Abstract
Immunotherapy with checkpoint inhibitors has revolutionized cancer therapy and is now the standard treatment for several different types of cancer, supported by favorable outcomes and good tolerance. However, it is linked to multiple immune manifestations, referred to as immune-related adverse events (irAEs). These adverse events frequently affect the skin, colon, endocrine glands, lungs, and liver. The gastrointestinal system is one of the most commonly affected organ systems and is responsible for the most frequent emergency visits resulting from irAEs. However, because immune checkpoint inhibitors are a recent addition to our arsenal of cancer drugs, many health-care providers remain unfamiliar with the management of irAEs. Gastroenterologists involved in the treatment of oncology patients who have received checkpoint inhibitors are currently encountering cases of abdominal pain, diarrhea, and other nonspecific symptoms that may be challenging to manage. This article reviews the gastrointestinal, hepatic, and pancreatic toxicities of checkpoint inhibitors and provides an approach to their diagnosis and recommended workup. It also highlights the management of irAEs according to their toxicity grading and specifically discusses the instances in which corticosteroids should be administered and/or the immune checkpoint inhibitors should be withheld.
Keywords: immune checkpoint inhibitor, immune-related adverse events, colitis, enterocolitis, liver toxicity, pancreatic toxicity
Introduction
Immune checkpoint inhibitors (ICIs), including inhibitors of programmed cell death protein-1 (PD-1), its ligand (PD-L1), and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), are currently the fourth pillar of cancer treatment and are increasingly used in numerous cancer types as monotherapy or as an adjunct to chemotherapy, radiotherapy, and surgery. Immune-related adverse events (irAEs) are well described in the literature and can affect any organ system [1]. The skin and gastrointestinal tract are the most commonly affected systems. Gastrointestinal irAEs can involve the bowels, liver, or pancreas, or can present as nonspecific symptoms such as nausea, vomiting, and abdominal pain. In most cases, the symptoms are mild, self-resolving with or without discontinuation of ICIs, and require only close monitoring. In cases where the symptoms are moderate to severe, there could be significant morbidity and impairment of the nutritional and volume status that might require hospitalization and can affect the patient’s eligibility to receive further cancer treatment [2].
Lower gastrointestinal toxicity
The most frequently seen gastrointestinal complications secondary to ICI therapy are diarrhea and colitis [3]. Both complications are more often seen secondary to therapy with CTLA-4 than PD-1 inhibitors [3]. The reported incidence of diarrhea and colitis in patients treated with ipilimumab, an anti-CTLA-4 antibody, is about 30% and 40%, respectively. In cases of colitis, mainly the descending colon is affected [4]. In some cases, enteritis without any colonic involvement can be seen, which may lead to small-bowel obstruction [4].
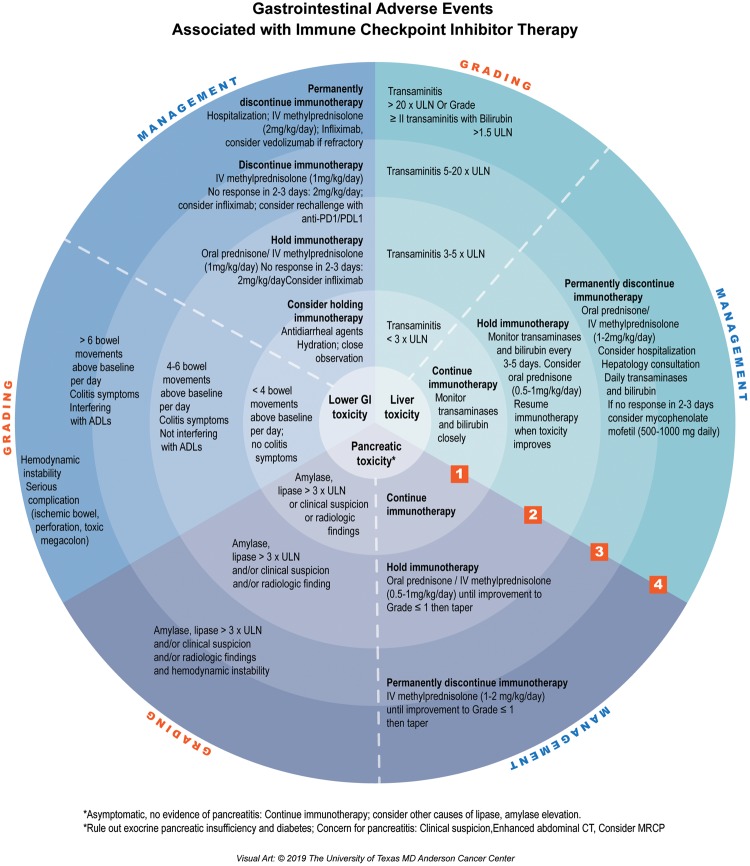
The diagnostic workup and management depend on the severity of the symptoms (Figure 1). Gastrointestinal irAEs are classified into four grades of increasing severity.
