Over the last ten years or so, it has become apparent that pericytes have the potential to differentiate into other cell types which may help in the repair and regeneration of tissue after injury. In fact, pericytes have been described as a precursor to mesenchymal stem cells. Their location at the interface between the microvasculature and the brain parenchyma means they are ideally positioned to initiate repair and regeneration in response to various factors. In this perspective, we will highlight how pericytes have stem cell potential alongside their role in regulating processes, such as angiogenesis and inflammation, and discuss how pericytes could be harnessed to promote tissue repair in the brain (Figure 1).
Figure 1.
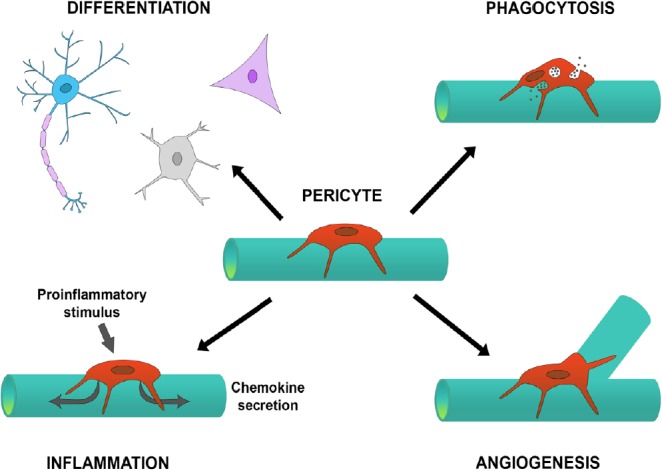
Pericytes have multiple properties that can lead to repair of tissue.
Pericytes residing on blood vessels can drive angiogenesis, mediate the inflammatory response, have stem cell properties by differentiating into other cell types, and can phagocytose material to clean up debris. The coordination of these responses can aid in the repair and regeneration of injured or diseased tissue, with evidence showing that this can occur in the brain.
Pericytes and their stem cell potential in the brain: Pericytes are multi-functional cells located within the basement membrane that surrounds capillaries throughout the body. They can regulate blood flow, are involved in angiogenesis and inflammation, and display stem cell-like properties. Within the brain, pericytes form part of the neurovascular unit and help maintain the blood-brain barrier (BBB).
A recurring feature in the pericyte literature is the ability of pericytes to act as stem cells and differentiate into multiple cell types. Some authors use the terms “pericyte” and “mesenchymal stem cell” interchangeably, while others argue that pericytes give rise to mesenchymal stem cells and are in fact the only true pluripotent stem cells in adult vertebrates (Davidoff, 2019).
The question of whether pericytes are indeed stem cells has been hotly debated in recent years with no consensus yet reached. It is generally accepted that pericytes share many features and markers of mesenchymal stem cells, but as both populations lack definitive markers and are heterogeneous, it is very difficult to demonstrate whether they are truly one and the same. Both in vivo and in vitro, pericytes appear to express markers typical of mesenchymal stem cells, such as CD44, CD73, CD90 and CD105. In addition, pericytes have been shown to have the potential to differentiate into a wide variety of cell types including chrondrocytes, osteoblasts, fibroblasts and smooth muscle cells. In the brain, pericytes have been shown to differentiate into neural and vascular cells (Nakagomi et al., 2015).
Within the periphery at least, the prevailing view is that pericytes are likely the in vivo precursors of mesenchymal stem cells and as such constitute an organ- and tissue-independent pool of pluripotent cells that reside in the microvasculature, and can respond to insult or injury in tissue-appropriate ways depending on cues from their surroundings (Yianni and Sharpe, 2018). However, one study suggested that while pericytes (defined as Tbx18-expressing cells) differentiate into multiple cell types in vitro, they do not contribute to other cell lineages in vivo either in physiological or pathological conditions (Guimaraes-Camboa et al., 2017).
Role of pericytes in repair: The location of pericytes at the interface between tissue cells and the vasculature places them in an ideal position to respond to injury. Their central location allows them to act as a paracrine signalling hub in which they receive signals from within the tissue itself or from the circulation. Pericytes have many important roles in the repair process including driving angiogenesis and vascular repair, mediating the influx of leukocytes and the inflammatory response, and guiding the removal of toxic species from the tissue. In addition to their potential stem cell activity outlined above, pericytes appear to play a critical role in the repair process of the brain and in the periphery.
