Keywords: cerebral ischemia, cognitive function, CXCR7, dendritic development, dentate gyrus, doublecortin, neurogenesis, neutralizing antibody, stroke, stromal cell-derived factor-1
Abstract
Stromal cell-derived factor-1 and its receptor CXCR4 are essential regulators of the neurogenesis that occurs in the adult hippocampal dentate gyrus. However, the effects of CXCR7, a new atypical receptor of stromal cell-derived factor-1, on hippocampal neurogenesis after a stroke remain largely unknown. Our study is the first to investigate the effect of a CXCR7-neutralizing antibody on neurogenesis in the dentate gyrus and the associated recovery of cognitive function of rats in the chronic stage of cerebral ischemia. The rats were randomly divided into sham, sham + anti-CXCR7, ischemia and ischemia + anti-CXCR7 groups. Endothelin-1 was injected in the ipsilateral motor cortex and striatum to induce focal cerebral ischemia. Sham group rats were injected with saline instead of endothelin-1 via intracranial injection. Both sham and ischemic rats were treated with intraventricular infusions of CXCR7-neutralizing antibodies for 6 days 1 week after surgery. Immunofluorescence staining with doublecortin, a marker for neuronal precursors, was performed to assess the neurogenesis in the dentate gyrus. We found that anti-CXCR7 antibody infusion enhanced the proliferation and dendritic development of doublecortin-labeled cells in the dentate gyrus in both ischemic and sham-operated rats. Spatial learning and memory functions were assessed by Morris water maze tests 30–32 days after ischemia. CXCR7-neutralizing antibody treatment significantly reduced the escape latency of the spatial navigation trial and increased the time spent in the target quadrant of spatial probe trial in animals that received ischemic insult, but not in sham operated rats. These results suggest that CXCR7-neutralizing antibody enhances the neurogenesis in the dentate gyrus and improves the cognitive function after cerebral ischemia in rats. All animal experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of China Medical University (CMU16089R) on December 8, 2016.
Chinese Library Classification No. R453; R363; R364
Introduction
The subgranular zone of the dentate gyrus (DG) and the subventricular zone of the olfactory bulb are two brain regions that all through life produce new neurons that go on to form synaptic contacts with the existing neural circuitry (Kempermann et al., 2015). Adult neurogenesis of the granule neurons in DG correlates with learning and memory and includes proliferation of neural progenitor cells, migration, differentiation and network integration of the new neurons. Many types of physiological and pathological stimulation influence the adult neurogenesis, including exercise (Liu and Nusslock, 2018), exogenous insult induced brain injury and stroke (Jin et al., 2001; Cheng et al., 2017).
Stromal cell-derived factor-1 (SDF-1) is a chemoattractant chemokine that has six variants (Peyvandi et al., 2018). SDF-1α is the main isoform that exists in many cell types and tissues in the central nervous system (Guyon, 2014). SDF-1 plays important roles in cell proliferation, migration, differentiation and maturation, as well as angiogenesis after ischemic stroke (Li et al., 2014). Many reports indicate that SDF-1 and its receptor, CXCR4, can regulate neurogenesis in the DG, in both normal and ischemic animals (Cui et al., 2013; Schultheiss et al., 2013; Zhao et al., 2015). Recently, it has been reported that SDF-1 also binds to CXCR7 (also known as RCD1 or ACKR3), a new atypical chemokine receptor of SDF-1, with a 10-fold greater affinity than CXCR4. CXCR7 is widely expressed in neural stem progenitor cells, immature neurons and mature granule neurons in the DG, pyramidal cells in CA3 as well as blood vessels in the adult brain (Balabanian et al., 2005; Schonemeier et al., 2008; Luker et al., 2010; Banisadr et al., 2015). A study revealed that the expression levels of both SDF-1 and CXCR7, but not CXCR4, are increased in the peri-infarction area of ischemic cerebral cortex (Zhang et al., 2018), suggesting that SDF-1 binds to CXCR7, rather than CXCR4, to modulate repair of the brain after a stroke.
This study explored the effect of anti-CXCR7 antibody infusion on the proliferation and dendritic development of immature neurons in the DG as well as on the recovery of cognitive functional in rats during the chronic stage following focal ischemia.
Materials and Methods
Animals
Forty adult male Wistar rats aged 8 weeks weighing 200–250 g were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China (license No. SCXK (Liao) 2008-0005). Rats were randomly divided into sham group (sham operation, n = 8), sham + anti-CXCR7 group (another sham operated group but treated with anti-CXCR7 antibody, n = 8), ischemia group (n = 12), and ischemia + anti-CXCR7 group (rats were treated with anti-CXCR7 antibody after ischemia, n = 12). The rats were housed in groups of up to five rats per cage and allowed access to food and water freely in an environment with controlled humidity and temperature.