Figure 1.
Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy
In grade 1, diarrhea is mild, with up to four stools per day over baseline or mildly higher ostomy output from baseline [3, 4]. The diagnostic investigation at this stage includes only stool microscopy, culture, and sensitivity (MCS). It is essential to exclude infectious causes of diarrhea such as Clostridium difficile or other pathogens [4]. Mild diarrhea is managed symptomatically with electrolyte-replacement therapy, oral rehydration, and antidiarrheal drugs (e.g. loperamide) [3, 5].
Grade 2 diarrhea is of moderate intensity, with four to six stools per day over baseline or a moderate increase in ostomy output [3, 4]. Stool MCS is the only diagnostic workup required at this stage [3]. The treatment of grade 2 diarrhea involves fluid-replacement therapy along with high-dose corticosteroids [3]. Management with corticosteroids, oral prednisolone (1 mg/kg), or intravenous methylprednisolone (1 mg/kg per day) is usually needed if the diarrhea persists for longer than 5 days. Pharmacologic treatment is continued until symptoms improve and the patient’s condition stabilizes [3].
Grades 3 and 4 diarrhea are considered severe. They are defined as seven or more stools per day over baseline, along with fecal incontinence [3]. The patient may present with symptoms such as abdominal pain, rectal bleeding, and mucus in stool [3]. This stage of diarrhea can be life-threatening and may lead to bowel perforation [4]. The diagnostic workup includes stool MCS and colonoscopy; the latter is mainly indicated if colitis is suspected or if the symptoms of diarrhea persist despite corticosteroid treatment [3]. Endoscopy usually shows inflammatory changes—such as erythema, inflammatory exudates, granularity, loss of vascularity, and ulcerations—anywhere along the gastrointestinal tract. Even in the absence of gross changes, biopsies may show mixed inflammatory cell infiltrates in the lamina propria, neutrophilic cryptitis, crypt abscesses, and glandular destructions or erosions of the mucosal surface; these features sometimes overlap with endoscopic findings of inflammatory bowel disease [6, 7]. Of note, the degree of diarrhea is not associated with endoscopic findings. However, colonic ulcerations on endoscopy are predictive of steroid-refractory ICI-related colitis [8]. Fecal lactoferrin and fecal calprotectin can help differentiate between an infectious and an inflammatory etiology of the diarrhea/colitis, and can be used to monitor disease activity and response to treatment [9]. Grades 3 and 4 diarrhea require hospital admission and prompt initiation of fluid-replacement therapy. In severe diarrhea, corticosteroid treatment with methylprednisolone (1–2 mg/kg per day) should be administered intravenously until the patient’s condition stabilizes [3, 5]. Immunosuppressive agents such as infliximab are indicated if no improvement is seen with intravenous methylprednisolone [2, 10]. Some case reports and case series of steroid-refractory grades 3 and 4 diarrhea describe the successful and safe use of budesonide, vedolizumab, or aminosalicylates such as mesalamine as second-line immunosuppressive therapy, as well as the use of fecal microbiota transplantation as a third-line therapy [8, 11].
