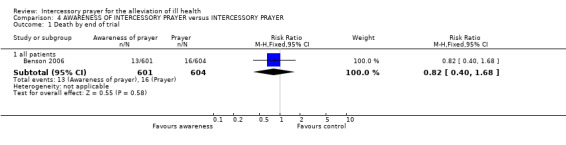
Comparison 4 AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER, Outcome 1 Death by end of trial.
4.2. Analysis.
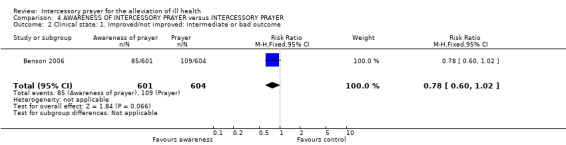
Comparison 4 AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER, Outcome 2 Clinical state: 1. Improved/not improved: intermediate or bad outcome.
4.3. Analysis.
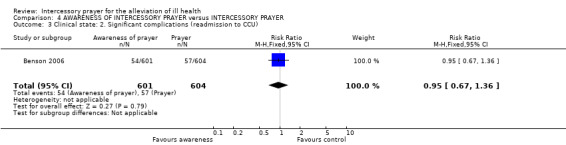
Comparison 4 AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER, Outcome 3 Clinical state: 2. Significant complications (readmission to CCU).
4.4. Analysis.
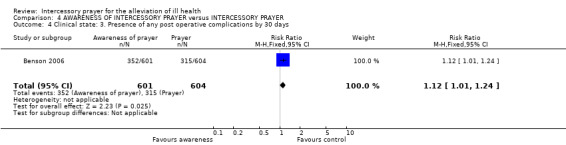
Comparison 4 AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER, Outcome 4 Clinical state: 3. Presence of any post operative complications by 30 days.
4.5. Analysis.
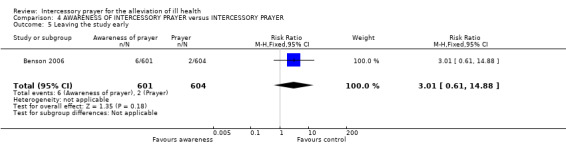
Comparison 4 AWARENESS OF INTERCESSORY PRAYER versus INTERCESSORY PRAYER, Outcome 5 Leaving the study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aviles 2001.
| Methods | Allocation: randomised (stratified by sex, age, diagnosis and general condition on hospital discharge). Blindness: double (participants and care givers blinded to assignment). Duration: 26 weeks. Consent: given. | |
| Participants | Diagnosis: cardiovascular disease. N=799. Age: >18 years. Sex: 476M, 323F. History: recently discharged from Coronary Care Unit. Exclusions: those unable to give consent, discharged to another hospital, unavailable for long‐term follow‐up | |
| Interventions | 1. Intercessory prayer: standard medical care + IP (minimum once per week by individuals or groups. No specific instructions on the contents of prayers). N=400.
2. Standard medical care. N=399. Intercessors had no contact with their assigned patients. |
|
| Outcomes | Death.
Clinical state: good/poor, complications.
Service use: rehospitalisation, emergency department visit .
Leaving the study early. Unable to use Quality of life: SF‐36 (no data). Clinical state: complications (event‐free survival ‐ not possible to extract from graph). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Telephone system, fully concealed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, care‐givers and interviewers blind to group of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Routine data ‐ fully ascertained. |
| Selective reporting (reporting bias) | Unclear risk | Quality of life data not reported. |
| Other bias | Unclear risk | Researchers' prior beliefs not clear. |
Benson 2006.
