Abstract
Neuronal mitochondrial dysfunction increases inflammatory mediators and leads to free radical generation and anti-oxidant enzymatic alterations, which are major neuropathological hallmarks responsible for autism. Mitochondrial dysfunction in autism is associated with decreased ATP levels due to reduced levels of cyclic adenosine monophosphate. Rat models of autism were established by intracerebroventricular injection of propionic acid. These rat models had memory dysfunction, decreased muscle coordination and gait imbalance. Biochemical estimation of propionic acid-treated rats showed changes in enzyme activity in neuronal mitochondrial electron transport chain complexes and increases in pro-inflammatory cytokines, oxidative stress and lipid biomarkers. Oral administration of 10, 20 and 30 mg/kg adenylate cyclase activator forskolin for 15 days reversed these changes in a dose-dependent manner. These findings suggest that forskolin can alleviate neuronal mitochondrial dysfunction and improve neurological symptoms of rats with autism. This study was approved by the RITS/IAEC, SIRSA, HARYANA on March 3, 2014 (approval No. RITS/IAEC/2014/03/03).
Keywords: adenylate cyclase, ATP, autism, forskolin, mitochondrial dysfunction, neuroinflammation, oxidative stress, propionic acid
Chinese Library Classification No. R453; R364; R749
Introduction
Autism is a severe and pervasive behavioural neurodevelopmental disorder (Choudhuryet al., 2012) characterized by impaired social behaviour and communication, repetitive behaviors (Myers et al., 2007), and sensory changes (Al-Lahham et al., 2010). Pathological hallmarks of autism include mitochondrial dysfunction, oxidative stress, neuroinflammation, neuroexcitation, abnormal synapse formation, over expression of glial cells in autism-related brain regions (the cerebellum, cerebral cortex, amygdala and hippocampus), neurotransmitter abnormalities (including decreased gamma amino butyric acid, increased levels of glutamate, enhanced dopamine level and dysregulation of serotonergic system and oxytocin in the brain) (McDougle et al., 2005; Al-Gadani et al., 2009; Morgan et al., 2010). Bowel dysfunction and gastrointestinal disturbances have been reported in patients with autism (de Theije et al., 2011).
Chemical toxins such as valproic acid and propionic acid play an important role in the neurodevelopment of autism (Sandhya et al., 2012). If a mother is exposed to thalidomide, a sedative or anti-vomiting medication, during pregnancy, then the offspring has the risk of limb deformities and autism-like behavioral disorders (Strömland et al., 1994; Lawler et al., 2004; Ragini et al., 2011). Propionic acid (PPA) is a food preservative (Zárate et al., 2004) that passes the lipid bilayer of cell membrane and is responsible for intracellular acidification, leading to neurotransmitter alterations (including biosynthesis and release of dopamine, glutamate and serotonin), and mainly affecting behavioral function (Brockand Buckel, 2004; Gupta et al., 2008). PPA inhibits Na+/K+ ATPase activity, increases neuroexcitation which can enhance neuronal depolarization, leading to impaired locomotor activity (Wyse et al., 1998), and also promotes intracellular calcium release. PPA can affect mitochondrial metabolism, and increase oxidative stress and synaptic transmission in the brain of individuals with autism (Nakao et al., 1992). PPA induction hampers cellular metabolites in tri-carboxylic acid cycle where it interferes with the conversion of succinyl co-A to succinate for generation of FADH2 which is consumed by the complex-II, further impairs the ATP synthesis, resulting in mitochondrial dysfunction, and interferes with overall cellular metabolism (Macfabe, 2013). Administration of PPA to rodents resulted in CNS lesions that selectively target right lateral ventricle, striatum, cortex, cerebellum, hippocampus and amygdala especially in autism (Xu et al., 2005).
In autism, mitochondrial functioning altered specially in dorsal prefrontal cortex that is associated with language and neuropsychological deficits, mental retardation, learning disabilities, seizures and gastrointestinal disturbances (Minshew et al., 1993; Aliev et al., 2009).
Cyclic adenosine mono-phosphate (cAMP) is recognized as universal cell regulator and is generated by catalysis of the adenylcyclase (AC) from ATP (Lee et al., 2013; Li et al., 2018). Immune function, growth, differentiation, gene expression and metabolism need cAMP signaling. cAMP activates cAMP dependent protein kinase (cAMP/PKA), which results in phosphorylation of CREB (Schenk and Snaar-Jagalska, 1999). CREB is a constitutively expressed transcriptive agent in the nuclei of hippocampal neurons, in the inner mitochondrial compartment as well as in the nucleus (Cammarota et al., 1999).
