Abstract
Stroke persists as a global health and economic crisis, yet only two interventions to reduce stroke-induced brain injury exist. In the clinic, many patients who experience an ischemic stroke often further suffer from retinal ischemia, which can inhibit their ability to make a functional recovery and may diminish their overall quality of life. Despite this, no treatments for retinal ischemia have been developed. In both cases, ischemia-induced mitochondrial dysfunction initiates a cell loss cascade and inhibits endogenous brain repair. Stem cells have the ability to transfer healthy and functional mitochondria not only ischemic neurons, but also to similarly endangered retinal cells, replacing their defective mitochondria and thereby reducing cell death. In this review, we encapsulate and assess the relationship between cerebral and retinal ischemia, recent preclinical advancements made using in vitro and in vivo retinal ischemia models, the role of mitochondrial dysfunction in retinal ischemia pathology, and the therapeutic potential of stem cell-mediated mitochondrial transfer. Furthermore, we discuss the pitfalls in classic rodent functional assessments and the potential advantages of laser Doppler as a metric of stroke progression. The studies evaluated in this review highlight stem cell-derived mitochondrial transfer as a novel therapeutic approach to both retinal ischemia and stroke. Furthermore, we posit the immense correlation between cerebral and retinal ischemia as an underserved area of study, warranting exploration with the aim of these treating injuries together.
Keywords: laser Doppler, MCAO, mesenchymal stem cells, mitochondrial network, mitochondrial transfer, ophthalmic artery, optic nerve, oxygen-glucose deprivation, regenerative medicine, retinal ganglion cells, visual impairment
Introduction
Stroke remains one of the greatest causes of mortality and morbidity in the United States and imposes an immense economic burden, projected to total 240 billion dollars annually by 2030 (Ovbiagele et al., 2013; Benjamin et al., 2019). Despite this, there exist only two approved treatment options, tissue plasminogen activator (tPA) and endovascular thrombectomy, but short therapeutic windows and high risk of additional damage limit their applicability (Gumbinger et al., 2014; Saver et al., 2016; Kaesmacher et al., 2017; Li et al., 2017). Ischemic stroke, which comprises 87% of all cases, involves a reduction of blood flow which leads to oxygen and nutrient deprivation and thus cell death in the brain, spinal cord, or retina (Sacco et al., 2013; Benjamin et al., 2019). Although neurological deficits are the typical examples of stroke sequelae, these deficits are compounded by stroke-induced visual impairments in about 92% of cases (Rowe et al., 2017) likely arising from middle cerebral artery occlusion (MCAO). Additionally, these deficits may persist for many months after stroke onset (Rowe et al., 2017). Visual impairments contribute to post-stroke disability statistics and interfere with quality of life and functional recovery (Sand et al., 2013). The retina is directly dependent upon the central nervous system and thus mirrors many of the same symptoms as the brain after ischemic stroke (Minhas et al., 2012). Retinal ischemia – the kind of ischemia specific to retinal cell loss and optic nerve damage – may often co-occur with cerebral ischemia and is responsible for many of these impairments (Osborne et al., 2004; Brown and Vasudevan, 2014; Lee et al., 2014). While the overlapping pathology uniting these two disorders is not fully understood, it may involve mitochondrial dysfunction (Moskowitz et al., 2010; Zhao et al., 2013; Prentice et al., 2015; Nguyen et al., 2019).
Mitochondria rely on blood flow to provide oxygen and glucose and function by repackaging these ingredients into forms usable by the rest of the cell. The reduced blood flow experienced during ischemic stroke inhibits normal mitochondrial functioning and thus reduces the usable energy in infarct area (Vosler et al., 2009). A cell loss cascade constitutes one of the manifold effects of this “power outage” and contributes to stroke pathology (Vosler et al., 2009; Hayakawa et al., 2018). This outage may lead to damage of the retinal ganglion cells and the optic nerve in retinal ischemia cases (Borlongan et al., 2015; Nguyen et al., 2019). Understanding and targeting this cascade is an emerging area of research. Astrocytes exhibit an innate capacity to transfer healthy mitochondria to endangered cells in ischemic areas (Hayakawa et al., 2018). While this alone is not enough to abrogate the cell loss cascade, it provides a prototype that may be copied and enhanced by stem cell transplants (Vosler et al., 2009; Kaneko et al., 2014; Berridge et al., 2016; Borlongan et al., 2019). Attenuating mitochondrial dysfunction through regenerative medicine represents an attractive approach for treating cerebral and retinal ischemia.