Angiogenesis, the formation of new blood vessels, is a critical part of the tissue repair process. When injury occurs, which can damage both the tissue cells and the vasculature, new blood vessels can help supply the recovering area with critical nutrients to enable the reestablishment of that tissue. Pericytes can migrate to newly formed endothelial sprouts to help stabilise the vessel and make them functional but it is also possible that vessel formation can be driven by pericytes prior to endothelial cell involvement (Ribatti et al., 2011). Multiple signalling factors are involved in pericyte-driven angiogenesis including platelet-derived growth factor B, Angiopoietin, vascular endothelial growth factor and Notch (Ribatti et al., 2011), with loss of endothelial platelet-derived growth factor B leading to pericyte loss and microvascular abnormalities (Bjarnegard et al., 2004). In the brain, the knockdown of platelet-derived growth factor receptor β signaling can lead to pericyte loss, microhaemorrhages and a disrupted BBB (Bjarnegard et al., 2004), demonstrating the importance of pericytes to maintaining vascular stability and function.
A critical component of the tissue repair process is inflammation. The brain has specialised resident immune cells called microglia, but also relies on the influx of circulating leukocytes to aid in clean-up and repair after injury. Experiments in human cultures have shown that pericytes have substantial immunological complexity which contributes to propagation and resolution of inflammation (Rustenhoven et al., 2017). Pericytes express and release cytokines and chemokines such as CCL2, CXCL8 and interleukin-1β as well as adhesion molecules to attract and direct leukocytes and activate glial cells in the brain (Rustenhoven et al., 2017). In vivo, pericytes are the first-responders to systemic inflammation in the brain, releasing chemokines to coordinate changes in brain function and directing the passage of extravasated leukocytes (Duan et al., 2018). In addition, pericytes can alter BBB properties in response to inflammatory stimuli to enable the passage of leukocytes into the brain (Rustenhoven et al., 2017). Pericytes also appear to have MHCII complexes that could facilitate antigen uptake and presentation, but lack the co-stimulatory molecules to activate naïve T cells, and so pericytes likely influence adaptive immunity through the reactivation of T cells that have already been primed by other antigen presenting cells (Rustenhoven et al., 2017).
Another aspect of pericyte function is the protection of the brain through clearance of toxic species. Following ischemia, pericytes can differentiate into multiple cell types including glial cells (Nakagomi et al., 2015) enabling phagocytosis of cellular debris to clean up the damaged region. However, even resident pericytes on blood vessels can exhibit phagocytosis, as they are able to clear aggregated amyloid beta through an low-density lipoprotein receptor-related protein-1-mediated mechanism (Ma et al., 2018). Therefore, pericytes can help prevent the build-up of waste and debris in the brain.
Could pericytes be used as a type of stem cell treatment? The versatile and multipotent nature of pericytes makes them attractive as a therapeutic option to facilitate repair, particularly through their roles in angiogenesis, phagocytosis and inflammation. A number of research groups have explored this idea in the brain using experimental models. Fibroblast-derived pericytes were implanted into the brains of an aged Alzheimer’s disease mouse model (APP/PS1) where they led to increased cerebral blood flow and clearance of toxic amyloid beta thereby reducing plaque burden (Tachibana et al., 2018). In addition, an abstract published in 2017 reported that administration of adipose-derived perivascular stem cells (CD146+, neural glial antigen 2+, platelet-derived growth factor receptor beta+) to rats undergoing middle cerebral artery occlusion showed improved somatosensory recovery and increased capillary density in the ischemic border zone 28 days later (Ogay et al., 2017). The administration of pericytes for a regenerative approach has also been utilized in peripheral tissues such as the heart and bone.
One of the issues for pericyte delivery to the brain, and one which has limited the use of stem cell therapy in the past, is the difficulty in crossing the BBB. Therefore, strategies must be developed to enable exogenously administered pericytes to readily enter the brain and exert any reparative effects if they are going to be a viable therapeutic option. A recent study attempted to achieve this by using mannitol in canines to transiently open the BBB and allow autologous pericytes derived from adipose tissue to enter the brain following intra-arterial injection (Youn et al., 2015). They showed that transient opening of the BBB with mannitol resulted in a significantly greater number of pericytes entering the brain from the circulation, indicating that there is potential with this technique.