Diterpenoid Forskolin (FSK) obtained from Coleus forskohlii, upregulates adenyl cyclase by increasing intracellular cAMP and leading to various physiological effects (Mehan et al., 2017). At present, many drugs are available but they provide only symptomatic relief and do not inhibit the progression of autism. Therefore, therapeutic approaches for new pharmacological interventions remained unclear. Mitochondrial dysfunction occurs mostly in the autistic individuals, due to which there is decreased level of cAMP responsible for low production of ATP (Song et al., 1997). Moreover, increased cAMP secondary messenger level or CREB phosphorylation can be obtained with direct adenyl cyclase activator FSK. Therefore, in this study, we investigated the neuroprotective potential of FSK in PPA-intoxicated rats.
Materials and Methods
Experimental animals
Wistar rats of either gender, weighing 220–250 g, aged 8–9 months, were obtained from the Animal House of Rajendra Institute of Technology & Sciences (RITS) (Sirsa, Haryana, India) and included in this study. These animals were kept in polyacrylic cages with a wire mesh roof and soft sand under specific situations of 12-hour dark/light cycle, ad libitum food and water at 23 ± 2°C. The study protocol was approved by the RITS/IAEC, SIRSA, HARYANA (RITS/IAEC/2014/03/03) on March 3, 2014 and conducted in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. Animals were acclimated to laboratory situations before experimentation.
Drug treatment and experiment schedule
PPA was procured from Sigma-Aldrich (Delhi, India) and FSK was provided as ex-gratia sample by BAPEX, Rajasthan, India. Analytical grade chemicals were used apart from PPA and FSK. Solutions of the drugs and chemicals were freshly prepared. 0.26 M PPA was prepared and 6.0 µL/kg PPA was given by intracerebroventricular injection for 8 successive days. A mixture of water and 2% ethanol was used to solubilize FSK and administered orally (p.o.). In this study, rats were randomly divided into six groups, with six animals per group. Experimental protocol schedule and parameter evaluation are presented in Table 1 and Figure 1.
Table 1.
Experimental grouping of animals
| Group | Pharmacological interventions | Dose | Route | Duration |
|---|---|---|---|---|
| 1 | Normal control | – | – | 22 days |
| 2 | FSK only | 30 mg/kg per day | p.o. | 22 days |
| 3 | PPA control | 6 µL/kg per day | i.c.v. | 8 days |
| 4 | FSK+PPA | 10 mg/kg per day | p.o. | 22 days |
| 6 µL/kg per day | i.c.v. | 8 days | ||
| 5 | FSK+PPA | 20 mg/kg per day | p.o. | 22 days |
| 6 µL/kg per day | i.c.v. | 8 days | ||
| 6 | FSK+PPA | 30 mg/kg per day | p.o. | 22 days |
| 6 µL/kg per day | i.c.v. | 8 days |
FSK: Forkolin; PPA: propionic acid.
Figure 1.
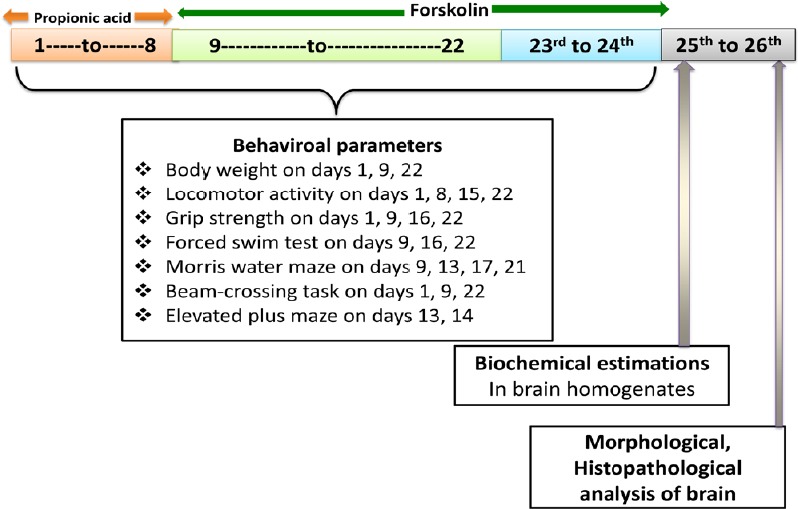
Experimental design (behaviroal and biochemical measurements)
Establishment of rat models of autism induced by intracerebroventricular injection of PPA
Rats were anesthetized by intraperitoneal injection of 75 mg/kg ketamine. The body of anesthetized rat was placed on warm pad with the head positioned in the stereotactic frame (Stoelting Co., Wood Dale, IL, USA). A hole was drilled through the parietal bone to access the right lateral cerebral ventricle (stereotactic coordinates: antero-posterior from bregma 1.3 mm, medio-lateral from mid-sagittal suture 1.8 mm, dorso-ventral from the skull 3.0 mm) (Thomas et al., 2012) and a cannula of 2.5 cm in length was inserted in the burr hole and the cannula was locked using plastic earpin. On day 1 to day 8, PPA (4.0 μL of 0.26 M solution) was injected through a hypodermic needle with 0.4 mm external diameter attached to a 10 μL of Hamilton microliter syringe into the left lateral cerebral ventricle within 10 minutes (1 μL/min). After injection, the Hamilton microneedle was left in place for 5 minutes to facilitate diffusivity of drug in CSF (MacFabe et al., 2008; Shultz et al., 2008; Thomas et al., 2010).