All animal experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of China Medical University (CMU16089R) on December 8, 2016. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Precautions were taken to minimize the number and suffering of animals used in each experiment.
Endothelin-1 stroke models
The sequence of experimental protocol is summarized in Figure 1. Anesthesia was conducted by isoflurane inhalation (using compressed air as carrier at 4.5% and then maintained at 1–2%. Endothelin-1 (ET-1, 100 μg) (Tocris, MN, USA) was dissolved in 200 μL sterile saline. In accordance with the previous study (Windle et al., 2006), ET-1 was administrated stereo-tactically with a Hamilton microsyringe into the brain at coordinates (three sites represent the stereotactic region of the striatum and the motor cortex): (1) anteroposterior (AP) +0.7 mm, mediolateral (ML) +2.2 mm, dorsoventral (DV) –2.0 mm; (2) AP +2.3 mm, ML +2.5 mm, DV –2.5 mm; (3) AP +0.7 mm, ML +3.8mm, DV –5.8 mm (2 μL per site). Saline, instead of ET-1, was injected at the same coordinates in the sham-operated rats. The ischemia model was considered to be successful if the right upper limb of the rat flexed to the chest when it was overhung by its tail, or rotated to the right while walking, when observed 24 hours later.
Figure 1.
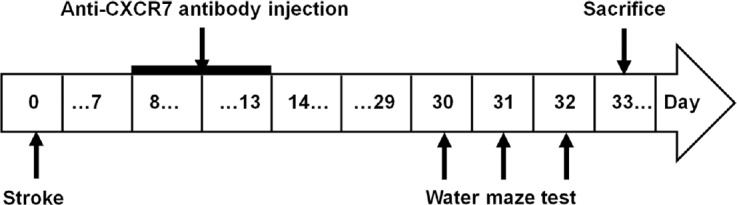
Study design.
The arrows indicate the timing (days) of stroke induction, anti-CXCR7 antibody injection, behavioral tests, and sacrifice.
Intraventricular administration of anti-CXCR7 antibody
Seven days after the ischemia and sham surgery, to neutralize CXCR7 signaling was neutralized by the anti-CXCR7 antibody (anti-GPCR-RDC-1, Abcam, Cambridge, UK) (0.5 μg/μL), diluted in sterile saline, and injected stereo-tactically into the lateral ventricle (AP –0.8 mm, ML +1.5 mm) via a microinjection system (RWD Life Science Co., Ltd., Shenzhen, China) once a day for 6 consecutive days (3.5 μL per day).
Morris water maze test
On post-ischemia days 30–32, the learning and memory functions were evaluated in a spatial navigation trial using the Morris water maze test, as described previously (Chuansheng et al., 2009). Escape latency (time to reach the platform within 120 seconds) was used to evaluate the acquisition of the water maze task. Eight rats from each group were tested (Figure 1). On the last day of the test period, the platform was removed to conduct the spatial probe trail. The rats were allowed to swim in the pool within 60 seconds, and the time percentage spent in the target quadrant was analyzed to allow semi-quantification of the search pattern of the rats (Zhao et al., 2013a, 2015). All behavioral analysis was performed blindly.
Tissue preparation and immunofluorescence staining
Thirty-three days after stroke, the animals were euthanized by inhalation of isoflurane until there was no response to a tail pinch. They were then perfused transcardially with physiological saline, then 4% paraformaldehyde before decapitation. The brains were dissected out and post-fixed in 4% paraformaldehyde at 4°C overnight, then dehydrated in 30% sucrose solution until the brain sank. Finally the samples were cut into 35-μm-thick coronal sections with a cryostat (Model CM 1950; Leica, Munich, Germany). Free-floating sections were stained by immunofluorescent staining in accordance with a previous study (Sebastian et al., 2007). The brain slices were blocked in 5% goat serum (diluted with blocking buffer) for 90 minutes, followed by incubation with Guinea pig anti-DCX antibody (1:500; Millipore, Billerica, MA, USA) overnight at 4°C. After washing with 0.01 M phosphate buffered saline for 10 minutes, all sections were incubated with Fluor 488 conjugated goat anti-guinea pig IgG (1:400; Invitrogen, Carlsbad, CA, USA) for 2 hours in the dark. Afterwards, all sections were mounted with antifade mounting medium (Beyotime Biotechnology, Beijing, China). All immunohistochemistry analysis was performed blindly.