| Methods | Allocation: randomised (serially numbered opaque envelopes). Blindness: all staff and researchers blind to allocation, participants either uncertain of intervention or knew receiving IP. Duration: 30 days after CABG. Consent: given. | |
| Participants | Diagnosis: people scheduled to receive non‐emergency CABG. N=1802. Age: >18 years, mean ˜ 63 years. Sex: 1293M, 509F. Exclusions: scheduled for emergent CABG, CABG more than 14 days after enrolment, other planned surgery within 30 days of CABG, minimally invasive CABG, ongoing chest pain, unstable angina or CABG with planned valve replacement, stent, angioplasty or carotid endarterectomy. | |
| Interventions | 1. Intercessory prayer: standard care + IP with participants uncertain if receiving IP (daily prayer by 3 Christian groups given specific phrase to add onto to their study prayer). N=604. 2. Intercessory prayer: standard care + IP with participants aware receiving IP (prayer as above). N=601. 3. Standard care: standard care + participants uncertain if receiving IP. N=597. | |
| Outcomes | Death. Clinical state: major event, complications. Service use: re‐admission to hospital. Leaving the study early. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised ‐ no further details. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Carers and researchers unaware of group of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clearly described. |
| Selective reporting (reporting bias) | Low risk | Reporting rather un‐selective, too many outcomes reported. |
| Other bias | High risk | Several authors worked in institutions likely to be sympathetic to positive outcome of prayer. |
Byrd 1988.
| Methods | Allocation: randomised (computer generated list). Blindness: double (participants, care‐givers and researchers blind to assignment. Duration: unclear ("for the remainder of admission" ‐ mean number of days in hospital ˜ 8, SD 8.8). Consent: given. | |
| Participants | Diagnosis: congestive heart failure (129), cardiomegaly (126), acute myocardial infarction (109). N=393. Age: mean ˜ 59 years. Sex: 265M, 128F. History: just admitted to Coronary Care Unit. | |
| Interventions | 1. Intercessory prayer: standard medical care + IP (by 'born again multi‐denominational Christians' outside hospital, daily by 3‐7 intercessors until discharge). N=192.
2. Standard medical care. N=201. Intercessors had no contact with their assigned patients. |
|
| Outcomes | Death. Clinical state: good/poor, complications. Service use: number of days in hospital, number of days in CCU, readmission to CCU. Leaving the study early. | |
| Notes | Multiple complications presented. Independent collaborator (Evandro Coutinho), blinded to data, selected 'Re‐admissions to CCU' as proxy for 'Complications'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Those rating outcomes not aware group of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Full reporting. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes clearly reported, but many secondary outcomes also emphasised in the trial's report. |
| Other bias | High risk | Clear expression of prior belief in the positive effects of prayer. |
Collipp 1969.
| Methods | Allocation: randomised (not described). Blindness: triple (care givers and participants not told of intervention, those praying unaware of their participation in study). Duration: 15 months. | |
| Participants | Diagnosis: leukaemia (16 lymphatic, 2 myelogenous). N=18. Age: mean ˜ 7 years. Sex: 10M, 8F. | |
| Interventions | 1. Intercessory prayer: standard medical care + IP (one protestant family praying daily with weekly reminder and frequent discussions ). N=10. 2. Standard medical care. N=8. | |
| Outcomes | Death.
Leaving the study early. Unable to use ‐ Clinical state: improved/not improved (no individual group data) Quality of life: adjustment (no individual group data). |
|
| Notes | Standard medication: between 2 and 5 drugs (different combinations of methotrexate, 6‐mercaptopurine, vincristine, prednisone, daunomycin, bis‐chlorethyl‐nitrosourea, cytosinearabioside, tryptophane mustard and fluorinated progesterone). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly selecting names. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Children with leukaemia and their parents did not know they were in a trial, all physicians blinded, those praying unaware of participation in study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For usable outcomes all data reported. |
| Selective reporting (reporting bias) | High risk | Clinical state and quality of life data poorly reported. |
| Other bias | High risk | Concluded article with statement "every physician has prescribed this remedy [prayer] and nearly every physician has seen it succeed" ‐ bias likely. |
Harris 1999.
| Methods | Allocation: randomised (chaplain's secretary "randomly assigned" to group based on last digit of medical record number). Blindness: double. Duration: ˜ 28 days (focus of prayer for 28 days, follow‐up period unclear). Consent: not stated. | |
| Participants | Diagnosis: any person sick enough to be admitted to Coronary Care Unit. N = 1013. Age: mean ˜ 66 years. Sex: 642M, 371F. History: recent admission to CCU. Exclusions: those admitted for cardiac transplantation. | |
| Interventions | 1. Intercessory prayer: standard medical care + IP (daily for 28 days). N=484. 2. Standard medical care. N=529. | |
| Outcomes | Death.
