Keywords: cortical neuron, ischemia, neurotrophin, oxygen-glucose deprivation, precursor protein of brain-derived neurotrophic factor, proprotein convertase, proprotein convertase 1/3
Abstract
Brain-derived neurotrophic factor (BDNF) has robust effects on synaptogenesis, neuronal differentiation and synaptic transmission and plasticity. The maturation of BDNF is a complex process. Proprotein convertase 1/3 (PC1/3) has a key role in the cleavage of protein precursors that are directed to regulated secretory pathways; however, it is not clear whether PC1/3 mediates the change in BDNF levels caused by ischemia. To clarify the role of PC1/3 in BDNF maturation in ischemic cortical neurons, primary cortical neurons from fetal rats were cultured in a humidified environment of 95% N2 and 5% CO2 in a glucose-free Dulbecco’s modified Eagle’s medium at 37°C for 3 hours. Enzyme-linked immunosorbent assays and western blotting showed that after oxygen-glucose deprivation, the secreted and intracellular levels of BDNF were significantly reduced and the intracellular level of PC1/3 was decreased. Transient transfection of cortical neurons with a PC1/3 overexpression plasmid followed by oxygen-glucose deprivation resulted in increased PC1/3 levels and increased BDNF levels. When levels of the BDNF precursor protein were reduced, the concentration of BDNF in the culture medium was increased. These results indicate that PC1/3 cleavage of BDNF is critical for the conversion of pro-BDNF in rat cortical neurons during ischemia. The study was approved by the Animal Ethics Committee of Wuhan University School of Basic Medical Sciences.
Chinese Library Classification No. R446; R741; Q421
Introduction
Ischemia is a common pathophysiological condition of the central nervous system (CNS). Brain-derived neurotrophic factor (BDNF) transcript levels rapidly increase in the cerebral cortex after ischemic insult (Cruz et al., 2018; Hu et al., 2019). However, the source of BDNF following ischemia is not well understood. In the CNS, both neurons and astrocytes can produce BDNF. Furthermore, astrocytes are more abundant than neurons. Glial fibrillary acid protein-positive astrocytes stain for BDNF after ischemia (Himeda et al., 2007) and oxygen-glucose deprivation (OGD) enhances BDNF expression in active astrocytes (Chen et al., 2015). Therefore, we speculated that the increase in BDNF transcript levels during ischemia mainly results from expression in active astrocytes; however, changes in BDNF expression in cortical neurons during ischemia are unclear.
BDNF has robust effects on synaptogenesis, neuronal differentiation and synaptic transmission and plasticity (Habtemariam, 2018; Shi et al., 2019). The maturation of BDNF is a complex process. BDNF is initially synthesized in a precursor form, and a series cleavage events are required to produce mature BDNF (m-BDNF). The BDNF precursor protein (pro-BDNF) is cleaved by either intracellular serine proteases, such as proprotein convertase 1/3 (PC1/3) and/or furin, or by extracellular proteases, such as plasmin, which is activated by tissue plasminogen activator, and/or matrix metallopeptidases (Lee et al., 2019). The significance of pro-BDNF cleavage in the CNS has been reported (Matsumoto et al., 2008; Yang et al., 2009). These two reports suggest that the cleavage of pro-BDNF is regulated in a highly specific manner and depends on the cellular context. In addition, pro-BDNF can have opposing effects on neuronal structure and synaptic plasticity by binding to the p75 receptor (Fleitas et al., 2018). Thus, the relative levels of pro-BDNF and m-BDNF are likely to play important roles in regulating brain structure and function.
PC1/3 expression is restricted to endocrine and neuroendocrine tissues (Seidah, 2011) and PC1/3 has a key role in the cleavage of protein precursors that are directed to regulated secretory pathways. In the CNS, PC1/3 immunoreactive signals localize to nerve terminals and the trans-Golgi network (Winsky-Sommerer et al., 2000). It is unclear whether PC1/3 mediates the change in BDNF expression during ischemia. We previously showed that furin mediates BDNF up-regulation in reactive astrocytes induced by OGD (Chen et al., 2015); therefore, we speculated that PC1/3 mediates the change in BDNF expression in neurons during ischemia. We used cultured rat cortical neurons to explore the impact of PC1/3 on BDNF expression in cortical neurons following OGD.
