Keywords: assessment, cognitive impairment, community, dementia, follow-up, Mini-Mental Status Examination Scale, model, new basis, prevention, prognostic factors
Abstract
The risk of dementia increases in patients with cognitive impairment. However, it is not clear what factors contribute to the onset of dementia in those with cognitive impairment. In this prospective cohort study, we will investigate the every-five-year incidence of cognitive impairment and prognostic factors for cognitive impairment. The Jidong cognitive impairment cohort was established from April 2012 to August 2015, during which we recruited 5854 healthy participants (55.1% male) older than 45 years (mean, 57 years). Participants received a health examination in the Staff Hospital, Jidong Oilfield Branch, China National Petroleum Corporation. Baseline data and blood samples were collected. Cognitive impairment was evaluated using the Mini-Mental State Examination, and was defined as a Mini-Mental State Examination score of less than 24. Dementia was assessed using the criteria of Diagnostic and Statistical Manual of Mental Disorders (Fourth edition), the International Working Group criteria, and the Mini-Mental State Examination score. The follow-up will continue until December 2024, during which a prognostic model will be constructed. The primary outcome is the presence/absence of dementia and the secondary outcome is quality of life. Baseline screening results showed the following: (1) Cognitive impairment was apparent in 320 participants (5.5%). These participants will be excluded from the Jidong cohort study, and the remaining participants will be followed up. (2) Of the 320 participants with cognitive impairment, there was a significantly higher prevalence of illiteracy than other education levels (35.9%, P < 0.05). Age, arterial hypertension, alcohol consumption, and passive smoking differed significantly between the cognitive impairment and healthy groups (P < 0.05). Multivariate logistic regression models showed that age (odds ratio [OR] = 1.059, 95% confidence interval [CI]: 1.044–1.074) and arterial hypertension (OR = 1.665, 95% CI: 1.143–2.427) were risk factors for mild cognitive impairment. With the increase of educational level (illiteracy, primary school, junior high school, high school, university, and above), cognitive impairment gradually decreased (OR < 1, P < 0.05). (3) This cohort study has initially screened for several risk factors for cognitive impairment at baseline, and subsequent prospective data will further describe, validate, and evaluate the effects of these risk factors on cognitive impairment and dementia. These results can provide clinical evidence for the early prevention of cognitive impairment and dementia. The study was approved by the Ethics Committee of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, Staff Hospital, Jidong Oilfield Branch, China National Petroleum Corporation on July 12, 2013 (approval No. 2013 YILUNZI 1).
Chinese Library Classification No. R441; R445; R741
Introduction
Dementia is one of the largest public health issues, with an estimated 9.9 million new cases worldwide reported in 2015 (Li et al., 2017; Lin, 2018). Dementia is a chronic and progressive clinical syndrome characterized by a decline in memory and other cognitive dysfunctions, which cannot be cured (Chen et al., 2018; White et al., 2018; Maiese, 2019). Cognitive impairment affects people’s daily activity and social abilities, and has been reported to have a strong relationship with dementia (Ijaopo, 2017; Das et al., 2019; Manzano Palomo et al., 2019). More than 10% of patients with mild cognitive impairment (MCI) are diagnosed with dementia every year (Wang et al., 2018). It has been reported that more than 10% of people over 65 years old experience MCI, and 15% of these cases progress to dementia every year. Thus, people with MCI are 10 times more likely to develop dementia than healthy people within the same age range (Tervo et al., 2004).
MCI is the transitional stage between normal cognitive function and dementia, and has similar characteristics to the early stage of Alzheimer’s disease (Liu et al., 2019; Mondragón et al., 2019). The annual risk of Alzheimer’s disease in patients with MCI is as high as 7% to 20% (Petersen et al., 1999). Once cognitive dysfunction occurs, patients are at increased risk of developing dementia (Busse et al., 2006). In the past few decades, many studies have investigated the risk factors for dementia, including neuropsychiatric symptoms (Gunther et al., 2012; Morandi et al., 2012; Forrester et al., 2016; Ismail et al., 2016), hypertension, diabetes, and dyslipidemia (Cherbuin et al., 2009; Solomon et al., 2009; Ott et al., 2010; Tanokashira et al., 2019). However, prevention, detection and intervention of dementia in the early stage are not yet fully understood (Rosato et al., 2019). It is still unclear what prognostic factors affect the outcome of dementia in subjects with cognitive impairment.
Little comprehensive information about the prognosis of MCI on a cohort of people with cognitive impairment has been made available. It is therefore necessary to further verify the risk factors of dementia and explore novel biomarkers that could influence the prognosis of MCI. Therefore, cognitive impairment cohort study was established to investigate the prevalence of MCI, the every-five-year incidence of MCI, and prognostic factors for MCI for the early prevention and preclinical warning of dementia.
Participants and Methods
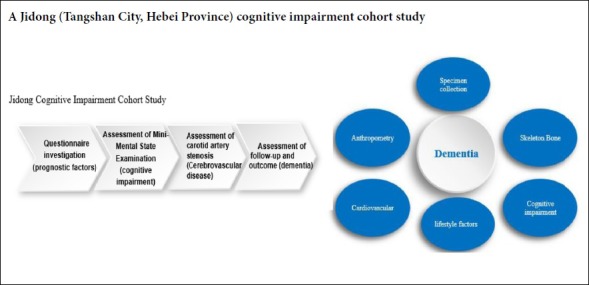
Study design
The Jidong cognitive impairment cohort study is a community-based, prospective, long-term observational cohort study to evaluate prognostic factors of cognitive impairment. In this study, all participants were evaluated at baseline and divided into two groups according to Mini-Mental State Examination (MMSE) scores: cognitive impairment group and the normal group (Albanna et al., 2017; Sokołowska et al., 2018). Prognostic risk factors for cognitive impairment were screened by clinicians and well-trained investigators.
Local government attaches great importance to and encourage the participants. This study also received support and cooperation from local participants. Hospitals with participants in physical examination have better medical conditions and convenient transportation, which further facilitates the participation of participants. According to the survey data over five years, the prevalence of hypertension and hyperlipidemia in the cohort study is high, which promotes the development of cardiovascular and cerebrovascular diseases. There are unified physical examination and stable personnel every year, which have enough eligible participants. The two large groups have a unified medical record system, which can query each participant’s death, disease and other clinical information. All participants underwent extensive laboratory, clinical, and environmental exposure measurements to identify clinical, biological, and environmental factors associated with MCI. Blood samples were collected at designated physical examination hospitals every year, which had abundant research data.