Measurement of infarct volume
To measure the infarct volume, 33 days after cerebral ischemia, brain sections from 4.5 mm anterior to the bregma to 7.5 mm posterior to the bregma were collected and stained with Nissl staining (Baldauf and Reymann, 2005). Sections on both the ipsilateral and contralateral hemispheres were measured with 1.51w ImageJ software (NIH, Bethesda, MD USA). The total infarct volume was calculated by multiplying the infarction areas (contralateral cortex area – spared ipsilateral cortex area) with the distance between sections (Swanson et al., 1990). The analysis was performed blindly.
Quantification of the proliferation and dendritic development of DCX-labeling cells in the DG
At 33 days after focal cerebral ischemia, immunofluorescence staining with doublecortin (DCX), a marker for neuronal precursors, was conducted to assess the neurogenesis in DG. Five sections in every sixth slice through the DG of each rat were viewed on a confocal microscope (FV 1000; Olympus, Tokyo, Japan) with a 10× objective. The number of DCX-labeling cells on every section was counted by Cell Counter (NIH ImageJ) and averaged. Three DCX-labeling cells that had largely intact dendritic trees were randomly selected from each section. Z-series stacks of confocal pictures were obtained under a 40× objective. The total dendritic length of the DCX-labeling cells was measured by reconstructing and tracing the images using NIH ImageJ software (Zhao et al., 2015).
Statistical analysis
All data are presented as the mean ± SEM. Statistical analyses were conducted using SPSS 21.0 software (IBM, Chicago, NY, USA). The number and the total dendritic length of the DCX-labeling cells, as well as the data in the probe trail, were analyzed by one-way analysis of variance followed by the least significant difference and Dunnett T3 post hoc test. The data of the spatial navigation trial were analyzed with analysis of variance for repeated measures followed by the least significant difference post hoc tests. The differences in infarct volume were analyzed using paired sample t-test. P < 0.05 was considered statistically significant.
Results
Anti-CXCR7 antibody improves cognitive behavioral recovery in water maze test after cerebral ischemia
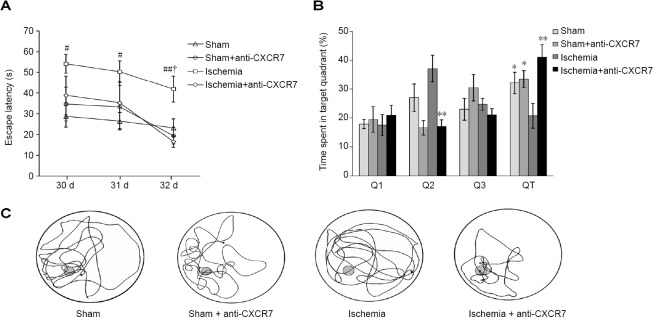
The results of the spatial navigation trials showed an overall group effect in escape latency (F[3, 28] = 8.329; P = 0.001), but the difference in swimming speed was not significant (F[3, 28] = 0.566; P > 0.05), indicating that different abilities of animals in the trial were not the result of motor impairments. All groups initially demonstrated higher escape latencies, which gradually reduced over time. The escape latency of ischemia group was significantly longer than either in the sham group (P < 0.01) or sham + anti-CXCR7 group (P < 0.05). Administration of CXCR7-neutralizing antibody significantly reduced the escape latency in the ischemic rats (P < 0.05). The results of escape latency showed no difference between the sham and anti-CXCR7 antibody-treated sham rat groups (P > 0.05; Figure 2A). In the spatial probe trial, the ischemic rats spent significantly more time in the target quadrant after CXCR7-neutralization (P < 0.01). There was no significant difference between the sham group and sham + anti-CXCR7 antibody group results of the probe trial (P > 0.05; Figure 2B). These differences in the learning and memory abilities to find the platform were reflected in the swim paths (Figure 2C).
Figure 2.
Behavioral assessments.
(A) Escape latency in the spatial navigation trial: The data are analyzed with repeated measures one-way analysis of variance followed by the least significant difference post hoc tests. (B) Data from the spatial probe trial showed that the time (%) spent in the target quadrant (QT). The difference between groups was analyzed by one-way analysis of variance test followed by the least significant difference (Q2 and QT), and Dunnett T3 (Q1 and Q3) post hoc tests (Q1 and Q3). (C) Representative swimming pattern from the probe trail test: Data of (A) and (B) are expressed as the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, vs. ischemia groups; #P < 0.05, ##P < 0.01, vs. sham group; †P < 0.01, vs. ischemia + anti-CXCR7 group.
Anti-CXCR7 antibody has no effect on the infarct volume after cerebral ischemia in rats
Among the 24 rats that were injected with ET-1, 6 rats were excluded, 3 died before testing and another 3 failed the ischemia model criteria. Nissl staining (Figure 3) was used 33 days after the ischemic insult and the infarct volumes were measured. The results showed the difference between the ischemia group (127.90 ± 12.86 mm3) and the ischemia + anti-CXCR7 group (108.70 ± 15.55 mm3) was not significant (P > 0.05; Figure 3). No damage was observed in either the sham group or the sham + anti-CXCR7 group.