This review was compiled using PubMed with sources within the last ten years. If the topic did not have relevant information within the last ten years, we used the most recent paper. In this review, we probe the relationship between retinal ischemia and stroke as simulated by MCAO, a popular stroke animal model (Borlongan et al., 1998a, b; Ishikawa et al., 2013a, b; dela Pena et al., 2015), and oxygen-glucose deprivation (OGD), an in vitro model of ischemia (Kaneko et al., 2014). Furthermore, we examine the therapeutic potential of stem cell-mediated mitochondrial transfer. Lastly, we discuss the methodological implications uncovered by examination of retinal and cerebral ischemia’s coincident pathologies and recent technological advances.
Middle Cerebral Artery Occlusion Models of Retinal Ischemia
Due to the close anatomic proximity of the MCA to the ophthalmic artery, the filament used to occlude the MCA may also induce retinal ischemia (Block et al., 1997; Steele et al., 2008; Allen et al., 2014; Borlongan et al., 2015; Nguyen et al., 2019). The hemodynamic, histopathological, and behavioral symptoms of retinal ischemia overlap markedly with those of ischemic stroke. For example, retinal laser Doppler readings closely approximate brain cerebral blood flow at baseline during perfusion, the drop in blood flow during MCAO, and the return to baseline post-reperfusion 3 and 14 days after stroke (Borlongan et al., 2015; Nguyen et al., 2019). During the acute phase of stroke, MCAO reduces circulation to both the ipsilateral cerebral hemisphere and ipsilateral eye by at least 80% compared to the baseline (Borlongan et al., 2015; Taninishi et al., 2015; Nguyen et al., 2019). While blood flow in the retina restores 5 minutes faster than hemispheric blood flow after reperfusion, this difference in their reperfusion profiles can likely be attributed to the extensive vascularity of the retina (Shih et al., 2014; Hui et al., 2017). Additionally, deficient collateral circulation in the retina likely balances their reperfusion for up to 3 days post-insult (Allen et al., 2016; Ritzel et al., 2016; Nguyen et al., 2019).
Similar to neurological and cognitive deficits associated with general stroke, visual impairments resulting from retinal ischemia are linked to the overall deficient blood flow to the eye, the resulting series of apoptotic events, and—as a consequence of this ischemia-induced oxidative stress—mitochondrial dysfunction in retinal ganglion cells (Borlongan et al., 2015; Russo et al., 2018; Yang et al., 2018; Nguyen et al., 2019). At 3 and 14 days post-MCAO, immunohistochemical staining techniques have measured reduced optic nerve width and increased ganglion cell loss in the ipsilateral eye coinciding with mitochondrial dysfunction (Borlongan et al., 2015; Nguyen et al., 2019). Indeed, retinal damage worsens up to 14 days after stroke, indicating that degenerative changes to cellular and mitochondrial structure following the initial insult continuously exacerbate neurodegeneration (Steele et al., 2008; Allen et al., 2014; Ritzel et al., 2016; Nguyen et al., 2019).
Furthermore, behavioral tests have evaluated the extent of visual deficits in stroke animals (Borlongan et al., 2015; Nguyen et al., 2019). After MCAO, most animals present varying degrees of visual deficits that can impede their ability to recognize visual cues. Stroke rats exhibit increased eye closure as well as a diminished response to light evidenced by their poorer performance on the light stimulus avoidance test compared to controls (Borlongan et al., 2015). Functional deficits such as electroretinogram alterations (Block et al., 1992, 1997; Block and Sontag, 1994), retinal cell loss (Steele et al., 2008; Allen et al., 2014), and retinal gliosis (Block et al., 1997) have also been observed in post-MCAO animals.