Materials and Methods
Primary cortical neuron culture
Embryonic day 18–20 embryos were isolated from pregnant Sprague-Dawley rats [n = 3, provided by Hubei Provincial Center for Disease Control and Prevention, China, license No. SYXK(E)2017-0065]. The study was approved by the Animal Ethics Committee of Wuhan University School of Basic Medical Sciences. Brains were dissected from the embryos and the cortices were isolated and then dissociated with papain and DNase using previously published methods (Brewer, 1995). Cell suspensions were plated in poly-L-lysine-coated 6-well plates at a density of 500 cells/mm2. Cultured neurons were maintained in serum-free Neurobasal medium (Life Technologies, New York, NY, USA) at 37°C in a humidified incubator with 5% CO2. The growth medium was changed twice a week. At 9 days in vitro, the growth medium was changed to Neurobasal medium supplemented with B27. To determine the proportion of neurons at 10 days in vitro, the cultured cells were double-immunostained with antibodies against microtubule-associated protein 1 (a neuron marker) and against glial fibrillary acid protein (an astrocyte marker). Cells were 80–90% microtubule-associated protein 1-positive and 5–10% glial fibrillary acid protein-positive (data not shown). The cultured cells were used for experiments at 10–14 days in vitro.
Exposure of neurons to OGD
To imitate ischemic conditions in vitro, the cultured cortical neurons were exposed to OGD as described previously (Furuichi et al., 2005). Briefly, cortical neurons were seeded in 6-well plates. When cells reached 60–70% confluency, the culture medium was changed to Dulbecco’s modified Eagle’s medium without glucose and pre-equilibrated with 95% N2 and 5% CO2. Cells were maintained in a humidified airtight chamber (Billups-Rothberg, Del Mar, CA, USA) with 5% CO2 and 95% N2 at 37°C for 3 hours. At the end of the OGD treatment, cells were maintained in Neurobasal medium supplemented with B27. Control cultures were incubated with normal Dulbecco’s modified Eagle’s medium at 37°C in 5% CO2 and 95% air for 3 hours. Trypan blue staining showed that all neurons were alive after 3 hours of OGD.
Cell transfection
Cortical neurons at 60–70% confluency were transiently transfected using FuGENE HD transfection reagent (Promega, Madison, WI, USA). Transfections using pPC1/3 and pIRES2-EGFP plasmids (provided by the Fifth Affiliated Hospital of Sun Yat-sen University, China) were performed in six-well plates. pro-BDNF, BDNF and PC1/3 levels were measured in cell lysates and culture supernatants at 0, 12, 24 and 48 hours after transfection.
Enzyme-linked immunosorbent assay
BDNF was detected using an enzyme-linked immunosorbent assay according to a previously published method (Chen et al., 2015). Identical volumes (300 μL) of culture supernatant were centrifuged at 1000 × g at 4°C for 20 minutes. The BDNF Emax immunoassay (ELabscience, Wuhan, China) was performed according to the manufacturer’s protocol. BDNF concentrations were estimated from a standard curve and are expressed as pg/mL protein. The intra- and inter-assay coefficients of variation were less than 10%.
Western blot assay
After different reperfusion times following 3 hours of OGD, protein samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gels (for PC1/3) or 15% sodium dodecyl sulfate-polyacrylamide gels (for BDNF and pro-BDNF) and then electrotransferred to polyvinylidene fluoride membranes. The membranes were incubated with mouse anti-BDNF (1:400, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-pro-BDNF (1:400, Abcam, Cambridge, UK), rabbit anti-PC1/3 (1:1000, Abcam) and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (1:1000, Kang-chen Biological Technology Co., Ltd., Shanghai, China) at 4°C overnight. The blots were incubated with appropriate peroxidase-conjugated mouse/rabbit secondary antibodies (1:1000; Santa Cruz Biotechnology) for 1 hour at room temperature after repeated washes. Immunoreactivity was detected using enhanced chemiluminescence assays (Thermo Scientific, Waltham, MA, USA). The relative expression of each protein was determined by densito-metric analysis using LabWorks 4.0 software (UVP Ltd., Cambridge, UK).