Upper gastrointestinal toxicity
Nonspecific upper gastrointestinal toxicity (GIT) can occur as an isolated presenting symptom, but more frequently coexists with lower GIT [1, 6]. The most frequent symptoms are nausea/vomiting (36% of patients) [12] and abdominal pain (83%) [12]. Diagnosis can be challenging, given that patients on ICI therapy are often receiving concurrent cancer therapies, suffering from the cumulative effect of previous treatment lines, or having cancer progression—any of which can cause nausea and/or vomiting. Additionally, feeding-tube problems [13] in cancer patients receiving ICI therapy can be a symptom of nonspecific upper GIT. GIT has been observed to be more common after anti-CTLA-4 therapy than after anti-PD1 and anti-PD-L1 therapy [14, 15]. Patients receiving anti-PD-L1 agents have lower rates of nausea and vomiting than patients receiving other chemotherapeutics (odds ratio [OR] 0.293, 95% confidential interval [CI] 0.225–0.380 and OR 0.206, 95% CI 0.122–0.348, respectively) [1]. Rates of grades 3–4 nausea (0.7%), decreased appetite (0.6%), and vomiting (0.5%) were also lower in patients receiving anti-PD-L1 than in those receiving other chemotherapeutics; however, these observations were not statistically significant [1]. The involvement of limited areas of the GI tract such as the duodenum, stomach, ileum, or colon suggests an underlying immune mechanism directed toward epitopes specific to this location [6, 12]. Endoscopic examination can show gastritis, duodenitis (frequently without Helicobacter pylori infection), esophageal or gastric ulcers, ileitis, or enterocolitis [12, 16]. ICI-induced enterocolitis typically develops after 6–7 weeks of treatment [17, 18]. It can be serious enough to lead to hospitalization, perforation, or death in 5%, 1%, and 0.8% of patients, respectively, after ipilimumab-induced enterocolitis [17]. In one study, 5 of 25 patients with anti-CTLA-4-related enterocolitis were shown to have ileitis (3 had ulceration and 2 had erythema) on ileocolonoscopy [12]. The grading system for enterocolitis and colitis is based on the Common Toxicity Criteria for Adverse Events (CTCAE) [19].
Furthermore, immune-induced pancreatitis [20], hepatitis [21], diabetic ketoacidosis [22], and disease progression need to be considered in patients presenting with abdominal pain after ICI therapy.
Patients with bowel perforation [23, 24] as a complication of immune-related colitis [25] may also present with abdominal pain as the sole symptom. Other irAEs such as pericarditis/myocarditis [26, 27], meningitis [28], cerebellitis [29], pancreatitis [20], or hepatitis [21] can manifest as nausea, vomiting, or epigastric pain, and thus can mimic upper GIT.
ICI therapy was found to be associated with immune-mediated enterocolitis through the initiation of a severe and extensive form of inflammatory bowel disease [12]. In fact, the tolerance to self-antigens was found to be severely compromised after ICI therapy due to the decrease in the production of inhibitory cytokines (e.g. interleukin-10 and tumor growth factor-β1) [6, 12, 30]. Such underlining pathology can be visualized on biopsy as chronic duodenitis, chronic gastritis with rare granulomas, loss of vascularity, ulcerations, and acute interstitial and crypt inflammation [1, 12, 16]. The unique ICI-induced immune modulation makes the serologic biomarker profile distinct from those of classic inflammatory bowel disease and graft-vs-host reactions [31, 32]. Interestingly, genetic polymorphism (especially the pathways involved in polyamine transport [33] and CTLA-4 allele polymorphisms [6, 16]) and microbiome profile may increase the risk of GIT after ICI therapy [6, 32]. Microbiota maintains the balanced immune environment through stimulating T regulatory cells and anti-inflammatory cytokines [6, 34]. Additionally, gut microbiota plays a role in the resolution of the immune-induced inflammatory bowel disease through promoting the synthesis of water-soluble B vitamins [6].