Behavioral parameters
Assessment of memory and cognitive performance
According to a previous study (Morris, 1984), the Morris water maze was performed on days 9, 13, 17 and 21 of the experimental scheme to assess the spatial learning and memory of rodents. A circular tank was divided into four quadrants and the platform was submerged underneath 2 cm in water in fourth quadrants. All rats received four consecutive days of training, with each trial having a ceiling time of 120 seconds and a trial interval of approximately 30 seconds. The rat had to swim until it climbed onto the platform. After climbing onto the platform, the animal remained there for 30 seconds before the commencement of the next trial. If the rat failed to reach the escape platform within the maximally allowed time of 120 seconds, it was guided with the help of a rod and allowed to remain on the platform for 30 seconds. The time taken by rats to locate the hidden platform (latency in seconds) and the time spent in target quadrant zone (TSTQ) was recorded. The TSTQ represents the degree of memory consolidation in animals after learning (Kaur at al., 2015).
Assessment of spontaneous locomotion
Locomotion was evaluated on days 1, 8, 15 and 22. Each animal’s locomotion activity was analyzed using actophotometer (INCO, Ambala, India)(counts per 5 minutes)(Singh et al., 2015).
Assessment of grip strength
Grip strength of animals was measured using grip strength meter (Chatillon, USA) (Olsson et al., 1995). Grip strength allows the neuromuscular function of rats by determining the maximal peak force developed by fore limb to measure grip strength. A rat was held by the tail and lowered towards the apparatus observed in percent fall of time until it grabbed a handle with both front paws. Rats were gently pulled back until they released their grip from the handle. The maximum force exerted by rodent instantly on immediate discharge of the paw grip from the grip stage was measured in percent fall of time as a degree of grip strength.
Assessment of memory retention
Elevated plus maze (EPM) (Kumar et al., 2007) was used to assess working memory in rodents. EPM task was performed on days 21 and 22. Acquisition and retention memory processes were assessed using the EPM. On day 21, acquisition transfer latency (TL1) was measured by placing rats individually at the end of one open arm facing away from central platform and the time took to move from the open arm to either of the enclosed arm with all its four legs cross to the enclosed arm was recorded. When the rat did not enter the enclosed arm for 90 seconds, it was gently pushed on the back into the enclosed arm and the transfer latency was assigned 90 seconds. The rat was allowed to move freely in the plus maze regardless of open and closed arms for 20 seconds after measurement of transfer latency. On day 22, retention transfer latency (TL2) was recorded in the same manner as in the acquisition trial. Usually, transfer latency was shortened on the second day. The percentage memory retention [(TL1 – TL2) × 100/TL1] was calculated. The maze was cleaned after each trial.