Statistical analysis
Data from independent experiments, each performed in triplicate, were pooled. Data are presented as the mean ± SEM. A two-tailed Student’s t-test was applied for comparisons between the control and OGD groups, and one-way analysis of variance was applied for multiple comparisons. All statistical analyses were conducted using SPSS 11.0 software (SPSS, Chicago, IL, USA). A P value < 0.05 indicates significant difference.
Results
Changes in BDNF levels in cultured cortical neurons exposed to OGD
We used OGD to mimic ischemic conditions in cultured cortical neurons. BDNF levels were estimated in cell lysates by western blotting and in culture medium by enzyme-linked immunosorbent assays after reperfusion for 0, 12, 24, 48 and 72 hours following OGD for 3 hours. At all times evaluated, the concentration of BDNF in the culture medium was reduced; there were significant differences after reperfusion for 24 and 48 (P < 0.05) and 72 hours (P < 0.01). Intracellular BDNF expression was also significantly decreased after reperfusion for 12 and 24 (P < 0.05) and 48 and 72 hours (P < 0.01; Figure 1).
Figure 1.
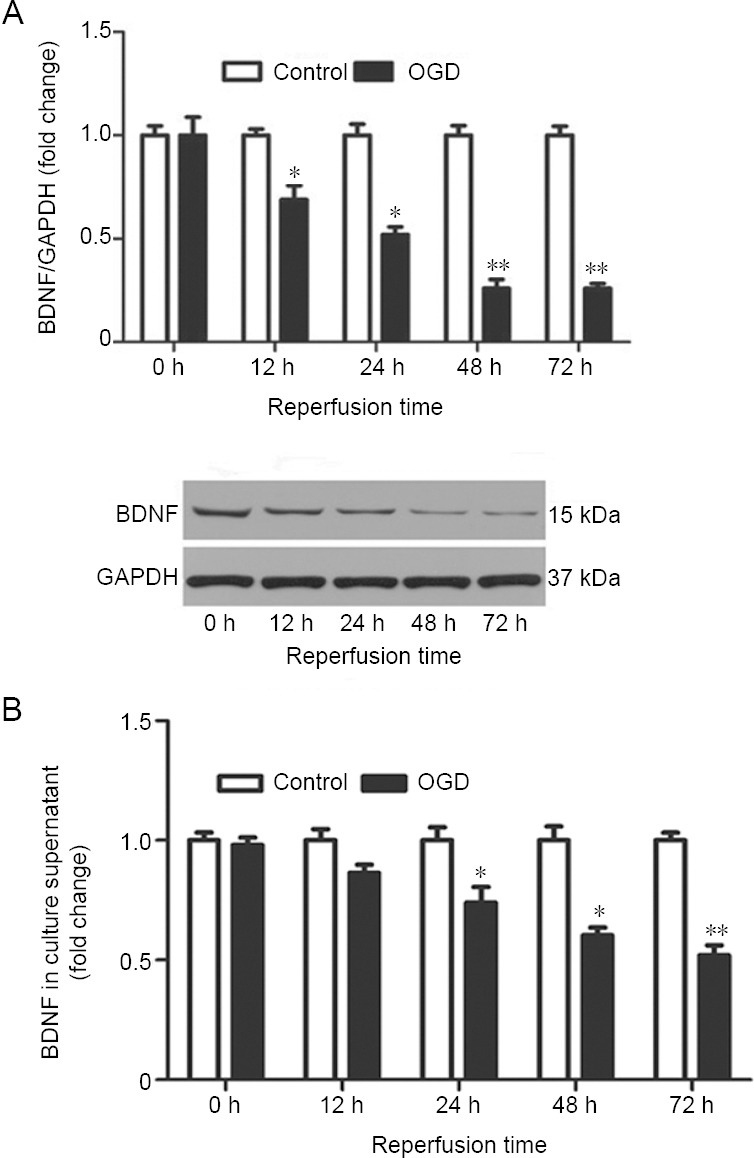
Change in BDNF levels after reperfusion for different times following OGD.