In addition to MCAO in vivo, exposure of retinal pigmented endothelial (RPE) cells to OGD has modeled retinal ischemia in vitro. In RPE cell cultures, use of immunocytochemical techniques reveals that OGD insult likewise decreases RPE cell survival (Nguyen et al., 2019). Moreover, gauging respiratory output with the Seahorse analyzer demonstrates that OGD results in mitochondrial dysfunction, characterized by diminished respiratory function, as well as a decreased number of mitochondrial networks and an elevated quantity of isolated, spherical mitochondria in retinal cell mitochondria (Nguyen et al., 2019). Mitochondrial networks are crucial in preserving the mitochondrial DNA integrity, respiratory capacity, and response to cellular stress (Nguyen et al., 2019). The interconnected morphology of the mitochondrial network is contingent on the dynamic balance of mitochondrial fusion and fission, and imbalance towards the latter produces fragmented, spherical mitochondria which are more likely to be damaged by oxidative stress (Nguyen et al., 2019).
While retinal cell death both in vivo and in vitro are evidently linked with mitochondrial dysfunction, the capacity of stem cell transplants to transfer their healthy mitochondria to ischemic retinal cells represents a novel restorative aspect of regenerative medicine.
Stem Cell Therapy Ameliorates Retinal Ischemic Pathology via Mitochondria Transfer
Stem cells confer a wide variety of neuroprotective, anti-inflammatory, and neuroregenerative effects, but, in particular, their ability to convey healthy mitochondria to endangered cells in ischemic areas posits them as an attractive therapeutic approach (Russo et al., 2018; Nguyen et al., 2019). After intravenous administration of mesenchymal stem cells (MSCs) in MCAO rats, cellular and optic nerve injuries display positive trends on day 3 and significant recovery by day 14 (Nguyen et al., 2019). In vitro, cultures exposed to OGD likewise exhibit retinal cell loss, which is attenuated when RPE cells are co-cultured with MSCs (Nguyen et al., 2019). Specifically, RPE cells subjected to OGD and co-cultured with MSCs exhibit increased cell viability, cell proliferation, and mitochondrial networks compared to the OGD-only control (Nguyen et al., 2019). Furthermore, OGD conditions alter mitochondrial dynamics via upregulation of Drp1 (fission protein) and downregulation of Mfn2 (fusion protein) (Zuo et al., 2014; Flippo et al., 2018; Nguyen et al., 2019). Evidence indicates that MSCs can significantly replenish Mfn2 expression but not that of Drp1, and using JC-1 mitochondrial membrane dye and live cell imaging reveals that MSCs do indeed transfer healthy mitochondria to injured retinal cells and decrease mitochondrial membrane depolarization (Zuo et al, 2014; Chen et al., 2017; Shi et al., 2017; Flippo et al., 2018; Nguyen et al., 2019). In light of these findings, stem cell-mediated mitochondrial repair represents a promising approach to mitigating visual impairments following cerebral and retinal ischemia.
When faced with cerebral and retinal ischemia, the earlier its symptomology is detected and treatment is implemented, the more favorable the outcome is for the patient (Biousse et al., 2018). Due to the vasculature and collateral nature in the brain and retina, their staggered reperfusion timing may affect the dispersal of healthy mitochondria from stem cells. Interestingly, optimizing the timing and delivery of stem cells alongside the current tPA treatment could enhance the functional outcome for stem cell mitochondrial transfer therapy in retinal ischemia.