Assessment of neuromuscular coordination
Beam-crossing task (BCT) (Tomkins et al., 2007) was performed to observe the gait dysfunction and measure total counts of foot slips. Rats were allowed to travel on beam from one side to another side and total number of foot slips was counted. Acquisition and retention memory processes were assessed using the EPZ. EPZ was used to assess working memory in rodents. EPM task was performed on days 21 and 22. On day 21, acquisition transfer latency (TL1) was recorded by placing rats individually at the end of one open arm facing away from central platform and the time took to move from the open arm to either of the enclosed arm with all its four legs cross to the enclosed arm was recorded. When the rat did not enter the enclosed arm for 90 seconds, it was gently pushed on the back into the enclosed arm and the transfer latency was recorded as 90 seconds. The rat was allowed to move freely in the plus maze regardless of open and closed arms for 20 seconds after the measurement of transfer latency. On day 22, retention transfer latency (TL2) was recorded in the same manner as in the acquisition trial. Usually, transfer latency was shortened on the second day, and relative to the first, percentage memory retention [(TL1 – TL2) × 100/TL1] was calculated. Additionally motor performance of rats in terms of neurological scores was scored on a scale ranging from 0 to 4. A score of 0 was assigned to animal that could readily transverse the beam. Scores 1, 2 and 3 were given to the animals that demonstrated mild, moderate and severe impairment, respectively. Score 4 was assigned to the animals completely unable to walk on beam. The maze was cleaned after each trial.
Assessment of depressive behaviors
Forced swim test (FST) was well established to determine rat depressive behaviors. The first trial was performed for 15 minutes followed by the second trial 24 hours later, with an exposure for 5 minutes which represents the duration of immobility (Abelaira et al., 2013).
Estimation of biochemical parameters in rat brain
Rat brain homogenate preparation
On day 22, rats were decapitated, and their brains were removed and cleaned with isotonic cold saline solution. The brains were then homogenized with 10 times (w/v) 0.1 M cold phosphate buffer (7.4), homogenated and centrifuged at 10,000 × g for 20 minutes. Supernatants were separated and aliquots were used for neurochemical measurements.
Measurement of protein content
Agappe protein estimation kit (Agappe Diagnostics, Delhi, India) was used to evaluate the protein content using Biuret method.
Measurement of enzymatic activity of mitochondrial-ETC complexes
Mitochondrial ETC complex-I (NADPH dehydrogenase) enzyme assay: Complex-I activity was measured by the oxidation rate of NADH at 340 nm in an assay medium for duration of 3 minutes at 37°C. Complex-I activity was expressed as % control (Höglinger et al., 2003).
Mitochondrial ETC complex-II (succinate dehydrogenase/SDH) enzyme assay: 0.3 mL of sodium succinate solution was mixed with 50 μL of homogenate and absorbance at 490 nm was measured with ultraviolet spectrophotometer. Data was expressed as INT reduced µmol/mg tissue protein (Bhateja et al., 2012).
Mitochondrial ETC complex-IV (cytochrome oxidase) enzyme assay: 0.3 mM reduced cytochrome c was mixed with 75 mM phosphate buffer. Reaction was further initiated by the mixing solubilised brain homogenate mitochondrial sample and absorbance at 550 nm was measured with ultraviolet spectrophotometer (Sottocasa et al., 1967).
Mitochondrial ETC complex-V (ATPase) enzyme assay: To inactivate ATPase, aliquots of mitochondrial sample were sonicated with perchloric acid (0.1 N) and centrifuged at 14,000 × g for 5 minutes at 4°C. 1 N NaOH was used to neutralize the sample containing ATP at –80°C. Complex-V enzyme assay was performed by high performance liquid chromatography at 254 nm (Ramanatha et al., 2012).
Measurement of AChE levels
According to a previous study by Ellman et al. (1961), measurement of acetyl cholinesterase (AChE) enzyme level was measured. The absorbance was assessed at 412 nm. AchE enzyme level was indicated as µM/mg protein.
LDH enzymatic assay
Biochemical estimation kit (Trans Asia, Delhi, India) was utilized to assess lactate dehydrogenase (LDH) enzymatic activity in rat brain homogenate indicated as IU/L (Searey, 1969).
Measurement of malondialdehyde levels
The measurement of malondialdehyde (MDA) in brain homogenate was estimated according to a previous study (Wills, 1996). The levels of MDA were expressed as nmol/mg tissue protein.
Measurement of reduced glutathione levels
Measurement of reduced glutathione (GSH) levels in the brain were measured according a previous report (Ellman et al., 1959). Results were calculated and expressed as µmol/mg tissue protein.
Measurement of nitrite concentration
Using Greiss reagent, accumulation of nitrite in the homogenate was assayed as given by Green et al. (1982). Nitrite concentrations were determined by spectrophotometric analysis at 540 nm, where standard curve was plotted by sodium nitrite and values were expressed as µM/mg protein (Tietze 1969).