Ethics statement
The study was performed in accordance with the guideline of the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects (2013). Approval was obtained from the Ethics Committee of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, Staff Hospital, Jidong Oilfield Branch, China National Petroleum Corporation on July 12, 2013 (approval No. 2013 YILUNZI 1). These approvals will be renewed every five years. Written informed consent was obtained from all participants.
Study setting and population
From April 2012 to August 2015, participants were recruited via posters in the Physical Examination Department of the Hospital. The basic information including life style and medicine history of participants was reviewed, and we contacted eligible participants. Participants were recruited from Kailuan and Caofeidian communities in Tangshan City, Hebei Province, China. Caofeidian located in the south of Tangshan City, and has a population of 2.687 million. There was no significant difference in baseline between Kaiuan and Caofeidian communities, we found the baseline of Kailuan and Caofeidian community and found that passive smoking had no significant difference in Caofeidian community, and other factors were consistent with the baseline of the Jidong cognitive impairment cohort study. The participants were recruited from Jidong cohort study, which included 12,096 employees and retirees. A sample of 6433 subjects aged 45 years or older was randomly selected, and 5854 subjects voluntarily participated in the study.
Inclusion criteria
The inclusion criteria of the study were as follows: aged ≥ 45 years old, no history of dementia, available questionnaire of cognitive impairment cohort investigation and signed informed consent provided, and blood sample information of the corresponding questionnaire investigation available.
Exclusion criteria
The exclusion criteria of the study were as follows: presence of cerebrovascular diseases, including coronary heart disease, stroke, transient ischemic attack, atrial fibrillation, heart failure, history of any type of cancer, and unavailable questionnaire.
Questionnaire investigation
All participants participated in the health information questionnaire with the assistance of a well-trained research assistant. The questionnaire collected information about demographics, lifestyle, and medical history (Table 1). The demographic information included sex, age, and educational level, which was classified as “illiteracy”, “primary school”, “junior high school”, “high school”, or “university and above”. Data about lifestyle included a history of smoking, passive smoking, and drinking. The medical history included the history of hypertension, hyperlipidemia, diabetes and cerebrovascular diseases, including stroke and transient ischemic attack, heart failure, atrial fibrillation, and myocardial infarction.
Table 1.
Physical examination of the Jidong cognitive impairment cohort study
| Test | Components |
|---|---|
| Specimen collection | Fasting blood |
| Anthropometry | Height, weight, waist and hip circumference |
| Participant break | Refreshment break with food provided |
| Cardiovascular | Carotid artery sonography, 12-lead electrocardiogram, and vascular profiling (blood pressures and pulse wave velocity) |
| Respiratory | Obstructive spirometry |
| Skeleton bone | Density examination |
| Gynecology (Female) | Gynecologic examination, pap smear, ultrasound, pelvic |
| Cognitive impairment | Mini-Mental State Examination |
| Dementia | Mini-Mental State Examination, clinical diagnosis |
The MMSE
MMSE scores were evaluated annually. The MMSE was a global cognitive screening instrument that measures cognitive functions, including orientation, attention, calculation, recall, language processing, and constructional praxis (Albanna et al., 2017; Sokołowska et al., 2018). Cognitive impairment was evaluated using the Chinese version of the MMSE, which had performed well in clinical studies in China (Li et al., 2018; Sun et al., 2019). The MMSE contained 30 questions, each of which is scored as 0 or 1. The total MMSE score thus ranged from 0 to 30 points. Participants with an MMSE score of ≥ 24 were considered as having a normal cognitive function. MMSE scores of 20–23 indicated MCI, MMSE scores of 10–20 indicated moderate cognitive impairment, and < 10 points indicated dementia (Patel et al., 2003; Gong et al., 2018).
Physical examination
A physical examination was performed annually. Weight, height, waist circumference, and hip circumference were measured. Blood pressure was determined to the nearest 2 mmHg using a mercury sphygmomanometer. Three systolic blood pressure and diastolic blood pressure readings were collected to calculate the average as the final blood pressure. Blood samples of participants were collected from the antecubital vein in the morning under fasting conditions. They were stored in vacuum tubes containing ethylenediaminetetraacetic acid and coagulation tubes. The hematological tests were conducted on fresh samples at the Central Laboratory of Kailuan General Hospital and Jidong Oil-Field Hospital, China. The fasting blood glucose was measured using the hexokinase/glucose-6-phosphate dehydrogenase method and cholesterol and triglyceride concentrations were determined by enzymatic methods (Mind Bioengineering Co., Ltd., Shanghai, China). The levels of biochemical markers, including cholesterol, blood glucose, triglyceride, insulin, high-density lipoproteins, low-density lipoproteins, alanine aminotransferase, and creatinine, were measured using an auto-analyzer (Hitachi, Tokyo, Japan). Finally, the blood samples were processed and separated on site and stored in a biospecimen bank at –80°C.
Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg. Diabetes was defined as a fasting glucose concentration ≥ 1260 mg/L or a non-fasting glucose concentration ≥ 1998 mg/L. Dyslipidemia was defined as a serum triglyceride ≥ 1500 mg/L, low-density lipoprotein cholesterol ≥ 1300 mg/L, or high-density lipoprotein cholesterol ≤ 400 mg/L. Any use of current medication or self-reported disease history was also used for the medical diagnoses.
Assessment of carotid artery stenosis
All participants underwent a bilateral carotid duplex ultrasound (EME Companion, Nicolet, Madison, WI, USA), which is the standard diagnostic examination for carotid stenosis. Participants with carotid stenosis (> 50%) were graded into the following strata according to the recommendations of radiologists and the results: 50–69% stenosis, ≥ 70% stenosis but less than near occlusion (Grant et al., 2003). The participants with severe carotid artery stenosis were advised to undergo further examinations such as CT, MRI, and angiography examinations. The bilateral carotid duplex ultrasound was evaluated annually.