The process of isolating, culturing and testing functionality of pericytes is critical for their success as a cellular therapy. Many methods have been developed to obtain functional pericytes from a range of tissues including skeletal muscle, adipose tissue and the placenta. Functional in vitro assays such as the stabilisation of endothelial networks will need to be used to confirm the functionality of isolated pericytes. Autologous pericyte cellular therapies present an attractive proposition given that they could be administered back to the same patient, thereby avoiding the problems of immune rejection that plague non-autologous cellular therapy.
Nomenclature: Unfortunately, the pericyte field has been complicated by confusing nomenclature, with multiple names for pericytes being used interchangeably. For example, pericytes have been called perivascular cells, perivascular stem cells and mesenchymal vascular stem cells. So, is everyone talking about the same cell type? The heterogeneity of pericytes and inconsistent results with cellular markers has meant that researchers could be labeling these as different cell types when they may in fact be the same. Therefore, to move forward as a field, we must determine what is specifically meant by a pericyte and remain consistent in our definition while being aware that there likely exist many sub-types of pericytes which may explain the differential findings reported in the literature.
Conclusion: While the jury is still out on whether pericytes function as stem cells in vivo or whether their ability to form other cell types is merely a function of culture conditions and/or artificial manipulation, this may actually be irrelevant to their potential for therapeutics. There is little doubt that pericytes exist within the microvasculature of all tissues, so there is potential to harness their stem cell properties to promote regeneration and repair even it requires some manipulation either in vitro or in vivo. The potential of pericyte therapy for many brain diseases is promising, and future research will determine whether differentiation of endogenous pericytes or exogenous administration of pericytes is the optimal strategy.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 2.Davidoff MS. The pluripotent microvascular pericytes are the adult stem cells even in the testis. Adv Exp Med Biol. 2019;1122:235–267. doi: 10.1007/978-3-030-11093-2_13. [DOI] [PubMed] [Google Scholar]
- 3.Duan L, Zhang XD, Miao WY, Sun YJ, Xiong G, Wu Q, Li G, Yang P, Yu H, Li H, Wang Y, Zhang M, Hu LY, Tong X, Zhou WH, Yu X. PDGFRbeta cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron. 2018;100:183–200e188. doi: 10.1016/j.neuron.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359e345. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Q, Zhao Z, Sagare AP, Wu Y, Wang M, Owens NC, Verghese PB, Herz J, Holtzman DM, Zlokovic BV. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-beta42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener. 2018;13:57. doi: 10.1186/s13024-018-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A, Matsuyama T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33:1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 7.Ogay V, Kumasheva V, Baidosova S, Li Y, Shpekov A, Makhambetov Y, Kaliyev A, Zhetpisbayev B, Olzhayev F, Ramankulov Y. Adipose-derived perivascular stem cells promote sensorimotor recovery after ischemic stroke in rats. J Biotechnol. 2017;256:S116. [Google Scholar]
- 8.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 9.Rustenhoven J, Jansson D, Smyth LC, Dragunow M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci. 2017;38:291–304. doi: 10.1016/j.tips.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Tachibana M, Yamazaki Y, Liu CC, Bu G, Kanekiyo T. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-beta pathology in amyloid model mice. Exp Neurol. 2018;300:13–21. doi: 10.1016/j.expneurol.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yianni V, Sharpe PT. Molecular programming of perivascular stem cell precursors. Stem Cells. 2018;36:1890–1904. doi: 10.1002/stem.2895. [DOI] [PubMed] [Google Scholar]
- 12.Youn SW, Jung KH, Chu K, Lee JY, Lee ST, Bahn JJ, Park DK, Yu JS, Kim SY, Kim M, Lee SK, Han MH, Roh JK. Feasibility and safety of intra-arterial pericyte progenitor cell delivery following mannitol-induced transient blood-brain barrier opening in a canine model. Cell Transplant. 2015;24:1469–1479. doi: 10.3727/096368914X682413. [DOI] [PubMed] [Google Scholar]