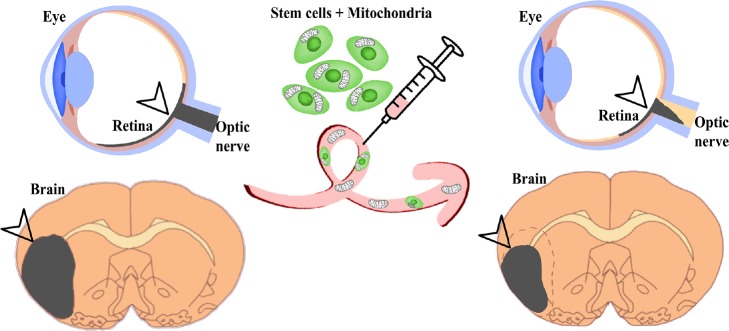
Overall, treatment with mesenchymal stem cells —whether via intravenous transplantation in vivo or co-culture with RPE cells in vitro—evidently ameliorates mitochondrial structure and function post-ischemia, likely because the exogenous MSCs transfer healthy mitochondria to endangered retinal cells (Figure 1).
Figure 1.
Stem cell therapy ameliorates stroke-induced retinal ischemia.
Mitochondrial transfer provides a mechanism by which stem cells may alleviate stroke pathology in the retina, optic nerve, and brain. Arrowhead and dark line indicate cell death/infarct area.
Methodological Implications
Close examination of animal stroke models raises several methodological implications for future stroke research. For one, that visual impairments have been closely associated with ischemic stroke and MCAO raises questions about the validity of several standard animal behavioral tests. Instead of purely measuring stroke-induced neurological deficits, it is possible that the MCAO subjects are performing poorly simply because their vision is impaired (Borlongan et al., 2008). As such, the varying results from behavioral tests may be due to visual impairment, the injured stroke brain, or a combination of the two. If the changes in behavior observed are due to visual deficits, then the validity of those neurological tests are undermined. For example, the Morris Water Maze is a standard cognitive test that demands animals to use visual cues along with memory of the hidden platforms. A poor performance on this test may be due to the animal not being able to see the environment clearly rather than the stroke brain causing the dysfunction. Therefore, one cannot definitively conclude whether the injured brain or the visual impairment caused the animal’s performance. Thus, further study of visual impairment as a possible mediator for post-MCAO functional score decline is needed. Additionally, this uncertainty may warrant the development of additional functional assessments that do not depend on visual acuity.
Recent studies have also highlighted the methodological implications of laser Doppler as a safe, noninvasive method to observe the hemodynamics of the eye (Borlongan et al., 2015). Laser Doppler provides a sensitive tool for monitoring blood flow, thus it serves as a reliable device outside of its traditional use to measure intracranial hemodynamics after MCAO (Borlongan et al., 2015). In comparison to other methods, laser Doppler allows for a less invasive reading of the eye and does not compound the trauma experienced by the stroke animal. Furthermore, this technique lessens the chance for infection due to its minimally invasive nature. Due to their high comorbidity, American Heart Association/American Stroke Association guidelines recommend immediate brain imaging upon retinal ischemia diagnosis (Furie et al., 2011). This practice can be translated to the clinic by providing a quick assessment of the patient’s eye and initiating treatment interventions sooner. This is especially critical for the current standard of care, tPA, which is limited to 4.5 hours after stroke (Gumbinger et al., 2014). Thus, laser Doppler could be translated to the clinic immediately, potentially preventing the death and disability of millions of stroke patients.
