Major depressive disorder (MDD) is a common psychiatric condition characterized by two main symptoms, low mood and anhedonia. About 15–30% of people suffering from MDD do not respond to standard-of-care antidepressants, e.g., the serotonin re-uptake inhibitors (SSRI), and are considered affected by Treatment-Resistant Depression (TRD). The neurobiology of this condition is presently unknown. Recent attempts of developing novel treatments for TRD have been driven by four major breakthroughs: (1) Increasing dopaminergic neurotransmission improves TRD symptoms; (2) Anhedonia occurs when central dopaminergic neurotransmission is low; (3) Enhanced neuroplasticity is critical for the action of antidepressants; (4) Ketamine shows antidepressant properties in TRD patients and triggers neuroplasticity in preclinical animal models. These breakthroughs are at the basis of a putative human translational cellular model for antidepressant agents that we are proposing in this article. The rationale is briefly described here.
Increasing dopaminergic neurotransmission improves TRD symptoms: Drugs that share the common mechanism of increasing dopaminergic neurotransmission were shown to improve symptoms in TRD patients as adjunctive treatment to SSRI (Dunlop and Nemeroff, 2007; Fawcett et al., 2016). Therefore, the dopaminergic system could be seen as a preferred substrate in targeting TRD patients with endogenously reduced dopaminergic transmission.
Anhedonia occurs when central dopaminergic neurotransmission is low: The second breakthrough was linking the symptom of anhedonia with defective function of the mesocorticolimbic dopaminergic system, a critical component of the reward pathway that controls the hedonic state. Anhedonia can be measured using both clinical scale and instrumental endpoints in humans and animals, and is extensively reviewed in Pizzagalli (2014). Pharmacologic agents blocking dopaminergic transmission enhance anhedonia, while reversal is seen with drugs increasing dopaminergic transmission in rodents and humans (Pizzagalli, 2014).
Enhanced neuroplasticity is critical for the action of antidepressants: The third breakthrough regards the evidence of reduced neuronal plasticity and neurotrophic factor signaling in prefrontal/cingulate cortex of rodents exposed to chronic stress and of humans with mood disorders. These effects were generally reverted by chronic dosing with SSRI (Duman et al., 2016). The lack of clinical effects of SSRI observed in TRD could be conceptualized as driven by reduced neuroplasticity that is unresponsive to SSRI. Since TRD is often characterized by a hypoactive dopaminergic system, it is tempting to suggest that a reduced neuroplasticity is also present in the mesocorticolimbic dopamine system. Therefore, triggering neuroplasticity in the dopaminergic system could help in re-engaging the brain areas modulated by this system, reverting the depressive syndrome of patients with TRD and, in particular, anhedonia.
Ketamine shows antidepressant properties in TRD patients and triggers neuroplasticity in preclinical animal models: The fourth breakthrough came with the evidence that a single intravenous infusion of ketamine, a racemic dissociative anaesthetic that blocks the N-methyl-D-aspartate glutamate receptor (NMDAR), produces rapid antidepressant effects, measurable at approximately 3 hours after infusion and lasting for 1–2 weeks (Zanos et al., 2018). The efficacy of such intervention was validated in several studies, to the point that the S-enantiomer of racemic ketamine was developed and approved as an intranasal twice-a-week treatment for MDD and TRD under the name of esketamine (https://www.fda.gov/media/121378/download). Preclinical studies in rodents showed that treatment with ketamine increased neuroplasticity in frontocortical/hippocampal circuits; these increases were associated with behavioral antidepressant-like effects (Duman et al., 2016). The proposed mechanism involves an NMDAR-dependent rapid attenuation of gamma-aminobutyric acid (GABA)ergic interneuron inhibitory drive to pyramidal neurons and an increased post-synaptic glutamate neurotransmission mediated by up-regulation of the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPAR). This increased AMPAR-mediated neurotransmission promotes synthesis and release of brain derived neurotropic factor (BDNF), a critical player in determining dendritic spine outgrowth via activation of the tropomyosin receptor kinase B (TrkB) receptor-dependent mitogen-activated protein kinase kinase (MEK)-extracellular regulated protein kinases (ERK) and protein kinase B (Akt)-mTOR pathways (Duman et al., 2016).
However, how the molecular and cellular mechanisms of action of ketamine translate in humans is only partially understood. Since ketamine is known to increase dopaminergic neurotransmissions in cingulate/prefrontal cortex (Kokkinou et al., 2018), we suggest that ketamine also induces neuroplasticity in the dopamine system, contributing to the delivery of antidepressant and anti-anhedonic effects in TRD patients.