Assessment of follow-up and outcomes
Procedure of follow-up
The duration of the follow-up will be 10 years and the study participants will be followed up via face-to-face interviews once every year in a routine medical examination until December 31, 2024, or until the occurrence of an outcome event as defined in this study. Interviewers will continue to collect data on cognitive impairment, demography, lifestyle, history of disease and basic physical symptoms, blood samples, anthropometry, dementia, and occurrence of other diseases such as cardiovascular diseases. Questionnaires were collected by trained researchers. Physical and clinical examinations were carried out by designated hospital professionals. Questionnaires and clinical physical examinations will be performed according to the same criteria as the baseline survey. Data on clinical outcomes will be extracted using a standard operational procedure follow-up system, including hospitalization reports and files from local general practitioners and medical specialists. The following data on chronic diseases will also be collected: hypertension, hyperlipidemia, diabetes, ischemic stroke, other neurodegenerative diseases, suboptimal health, chronic hepatitis, chronic kidney disease, and cancer.
Measures and outcomes
The primary outcome of this prospective observational cohort study was dementia, including senile dementia, vascular dementia, mixed dementia, and other types of dementia during a 10-year follow up. The every-five-year incidence of cognitive impairment will be recorded. Dementia will be diagnosed according to the criteria of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fourth edition (American Psychiatric Association (1994) DSM-IV Criteria) and the International Working Group (Winblad et al., 2004) criteria. All dementia records will be reviewed by certified neurologists and neuropsychologists. If no consensus on diagnosis is reached, the third higher-level expert will review the unclear diagnosis. The final diagnosis will be based on the clinical judgment of the consensus group.
The secondary outcome was the decline in quality of life during the 10-year follow up. The decline in quality of life was assessed using Quality of Life (QoL). QoL developed through beliefs, experiences, and expectations, which were subjective phenomena (Bubien et al., 1996; Testa et al., 1996). QoL included multiple dimensions such as individual perception, experiences, beliefs and expectations (Reynolds et al., 2008).
The MMSE score is used to assess cognitive functioning and the occurrence of relevant diseases. The MMSE score is influenced by age and years of education; participants with higher educational levels perform better than those with lower levels, and cognitive abilities decline with age (Carpinelli Mazzi et al., 2019), which leads to a decline in quality of life.
Quality control
All investigators and clinicians were trained in all items including the questionnaires and all aspects of measurements about laboratory, clinical, and environmental exposure measurements (using standardized techniques). Training was conducted within the laboratories of each participating investigator under the supervision of experienced staff, until the required standard of testing and competency was achieved. During the follow-up, all items will not only be randomly sampled, but also regularly monitored centrally to assess the quality of items, the time delay of blood processing, and the accuracy of data. In addition, the staff of the third party (Rehabilitation Medical Technology Development Company) will also monitor the quality control of the follow-up work.
Statistical analysis
All the data were examined to ensure the accuracy of data and analyzed using SAS software (version 9.1; SAS Institute, Cary, NC, USA). First, descriptive statistical methods were used to reveal the distribution of baseline characteristics, including demographic variables, lifestyle, cognitive function, and risk factors. Continuous variables are expressed as the mean ± SD. Categorical variables are expressed as counts (percentages). Second, chi-square tests were used to compare categorical variables between groups. The significance level (two-sided) was α = 0.05. Changes from baseline to yearly follow-up in prognosis risk factors of cognitive impairment, sociodemographic factors, and dementia was measured. Relationships between variables of interest will be investigated using relevant statistical models. We will apply survival analysis, logistic regression, linear regression to measure the changes from baseline to yearly follow-up in risk factors, the primary or secondary outcomes, and sociodemographic factors. These analyses will focus on the factors that influence cognitive decline and the incidence of dementia.
Results
Based on the inclusion and exclusion criteria, 5854 participants were included into Jidong cognitive impairment cohort study (Figure 1). The mean age of the participants was 57.4 ± 9.5 years and 55.1% of participants were male. Baseline characteristics of participants are summarized in Table 2. Among the study population, 39.2% of the participants completed junior high school, 67.0% never smoked, and 69.4% never drank alcohol.
Figure 1.
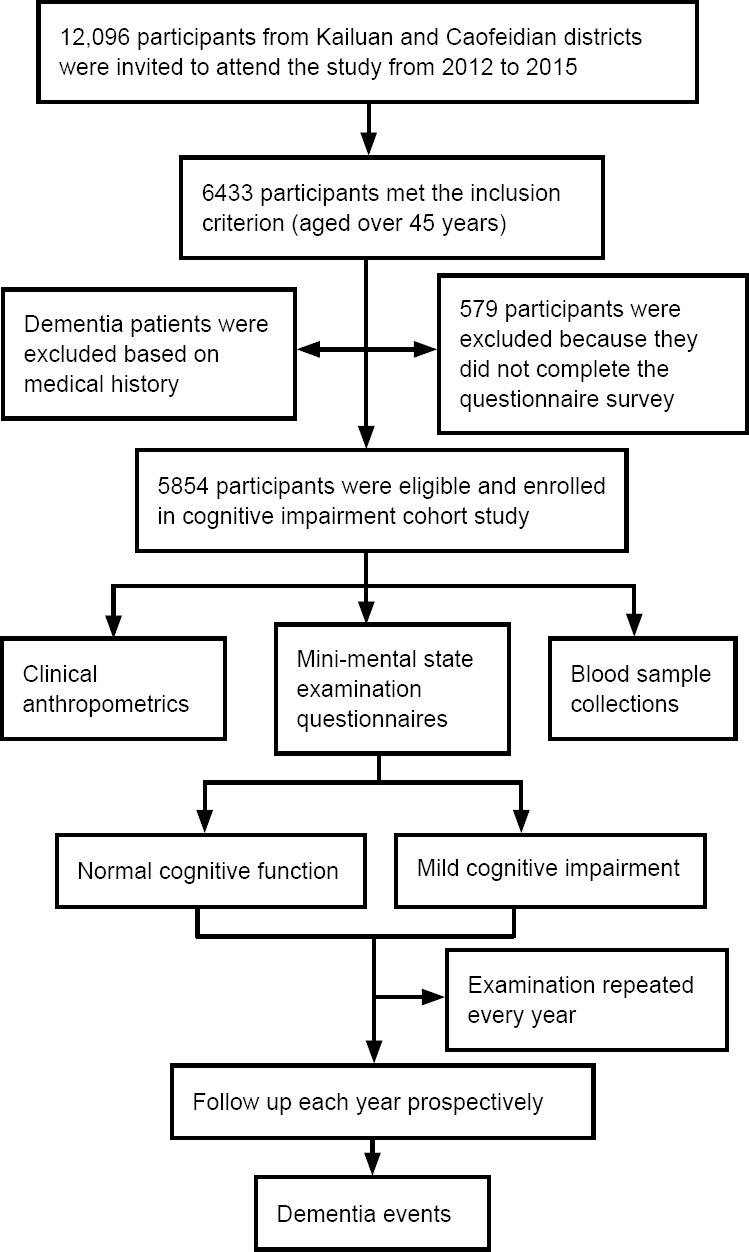
Flow chart of the Jidong cognitive impairment cohort study.