Clinical state: good/poor, complications.
Leaving the study early. Unable to use ‐ Clinical state: MAHI‐CCU score (no SD). Service use: length of hospital stay (no SD). |
|
| Notes | Multiple complications presented. Independent collaborator (Dr Evandro Coutinho), blinded to data, selected 'Re‐admissions to CCU' as proxy for 'Complications'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned on basis of record number. |
| Allocation concealment (selection bias) | Low risk | Adequate. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blind, CCU staff, data collectors and statisticians blind to group of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Over‐reporting of outcomes that were not primary. |
| Other bias | Unclear risk | No clear indication that researchers had strong prior beliefs. |
Joyce 1964.
| Methods | Allocation: unsure (allocation by spin of a coin, matched for sex, age and primary diagnosis). Blindness: double. Duration: 6 months. | |
| Participants | Diagnosis: rheumatoid arthritis (17), ankylosing spondylitis (5), osteoarthritis (2), scleroderma (1), personality problems (5), depression (1), obsessional neurosis (1), anxiety neurosis (1), learning disability (2), schizophrenia (1), unknown (2). N=38. Age: 23 ‐78 years, mean ˜ 51 years. Sex:10M, 28F. History: chronic stable or progressively deteriorating illnesses. | |
| Interventions | 1. Intercessory prayer: Standard medical care + IP (15 minutes per day for about 15 hours during trial). N=19. 2. Standard medical care. N=19. | |
| Outcomes | Clinical state: good/poor (Clinical State Scale), positive/negative attitude (Attitude Scale). Unable to use ‐ Leaving the study early: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Spin of a coin. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants not aware of participation in trial, rater unaware of group of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clearly reported clinical state. |
| Selective reporting (reporting bias) | High risk | Did not clearly report loss to follow up. |
| Other bias | Low risk | Undertaken by two researchers, one of whom started with the belief that prayer 'worked' and the other that it did not ‐ no clear other biases. |
Krucoff 2001.
| Methods | Allocation: randomised (via on site envelopes). Blindness: double (prayer groups described). Duration: 6 months (after hospitalisation). Consent: given. | |
| Participants | Diagnosis: About to undergo invasive diagnostic angiography or PCI N=150 (but only 127 were analysed). Age: mean ˜ 63 years. Sex: 149M, 1F. History: chest pain at rest with or without acute electrocardiographic changes. | |
| Interventions | 1. Intercessory prayer: standard medical care + IP (8 different denominations carried out daily prayers except for Jewish prayer placed on Western Wall). N=24. 2. Standard care: standard medical care given on cardiac unit for those undergoing invasive PCI. N=27. 3. Stress/relaxation + standard medical care. N=28. 4. Touch therapy + standard medical care. N=24. 5. Imagery + standard medical care. N=24. | |
| Outcomes | Death. Clinical state: good/poor, complications. Leaving the study early. | |
| Notes | Prayer and standard group data used only for this review. Prayer and standard therapy assignments were double blind. Not possible with other groups due to 'hands on' nature of interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised ‐ no further details. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, family, staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear ascertainment of outcome data. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | Unclear risk | Researchers' prior beliefs unclear. |
Leibovici 2001.
| Methods | Allocation: randomised (random number generator to split the two groups and then coin toss to decide allocation). Blindness: double. Duration: until discharge. | |
| Participants | Diagnosis: blood stream infection. N=3,393. Age: mean ˜ 72 years. Sex: 1785M, 1608F History: hospitalised. | |
| Interventions | 1. Intercessory prayer*: standard medical care + IP (one short daily prayer for entire group). N=1691. 2. Standard medical care. N=1702. | |
| Outcomes | Death.