Similar to the treatment of lower GIT, corticosteroid therapy may be needed to manage upper GIT, followed by infliximab for refractory cases [12]. Patients treated with anti-CTLA-4 agents should be advised to avoid NSAIDs [16]. On the other hand, steroids induce complete clinical remission in 37% of the patients with immune-mediated enterocolitis after anti-CTLA-4 therapy [12].
Hepatitis
Immune-mediated hepatitis related to ICIs is an emerging entity [1]. The incidence of immune-related hepatotoxicity is wide-ranging in the literature and depends on the type of immunotherapy administered (anti-CTLA-4 or anti-PD-1/anti-PD-L1) and the sampled study population. In general, hepatotoxicity occurs in 2%–10% of patients treated with ICIs. Most cases are mild and resolve with discontinuation of therapy. Severe hepatic failure is less common but may occur weeks to months after immune therapy [1, 4, 35]. Concurrent treatment with nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4), even at standard doses, increases the risk of hepatotoxicity up to 37%, and to 15% for high-grade toxicity [1, 10, 36]. Liver-related laboratory abnormalities, especially of high grade, are also common when ICIs are given in combination with other agents such as vemurafenib.
ICI-induced liver injury presents classically as an asymptomatic elevation of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) on routine laboratory testing, with or without increased bilirubin or fever, 6–14 weeks after starting ICI treatment, although earlier or later adverse events may be seen [1, 24, 35, 36]. For that reason, liver-function tests should be included in the workup of patients presenting with fever during ICI treatment [35]. When symptomatic, immune-mediated hepatotoxicity can be subtle or can mimic hepatitis or liver failure. These symptoms range from lack of appetite, dizziness, jaundice, nausea, and vomiting, to abdominal pain, dark urine, and excessive bleeding, or bruising [1].
ICI-induced hepatotoxicity seems to be an immune-mediated process because it carries histologic similarities to classic autoimmune hepatitis, according to a recent histology study by Zen et al. [37]. Typical findings on liver biopsy are lobular hepatitis and infiltrating CD3+ or CD8+ T lymphocytes, positively responding to corticosteroids [36, 37]. However, contrary to a usual immune-mediated phenomenon, there is a lack of serological autoimmune abnormalities such as antinuclear antibody and immunoglobulin G elevations. Furthermore, on immunostaining, there are rare CD20+ or CD4+ lymphocytes, also in contrast to classic autoimmune hepatitis findings and possibly explaining the less zone-selective hepatocyte necrosis that might not require the strong activation of helper T-cells and immunoglobulin production. This pattern of necrosis may also be explained by the fact that PD-1 and CTLA-4 are expressed by CD8+ cytotoxic T lymphocytes. Therefore, the interaction between CD4+ helper T-cells and B-cells may be activated less frequently in ICI-related liver injury than in autoimmune hepatitis [37, 38].
Liver enzymes are usually evaluated prior to immune therapy and before every cycle of treatment, with regular clinical evaluation for symptoms and signs of liver toxicity [1, 4, 36].
The diagnosis of ICI-induced hepatotoxicity remains a process of exclusion, especially in the absence of specific biomarkers. Liver injury related to viral infections, alcohol and medications, or liver metastatic disease should be excluded [3, 4, 38]. If the clinical presentation is suggestive of autoimmune hepatitis, antinuclear antibodies, anti-smooth muscle antibodies, and anti-neutrophil cytoplasmic antibodies can be tested [1]. Imaging such as computed tomography or ultrasonography to assess for possible thromboembolic or obstructive etiology should be performed. Nonspecific findings such as hepatomegaly, edema, periportal lymphadenopathy, or attenuated liver parenchyma may be evident on imaging, according to disease severity [1]. In complicated or unclear cases, liver biopsy can be considered as a tool to confirm other etiologies of liver injury [4, 36]. However, initiation of therapy should not be delayed for serological or histologic workup [36].
Grading of immune-related hepatitis depends mostly on the degree of elevation of ALT and AST and the presence of elevated levels of bilirubin. The grade guides management recommendations and algorithms (Figure 1).