Table 2.
Baseline characteristics of participants in Jidong cognitive impairment cohort study
| Characteristics | Total | Male | Female | P* |
|---|---|---|---|---|
| n | 5854 | 3223 | 2631 | |
| Age (yr) | 57.4±9.5 | 58.4±10.1 | 56.1±8.6 | < 0.001 |
| Education | < 0.001 | |||
| Illiteracy | 509 (8.7) | 236 (7.3) | 273 (10.4) | |
| Primary school | 664 (11.3) | 364 (11.3) | 300 (11.4) | |
| Junior high school | 2297 (39.2) | 1348 (41.8) | 949 (36.1) | |
| High school | 1221 (20.9) | 579 (18.0) | 642 (24.4) | |
| University and above | 1163 (19.8) | 696 (21.6) | 467 (17.8) | |
| Previous history of disease | ||||
| Arterial hypertension | 603 (10.3) | 419 (13.0) | 184 (7.0) | < 0.001 |
| Diabetes | 175 (3.0) | 106 (3.2) | 69 (2.6) | 0.136 |
| Dyslipidemia | 435 (7.4) | 258 (8.0) | 177 (6.7) | 0.064 |
| Smoking | < 0.001 | |||
| Never | 3923 (67.0) | 1391 (43.1) | 2532 (96.2) | |
| Current | 1598 (27.3) | 1508 (46.8) | 90 (3.4) | |
| Former (quit smoking for less than 12 months) | 91 (1.6) | 87(2.7) | 4 (0.2) | |
| Former (quit smoking for more than 12 months) | 242 (4.1) | 237 (7.4) | 5 (0.2) | |
| Passive smoking | 1158 (19.8) | 540 (16.8) | 618 (23.5) | < 0.001 |
| Alcohol consumption | < 0.001 | |||
| Never | 4065 (69.4) | 1570 (48.7) | 2495 (94.8) | |
| Less than two standard drinks | 1211 (20.7) | 1099 (34.1) | 112 (4.3) | |
| Equal or more than two standard drinks | 472 (8.1) | 463 (14.4) | 9 (0.3) | |
| Drinking, but not quantities | 106 (1.8) | 91 (2.8) | 15 (0.6) |
Data are expressed as the n (%). *The results were analyzed by the chi-square test. For ranked data, Wilcoxon rank sum test was used for two groups of data and Kruskal-Wallis test was used for multiple groups of data.
Table 3 shows the distributions of factors about cognitive impairment among participants with or without cognitive impairment. The prevalence of MCI in the study population was 5.5%. The age, educational level, drinking, passive smoking, and blood pressure differed significantly between the cognitive impairment and normal groups (P < 0.05). The prevalence of cognitive impairment significantly increased with age. Participants with a lower educational level had a higher prevalence of cognitive impairment. There were no significant between-group differences in sex, diabetes mellitus, dyslipidemia, or smoking.
Table 3.
Factor distribution in participants with or without cognitive impairment
| Characteristics | Total | Cognitive impairment | Non-cognitive impairment | P* |
|---|---|---|---|---|
| n | 5854 | 320 | 5534 | |
| Age (yr) | < 0.001 | |||
| 45–55 | 2641 | 60 (2.3) | 2581 (97.7) | |
| 55–65 | 2025 | 117 (5.8) | 1908 (94.2) | |
| 65–75 | 817 | 67 (8.2) | 750 (91.8) | |
| > 75 | 371 | 76 (20.5) | 295 (79.5) | |
| Sex | 0.196 | |||
| Male | 3223 | 165 (5.1) | 3058 (94.9) | |
| Female | 2631 | 155 (5.9) | 2476 (94.1) | |
| Education | < 0.001 | |||
| Illiteracy | 273 | 98 (35.9) | 175 (64.1) | |
| Primary school | 324 | 55 (17.0) | 269 (83.0) | |
| Junior high school | 2397 | 134 (5.6) | 2263 (94.4) | |
| High school | 1521 | 23 (1.5) | 1498 (98.5) | |
| University and above | 1339 | 10 (0.7) | 1329 (99.3) | |
| Previous history of disease | ||||
| Arterial hypertension | 603 | 50 (8.3) | 553 (91.7) | 0.001 |
| Diabetes mellitus | 175 | 15 (8.6) | 160 (91.4) | 0.067 |
| Dyslipidemia | 435 | 27 (6.2) | 408 (93.8) | 0.480 |
| Smoking | 0.077 | |||
| Never | 3923 | 222 (5.7) | 3701 (94.3) | |
| Current | 1598 | 73 (4.6) | 1525 (95.4) | |
| Former (quit smoking for less than 12 months) | 91 | 9 (9.9) | 82 (90.1) | |
| Former (quit smoking for more than 12 months) | 242 | 16 (6.6) | 226 (93.4) | |
| Passive smoking | 1122 | 41 (3.7) | 1081 (96.3) | < 0.001 |
| Alcohol consumption | < 0.001 | |||
| Never | 4065 | 249 (6.1) | 3816 (93.9) | |
| Less than two standard drinks | 1211 | 46 (3.8) | 1165 (96.2) | |
| Equal or more than two standard drinks | 472 | 22 (4.7) | 450 (95.3) | |
| Drinking, but not quantities | 106 | 3 (2.8) | 103 (97.2) |
Data are expressed as the n (%). *The results were analyzed by the chi-square test. For ranked data, Wilcoxon rank sum test was used for two groups of data and Kruskal-Wallis test was used for multiple groups of data.
The multivariate logistic regression model showed that age (OR = 1.059, 95% CI = 1.044–1.074) and arterial hypertension (OR = 1.665, 95% CI = 1.143–2.427) were risk factors for MCI. Taking the ‘illiteracy’ educational level as a reference, odds ratio values of primary school (OR = 0.425, 95% CI = 0.254–0.711), junior middle school (OR = 0.152, 95% CI = 0.096–0.241), high school (OR = 0.057, 95% CI = 0.032–0.103), university and above (OR = 0.029, 95% CI = 0.014–0.062) reduced significantly, which indicates that with the increase in educational level, cognitive impairment gradually decreased (OR < 1, P < 0.05) (Table 4).