Conclusion
Ischemic stroke continues to be a devastating disease worldwide, and stroke patients often suffer from further complications such as visual impairment. Retinal ischemia constitutes one major source of stroke-related visual impairments, as blood flow in the ophthalmic artery often becomes restricted during cerebral ischemia because of its anatomical juxtaposition with the MCA. Consequently, use of MCAO in rodent models of stroke may not only cause ischemic insult in the brain, but also in the retina. Laser Doppler represents an effective approach to monitoring hemodynamics in these organs and reveals that perfusion rates in the ipsilateral cerebral hemisphere and the ipsilateral eye mirror each other before, during, and after an ischemic insult (Borlongan et al., 2015; Nguyen et al., 2019). Along with this parallel in blood flow alteration, the pathology of retinal ischemia overlaps with that of stroke in various other ways. Particularly, mitochondrial dysfunction significantly contributes to retinal cell death, and thus visual deficits, in retinal ischemia victims (Osborne, 2010; Park et al., 2011; Nguyen et al., 2019). To this end, the transfer of healthy mitochondria from stem cell transplants to endangered retinal cells has emerged as an auspicious therapeutic approach. In this review, we highlighted the current preclinical evidence that exogenous MSCs mitigate cellular degeneration in both MCAO rats and OGD-RPE cell cultures via mitochondrial transfer, as enhanced survival of retinal cells coincides with repaired mitochondrial structure and function (Nguyen et al., 2019). In addition to these promising findings, we have noted that forthcoming stroke investigations should consider the propensity of MCAO to induce retinal ischemia in addition to stroke, as this consequence may necessitate refining traditional behavioral tests of stroke rats to more precisely determine the extent to which cognitive—as opposed to visual—impairments contribute to functional declines. Future research must also clarify how mitochondrial transfer fits in the broader scheme of stem cell-mediated cell survival improvement mechanisms, including various bystander effects (Chau et al., 2016; Stonesifer et al., 2017; Nguyen et al., 2019). Furthermore, optimizing the delivery route and timing of stem cell transplantation, as well as combining stem cell-mediated mitochondrial transfer with tPA, may further ameliorate cell loss and visual impairments and promote functional recovery, all of which warrants further investigation.
Footnotes
Conflicts of interest: CVB was funded and received royalties and stock options from Astellas, Asterias, Sanbio, Athersys, KMPHC, and International Stem Cell Corporation; and also received consultant compensation for Chiesi Farmaceutici. He also holds patents and patent applications related to stem cell biology and therapy. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Financial support: This work was funded by the National Institutes of Health (NIH) R01NS071956, NIH R01NS090962, NIH R21NS089851, NIH R21NS094087, and Veterans Affairs Merit Review I01 BX001407 (all to CVB).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was funded by the National Institutes of Health (NIH) R01NS071956, NIH R01NS090962, NIH R21NS089851, NIH R21NS094087, and Veterans Affairs Merit Review I01 BX001407 (all to CVB).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Allen RS, Sayeed I, Cale HA, Morrison KC, Boatright JH, Pardue MT, Stein DG. Severity of middle cerebral artery occlusion determines retinal deficits in rats. Exp Neurol. 2014;254:206–215. doi: 10.1016/j.expneurol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MV, McConnell MJ, Grasso C, Bajzikova M, Kovarova J, Neuzil J. Horizontal transfer of mitochondria between mammalian cells: beyond co-culture approaches. Curr Opin Genet Dev. 2016;38:75–82. doi: 10.1016/j.gde.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: Follow the guidelines! Ophthalmology. 2018;125:1597–1607. doi: 10.1016/j.ophtha.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Block F, Schwarz M, Sontag KH. Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neurosci Lett. 1992;144:124–126. doi: 10.1016/0304-3940(92)90731-l. [DOI] [PubMed] [Google Scholar]
- 6.Block F, Sontag KH. Differential effects of transient occlusion of common carotid arteries in normotensive rats on the somatosensory and visual system. Brain Res Bull. 1994;33:589–593. doi: 10.1016/0361-9230(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 7.Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M. Retinal ischemia induced by the intraluminal suture method in rats. Neurosci Lett. 1997;232:45–48. doi: 10.1016/s0304-3940(97)00575-2. [DOI] [PubMed] [Google Scholar]