Leaving the study early. Unable to use ‐ Clinical state: duration of fever (no mean, SD). Service use: length of hospital stay (no mean, SD). |
|
| Notes | *Prayer took place 4‐10 years after the clinical outcomes had been recorded i.e. retroactive prayer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Toss of coin. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Did not obtain consent from participants to maintain blindness. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Full record ascertainment. |
| Other bias | Unclear risk | Researchers' prior belief is unclear. |
Walker 1997.
| Methods | Allocation: randomised (computer algorithm based on 4 randomisation variables). Blindness: double. Duration: 6 months. | |
| Participants | Diagnosis: Primary diagnosis of alcohol abuse or dependence. N=40. Age: mean ˜ 34 years. Sex: 29M, 11F. History: no psychiatric or organic impairment. | |
| Interventions | 1. Intercessory prayer: standard care + IP (volunteers with 5 years experience prayed daily for 6 months, prayer was nondirective). N=22. 2. Standard care. N=18. | |
| Outcomes | Leaving the study early. Unable to use ‐ Average monthly SDU (no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double, untested. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Good reporting of loss to follow up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, poor reporting of continuous data. |
| Other bias | Low risk | No clear other bias. |
IP: Intercessory Prayer CABG: coronary artery bypass graft CCU: Coronary Care Unit. PCI: Percutaneous Coronary Intervention SDU: Standard Drinking Units
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbot 2000 | Allocation: not randomised, a review. |
| Cha 2001 | Allocation: randomised (computer codes).
Diagnosis: current IVF treatment, N=199, age 26‐46 years, women, consecutively treated with IVF‐ET over a 4 month period.
Interventions: Standard IVF treatment + intercessory prayer (within 5 days of hormone treatment, daily throughout course of treatment) vs. standard medical care.
Outcomes: Pregnancy, leaving the study early. Excluded because of Feedback, after we learnt of controversy and that the Journal of Reproductive Medicine had removed the study from its web site. One of the study authors was added to the author list without their knowledge, and disowns the study (web link ‐ accessed February 2009). The lead author has not been possible to contact. |
| Conti 1999 | Allocation: randomised. Participants: people receiving haemodialysis. Interventions: intercessory prayer or positive visualization versus combination of intercessory prayer with expectation of PV or PV with expectation of IP, not standard care. |
| Galton 1883 | Allocation: not randomised, case control study, life expectancy of monarchs (much prayed for group) versus other professionals. |
| Green 1993 | Allocation: randomised. Participants: hospitalised neurosurgical pituitary patients. Intervention: Intercessory prayer plus enhanced expectations versus intercessory prayer with normal expectations. |
| Greyson 1997 | Allocation: randomised. Participants: people with 'major depression receiving traditional treatment'. Interventions: 'distance healing', LeShan meditation technique, not intercessory prayer. |
| Harrison 1999a | Allocation: randomised. Participants: college students. Interventions: intercessory prayer and personality factors. Outcomes: combined effects of personality factors and prayer on college success. |
| Lilliston 1981 | Allocation: not randomised, case control study. |
| Lilliston 1982 | Allocation: not randomised, case control study. |
| O'Mathuna 1999 | Allocation: not randomised, a review. |
| Sicher 1998 | Allocation: randomised. Participants: people with AIDS. Intervention: 'distance healing', not specifically intercessory prayer. |
| Toth 1999 | Allocation: not randomised, a review. |
| Wiesendanger 2001 | Allocation: not randomised, a review. |
| Wirth 1994a | Allocation: randomised. Participants: those with full thickness dermal wounds. Interventions: non‐contact therapeutic touch versus non‐contact therapeutic touch and Rekiki and LeShan meditation along with intercessory prayer, not intercessory prayer alone. |
| Wirth 1994b | Allocation: randomised. Intervention: non‐traditional distant prayer versus none, not intercessory prayer. Outcomes: physiological measures. |
Differences between protocol and review
The original versions of this review did have a different protocol (Roberts 2000, Roberts 2007), which is reproduced in this version (Appendix 2). RevMan 5 (RevMan 2008) has necessitated improvements in methods in all Cochrane reviews and we have tried to comply with these without substantively diverging from the original intent of the earlier versions. However, this did mean using random‐effects analyses ‐ we think this is an overall improvement to the review without materially effecting the results of the review.