Table 4.
Relationship between cognitive impairment and risk factors
| Variable | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age* | 0.057 | 0.007 | 63.764 | < 0.001 | 1.059 | 1.044–1.074 |
| Educational level* | ||||||
| Illiteracy | Reference | |||||
| Primary school | –0.856 | 0.263 | 10.614 | 0.0011 | 0.425 | 0.254–0.711 |
| Junior high school | –1.886 | 0.235 | 64.317 | < 0.001 | 0.152 | 0.096–0.241 |
| High school | –2.863 | 0.302 | 89.747 | < 0.001 | 0.057 | 0.032–0.103 |
| University and above | –3.531 | 0.386 | 83.616 | < 0.001 | 0.029 | 0.014–0.062 |
| Arterial hypertension* | 0.51 | 0.192 | 7.052 | 0.008 | 1.665 | 1.143–2.427 |
| Passive smoking* | 0.089 | 0.192 | 0.212 | 0.645 | 1.093 | 0.750–1.593 |
| Alcohol consumption* | ||||||
| Never | Reference | |||||
| Less than two standard drinks | –0.513 | 0.187 | 7.525 | 0.006 | 0.599 | 0.415–0.864 |
| Equal or more than two standard drinks | 0.505 | 0.276 | 3.33 | 0.068 | 1.656 | 0.963–2.847 |
| Drinking, but not quantities | 0.099 | 0.459 | 0.047 | 0.828 | 1.105 | 0.449–2.717 |
*: Screening out meaningful single-factor indicators (P < 0.05). SE: Standard error; Wald: chi-square value; OR: odds ratio; CI: confidence interval.
Discussion
In 2012, the World Health Organization reported that dementia had become global public health issues (Cahill S, 2019). In China, approximately 30% of subjects over the age of 80 years suffer from dementia (Rocca et al., 1991; White et al., 1996). The previous population-based studies examined the rate of conversion of dementia among persons with MCI, and the rate of conversion from MCI to dementia was much higher than the normal cognitive population (Bozoki et al., 2001; Morris et al., 2001). Bennett et al. (2002) followed up 211 patients with MCI for 4–5 years and found that the rate of conversion from MCI to Alzheimer’s disease was 7.5% every year, which was 3.1 times higher than the normal cognitive population. Petersen et al. (1999) found that the rate of conversion from MCI to Alzheimer’s disease (10–12% every year) was 10 times higher than the normal cognitive population. Owing to methodological differences including different definitions and different scales, the annual conversion rates of dementia varied from 10% to 20% (Petersen et al., 2001). Little research has been conducted in China. Because the living habits and environment of people in each region will have different impact, it is of great significance to investigate cognitive impairment in China. The Jidong cognitive impairment cohort study is an exclusive cohort of patients with cognitive impairment in China. This study will attempt to explore the risk factors of MCI transition to Alzheimer’s disease for the early prevention of dementia.
At baseline, we found that age, drinking, passive smoking, and blood pressure were significantly associated with cognitive impairment. Women had a higher MCI rate than men, which indicates that women have a higher risk of developing dementia, whereas the differences in smoking, diabetes, and hyperlipidemia were not significant between the cognitive impairment and normal groups. The multivariate logistic regression models revealed that age and arterial hypertension were risk factors for MCI. With an increase in educational level, cognitive impairment decreased gradually. However, the participants in this study tended to be younger. In patients with cognitive impairment, there was no significant difference between patients with diabetes and hyperlipidemia in our study, because the awareness of illness in diabetes and hyperlipidemia patients is low. This study was not found the correlation between smoking and cognitive impairment, which needs to be further validated by a follow up. Previous work has found that cognitive impairment is significantly correlated with stroke and coronary heart disease (Lu et al., 2016; Petrova et al., 2018). Ballard et al. (2003) found that more than 30% of patients with stroke developed cognitive impairment within 3–15 months, and 9% of them developed dementia.
After years of follow-up, further longitudinal studies will provide evidence to reveal the prognostic factors of MCI. The Jidong cognitive impairment cohort study will characterize cognitive impairment and accurately estimate the incidence of dementia. This study contributes to the comprehensive assessment of objective health conditions as well as lifestyle and environmental factors. Our results support some previous studies reporting an association of age and hypertension with heart disease and cognitive impairment (Tervo et al., 2004; Iadecola et al., 2016; Stewart et al., 2019). Aging and hypertension were significant and independent risk factors for the conversion to MCI. MCI is associated with cardiovascular risk factors and contributes to the development of cardiovascular diseases (Cherbuin et al., 2009; Solomon et al., 2009). In contrast, a higher level of education was a protective factor for the conversion to dementia. A lower educational level was associated with higher chances of cognitive impairment. However, the blood samples were not measured at baseline, but we have collected them annually since 2015.
Compared with other cognitive impairment cohorts, the design and study population of these studies are presented in Table 5 (Vannier-Nitenberg et al., 2013; van Rijsbergen et al., 2013; Arntz et al., 2014; Palmer et al., 2015; van Rooij et al., 2015; Mauthner et al., 2016; Hooghiemstra et al., 2017; Soares et al., 2017; Aben et al., 2018; Song et al., 2019). Compared with previous studies, the sample size of the present study is larger, and the follow-up time will be longer. The middle-aged and elderly people over 45 years of age were selected, which is conducive to the continuation of follow-up. In this study, the diagnostic tool was the MMSE scale. From the Table 5, we can see that each study used different diagnostic tools. The MMSE scale can be used as a direct diagnostic tool. Some studies have also combined other scales to diagnose dementia, such as the Rey Auditory Language Learning Test and the Cognitive Failure Questionnaire. Furthermore, the diagnostic criteria between these different tools are different. According to the MMSE score, some studies consider the healthy group to be those with an MMSE score > 26, while in this study, the healthy group was considered to be those with an MMSE score ≥ 24. The outcome was also different between studies, and included dementia, stroke, cardiovascular events, and cognitive impairment. In addition, the diagnostic tools in this study need to be further improved, and the combination of other diagnostic tools can better assess cognitive impairment. During the follow-up in 2019, we used a combination of MMSE and Montreal Cognitive Assessment (MoCA).
Table 5.