- 8.Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, Hess DC, Kondziolka D. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–250. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlongan CV, Nguyen H, Lippert T, Russo E, Tuazon J, Xu K, Lee JY, Sanberg PR, Kaneko Y, Napoli E. May the force be with you: Transfer of healthy mitochondria from stem cells to stroke cells. J Cereb Blood Flow Metab. 2019;39:367–370. doi: 10.1177/0271678X18811277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlongan CV, Saporta S, Poulos SG, Othberg A, Sanberg PR. Viability and survival of hNT neurons determine degree of functional recovery in grafted ischemic rats. Neuroreport. 1998b;9:2837–2842. doi: 10.1097/00001756-199808240-00028. [DOI] [PubMed] [Google Scholar]
- 11.Borlongan C, Lee J, Tajiri N, Acosta S, Nguyen H. An eye-opener for stroke: Pathological consequences of reduced blood flow in the ipsilateral eye following transient middle cerebral artery occlusion in adult rats. Poster presented at: McCormick Place Convention Center; 2015. [Google Scholar]
- 12.Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998a;16:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- 13.Brown SM, Vasudevan A. Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol. 2014;158:1353. doi: 10.1016/j.ajo.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Chau M, Zhang J, Wei L, Yu SP. Regeneration after stroke: stem cell transplantation and trophic factors. Brain Circ. 2016;2:86–94. doi: 10.4103/2394-8108.186279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Zhang GP, Guo SL, Ding JQ, Lin JJ, Yang Q, Li ZY. Mfn2-mediated preservation of mitochondrial function contributes to the protective effects of BHAPI in response to ischemia. J Mol Neurosci. 2017;63:267–274. doi: 10.1007/s12031-017-0976-z. [DOI] [PubMed] [Google Scholar]
- 16.dela Pen~a IC, Yoo A, Tajiri N, Acosta SA, Ji X, Kaneko Y, Borlongan CV. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J Cereb Blood Flow Metab. 2015;35:338–346. doi: 10.1038/jcbfm.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flippo KH, Gnanasekaran A, Perkins GA, Ajmal A, Merrill RA, Dickey AS, Taylor SS, McKnight GS, Chauhan AK, Usachev YM, Strack S. AKAP1 protects from cerebral ischemic stroke by inhibiting Drp1-dependent mitochondrial fission. J Neurosci. 2018;38:8233–8242. doi: 10.1523/JNEUROSCI.0649-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 19.Gumbinger C, Reuter B, Stock C, Sauer T, Wietholter H, Bruder I, Rode S, Kern R, Ringleb P, Hennerici MG, Hacke W, Schlaganfall AG. Time to treatment with recombinant tissue plasminogen activator and outcome of stroke in clinical practice: Retrospective analysis of hospital quality assurance data with comparison with results from randomised clinical trials. BMJ. 2014;348:g3429. doi: 10.1136/bmj.g3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH. Extracellular mitochondria for therapy and diagnosis in acute central nervous system injury. JAMA Neurol. 2018;75:119–122. doi: 10.1001/jamaneurol.2017.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui F, Nguyen CT, He Z, Vingrys AJ, Gurrell R, Fish RL, Bui BV. Retinal and cortical blood flow dynamics following systemic blood-neural barrier disruption. Front Neurosci. 2017;11:568. doi: 10.3389/fnins.2017.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, Mimura O, Dezawa M, Kim SU, Borlongan CV. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013a;44:3473–3481. doi: 10.1161/STROKEAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa H, Tajiri N, Vasconcellos J, Kaneko Y, Mimura O, Dezawa M, Borlongan CV. Ischemic stroke brain sends indirect cell death signals to the heart. Stroke. 2013b;44:3175–3182. doi: 10.1161/STROKEAHA.113.001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaesmacher J, Kaesmacher M, Maegerlein C, Zimmer C, Gersing AS, Wunderlich S, Friedrich B, Boeckh-Behrens T, Kleine JF. Hemorrhagic transformations after thrombectomy: risk factors and clinical relevance. Cerebrovasc Dis. 2017;43:294–304. doi: 10.1159/000460265. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko Y, Tajiri N, Shojo H, Borlongan CV. Oxygen-glucose-deprived rat primary neural cells exhibit DJ-1 translocation into healthy mitochondria: a potent stroke therapeutic target. CNS Neurosci Ther. 2014;20:275–281. doi: 10.1111/cns.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157:1231–1238. doi: 10.1016/j.ajo.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Gao X, Yao Z, Feng X, He H, Xue J, Gao P, Yang L, Cheng X, Chen W, Yang Y. Permeability surface of deep middle cerebral artery territory on computed tomographic perfusion predicts hemorrhagic transformation after stroke. Stroke. 2017;48:2412–2428. doi: 10.1161/STROKEAHA.117.017486. [DOI] [PubMed] [Google Scholar]