Cells were exposed to OGD for 3 hours and then subjected to reperfusion for 0, 12, 24, 48, or 72 hours. (A) Intracellular BDNF (ratio to levels at 0 hour) was detected by western blotting. The illustrated western blot is of the OGD group. (B) The concentration of BDNF in the culture medium was assayed with an enzyme-linked immunosorbent assay. Data are expressed as the mean ± SEM (ratio to levels at 0 hour), and were analyzed by Student’s t-test. Values are from three independent experiments. *P < 0.05, **P < 0.01, vs. control group. BDNF: Brain-derived neurotrophic factor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; OGD: oxygen-glucose deprivation.
Down-regulation of PC1/3 by OGD in cultured cortical neurons
PC1/3 and furin can cleave pro-BDNF (Yang et al., 2009) and furin levels increase during hypoxia (McMahon et al., 2005; Chen et al., 2015). We also found that furin expression was up-regulated in cortical neurons subjected to OGD (data not shown). Therefore, we measured changes in PC1/3 expression in cultured cortical neurons following OGD. PC1/3 expression was estimated by western blotting after reperfusion for 0, 12, 24, 48 and 72 hours following 3 hours of OGD. PC1/3 expression in cultured cortical neurons was down-regulated after OGD; there was significant down-regulation after reperfusion for 12 (P < 0.05) and 48 and 72 hours (P < 0.01; Figure 2). In cultured cortical neurons subjected to OGD, PC1/3 and BDNF expression were simultaneously down-regulated.
Figure 2.
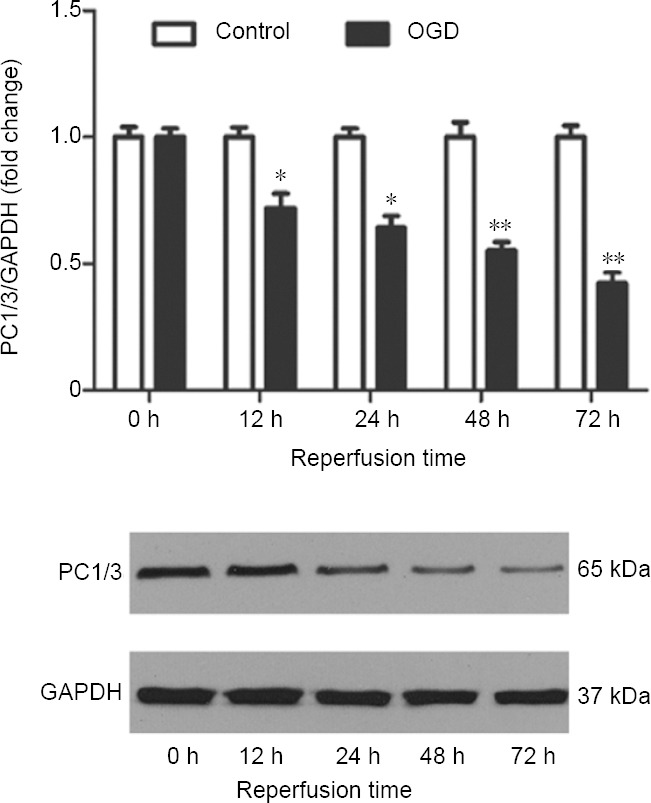
Western blot analysis of PC1/3 in cultured cortical neurons after OGD for 3 hours and reperfusion for various times.
The illustrated western blot is of the OGD group. Data are expressed as the mean ± SEM (ratio to levels at 0 hour), and were analyzed by Student’s t-test. Values are presented as the fold change relative to control values and are from three independent experiments. *P < 0.05, **P < 0.01, vs. control group. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; OGD: oxygen-glucose deprivation; PC1/3: proprotein convertase 1/3.