The introduction of human inducible pluripotent stem cells (iPSC) that can be differentiated into neurons has provided the possibility to investigate some aspects of the molecular and cellular mechanism of actions of drugs, adding a novel methodology to the translational tool box (Cavalleri et al., 2018; Collo et al., 2018a). Accordingly, human iPSC-derived neurons together with pharmacodynamic signals, soluble biomarkers, genetic risk profile and neuroimaging can work in parallel, complementing each other, to generate a better understanding of the therapeutic relevance of neuroactive pharmacological agents, providing an integrated translational approach for drug development.
In our laboratory, we developed a human iPSC-derived model of dopaminergic neurons to study the molecular aspects of structural neuroplasticity produced by ketamine. Initially, we paralleled the studies on iPSC-derived human dopaminergic neurons with primary cultures of mouse mesencephalic dopaminergic neurons, according to standardized protocols (Cavalleri et al., 2018; Collo et al., 2018a). In vitro pharmacological tests were performed by incubating dopaminergic neurons with ketamine at various concentrations and exposure times (e.g., 0.1–1.0 μM for 1-hour exposure) compatible with the dosing regimen used in the clinics (Zanos et al., 2018). Consistent effects of ketamine on dendrite outgrowth and soma size were observed 3 days after 1 hour of exposure, a time considered relevance in modelling the sustained clinical antidepressant effects of ketamine observed few days after infusion. We also showed that ketamine induces structural neuralplasticity in human dopaminergic neurons by activating the PI3K-Akt-mTORC1 pathways via a BDNF/TrkB-dependent mechanism (Cavalleri et al., 2018), the same mechanisms previously shown in mouse frontocortical/hippocampal neurons (Duman et al., 2016). Both rapid phosphorylation of mTOR-dependent p70S6 kinase and long-term structural plasticity were blocked by the PI3K inhibitors LY294002 and mTORC1 inhibitor rapamycin. Immuno-neutralization of BDNF, inhibition of TrkB receptors and blockade of MEK-ERK signaling likewise prevented ketamine-induced structural plasticity measured 3 days after exposure, confirming the involvement of BDNF/TrkB signaling in the activation of the mTOR pathway also in human dopaminergic neurons (Cavalleri et al., 2018). These effects of ketamine were abolished by the AMPAR antagonists NBQX and GYKI52466, as previously shown in mice (Duman et al., 2016). Intriguingly, ketamine-induced neuroplasticity on human dopaminergic neurons required functionally intact dopaminergic D3 receptors (D3R), since these effects were abolished by pretreatment with selective D3R antagonists (Cavalleri et al., 2018). Moreover, activation of D3R using D3R-preferential agonist pramipexole produced structural plasticity in dopaminergic neurons at concentration compatible with their clinical therapeutic use in TRD patients (Fawcett et al., 2016; Collo et al., 2018a). The clinical relevance of these molecular and cellular effects on neuroplasticity are partially supported by neuroimaging studies that indicate an association between anhedonia and defective prefrontal, striatal and orbitofrontal circuits, known terminal fields of the ascending mesocortical dopaminergic system. These defective circuits were partially reverted to normality in TRD patients 24 hours after ketamine infusion (Abdallah et al., 2016). Similar effects were obtained after 6-week daily dosing with add-on pramipexole in patients with mood disorders (Mah et al., 2011).