- 28.Minhas G, Morishita R, Anand A. Preclinical models to investigate retinal ischemia: advances and drawbacks. Front Neurol. 2012;3:75. doi: 10.3389/fneur.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen H, Lee JY, Sanberg PR, Napoli E, Borlongan CV. Eye opener in stroke: Mitochondrial dysfunction and stem cell repair in retinal ischemia. Stroke. 2019;50:2197–2206. doi: 10.1161/STROKEAHA.119.025249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen H, Zarriello S, Coats A, Nelson C, Kingsbury C, Gorsky A, Rajani M, Neal EG, Borlongan CV. Stem cell therapy for neurological disorders: a focus on aging. Neurobiol Dis. 2019;126:85–104. doi: 10.1016/j.nbd.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Osborne NN. Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757. doi: 10.1016/j.exer.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 35.Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Opthamol Vis Sci. 2011;52:2837–2843. doi: 10.1167/iovs.09-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prentice H, Modi JP, Wu JY. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:964518. doi: 10.1155/2015/964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritzel RM, Pan SJ, Verma R, Wizeman J, Crapser J, Patel AR, Lieberman R, Mohan R, McCullough LD. Early retinal inflammatory biomarkers in the middle cerebral artery occlusion model of ischemic stroke. Mol Vis. 2016;22:575–588. [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe FJ. Stroke survivors’ views and experiences on impact of visual impairment. Brain Behav. 2017;7:e00778. doi: 10.1002/brb3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo E, Napoli E, Borlongan CV. Healthy mitochondria for stroke cells. Brain Circ. 2018;4:95–98. doi: 10.4103/bc.bc_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sand KM, Midelfart A, Thomassen L, Melms A, Wilhelm H, Hoff JM. Visual impairment in stroke patients–a review. Acta Neurol Scand Suppl. 2013;196:52–56. doi: 10.1111/ane.12050. [DOI] [PubMed] [Google Scholar]
- 42.Saver JL, Goyal M, Van der Lugt AA, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, Cardona P. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316:1279–1289. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Yi C, Li X, Wang J, Zhou F, Chen X. Overexpression of Mitofusin2 decreased the reactive astrocytes proliferation in vitro induced by oxygen-glucose deprivation/reoxygenation. Neurosci Lett. 2017;639:68–73. doi: 10.1016/j.neulet.2016.12.052. [DOI] [PubMed] [Google Scholar]
- 44.Shih YY, De La Garza BH, Huang S, Li G, Wang L, Duong TQ. Comparison of retinal and cerebral blood flow between continuous arterial spin labeling MRI and fluorescent microsphere techniques. J Magn Reson Imaging. 2014;40:609–615. doi: 10.1002/jmri.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steele EC, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008;39:2099–2104. doi: 10.1161/STROKEAHA.107.504357. [DOI] [PubMed] [Google Scholar]
- 46.Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taninishi H, Jung JY, Izutsu M, Wang Z, Sheng H, Warner DS. A blinded randomized assessment of laser Dopp:ler flowmetry efficacy in standardizing outcome from intraluminal filament MCAO in the rat. J Neurosci Methods. 2015;241:111–120. doi: 10.1016/j.jneumeth.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Vosler PS, Graham SH, Wechsler LR, Chen J. Mitochondrial targets for stroke: focusing basic science research toward development of clinically translatable therapeutics. Stroke. 2009;40:3149–3155. doi: 10.1161/STROKEAHA.108.543769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–275. doi: 10.1016/j.redox.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Yu B, Xiang YH, Han XJ, Xu Y, So KF, Xu AD, Ruan YW. Changes in retinal morphology, electroretinogram and visual behavior after transient global ischemia in adult rats. PLoS One. 2013;8:e65555. doi: 10.1371/journal.pone.0065555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo W, Zhang S, Xia CY, Guo XF, He WB, Chen NH. Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: the role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology. 2014;86:103–115. doi: 10.1016/j.neuropharm.2014.07.002. [DOI] [PubMed] [Google Scholar]