Grade 1 is usually an asymptomatic elevation of AST or ALT less than three times the upper normal limit without a significant elevation in bilirubin value (<1.5 times the upper normal limit) [3]. Liver-enzyme tests should be repeated prior to each infusion and/or weekly. No treatment is recommended and immunotherapy can be continued as long as the patient remains asymptomatic.
Grade 2 is defined as an elevation of AST or ALT of more than three times the upper normal limit. It requires temporarily withholding ICIs and close monitoring of liver enzymes until they normalize, with subsequent weekly assessments. If no improvement of transaminitis is seen after 3–5 days, prednisone (0.5–1 mg/kg/day) should be considered. Special consideration to discontinue hepatotoxic drugs should be made [1, 3]. According to the National Comprehensive Cancer Network management guidelines, any significant transaminitis (>grade 1) with elevation of bilirubin to more than 1.5 times the upper normal limit should be managed aggressively.
Grade 3 hepatitis is defined as severe transaminitis with AST or ALT increased to more than five times the upper normal limit, whereas grade 4 is characterized by life-threatening transaminitis with AST or ALT increased to more than 20 times the upper normal limit. Grades 3 and 4 hepatotoxicity require permanent discontinuation of ICI, expedited initiation of corticosteroids (1–2 mg/kg per day of oral prednisone or intravenous methylprednisolone), and daily liver-enzyme testing. If no improvement of transaminitis is seen after 3–5 days, consideration of other immunosuppressants such as mycophenolate mofetil (500–1,000 mg twice daily) is advised. Because of the risk of idiosyncratic liver failure, infliximab—the gold-standard immunosuppressant in other ICI irAEs—is not indicated for immune-related hepatitis [39]. The use of other immunosuppressive therapies has been reported, such as anti-thymocyte globulin, tacrolimus, and, in extreme cases, plasma exchange [36, 40]. Although the optimal duration of corticosteroid use is uncertain, they are usually tapered over 4–6 weeks, with re-escalation if required.
Pancreatic toxicity
Pancreatic toxicity associated with ICI therapy is uncommon, occurring in <2% of patients, and usually presents as a transient asymptomatic increase in lipase or amylase [1, 41]. There is ambiguous evidence in the literature regarding the true incidence, clinical characteristics, and optimal management of ICI-induced pancreatic injury (ICIPI) [1, 41]. Because the significance of isolated elevated amylase and lipase levels is still vague [4, 38], routine monitoring of pancreatic enzymes in asymptomatic patients is not recommended unless pancreatitis is suspected [1]. Acute pancreatitis is rare [1, 6, 41, 42] and patients may present with a typical pancreatitis picture or with isolated symptoms of nausea, vomiting, fever, epigastric pain, or diarrhea [41]. Diagnosing acute pancreatitis requires the presence of two of the following three criteria: epigastric pain, elevated serum lipase (at least three times the upper normal limit; Figure 1), and findings of acute pancreatitis on imaging [43]. Unlike autoimmune pancreatitis, immune-related pancreatitis is subacute and computed tomography may show only pancreatic edema. ICIPI may also manifest as chronic pancreatitis or exocrine or endocrine pancreatic insufficiency. Pancreatic exocrine insufficiency with or without pancreatitis may manifest as irregular stools with diarrhea, discoloration of feces, and weight loss despite good appetite. Scatoscopy showing decreased level of fecal elastase (<15 mg/g feces) is suggestive of pancreatic exocrine insufficiency and treatment requires oral pancreatic-enzyme replacement [44, 45]. Cases of new-onset diabetes that require lifelong therapy and diabetic ketoacidosis are well described in the literature, with a 1% incidence rate among patients treated with ICIs [44–47].