Figure 3.
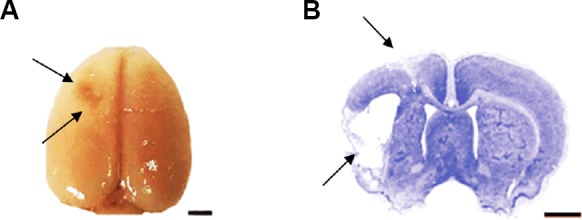
Overview of the cerebral infarct in ischemic rats.
(A) Gross assessment of the ischemic lesion 33 days after ischemia onset. (B) Nissl staining of typical infarct area. Arrows point to the infarction. Scale bars: 2 mm.
Anti-CXCR7 antibody enhances the proliferation and dendritic development of DCX-labeling cells in the DG
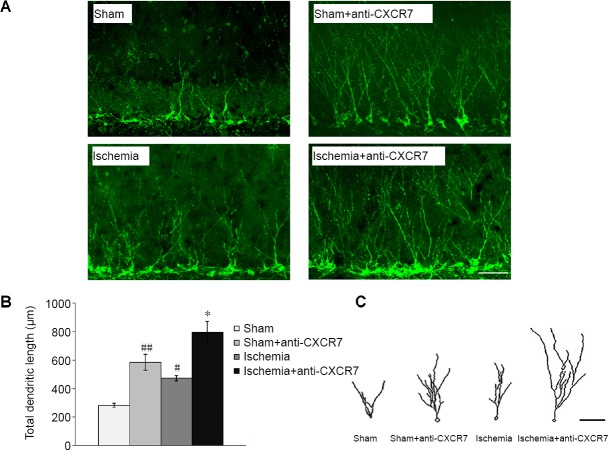
The number of DCX-labeled cells, which indicates immature neurons in the DG, was augmented significantly on day 33 after initiation of ischemia compared with the sham group (P < 0.01). Treatment with anti-CXCR7 antibody, which neutralizes CXCR7, increased the proliferation of DCX-positive cells both after ischemia (P < 0.05) and in the sham operated rats (P < 0.05) compared with untreated rats (Figure 4). The total dendritic length of DCX-positive cells from the ischemia group was significantly greater than that of the sham group (P < 0.05). The administration of anti-CXCR7 antibody enhanced the total dendritic length of DCX-labeled cells in the DG of both ischemic rats (P < 0.01) and sham-operated rats (P < 0.01) compared with without treatment (Figure 5).
Figure 4.
Proliferation of DCX-positive cells in the DG.
(A) At 33 days after focal cerebral ischemia, immunofluorescence images of DCX-positive cells in the DG. Scale bar: 200 μm. (B) Numbers of DCX-positive cells in the DG (10× objective). Data are expressed as the mean ± SEM (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. ischemia group; ##P < 0.01, vs. sham group. DCX: Doublecortin; DG: dentate gyrus.
Figure 5.
Dendrite tracing of DCX-positive cells in DG.
(A) At 33 days after focal cerebral ischemia, immunofluorescence images of DCX-positive cells in the DG (green for DCX, Fluor 488 conjugated goat anti-guinea pig IgG). Scale bar: 50 μm. (B) Quantification of total dendritic length of DCX-positive cells (40× objective): Data are expressed as the mean ± SEM (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. ischemia group; #P < 0.05, ##P < 0.01, vs. sham group. (C) Representative reconstructed drawings of DCX-positive cells in the DG (simulation with NIH ImageJ software). Scale bar: 50 μm. DCX: Doublecortin; DG: dentate gyrus.
Discussion
In this study, the antibody neutralizing CXCR7 was administrated to rats 1 week after the onset of ischemia and repeated daily for 6 consecutive days. The results demonstrated for the first time that anti-CXCR7 antibody enhanced the proliferation and dendritic development of immature neurons in the DG, in both the sham-operated rats and rats conducted with focal cerebral ischemia, and improved the recovery of cognitive function in the chronic phase of cerebral ischemia.