We did not anticipate a trial of retrospective prayer and only having discovered the Leibovici 2001 study did we really consider how to handle these data. We decided to include this study in a separate comparison for the 2010 update.
Contributions of authors
Irshad Ahmed ‐ protocol preparation, study selection, data extraction, review completion.
Steve Hall ‐ protocol preparation, study selection, data extraction, review completion.
Leanne Roberts ‐ protocol preparation, study selection, data extraction, review completion and maintenance, update study selection, update review completion
Andrew Davison ‐ update research, update review completion
Sources of support
Internal sources
NHS Executive, Anglia and Oxford, UK.
Diocese of Oxford, UK.
External sources
Cochrane Schizophrenia Group, UK.
Declarations of interest
The reviewers are of mixed backgrounds, including Christianity and Islam.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Aviles 2001 {published data only}
- Aviles JM, Whelan E, Hernke DA, Williams BA, Kenny KE, O'Fallon M, Kopecky SL. Intercessory prayer and cardiovascular disease progression in a coronary care unit population: a randomized controlled trial. Mayo Clinical Proceedings 2001;76:1192‐8. [DOI] [PubMed] [Google Scholar]
Benson 2006 {published data only}
- Benson H, Dusek JA, Sherwood JB, Lam P, Bethea CF, Carpenter W, Levitsky S, Hill PC, Clem DW, Jain MK, Drumel D, Kopecky SI, Mueller PS, Marek D, Rollins S, Hibberd PI. Study of the therapeutic effects of intercessory prayer (STEP) in cardiac bypass patients: A multicenter randomised trial of uncertainty and certainty of receiving intercessory prayer. American Heart Journal 2006;151:934‐42. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Sherwood JB, Friedman R, Myrers P, Bethea CF, Levitsky S, Hill PC, Jain MK, Kopecky SZ, Mueller PS. Lam P, Benson H, Hibberd PL. Study of the therapeutic effects of intercessory prayer (STEP) study design and research methods. American Heart Journal 2002;143(4):577‐84. [DOI] [PubMed] [Google Scholar]
Byrd 1988 {published data only}
- Byrd RC. Positive therapeutic effects of intercessory prayer in a coronary care unit population. Southern Medical Journal 1988;81:826‐9. [DOI] [PubMed] [Google Scholar]
Collipp 1969 {published data only}
- Collipp PJ. The efficacy of prayer: a triple‐blind study. Medical Times 1969;97:201‐4. [PubMed] [Google Scholar]
Harris 1999 {published data only}
- Harris W, Gowda M, Kolb J, Strychacz C, Vacek J, Jones P, Forker A, O'Keefe J, McCallister B. A randomized, controlled trial of the effects of remote intercessory prayer on outcomes in patients admitted to the coronary care unit. Archives of Internal Medicine 1999;159:2273‐8. [DOI] [PubMed] [Google Scholar]
Joyce 1964 {published data only}
- Joyce CRB, Welldon RMC. The objective efficacy of prayer. Journal of Chronic Disorders 1965;18:367‐77. [DOI] [PubMed] [Google Scholar]
Krucoff 2001 {published data only}
- Krocoff MW, Crater SW, Green CL, Maas AC, Seskevich JE, Lane JD, Loeffler KA, Morris K, Bashore TM, Koenig HG. Integrative noetic therapies as adjuncts to percutaneous intervention during unstable coronary syndromes: Monitoring and Actualization of Noetic Training (MANTRA) feasibility pilot. American Heart Journal 2001;142:760‐7. [DOI] [PubMed] [Google Scholar]
Leibovici 2001 {published data only}
- Leibovici L. Effects of remote, retroactive intercessory prayer on outcomes in patients with bloodstream infection: randomised controlled trial. BMJ 2001;323:1450‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1997 {published data only}