PC1/3 overexpression increases BDNF and reduces pro-BDNF levels
We attempted to determine whether the cleavage of secreted BDNF by PC1/3 is a factor determining whether BDNF is sorted into regulated secretory pathways. PC1/3 activity was increased by the transfection of pPC1/3, a plasmid that overexpresses PC1/3. When PC1/3 levels were increased (Figure 3A), the levels of BDNF were also increased. Intracellular BDNF levels were significantly increased 12 hours after transfection (P < 0.05), and continued to increase at 24 and 48 hours after transfection (P < 0.01; Figure 3B). At the same time, pro-BDNF levels were reduced (Figure 3C), and the down-regulation of pro-BDNF was significant at 24 hours (P < 0.05) and 48 hours (P < 0.01) after transfection. The concentration of BDNF in the culture medium was increased (Figure 3D). There was no significant difference in BDNF levels 12 hours after transfection compared with the control group but there were significant differences at 24 (P < 0.01) and 48 hours (P < 0.01) after transfection relative to the control group.
Figure 3.
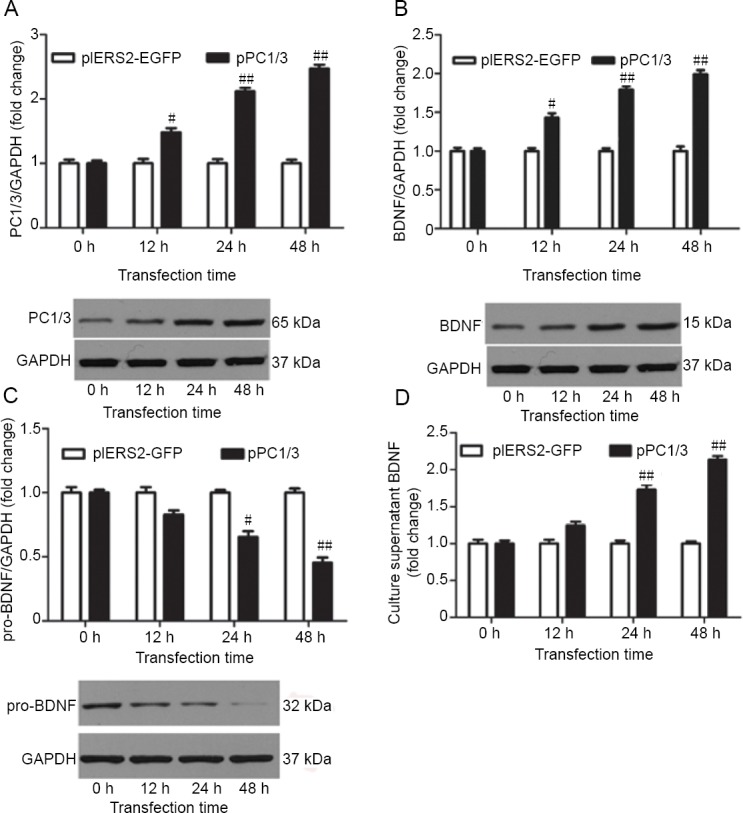
Effects of PC1/3 cleavage on BDNF expression.
BDNF expression was estimated at 0, 12, 24 and 48 hours after transfecting cortical neurons with pPC1/3. (A–C) Intracellular PC1/3 (A), BDNF (B) and pro-BDNF (C) were detected by western blotting. The illustrated western blots are of the OGD group. (D) BDNF in the culture medium was assayed by an enzyme-linked immunosorbent assay. Data are expressed as the mean ± SEM, and were analyzed by Student’s t-test. Values are presented as the percentage of control values and are from three independent experiments. #P < 0.05, ##P < 0.01, vs. pIRES2-EGFP group. BDNF: Brain-derived neurotrophic factor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; PC1/3: proprotein convertase 1/3; pIRES2-EGFP: pIRES2-EGFP plasmid; pPC1/3: pPC1/3 plasmid; pro-BDNF: precursor protein of BDNF.