Studies in rats with ischemia, epilepsy or those kept in an enriched environment have shown that SDF-1/CXCR4 is involved in both the increased proliferation of neural progenitor cells and their dendritic development in the DG of the hippocampus (Song et al., 2015; Zhao et al., 2015; Zhang et al., 2016). However, little is known about the function of CXCR7 on DG neurogenesis after stroke. Our data indicate there are beneficial effects of delayed, sustained CXCR7 neutralization on neurogenesis in DG and cognitive recovery in the late stage of cerebral ischemia. Our previous studies also showed that administration of other agents 1 week after ischemia induction regulated not only neurogenesis but also axonal regeneration in the chronic phase after focal ischemia induced by ET-1 (Sun et al., 2016, 2017; Xu et al., 2017). Therefore, the anti-CXCR7 antibody treatment in this study was also initiated 1 week after ischemia and continued for 6 days so that it could exert its effect in the chronic period after cerebral ischemia.
Our results showed that the proliferation and the dendritic growth of the immature neurons in the DGs of ischemic rats increased significantly after anti-CXCR7 antibody administration. A recent study showed that CXCR7 ablation caused an increase in proliferation of neural progenitor cells in the DG (Abe et al., 2018), which is in accordance with the results of the sham rats in our study. However, many studies indicated that SDF-1/CXCR7 provided neural progenitor cells with proliferation, migration and survival advantage in vitro (Abe et al., 2014; Chen et al., 2015; Merino et al., 2015; Wang et al., 2016). The conflicting results could be explained by the differences between the microenvironment in the cell culture medium and the brain tissue, where the level of SDF-1/CXCR4 and their function might also be different.
CXCR7, a new chemokine receptor that is expressed abundantly in the adult DG, is a scavenger for SDF-1 (Boldajipour et al., 2008; Abe et al., 2014). It negatively regulates SDF-1 functions and reduces the cell sensitivity towards SDF-1 (Naumann et al., 2010; Uto-Konomi et al., 2013). The CXCR7 antibody has been shown to be effective in preventing binding of the CXCR7 chemokine ligands SDF-1 to their cognate receptor, the inhibitory effect is even more efficient than the small molecule CXCR7 inhibitor CCX771 (D’Huys et al., 2018). However, this blocking does not inhibit the function of SDF-1 in all circumstances (Zheng et al., 2018, 2019). We hypothesized that the anti-CXCR7 antibody treatment induced the post-stroke reduction in CXCR7 expression thereby facilitating the extracellular SDF-1 to achieve a higher, more effective, concentration. This would create a more advantageous peri-infarcted microenvironment for the long-term survival of newborn neural cells,
Furthermore, the CXCR7 neutralizing antibody might exert its effect on the neurogenesis by interacting with CXCR4. CXCR7 negatively regulates CXCR4 through heterodimerization with CXCR4, inducing the internalization and degradation of CXCR4. The reduction in CXCR4 resulted in the suppression of SDF-1-CXCR4 induced cellular events (Sanchez-Alcaniz et al., 2011; Uto-Konomi et al., 2013). CXCR7 also affects the downstream signaling of CXCR4, including a weakening of calcium signaling and the Gαi-protein activation mediated by CXCR4 (Levoye et al., 2009). Our western blot results (to be published) showed anti-CXCR7 antibody treatment increased the CXCR4 expression in both ischemic and sham rats. These observations suggest that anti-CXCR7 antibody treatment reduces CXCR4 degradation, resulting in an improvement in the cell’s sensitivity to SDF-1 and its interaction with CXCR4. This promotes nerve regeneration and dendritic development in the hippocampal DG region, after focal cerebral ischemia. Elucidation of the dependency and exact effect of CXCR7 in neurogenesis after a stroke would require future studies that block the SDF-1/CXCR4 pathway, and use knockout (by genetic ablation) or knock down (via RNA silencing) of CXCR7 in vivo.
The focal cerebral ischemia induced by ET-1 in this study resulted in impairment in the performance of water maze test, which was reduced significantly by anti-CXCR7 antibody treatment. The improved cognitive functional recovery might be due to the enhanced hippocampal neurogenesis induced by anti-CXCR7 antibody, clearly shown by the results of immunofluorescent staining. Our results are in line with many previous studies that have favored the idea that promotion or suppression of hippocampal neurogenesis corresponds with improvement or deficits of learning and memory function, respectively (Winocur et al., 2006; Zhao et al., 2015; Li et al., 2018). It is interesting to note that the behavioral performance of ischemic rats improved after CXCR7 neutralization but that of the sham rats did not, indicating that there might be an upper threshold for performance improvement in non-damaged animals. It is known that the cognitive function recovery, after cerebral ischemia, depends on the activation of oxidative stress (Raz et al., 2010), signaling pathways, including notch signaling, nuclear factor-κB signaling and extracellular signal-regulated kinase pathway (Zhang et al., 2012; Feng et al., 2013; Li et al., 2013) and the release of some trophic factors and cytokines (Kiprianova et al., 1999; Gibson et al., 2005). After cortical infarcts, inflammatory processes and microglial activation occur in many brain areas, including the hippocampus, and can disturb the microenvironment of the neural progenitor cells in the DG (Stoll et al., 1998; Schroeter et al., 1999). Therefore, the discrepancy in the results of the Morris water maze test induced by neutralizing antibody might result from the change of microenvironment and the involved signaling pathway after stroke.