- Walker SR, Tonigan JS, Miller W, Comer S, Kahlich L. Intercessory prayer in the treatment of alcohol abuse and independence: A pilot investigation. Alternative Therapies in Health and Medicine 1997;3:79‐86. [PubMed] [Google Scholar]
References to studies excluded from this review
Abbot 2000 {published data only}
- Abbot NC. Healing as a therapy for human disease: A systematic review. The Journal of Alternative and Complementary Medicine 2000;6:159‐69. [DOI] [PubMed] [Google Scholar]
Cha 2001 {published data only}
- Cha KY, Writh DP, Lobo RA. Does prayer influence the success of in vitro fertilization‐embryo transfer: report of a masked, randomized trial. The Journal of Reproductive Medicine 2001;46:781‐7. [PubMed] [Google Scholar]
Conti 1999 {unpublished data only}
- Conti JM. The effects of intercessory prayer and transpersonal positive visualization on a hemodialysis population. Amherst, USA: Univ. of Massachusetts Amherst, 1999. [Google Scholar]
Galton 1883 {published data only}
- Galton F. Inquiries into human faculty and its development. London: Macmillan, 1883. [Google Scholar]
Green 1993 {unpublished data only}
- Green WM. The therapeutic effects of distant intercessory prayer and patient's enhanced positive expectations on recovery rates and anxiety levels of hospitalized neurosurgical pituitary patients: A double blind study. San Francisco, USA: California Institute of Integral Studies, 1993. [Google Scholar]
Greyson 1997 {published data only}
- Greyson B. Distance healing of patients with major depression. Journal of Scientific Exploration 1997;10:447. [http://www.jse.com/v10n4a1.html] [Google Scholar]
Harrison 1999a {published data only (unpublished sought but not used)}
- Harrison GM. Long distance intercessory prayer: personality factors of the prayor and the prayee and their effect on college success of the prayee. Spalding, UK: Univ. of Spalding, 1999. [Google Scholar]
Lilliston 1981 {published data only}
- Lilliston L, Brown PM. Perceived effectiveness of religious solutions to personal problems. Journal of Clinical Psychology 1981;37:118‐22. [DOI] [PubMed] [Google Scholar]
Lilliston 1982 {published data only}
- Lilliston L, Brown PM, Schliebe HP. Perceptions of religious solutions to personal problems of women. Journal of Clinical Psychology 1982;38:546‐9. [DOI] [PubMed] [Google Scholar]
O'Mathuna 1999 {published data only}
- O'Mathuna DP. Prayer Research. What are we measuring?. Journal of Clinical Nursing 1999;16:18‐21. [DOI] [PubMed] [Google Scholar]
Sicher 1998 {published data only}
- Sicher F, Targ E, Moore D, Smith H. A randomized double‐blind study of the effect of distant healing in a population with advanced AIDS. Western Journal of Medicine 1998;169(6):356‐63. [PMC free article] [PubMed] [Google Scholar]
Toth 1999 {published data only}
- Toth JC. Faith in God: Help for partners in pain. Journal of Christian Nursing 1999;16:19‐21. [DOI] [PubMed] [Google Scholar]
Wiesendanger 2001 {published data only}
- Wiesendanger H, Werthmuller L, Reuter K, Walach H. Chronically ill patients treated by spiritual healing improve in quality of life: Results of a randomised waiting‐list controlled study. The Journal of Alternative and Complementary Medicine 2001;7:45‐51. [DOI] [PubMed] [Google Scholar]
Wirth 1994a {published data only}
- Wirth DP, Barrett MJ. Complementary healing therapies. International Journal of Psychosomatics 1994;41:61‐7. [PubMed] [Google Scholar]
Wirth 1994b {published data only}
- Wirth DP, Cram JR. The psychophysiology of nontraditional prayer. International Journal of Psychosomatics 1994;41:68‐75. [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information.. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1995
- Anonymous. AIDS daily summary. http://cdnac.org:72/0/2/dec95/ads0621 (accessed on 30 Jan 1998).