When it occurs, ICIPI may present earlier than other irAEs, especially in patients receiving anti-CTLA-4 therapy [2, 41], and is usually treated conservatively with fluids and pain management [35, 42]. There is emerging evidence regarding the benefit of early (within 48 hours) aggressive intravenous hydration in patients with grade 3 or higher elevation in lipase, even in asymptomatic patients [41]. In the absence of symptoms, corticosteroids are not indicated, according to expert opinion [1, 41]. In symptomatic patients or in cases with severe elevation of pancreatic enzymes, workup for other etiologies of pancreatitis is warranted, including imaging. However, the decision to initiate steroids and to withhold or continue immunotherapy remains controversial and depends on the severity of the clinical picture and the degree of elevation of amylase and lipase. Therefore, the decision should only be made after discussion with the oncologist. Of note, corticosteroids and intravenous fluid use in ICIPI did not affect patients’ short-term outcomes in terms of normalization of pancreatic enzymes, clinical improvement, or duration of hospital stay, according to the largest ICIPI case series, which was done by Abu-Sbeih et al. [41]. However, there were fewer long-term adverse outcomes in terms of chronic pancreatitis, new-onset diabetes, or recurrence of lipase elevation in patients who received aggressive intravenous hydration [41]. Smoking and hyperlipidemia were associated with worse outcomes [41].
Future directions
Gastrointestinal toxicities are among the leading adverse events associated with ICI therapy. Upper and lower GIT, hepatitis, and pancreatitis frequently overlap and can be associated with other irAEs. irAEs most frequently lead to mild symptoms, not affecting cancer patients’ nutritional status, intravascular volume status, or ability to receive further immunotherapy. However, when they are severe, irAEs need to be treated aggressively with corticosteroids or other immunosuppressants, with at least temporary discontinuation of ICI therapy. Several guidelines from expert opinion are being published as our understanding and experience with the management of irAEs is expanded. The most critical action remains high clinical suspicion and diagnosis of these adverse events, with routine testing in some cases in order to expedite their recognition and collaborative management.
Authors’ contributions
P.C. and S.-C.J.Y. conceived of and designed the project. E.R., P.C., and M.K. contributed to the drafting of the manuscript. P.C., J.M., C.C., and S.-C.J.Y. contributed to the editing of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Acknowledgements
None.
Conflicts of interest
The authors declare that there is no conflict of interests in this study.
References
- 1. Brahmer JR, Lacchetti C, Schneider BJ. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. JCO 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sosa A, Lopez Cadena E, Simon Olive C. et al. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol 2018;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lomax AJ, McNeil C.. Acute management of autoimmune toxicity in cancer patients on immunotherapy: common toxicities and the approach for the emergency physician. Emerg Med Australas 2017;29:245–51. [DOI] [PubMed] [Google Scholar]
- 4. Kumar V, Chaudhary N, Garg M. et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simmons D, Lang E.. The most recent oncologic emergency: what emergency physicians need to know about the potential complications of immune checkpoint inhibitors. Cureus 2017;9:e1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cramer P, Bresalier RS.. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep 2017;19:3.. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Abu-Sbeih H, Mao E. et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis 2018;24:1695–705. [DOI] [PubMed] [Google Scholar]
- 8. Abu-Sbeih H, Ali FS, Alsaadi D. et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer 2018;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reddy HG, Schneider BJ, Tai AW.. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol 2018;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson DH, Zobniw CM, Trinh VA. et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer 2018;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi K, Abu-Sbeih H, Samdani R. et al. Can immune checkpoint inhibitors induce microscopic colitis or a brand new entity? Inflamm Bowel Dis 2019;25:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marthey L, Mateus C, Mussini C. et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blumenstein I, Shastri YM, Stein J.. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol 2014;20:8505–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morganstein DL, Lai Z, Spain L. et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2017;86:614–20. [DOI] [PubMed] [Google Scholar]
- 15. Osorio JC, Ni A, Chaft JE. et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berman D, Parker SM, Siegel J. et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010;10:1–10. [PMC free article] [PubMed] [Google Scholar]
- 17. Hodi FS, O'Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber JS, Kähler KC, Hauschild A.. Management of immune-related adverse events and kinetics of response with ipilimumab. JCO 2012;30:2691–7. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0: NIH; 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (30 November 2019, date last accessed).