The Nissl staining results showed that the infarct volume of ischemic rats was not significantly altered after anti-CXCR7 antibody treatment post cerebral ischemia induction. Many studies have demonstrated that changes in infarct size do not correlate with changes in functional outcome (Hattori et al., 2000; Zhao et al., 2015), which is consistent with our results. In this study, the administration of ET-1 resulted in the infarction of both the motor cortex and the striatum, but led to an impairment of the cognitive function in the ischemic rats. Our previous study and many other studies have also shown that the spatial learning function was impaired in a stroke model without any hippocampal damage (Hattori et al., 2000; Gibson et al., 2005; Zhao et al., 2013a, b, 2015). Spatial learning ability detected by the Morris water maze has been shown to depend on different brain regions, including the entorhinal and perirhinal cortices, as well as the cortex in the prefrontal lobe and cingulate gyrus, the neostriatum and even the cerebellum, acting together to integrate into the neural network (D’Hooge and De Deyn, 2001). The improvement of cognitive function here highlights the possibility of using anti-CXCR7 antibody as a late time point treatment strategy for the functional recovery in the chronic phase after focal cerebral ischemia.
In summary, neutralization of CXCR7 with a specific function-blocking antibody, commencing 1 week after ischemic operation, resulted in significantly enhanced neurogenesis in the hippocampal DG, in parallel with increased cognitive functional recovery. These effects, at a late stage of ischemia, could arise from the elevated levels of SDF-1 and CXCR4 resulting from CXCR7 neutralization. However, it should be noted that CXCR7 function may not be inhibited completely by the administration of the CXCR7 neutralizing antibody. Further study is needed to investigate the direct effect of CXCR7 on neurogenesis in DGs by knocking out or RNA silencing of the CXCR7 receptor, and in an environment without the interaction of the SDF-1/CXCR4 signaling pathway. Our results highlight the feasibility and importance of using an anti-CXCR7 antibody as a potential strategy to improve functional recovery in the chronic phase of cerebral ischemia and indicate the need to elucidate the underlying mechanisms.
Additional file: Open peer review reports 1 (104.2KB, pdf) and 2 (104.2KB, pdf) .
Acknowledgments
We are very grateful to professional English writing editor, Dr. Thomas Dunlop from University of Eastern Finland for editing this manuscript.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81401002 (to SSZ). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All animal experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of the China Medical University (approval No. CMU16089R) on December 8, 2016. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Cheung-Ter Ong, Chia Yi Christian Hospital, Taiwan, China; Aurel Popa-Wagner, University Medicine Rostock, Germany.
P-Reviewers: Ong CT, Popa-Wagner A; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Dawes EA, Pack M, Qiu Y, Song LP; T-Editor: Jia Y
Funding: This work was supported by the National Natural Science Foundation of China, No. 81401002 (to SSZ).
References
- 1.Abe P, Wüst HM, Arnold SJ, van de Pavert SA, Stumm R. CXCL12-mediated feedback from granule neurons regulates generation and positioning of new neurons in the dentate gyrus. Glia. 2018;66:1566–1576. doi: 10.1002/glia.23324. [DOI] [PubMed] [Google Scholar]
- 2.Abe P, Mueller W, Schütz D, MacKay F, Thelen M, Zhang P, Stumm R. CXCR7 prevents excessive CXCL12-mediated downregulation of CXCR4 in migrating cortical interneurons. Development. 2014;141:1857–1863. doi: 10.1242/dev.104224. [DOI] [PubMed] [Google Scholar]
- 3.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 4.Baldauf K, Reymann KG. Influence of EGF/bFGF treatment on proliferation, early neurogenesis and infarct volume after transient focal ischemia. Brain Res. 2005;1056:158–167. doi: 10.1016/j.brainres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Banisadr G, Podojil JR, Miller SD, Miller RJ. Pattern of CXCR7 gene expression in mouse brain under normal and inflammatory conditions. J Neuroimmune Pharm. 2015;1:26–35. doi: 10.1007/s11481-015-9616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Zhang M, Li Y, Xu D, Wang Y, Song A, Zhu B, Huang Y, Zheng JC. CXCR7 mediates neural progenitor cells migration to CXCL12 Independent of CXCR4. Stem Cells. 2015;33:2574–2585. doi: 10.1002/stem.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Wang H, Zhang X, Zhao S, Zhou Z, Mu X, Zhao C, Teng W. The role of SDF-1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Front Neurosci. 2017;11:590. doi: 10.3389/fnins.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuansheng Z, Jun W, Shanshan Z, Yingxue N. Constraint-induced movement therapy enhanced neurogenesis and behavioral recovery after stroke in adult rats. Tohoku J Exp Med. 2009;218:301–308. doi: 10.1620/tjem.218.301. [DOI] [PubMed] [Google Scholar]