General characteristics of studies on cognitive impairment cohort study
| Author | Sample size (n) | Age (yr) | Diagnostic tools | Outcome | Follow-up | Cohort location |
|---|---|---|---|---|---|---|
| van Rijsbergen et al. (2013) | 300 | Over 18 | Cognitive failures questionnaire | Stroke | 2009/2014 | Netherlands |
| Vannier-Nitenberg et al. (2013) | 620 | Over 65 | MMSE | Mild cognitive impairment and dementia | 2009/2013 | France |
| Arntz et al. (2014) | 1500 | 18–49 | Wechsler adult intelligence scale - fourth edition/Cognitive failure questionnaire | Death | USA | |
| Palmer et al. (2015) | 750 | Over 18 | MMSE/Rey auditory verbal learning test | Cardiovascular events | 2013-07/ 2014-04 | Italy |
| van Rooij et al. (2015) | 150 | Over 45 | Cognitive failures questionnaire | Cognitive impairment | Netherlands | |
| Mauthner et al. (2016) | 250 | Over 20 | Montreal cognitive assessment | Cognitive impairment | 2015-01/ 2020-06 | Swiss |
| Hooghiemstra et al. (2017) | 645 | Over 50 | MMSE | Vascular cognitive impairment | 2014/2019 | Dutch |
| Soares et al. (2017) | 1125 | Over 60 years | MMSE | Cognitive impairment | 2004/2011 | Brazil |
| Aben et al. (2018) | 350 | Over 50 | Montreal cognitive assessment | Cognitive recovery | 2017-01/ 2019-06 | Netherlands |
| Song et al. (2019) | 5854 | Over 45 | MMSE | Dementia | 2015/2024 | China |
MMSE: Mini-Mental State Examination.
Previous work has also reported some diseases to be associated with cognitive impairment, including Alzheimer’s disease, delirium, neurocognitive impairment, renal hemodialysis, acute ischemic stroke, transient cerebral ischemia, and type 2 diabetes. The advantages of our study are as follows: (1) we recruited participants over 45 years or older as study population. This could help to screen for cognitive impairment from an earlier time point. (2) Bilateral carotid duplex ultrasound was used to detect the degree of carotid artery stenosis. (3) Our questionnaires were administered face to face after investigators had received strict training, and follow-up surveys will be conducted every year. (4) We will collect blood samples every year to investigated the biomarkers associated with dementia.
The present study has some limitations. First, examinations of cognitive impairment should be carried out in the field involving cognition comprehensively such as orientation, recall, attention, calculation, language processing and constructional praxis. Different neuropsychological scales should be used to assess cognition. Additionally, the study sample was from Kailuan and Caofeidian communities, Tangshan city, in northern China, which might not represent the entire Chinese population. Moreover, the questionnaire might lead to recall bias. Nevertheless, we can reduce the recall bias by comparing and analyzing the results of the baseline survey and clinical examination.
This report elaborated the theoretical objectives, design, and baseline characteristics of Jidong cognitive impairment cohort study. This study was aimed to survey the baseline of Jidong cognitive impairment cohort study, especially the associations between prognostic factors of cognitive impairment and dementia in China, which was expected to provide evidence for the intervention of dementia in the future.
Additional file: Open peer review report 1 (110.1KB, pdf) .
Acknowledgments
We are grateful to the Kailuan and Caofeidian communities, Tangshan City, Hebei Province, China for its collaboration, especially the dedicated participants and all research staff involved in the study. We also thank Ruike Donghua Translational Medical Research Center Co., Ltd. for supporting the project.
Footnotes
Conflicts of interest: The authors confirm that there are no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 91749205 (to YZ), 81973112 (to YZ), 81973138 (to DL), 81903401 (to WJX); the Young Taishan Scholars Program of Shandong Province of China, No. tsqn20161046 (to WJX). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study was performed according to the guideline of the Declaration of Helsinki. Approvals were obtained from Ethics Committee of Kailuan General Hospital of Tangshan City and the Medical Ethics Committee, Staff Hospital, Jidong Oilfield Branch, China National Petroleum Corporation on July 12, 2013 (approval No. 2013 YILUNZI 1). These approvals will be renewed every 5 years. Written informed consent was obtained from each participant. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the STrengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Ruike Donghua Translational Medical Research Center Co., Ltd., China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Willian Orlando Castillo, Universidade de Sao Paulo, Brazil.
Funding: This study was supported by the National Natural Science Foundation of China, No. 91749205 (to YZ), 81973112 (to YZ), 81973138 (to DL), 81903401 (to WJX); the Young Taishan Scholars Program of Shandong Province of China, No. tsqn20161046 (to WJX).
P-Reviewer: Castillo WO; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Cason C, Stow A, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Aben HP, Reijmer YD, Visser-Meily JM, Spikman JM, de Bresser J, Biessels GJ, de Kort PLM. A role for new brain magnetic resonance imaging modalities in daily clinical practice protocol of the prediction of cognitive recovery after stroke (PROCRAS) Study. JMIR Res Protoc. 2018;7:e127. doi: 10.2196/resprot.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanna M, Yehya A, Khairi A, Dafeeah E, Elhadi A, Rezgui L, Kahlout SA, Yousif A, Uthman B, Al-Amin H. Validation and cultural adaptation of the arabic versions of the mini-mental status examination-2 and mini-cog test. Neuropsychiatr Dis Treat. 2017;13:793–801. doi: 10.2147/NDT.S126825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. DSM-IV Criteria. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC, USA: American Psychiatric Association; 1994. [Google Scholar]
- 4.Arntz RM, van Alebeek ME, Synhaeve NE, Brouwers PJ, van Dijk GW, Gons RA, den Heijer T, de Kort PLM, de Laat KF, van Norden AG, Vermeer SE, van der Vlugt MJ, Kessels RPC, van Dijk EJ, de Leeuw FE. Observational Dutch Young Symptomatic StrokE studY (ODYSSEY): study rationale and protocol of a multicentre prospective cohort study. BMC Neurol. 2014;14:55. doi: 10.1186/1471-2377-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard C, Rowan E, Stephens S, Kalaria R, Kenny RA. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke. 2003;34:2440–2444. doi: 10.1161/01.STR.0000089923.29724.CE. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 8.Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation. 1996;94:1585–1591. doi: 10.1161/01.cir.94.7.1585. [DOI] [PubMed] [Google Scholar]