Barnes 2004
- Barnes PM, Powell‐Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. http://nccam.nih.gov/news/report.pdf (accessed July 2008); Vol. 343. [PubMed]
Bender 2008
- Bender R, Bunce C, Clarke M, Gates S, Lange S, Pace NL, Thorlund K. Attention should be given to multiplicity issues in systematic reviews. Journal of Clinical Epidemiology 2008;61:857‐65. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Chibnall 2001
- Chibnall JT, Jeral JM, Cerullo MA. Experiments on distant intercessory prayer: God, science, and the lesson of Massah. Archives of Internal Medicine 2001;161(21):2529‐36. [PUBMED: 11718583] [DOI] [PubMed] [Google Scholar]
Choi 1997
- Choi T. Hippocrates. http://www.medscape.com/time/hippocrates/1997/v11.n05/h1105.3.fleming.html (accessed on 30 Jan 1998).
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceeding of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. Cape Town, 2000.
Deuteronomy 6:16
- Old Testament. The Holy Bible, New Revised Standard Version 1995.
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1995
- Egger M, Smith GD. Misleading meta‐analysis. BMJ 1995;310:752‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Hegel 2008
- Hegel GWF. Lectures on the philosophy of religion. Edited by PC Hodgson; translated by RF Brown, PC Hodgson, and JM Stewart with the assistance of HS Harris. Vol. 1: Introduction and the concept of religion, New York: Oxford University Press, 2008:258. [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. www.cochrane‐handbook.org. The Cochrane Collaboration.
Jadad 1996
- Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koenig 2000
- Koenig HG, McCullough M, Larson D. Handbook of Religion and Health. New York: Oxford University Press, 2000. [Google Scholar]
Larson 1997
- Larson D, Mathews D. Hippocrates. http://www.medscape.com/time/hippocrates/1997/v11.n05/h1105.3.fleming.html (accessed on 30 Jan 1998).
Leibovici 2002
- Leibovici L. Author's reply. BMJ 2002;324:1037. [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McCaffrey 2004
- McCaffrey AM, Eisenberg DM, Legedza AT, et al. Prayer for health concerns: results of a national survey on prevalence and patterns of use. Archives of Internal Medicine 2004;164(8):858‐62. [DOI] [PubMed] [Google Scholar]
OED 1989
- Simpson J, Weiner E (eds). Oxford English Dictionary. Oxford: Oxford University Press, 1989. [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Turner 2004
- Turner D. Faith, reason and the existence of God. Cambridge: Cambridge University Press, 2004. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Wittgenstein 1974
- Wittgenstein L. Tractatus Logico‐Philosophicus. Vol. 6.44, London: Routledge and Kegan Paul, 1974. [Google Scholar]
Xia 2007
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, Pinfold V, Takriti Y. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium; 2007; Oct 23‐27; Sao Paulo, Brazil. Sao Paulo, 2007.
References to other published versions of this review
Roberts 2000
- L Roberts, I Ahmed, S Hall. Intercessory prayer for the alleviation of ill health. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000368] [DOI] [PubMed] [Google Scholar]
Roberts 2007
- Roberts L, Ahmed I, Hall S. Intercessory prayer for the alleviation of ill health. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000368.pub2] [DOI] [PubMed] [Google Scholar]