- 10.Cui L, Qu H, Xiao T, Zhao M, Jolkkonen J, Zhao C. Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia. Restor Neurol Neurosci. 2013;31:239–251. doi: 10.3233/RNN-120271. [DOI] [PubMed] [Google Scholar]
- 11.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 12.D’Huys T, Claes S, Van Loy T, Schols D. CXCR7/ACKR3-targeting ligands interfere with X7 HIV-1 and HIV-2 entry and replication in human host cells. Heliyon. 2018;4:e00557. doi: 10.1016/j.heliyon.2018.e00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X, Yang S, Liu J, Huang JIA, Peng JUN, Lin J, Tao J, Chen L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 14.Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- 15.Guyon A. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci. 2014;8:65. doi: 10.3389/fncel.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- 17.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Csh Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiprianova I, Sandkühler J, Schwab S, Hoyer S, Spranger M. Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol. 1999;159:511–519. doi: 10.1006/exnr.1999.7109. [DOI] [PubMed] [Google Scholar]
- 20.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Huang R, Shetty RA, Thangthaeng N, Liu R, Chen Z, Sumien N, Rutledge M, Dillon GH, Yuan F, Forster MJ, Simpkins JW, Yang SH. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol Dis. 2013;59:18–25. doi: 10.1016/j.nbd.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Liu RZ, Zeng Q, Huang ZH, Zhang JD, Liu ZL, Zeng J, Xiao H. 3’-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion. Neural Regen Res. 2018;13:1561–1567. doi: 10.4103/1673-5374.237119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Huang J, He X, Tang G, Tang Y-H, Liu Y, Lin X, Lu Y, Yang G-Y, Wang Y. Postacute stromal cell–derived factor-1α expression promotes neurovascular recovery in ischemic mice. Stroke. 2014;45:1822–1829. doi: 10.1161/STROKEAHA.114.005078. [DOI] [PubMed] [Google Scholar]
- 24.Liu PZ, Nusslock R. Exercise and hippocampal neurogenesis: a dogma re-examined and lessons learned. Neural Regen Res. 2018;13:1354–1355. doi: 10.4103/1673-5374.235225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luker KE, Steele JM, Mihalko LA, Ray P, Luker GD. Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands. Oncogene. 2010;29:4599–4610. doi: 10.1038/onc.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merino JJ, Bellver-Landete V, Oset-Gasque MJ, Cubelos B. CXCR4/CXCR7 molecular involvement in neuronal and neural progenitor migration: focus in CNS repair. J Cell Physiol. 2015;230:27–42. doi: 10.1002/jcp.24695. [DOI] [PubMed] [Google Scholar]
- 27.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes H-G, Rot A, Thelen M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyvandi AA, Roozbahany NA, Peyvandi H, Abbaszadeh HA, Majdinasab N, Faridan M, Niknazar S. Critical role of SDF-1/CXCR4 signaling pathway in stem cell homing in the deafened rat cochlea after acoustic trauma. Neural Regen Res. 2018;13:154–160. doi: 10.4103/1673-5374.224382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raz L, Zhang QG, Zhou CF, Han D, Gulati P, Yang LC, Yang F, Wang RM, Brann DW. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS One. 2010;5:e12606. doi: 10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Alcaniz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, Lopez-Bendito G, Stumm R, Marin O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Schonemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–220. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- 32.Schroeter M, Jander S, Witte OW, Stoll G. Heterogeneity of the microglial response in photochemically induced focal ischemia of the rat cerebral cortex. Neuroscience. 1999;89:1367–1377. doi: 10.1016/s0306-4522(98)00398-4. [DOI] [PubMed] [Google Scholar]
- 33.Schultheiss C, Abe P, Hoffmann F, Mueller W, Kreuder AE, Schutz D, Haege S, Redecker C, Keiner S, Kannan S, Claasen JH, Pfrieger FW, Stumm R. CXCR4 prevents dispersion of granule neuron precursors in the adult dentate gyrus. Hippocampus. 2013;23:1345–1358. doi: 10.1002/hipo.22180. [DOI] [PubMed] [Google Scholar]