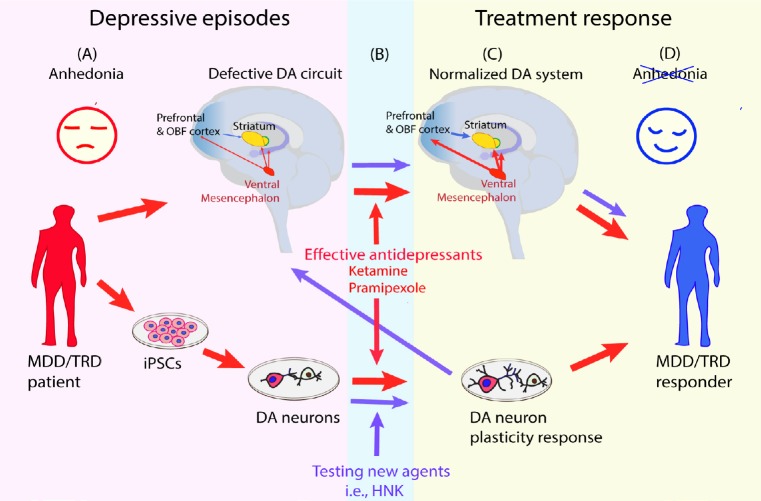
When considered together, these findings indicate that two therapeutic agents clinically effective on low mood and anhedonia in TRD patients (Fawcett et al., 2016; Zanos et al., 2018), showed effects on neuroimaging-defined anhedonia circuits (Abdallah et al., 2016; Mah et al., 2016) and, produced dendritic outgrowth in human iPSC-derived dopaminergic neurons at exposures compatible with the clinical effects (Cavalleri et al., 2018; Collo et al., 2018a). The essential features of this model are represented in Figure 1: parallel quantitative assessments are run in the same subject for clinical anhedonia, for neuroimaging signals generated from the neuroanatomical regions where the dopaminergic system is active and in iPSC-derived dopaminergic neuron neuroplasticity.
Figure 1.
Schematic representation of a proposed translational model implementing human iPSC-derived dopaminergic neurons to assess neural plasticity induced by pharmacological agents potentially active on anhedonia.
(A) MDD/TRD subjects with low mood and prevalent anhedonia are profiled with (upper red arrow) neuroimaging for hypofunctional mesocorticolimbic dopaminergic system assessing prefrontal, orbitofrontal and striatal brain areas, as proposed by Pizzagalli et al. (2014) and Abdallah et al. (2017). iPSCs (lover red arrow) are generated from somatic cells donated by the same subjects and are differentiated to reproduce the neuron phenotype of the circuits involved, in this case DA neurons. (B) Red arrow: ketamine and pramipexole, effective anti-anhedonic treatments studied in clinical and neuroimaging trials, are tested in human iPSC-derived DA neurons at doses and exposure time compatible with the clinical use. Purple arrow: HNK is tested as new pharmacologic agent in human iPSC-derived DA neurons for neural plasticity. (C) Red arrow: treatments with ketamine and pramipexole result in neuroimaging normalization, clinical improvement and dendritic outgrowth in human iPSC-derived dopaminergic neurons at exposures compatible with clinical use. Purple arrow: proposed neuroimaging study with HNK needed to confirm the translational relevance of the positive human iPSC-derived dopaminergic in vitro test. DA: Dopaminergic; HNK: (2R,6R)-hydroxynorketamine; iPSCs: inducible pluripotent stem cells; MDD: major depressive disorder; OBF: orbitofrontal cortex; TRD: treatment-resistant depression.
In order to formally validate the translational relevance of this model, further prospective studies that include pharmacokinetic and pharmacodynamic assessments in TRD patients will be required. The study design for this trial will include anhedonia and neuroimaging as clinical pharmacodynamic (PKPD) endpoint and neuroplasticity of iPSC derived neurons from the same TRD patients as in vitro pharmacodynamic endpoint. Preliminary PKPD estimates are already available for ketamine: data from PKPD behavioral studies in rodents, clinical trials in MDD/TRD patients, in vitro electrophysiological and morphological analysis in both mouse and human neurons provide a reasonable but still preliminary support to the proposed translational model (Collo et al., 2018a; Zanos et al., 2018; Shaffer et al., 2019).
Recently, an active metabolite of ketamine, (2R,6R)-hydroxynorketamine (HNK) was shown to produce antidepressant and anti-anhedonic effects in animal models (Zanos et al., 2018). Intriguingly, HNK does not bind to NMDAR but directly engages an AMPA-dependent mechanism (Shaffer et al., 2019). In human, HNK is slowing forming and reaches its peak at submicromolar concentrations (< 0.5 μM) 6–12 hours after administration, while the half-life of ketamine is about 2 hours (Zanos et al., 2018). Prediction from preclinical data indicates a pharmacological effective concentration in human at 0.1 μM (Shaffer et al., 2019). Interestingly, the preclinical behavioral effects of HNK are blocked by the AMPAR antagonist NQBX, pointing to the critical role for AMPAR-dependent BDNF-TrkB signaling (Zanos et al., 2018). In human iPSC-derived dopaminergic neurons, 6-hour exposure to 0.5 μM HNK produced dendritic outgrowth when measured 3 days after dosing, effects similar to those obtained for 1-hour exposure to 1.0 μM ketamine (Collo et al., 2018b). These effects were blocked by pretreatment with the AMPA receptor antagonists NBQX and GYKI52466 and by the mTOR pathway blocker, rapamycin (Collo et al., 2018b).