- 9.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. Br J Psychiatry. 2006;189:399–404. doi: 10.1192/bjp.bp.105.014779. [DOI] [PubMed] [Google Scholar]
- 10.Cahill S. WHO’s global action plan on the public health response to dementia: some challenges and opportunities. Aging Ment Health. 2019;2:1–3. doi: 10.1080/13607863.2018.1544213. [DOI] [PubMed] [Google Scholar]
- 11.Carpinelli Mazzi M, Iavarone A, Russo G, Musella C, Milan G, D’Anna F, Garofalo E, Chieffi S, Sannino M M, Illario M, De Luca V, Postiglione A, Abete P. Mini-Mental State Examination: new normative values on subjects in Southern Italy. Aging Clin Exp Res. 2019 doi: 10.1007/s40520-019-01250-2. doi:101007/s40520-019-01250-2. [DOI] [PubMed] [Google Scholar]
- 12.Chen YK, Xiao WM, Li W, Ni ZX, Liu YL, Xu L, Qu JF, Ng CH, Xiang YT. Microbleeds in fronto-subcortical circuits are predictive of dementia conversion in patients with vascular cognitive impairment but no dementia. Neural Regen Res. 2018;13:1913–1918. doi: 10.4103/1673-5374.239441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherbuin N, Reglade-Meslin C, Kumar R, Jacomb P, Easteal S, Christensen H, Sachdev P, Anstey KJ. Risk factors of transition from normal cognition to mild cognitive disorder: the PATH through Life Study. Dement Geriatr Cogn Disord. 2009;28:47–55. doi: 10.1159/000229025. [DOI] [PubMed] [Google Scholar]
- 14.Das BC, Dasgupta S, Ray SK. Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer’s disease. Neural Regen Res. 2019;14:1880–1892. doi: 10.4103/1673-5374.259604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester SN, Gallo JJ, Smith GS, Leoutsakos JS. Patterns of neuropsychiatric symptoms in mild cognitive impairment and risk of dementia. Am J Geriatr Psychiatry. 2016;24:117–125. doi: 10.1016/j.jagp.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Z, Li J, Zhong Y, Guan X, Huang A, Ma L. Effects of dexmedetomidine on postoperative cognitive function in patients undergoing coronary artery bypass grafting. Exp Ther Med. 2018;16:4685–4689. doi: 10.3892/etm.2018.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katanick S, Needleman L, Pellerito J, Polak JF, Rholl KS, Wooster DL, Zierler E. Carotid artery stenosis: gray-scale and doppler US diagnosis-society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 18.Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR, 3rd, Canonico A, Merkle K, Cannistraci CJ, Rogers BP, Gatenby JC, Heckers S, Gore JC, Hopkins RO, Ely EW. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooghiemstra AM, Bertens AS, Leeuwis AE, Bron EE, Bots ML, Rocca HB, de Craen AJM, van der Geest RJ, Greving JP, Kappelle LJ, Niessen WJ, van Oostenbrugge RJ, van Osch MJP, de Roos A, van Rossum AC, Biessels GJ, van Buchem MA, Daemen MJAP, van der Flier WM. The missing link in the pathophysiology of vascular cognitive impairment: design of the heart-brain study. Cerebrovasc Dis Extra. 2017;7:140–152. doi: 10.1159/000480738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A. Impact of hypertension on cognitive function: a scientific statement from the American heart association. Hypertension. 2016;68:e67–94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ijaopo EO. Dementia-related agitation: a review of non-pharmacological interventions and analysis of risks and benefits of pharmacotherapy. Transl Psychiatry. 2017;7:e1250. doi: 10.1038/tp.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, Aguera-Ortiz L, Sweet R, Miller D, Lyketsos CG. Neuropsychiatric symptoms as earlymanifes tations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Yang C, Wu S S, Nie Y, Zhang X, Lu M, Chu T, Shi F. Alterations of graphic properties and related cognitive functioning changes in mild Alzheimer’s disease revealed by individual morphological brain network. Front Neurosci. 2018;12:927. doi: 10.3389/fnins.2018.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Yuan J, Yang L, Qin W, Yang S, Li Y, Fan H, Hu W. The significant effects of cerebral microbleeds on cognitive impairment: An updated meta-analysis. PLoS One. 2017;12:e0185145. doi: 10.1371/journal.pone.0185145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CS. Revisiting the link between cognitive decline and masticatory impairment. BMC Geriat. 2018;18:5. doi: 10.1186/s12877-017-0693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu DQ, Li RM, Zhang MQ, Chen YY, Zhang HP. Meta-analysis of the effect of aerobic exercise on mild cognitive impairment in the elderly. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:5727–5731. [Google Scholar]
- 27.Lu D, Ren S, Zhang J, Sun D. Vascular risk factors aggravate cognitive impairment in first-ever young ischaemic stroke patients. Eur J Neurol. 2016;23:940–947. doi: 10.1111/ene.12967. [DOI] [PubMed] [Google Scholar]
- 28.Maiese K. Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural Regen Res. 2019;14:773–774. doi: 10.4103/1673-5374.249224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzano Palomo MS, Anaya Caravaca B, Balsa Bretón MA, Castrillo SM, Vicente AM, Castro Arce E, Alves Prez MT. Mild cognitive impairment with a high risk of progression to Alzheimer’s disease dementia (MCI-HR-AD): effect of souvenaid® treatment on cognition and 18F-FDG PET scans. Alzheimers Dis Rep. 2019;3:95–102. doi: 10.3233/ADR-190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauthner O, Claes V, Jeremy Walston J, Sandra Engberg S, BinetI I, Michael DickenmannI M, Golshayan D, Hadaya K, Huynh-Do UYEN, Calciolari S, Geest SD. Exploring frailty and mild cognitive impairment in kidney transplantation to predict biomedical, psychosocial, and health cost outcomes (GERAS): Protocol of a nationwide prospective cohort study. J Adv Nurs. 2016;73:716–734. doi: 10.1111/jan.13179. [DOI] [PubMed] [Google Scholar]