Discussion
The present study shows that BDNF expression is down-regulated in cultured cortical neurons after OGD. These results are consistent with previous findings (Ferrer et al., 2001; Himeda et al., 2007). Himeda et al. (2007) found that BDNF immunoreactivity was not significantly changed in hippocampal neurons for up to 2 days after transient cerebral ischemia, although BDNF immunoreactivity was significantly decreased in damaged hippocampal neurons. Moreover, BDNF was significantly up-regulated in hippocampal CA1 glial cells 5 days after ischemia (Himeda et al., 2007). In addition, we previously found that BDNF expression was enhanced after OGD in astrocytes (Chen et al., 2015). Among the neuroprotection mechanisms mediated by astrocytes is the release of neurotrophic factors by reactive astrocytes to sustain neuronal survival during hypoxia (Ishitsuka et al., 2012). However, the reason for BDNF down-regulation in neurons during ischemia has not been clarified.
To clarify the mechanism underlying the down-regulation of BDNF in neurons during ischemia, we mimicked ischemia in cortical neurons through OGD and measured BDNF cleavage, with a focus on PC1/3. Cultured cortical neurons were subjected to OGD, and PC1/3 levels were determined. Compared with the control group, PC1/3 levels were significantly decreased in the OGD group after reperfusion for 24 hours. Our results indicate that the change in PC1/3 levels was similar to the change in BDNF levels in cortical neurons after OGD. We, therefore, suggest that PC1/3 cleavage of BDNF is important in cortical neurons during ischemia.
The cleavage of neurotrophins by proprotein convertases, such as furin, may be a factor that determines whether a neurotrophin is transported into the constitutive or regulated secretory pathways in the trans-Golgi network (Matsumoto et al., 2008). Without furin cleavage, the precursor protein retains the sorting signal that directs the protein to the regulated pathway. When furin is up-regulated under certain conditions, more pro-BDNF will be cleaved by furin. However, during hypoxia, our results show that furin was up-regulated (data not shown), and at the same time, BDNF levels were down-regulated in cortical neurons. The reason for this is possibly related to OGD inducing a change in the expression of the furin-like proprotein convertase. PC1/3 is a key enzyme involved in the cleavage of protein precursors that are guided to the regulated secretory pathway, and PC1/3 immunoreactive signals were found in the trans-Golgi network and at nerve terminals in the CNS. Based on these findings, we speculate that PC1/3 has a major role in modulating BDNF levels in cortical neurons during OGD. Moreover, our results also show that PC1/3 was down-regulated in cortical neurons after OGD. The down-regulation of PC1/3 and BDNF was similar in cortical neurons after OGD.
To clarify the effect of PC1/3 cleavage on the maturation of BDNF in cortical neurons, pro-BDNF and BDNF levels were measured when PC1/3 activity was increased by PC1/3 overexpression. When PC1/3 expression was increased, pro-BDNF was down-regulated and BDNF was up-regulated. This indicates that PC1/3 has a major role in BDNF maturation in cortical neurons. In addition, previous reports showed that pro-BDNF may escape cleavage by furin and be transported into the regulated secretory pathway, where it is cleaved by PC1/3 and released in an activity-dependent manner (Seidah et al., 1996). Our results support the notion that neurons regulate neurotrophins by changing furin or furin-like proprotein convertase levels, which regulates the intracellular transport of the neurotrophins they produce (Mowla et al., 1999).
Intracellular pro-BDNF has different fates in vivo (Foltran and Diaz, 2016): intracellular cleavage and release of m-BDNF and secretion as pro-BDNF. In fact, pro-BDNF is the main secreted form although it is difficult to verify in vivo (Dieni et al., 2012). Regulation of the ratio between pro-BDNF and m-BDNF is important under pathological conditions (Lu et al., 2005). In addition, pro-BDNF can act in vivo to regulate neurite growth, spine formation and cell survival, effects that are all distinct from those of m-BDNF (Koshimizu et al., 2009; Yang et al., 2014). Therefore, we suggest that in ischemic cortical neurons, the ratio of pro-BDNF to BDNF is regulated by PC1/3. However, our study is a preliminary in vitro exploration of the relationship between BDNF and PC1/3 in neurons and the further studies should be performed in vivo.