- 20. Kohlmann J, Wagenknecht D, Simon JC. et al. Immune-related pancreatitis associated with checkpoint blockade in melanoma. Melanoma Res 2019;29:549–52. [DOI] [PubMed] [Google Scholar]
- 21. Tian Y, Abu-Sbeih H, Wang Y, Immune checkpoint inhibitors-induced hepatitis In: Naing A, Hajjar J (eds). Immunotherapy. Cham: Springer International Publishing, 2018, 159–64. [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Morgan A, Shah S. et al. Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy. Endocrinol Diabetes Metab Case Rep 2018;2018:18-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horvat TZ, Adel NG, Dang T-O. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. JCO 2015;33:3193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hryniewicki AT, Wang C, Shatsky RA. et al. Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J Emerg Med 2018;55:489–502. [DOI] [PubMed] [Google Scholar]
- 25. Som A, Mandaliya R, Alsaadi D. et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases 2019;7:405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Atallah-Yunes SA, Kadado AJ, Kaufman GP. et al. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol 2019;145:1527–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yun S, Vincelette ND, Mansour I. et al. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med 2015;2015:794842.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalakas MC. Neurological complications of immune checkpoint inhibitors: what happens when you ‘take the brakes off’ the immune system. Ther Adv Neurol Disord 2018;11:175628641879986. 1756286418799864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zurko J, Mehta A.. Association of immune-mediated cerebellitis with immune checkpoint inhibitor therapy. Mayo Clin Proc Innov Qual Outcomes 2018;2:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarhini AA, Zahoor H, Lin Y. et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berman D, Parker SM, Chasalow SD. et al. Potential immune biomarkers of gastrointestinal toxicities and efficacy in patients with advanced melanoma treated with ipilimumab with or without prophylactic budesonide. JCO 2008;26:3022. [Google Scholar]
- 32. de Souza HS, Fiocchi C.. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016;13:13.. [DOI] [PubMed] [Google Scholar]
- 33. Dubin K, Callahan MK, Ren B. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vetizou M, Pitt JM, Daillere R. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pallin DJ, Baugh CW, Postow MA. et al. Immune-related adverse events in cancer patients. Acad Emerg Med 2018;25:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haanen J, Carbonnel F, Robert C. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. [DOI] [PubMed] [Google Scholar]
- 37. Zen Y, Yeh MM.. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 2018;31:965–73. [DOI] [PubMed] [Google Scholar]
- 38. De Martin E, Michot JM, Papouin B. et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. [DOI] [PubMed] [Google Scholar]
- 39. Ghabril M, Bonkovsky HL, Kum C. et al. Liver injury from tumor necrosis factor-alpha antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol 2013;11:558–64.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Riveiro-Barciela M, Munoz-Couselo E, Fernandez-Sojo J. et al. Acute liver failure due to immune-mediated hepatitis successfully managed with plasma exchange: new settings call for new treatment strategies? J Hepatol 2019;70:564–6. [DOI] [PubMed] [Google Scholar]
- 41. Abu-Sbeih H, Tang T, Lu Y. et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J Immunother Cancer 2019;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikeuchi K, Okuma Y, Tabata T.. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer 2016;99:148–50. [DOI] [PubMed] [Google Scholar]
- 43. Grover S, Rahma OE, Hashemi N. et al. Gastrointestinal and hepatic toxicities of checkpoint inhibitors: algorithms for management. Am Soc Clin Oncol Educ Book 2018;13–9. [DOI] [PubMed] [Google Scholar]
- 44. Prasanna T, McNeil CM, Nielsen T. et al. Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy. Immunotherapy 2018;10:171–5. [DOI] [PubMed] [Google Scholar]
- 45. Hofmann L, Forschner A, Loquai C. et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 46. Stamatouli AM, Quandt Z, Perdigoto AL. et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maamari J, Yeung SJ, Chaftari PS.. Diabetic ketoacidosis induced by a single dose of pembrolizumab. Am J Emerg Med 2019;37:376.e1–e2. [DOI] [PubMed] [Google Scholar]