It is tempting to contextualize these HNK data into the translational working model described in Figure 1. In this cartoon, ketamine and pramipexole are considered as reference drugs: (1) they have published evidence of producing a treatment response in TRD/Mood Disorder patients by improving anhedonia; (2) they were shown to be capable of engagement and partial normalization of defective anhedonia neural circuits in TRD/Mood Disorder patients; (3) they produced in vitro structural neuroplasticity in iPSC-derived human dopaminergic neurons; (4) they are active in vitro at concentrations compatible with those of clinical studies. Notably, the full contextualization of HNK as novel agent with putative antianhedonic effects into this translational model is not complete. In fact, evidence of HNK as an independent therapeutic antidepressant for TRD is not available yet, clinical studies being in progress (https://clinicaltrials.gov/ct2/show/, NCT03977675). This holds true also for neuroimaging, whose evidence of HNK effects on anhedonia neural circuits are indirectly associated with the effects of ketamine infusion measured at 24 hours after dosing (Abdallah et al., 2016), when the actions of approximately 12 hours exposure to pharmacological active HNK levels could have exerted its effects on neural circuits (Zanos et al., 2018). While further characterization in iPSC-derived dopaminergic neurons from MDD/TRD patients are needed, in Figure 1 we report an initial attempt for translating the available HNK in vitro data into clinical studies. Currently, HNK is in development at the NCAT/NIH (https://ncats.nih.gov/chemtech/projects/active/ketamine).
In conclusion, we propose a translational approach driven by human biology in the attempt to conceptually linking molecular and cellular substrates with clinical and neuroimaging relevant information. The model is driven by human PKPD data from the pharmacological agent in consideration and focus on a neural circuit-based hypothesis of psychiatric disorders. Interestingly, the possibility to study the molecular and cellular aspects of disorders and the mechanism of action of psychoactive drugs offered by iPSC-derived neurons will be critical for driving the identification of novel targets.
This work is funded by Ministry of Education, University and Research (MIUR) ex-60% research fund University of Brescia, Italy. Emilio Merlo Pich is employee of Takeda Pharmaceutical International AG.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Francisco Capani, Fundación Instituto Leloir, Argentina; Suk Yu Yau, The Hong Kong Polytechnic University, Hong Kong, China.
P-Reviewers: Capani F, Yau SY; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Apel PJ, Ma J, CaAbdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, Mathew SJ, Mathalon DH. Prefrontal connectivity and glutamate transmission: Relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:566–574. doi: 10.1016/j.bpsc.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018;23:812–823. doi: 10.1038/mp.2017.241. [DOI] [PubMed] [Google Scholar]
- 3.Collo G, Cavalleri L, Bono F, Mora C1, Fedele S, Invernizzi RW, Gennarelli M, Piovani G, Kunath T, Millan MJ, Merlo Pich E, Spano P. Ropinirole and pramipexole promote structural plasticity in human iPSC-derived dopaminergic neurons via BDNF and mTOR signaling. Neural Plast. 2018a;2018:4196961. doi: 10.1155/2018/4196961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collo G, Cavalleri L, Chiamulera C, Merlo Pich E. (2R 6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018b;29:1425–1430. doi: 10.1097/WNR.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 5.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Fawcett J, Rush AJ, Vukelich J, Diaz SH, Dunklee L, Romo P, Yarns BC, Escalona R. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am J Psychiatry. 2016;173:107–111. doi: 10.1176/appi.ajp.2015.15060788. [DOI] [PubMed] [Google Scholar]
- 9.Mah L, Zarate CA, Jr, Nugent AC, Singh JB, Manji HK, Drevets WC. Neural mechanisms of antidepressant efficacy of the dopamine receptor agonist pramipexole in treatment of bipolar depression. Int J Neuropsychopharmacol. 2011;14:545–551. doi: 10.1017/S1461145710001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer CL, Dutra JK, Tseng WC, Weber ML, Bogart LJ, Hales K, Pang J, Volfson D, Am Ende CW, Green ME, Buhl DL. Pharmacological evaluation of clinically relevant concentrations of (2R, 6R)-hydroxynorketamine. Neuropharmacology. 2019;153:73–81. doi: 10.1016/j.neuropharm.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA, Jr, Gould TD. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–660. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]