In conclusion, PC1/3 and BDNF levels were reduced in cultured cortical neurons after OGD. The furin-like cleavage mechanism induced by OGD that processes BDNF in cortical neurons is different from that in reactive astrocytes. This study shows a new effect of PC1/3 on pro-BDNF cleavage in ischemic cortical neurons. The impact of BDNF and PC1/3 down-regulation in cortical neurons following OGD requires further study.
Acknowledgments
Thanks to Professor Xiao-Mou Peng, Department of Infectious Diseases, the Fifth Affiliated Hospital of Sun Yat-sen University, China for providing pPC1/3 and pIRES2-EGFP plasmids.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the National Nature Science Foundation of China, No. 81501053 (to YC). The funding body played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: All procedures were approved by the Animal Ethics Committee of Wuhan University School of Basic Medical Sciences.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Nature Science Foundation of China, No. 81501053 (to YC).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral, cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Zhang J, Deng M. Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen-glucose deprivation. J Neurosci Res. 2015;93:189–194. doi: 10.1002/jnr.23455. [DOI] [PubMed] [Google Scholar]
- 3.Cruz Y, García EE, Gálvez JV, Arias-Santiago SV, Carvajal HG, Silva-García R, Bonilla-Jaime H, Rojas-Castañeda J, Ibarra A. Release of interleukin-10 and neurotrophic factors in the choroid plexus: possible inductors of neurogenesis following copolymer-1 immunization after cerebral ischemia. Neural Regen Res. 2018;13:1743–1752. doi: 10.4103/1673-5374.238615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, Barde YA. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer I, Krupinski J, Goutan E, Marti E, Ambrosio S, Arenas E. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2001;101:229–238. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- 6.Fleitas C, Piñol-Ripoll G, Marfull P, Rocandio D, Ferrer I, Rampon C, Egea J, Espinet C. proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol Brain. 2018;11:68. doi: 10.1186/s13041-018-0411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foltran RB, Diaz SL. BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem. 2016;138:204–221. doi: 10.1111/jnc.13658. [DOI] [PubMed] [Google Scholar]
- 8.Furuichi T, Liu W, Shi H, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- 9.Habtemariam S. The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: new pharmacological concepts for old and new drugs. Neural Regen Res. 2018;13:983–984. doi: 10.4103/1673-5374.233438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himeda T, Tounai H, Hayakawa N, Araki T. Postischemic alterations of BDNF, NGF, HSP 70 and ubiquitin immunoreactivity in the gerbil hippocampus: pharmacological approach. Cell Mol Neurobiol. 2007;27:229–250. doi: 10.1007/s10571-006-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Guo TC, Zhang XY, Tian J, Lu YS. Paired associative stimulation improves synaptic plasticity and functional outcomes after cerebral ischemia. Neural Regen Res. 2019;14:1968–1976. doi: 10.4103/1673-5374.259618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, Kuroda J, Kamouchi M, Kitazono T. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83:352–359. doi: 10.1016/j.mvr.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, Son Y, Lee M, Moon C, Kim SH, Shin IS, Yang M, Bae S, Kim JS. Sodium butyrate prevents radiation-induced cognitive impairment by restoring pCREB/BDNF expression. Neural Regen Res. 2019;14:1530–1535. doi: 10.4103/1673-5374.255974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 17.McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 18.Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seidah NG. What lies ahead for the proprotein convertases? Ann N Y Acad Sci. 2011;1220:149–161. doi: 10.1111/j.1749-6632.2010.05883.x. [DOI] [PubMed] [Google Scholar]
- 20.Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Gao SL, Liu JD, Gu TX, Shi EW. Bone marrow mesenchymal stem cell exosomes alleviate oxygen-glucose deprivation/reperfusion injury in hippocampal neurons. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:3316–3322. [Google Scholar]
- 22.Winsky-Sommerer R, Benjannet S, Rovere C, Barbero P, Seidah NG, Epelbaum J, Dournaud P. Regional and cellular localization of the neuroendocrine prohormone convertases PC1 and PC2 in the rat central nervous system. J Comp Neurol. 2000;424:439–460. [PubMed] [Google Scholar]
- 23.Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7:796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