- 34.Sebastian J, Chunmei Z, Nicolas T, Clemenson GD, Yan L, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song C, Xu W, Zhang X, Wang S, Zhu G, Xiao T, Zhao M, Zhao C. CXCR4 antagonist AMD3100 suppresses the long-term abnormal structural changes of newborn neurons in the intraventricular kainic acid model of epilepsy. Mol Neurol Biol. 2015;53:1518–1532. doi: 10.1007/s12035-015-9102-9. [DOI] [PubMed] [Google Scholar]
- 36.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurol Biol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Zhou Z, Liu T, Zhao M, Zhao S, Xiao T, Jolkkonen J, Zhao C. Fluoxetine enhances neurogenesis in aged rats with cortical infarcts, but this is not reflected in a behavioral recovery. J Mol Neurosci. 2016;58:233–242. doi: 10.1007/s12031-015-0662-y. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Cheng X, Wang H, Mu X, Liang Y, Luo Y, Qu H, Zhao C. dl-3-n-butylphthalide promotes neuroplasticity and motor recovery in stroke rats. Behav Brain Res. 2017;329:67–74. doi: 10.1016/j.bbr.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 39.Swanson RA, Shiraishi K, Morton MT, Sharp FR. Methionine sulfoximine reduces cortical infarct size in rats after middle cerebral artery occlusion. Stroke. 1990;21:322–327. doi: 10.1161/01.str.21.2.322. [DOI] [PubMed] [Google Scholar]
- 40.Uto-Konomi A, McKibben B, Wirtz J, Sato Y, Takano A, Nanki T, Suzuki S. CXCR7 agonists inhibit the function of CXCL12 by down-regulation of CXCR4. Biochem Biophys Res Commun. 2013;431:772–776. doi: 10.1016/j.bbrc.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Xu P, Qiu L, Zhang M, Huang Y, Zheng J. CXCR7 participates in CXCL12-mediated cell cycle and proliferation regulation in mouse neural progenitor cells. Curr Mol Med. 2016;16:738–746. doi: 10.2174/1566524016666160829153453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, Corbett D. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Mu X, Wang H, Song C, Ma W, Jolkkonen J, Zhao C. Chloride co-transporter NKCC1 inhibitor bumetanide enhances neurogenesis and behavioral recovery in rats after experimental stroke. Mol Neurobiol. 2017;54:2406–2414. doi: 10.1007/s12035-016-9819-0. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Huang G, Liu H, Chang H, Wilson JX. Folic acid enhances Notch signaling, hippocampal neurogenesis, and cognitive function in a rat model of cerebral ischemia. Nutr Neurosci. 2012;15:55–61. doi: 10.1179/1476830511Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XQ, Mu JW, Wang HB, Jolkkonen J, Liu TT, Xiao T, Zhao M, Zhang CD, Zhao CS. Increased protein expression levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be associated with enhanced neurogenesis induced by environmental enrichment. Mol Med Rep. 2016;14:2231–2237. doi: 10.3892/mmr.2016.5470. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Zhang H, Lin S, Chen X, Yao Y, Mao X, Shao B, Zhuge Q, Jin K. SDF-1/CXCR7 chemokine signaling is induced in the peri-infarct regions in patients with ischemic stroke. Aging Dis. 2018;9:287–295. doi: 10.14336/AD.2017.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao S, Zhao M, Xiao T, Jolkkonen J, Zhao C. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke. 2013b;44:1698–1705. doi: 10.1161/STROKEAHA.111.000361. [DOI] [PubMed] [Google Scholar]
- 49.Zhao S, Qu H, Zhao Y, Xiao T, Zhao M, Li Y, Jolkkonen J, Cao Y, Zhao C. CXCR4 antagonist AMD3100 reverses the neurogenesis and behavioral recovery promoted by forced limb-use in stroke rats. Restor Neurol Neurosci. 2015;33:809–821. doi: 10.3233/RNN-150515. [DOI] [PubMed] [Google Scholar]
- 50.Zhao SS, Zhao Y, Xiao T, Zhao M, Jolkkonen J, Zhao CS. Increased neurogenesis contributes to the promoted behavioral recovery by constraint-induced movement therapy after stroke in adult rats. CNS Neurosci Ther. 2013a;19:194–196. doi: 10.1111/cns.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng J, Wang H, Zhou W. Modulatory effects of trophoblast-secreted CXCL12 on the migration and invasion of human first-trimester decidual epithelial cells are mediated by CXCR4 rather than CXCR7. Reprod Biol Endocrinol. 2018;16:17. doi: 10.1186/s12958-018-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng N, Liu W, Chen J, Li B, Liu J, Wang J, Gao Y, Shao J, Jia L. CXCR7 is not obligatory for CXCL12-CXCR4-induced epithelial-mesenchymal transition in human ovarian cancer. Mol Carcinogen. 2019;58:144–155. doi: 10.1002/mc.22916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.