- 31.Mondragón JD, Maurits NM. Functional neural correlates of anosognosia in mild cognitive impairment and alzheimer’s disease: a systematic review. Neuropsychol Rev. 2019;29:139–165. doi: 10.1007/s11065-019-09410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morandi A, Rogers BP, Gunther ML, Merkle K, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR, 3rd, Canonico A, Cannistraci CJ, Gore JC, Ely EW, Hopkins RO. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer’s disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 34.Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, van Duijn CM, Breteler MM. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the rotterdam study. Lancet. 1998;351:1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 35.Palmer SC, Ruospo M, Barulli MR, Iurillo A, Saglimbene V, Natale P, Gargano L, Murgo AM, Loy C, van Zwieten A, Wong G, Tortelli R, Craig JC, Johnson DW, Tonelli M, Hegbrant J, Wollheim C, Logroscino G, Strippoli GFM. COGNITIVE-HD study: protocol of an observational study of neurocognitive functioning and association with clinical outcomes in adults with end-stage kidney disease treated with haemodialysis. BMJ Open. 2015;5:e009328. doi: 10.1136/bmjopen-2015-009328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel M, Coshall C, Rudd AG, Wolfe CD. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clin Rehabil. 2003;17:158–166. doi: 10.1191/0269215503cr596oa. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the american academy of neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 39.Petrova MM, Shprakh VV, Kaskaeva DS, Eremina OV, Narkevich AN, Eremina SS. Prognostic methods of postoperative cognitive dysfunction in patients with ischemic heart disease after coronary bypass surgery under extracorporeal circulation. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118:81–86. doi: 10.17116/jnevro201811812281. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds MR, Ellis E. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19:762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocca WA, Hofman A, Brayne C, Breteler MM, Clarke M, Copeland JR, Dartigues F, Engedal K, Hagnell O, Heeren TJ, Jonker C, Lindesay J, Lobo A, Mann AH. The prevalence of vascular dementia in Europe: facts and fragments from 1980-1990 studies. EURODEM-Prevalence Research Group. Ann Neurol. 1991;30:817–824. doi: 10.1002/ana.410300611. [DOI] [PubMed] [Google Scholar]
- 42.Rosato M, Leavey G, Cooper J, De Cock P, Devine P. Factors associated with public knowledge of and attitudes to dementia: a cross-sectional study. PLoS One. 2019;14:e0210543. doi: 10.1371/journal.pone.0210543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soares WB, dos Santos EB, Bottino CMdC, Elkis H. Psychotic symptoms in older people without dementia from a Brazilian community-based sample: a seven years’ follow-up. PLoS One. 2017;12:e0178471. doi: 10.1371/journal.pone.0178471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokołowska N, Sokołowski R, Polak-Szabela A, Mazur E, Podhorecka M, Kędziora-Kornatowska K. Comparison of the effectiveness of the Montreal Cognitive Assessment 7, 2 and the Mini-Mental State Examination in the detection of mild neurocognitive disorder in people over 60 years of age. Preliminary study. Psychiatr Pol. 2018;52:843–857. doi: 10.12740/PP/68611. [DOI] [PubMed] [Google Scholar]
- 45.Solomon A, Kåreholt I, Ngandu T, Wolozin B, Macdonald SW, Winblad B, et al. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol Aging. 2009;30:1006–1009. doi: 10.1016/j.neurobiolaging.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Stewart RAH, Held C, Krug-Gourley S, Waterworth D, Stebbins A, 7, Chiswell K, Hagstrom E E, Armstrong PW, Wallentin L, White H. Cardiovascular and lifestyle risk factors and cognitive function in patients with stable coronary heart disease. J Am Heart Assoc. 2019;8:e010641. doi: 10.1161/JAHA.118.010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun D, Sun X, Xu Y, Wu T, Tao L. Superoxide dismutase activity and risk of cognitive decline in older adults: findings from the Chinese longitudinal healthy longevity survey. Exp Gerontol. 2019;118:72–77. doi: 10.1016/j.exger.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Tanokashira D, Fukuokaya W, Taguchi A. Involvement of insulin receptor substrates in cognitive impairment and Alzheimer’s disease. Neural Regen Res. 2019;14:1330–1334. doi: 10.4103/1673-5374.253535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tervo S, Kivipelto M, Hanninen T, Vanhanen M, Hallikainen M, Mannermaa A, Soininen H. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 50.Testa MA, Simonson DC. Assesment of quality-of-life outcomes. N Engl J Med. 1996;334:835–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 51.Thingstad P, Askim T, Beyer MK, Bråthen G, Ellekjær H, Ihle-Hansen H, Knapskog AB, Lydersen S, Munthe-Kaas R, Næss H, Pendlebury ST, Seljeseth YM, Saltvedt I. The Norwegian Cognitive impairment after stroke study (Nor-COAST): study protocol of a multicentre, prospective cohort study. BMC Neurol. 2018;18:193. doi: 10.1186/s12883-018-1198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Rijsbergen MW, Mark RE, de Kort PL, Sitskoorn MM. The COMPlaints After Stroke (COMPAS) study: protocol for a Dutch cohort study on poststroke subjective cognitive complaints. BMJ Open. 2013;3:e003599. doi: 10.1136/bmjopen-2013-003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Rooij FG, Tuladhar AM, Kessels RP, Vermeer SE, Góraj BM, Koudstaal PJ, Norris DG, de Leeuw FE, van Dijk EJ. Cohort study ON Neuroimaging, Etiology and Cognitive consequences of Transient neurological attacks (CONNECT): study rationale and protocol. BMC Neurol. 2015;15:36. doi: 10.1186/s12883-015-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vannier-Nitenberg C, Dauphinot V, Bongue B, Sass C, Rouch I, Beauchet O, Krolak-Salmon P, Fantino B. Early detection of memory impairment in people over 65 years old consulting at Health Examination Centers for the French health insurance: the EVATEM protocol. BMC Geriatr. 2013;13:55. doi: 10.1186/1471-2318-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Wang F, Liu J, Zhang Q, Lei P. Inverse relationship between baseline serum albumin levels and risk of mild cognitive impairment in elderly: a seven-year retrospective cohort study. Tohoku J Exp Med. 2018;246:51–57. doi: 10.1620/tjem.246.51. [DOI] [PubMed] [Google Scholar]
- 56.White AT, Merino RB, Hardin S, Kim S. Non-invasive, cost-effective, early diagnosis of mild cognitive impairment in an outpatient setting: pilot study. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:13–16. doi: 10.1109/EMBC.2018.8512268. [DOI] [PubMed] [Google Scholar]
- 57.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb D. Prevalence of dementia in older Japanese-American men in Hawaii-The Honolulu-Asia aging study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 58.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Ba¨ ckman L, Alber M, Almkvist O, Arai H, Basun H, Blennow K, Deleo M, Decarl C, Erkinjuntt C, Giacobini E, Graff C, Hardy J, Jack C, et al. Mild cognitive impairment-beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 59.World Medical Association. World medical association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