Measurement of superoxide dismutase activity
Measurement of superoxide dismutase (SOD) activity was measured according to the method of Misra and Frodvich (1972). The absorbance was spectrophotometrically quantified at 480 nm. Results are expressed as % control (Misra and Fridovich, 1972).
Measurement of catalase activity
Catalase activity was measured according to a previous method (Green et al., 1982). The final degradation rate of H2O2 was measured spectrophotometrically at 240 nm. Catalase activity was expressed as µM/H2O2 decomposed/min activity.
Measurement of neuroinflammatory markers in rat brain homogenate
KRISHGEN BioSystem (Bio-RAD, India) was used to measure tumor necrosis factor-α (TNFα), interleulin-1 (IL-1), and interleukin-6 (IL-6) levels according to the method of Singh and Kumar (2016).
Statistical analysis
All data are presented as the mean ± SEM and analyzed via GraphPad Prism 5.0 software for Windows (GraphPad Software, San Diego, CA, USA). Results were analyzed using two-way analysis of variance followed by Bonferroni test, one-way analysis of variance and Tukey’s post hoc multiple comparison analysis test. P < 0.05 was considered statistically significant.
Results
Effects of FSK on behaviors of rats with autism
Memory and cognitive performance
A significant increase in escape latency on day 9 was observed in PPA-treated rats compared to normal and FSK-administered rats (P < 0.05). There was no significant difference in increase in escape latency between normal and FSK-treated rats (P > 0.05).
On days 13 and 17, significant reduction in escape latency was observed in FSK30 mg/kg-treated autistic rats than in FSK 10 and 20 mg/kg-treated autistic rats (P < 0.05; Figure 2). TSTQ was performed on day 22. PPA-treated rats showed major reduction in TSTQ when compared to normal and FSK-treated rats (P < 0.05). Administration of 10 and 20 mg/kg FSK led to significant increase in TSTQ when compared with PPA-treated group (P < 0.05). Administration of 30 mg/kg FSK caused notable recovery in memory function when compared with 10 and 20 mg/kg FSK in PPA-intoxicated rats (P < 0.05; Figure 3).
Figure 2.
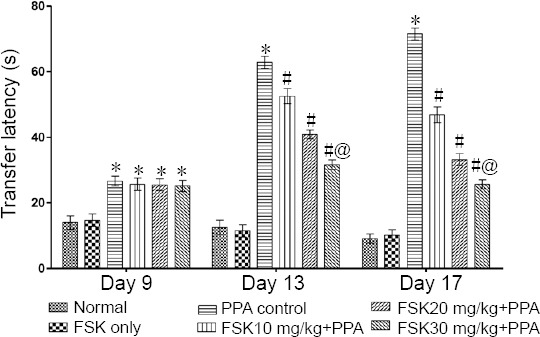
Effect of FSK on memory and cognitive performance in rats with autism induced by intracerebroventricular injection of PPA as shown by Morris water maze test results.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P <0.05, vs. PPA control; @P < 0.05, vs. FSK 10 mg/kg + PPA and FSK 20 mg/kg + PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Figure 3.
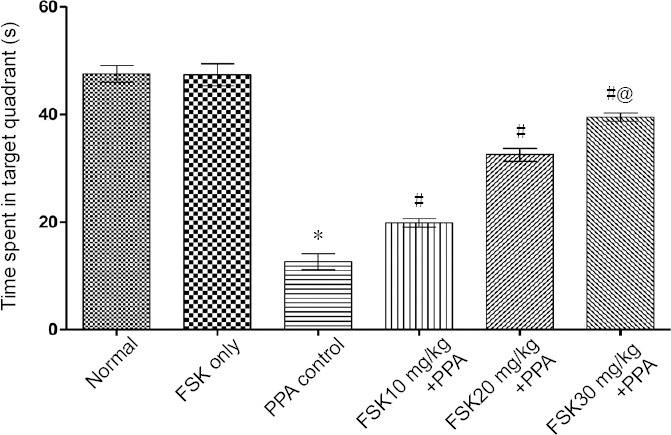
Effect of FSK on time spent in target quadrant zone in rats with autism induced by intracerebroventricular injection of PPA as shown by Morris water maze test results.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P <0.05, vs. PPA control; @P < 0.05, vs. FSK 10 mg/kg + PPA treatment and FSK 20 mg/kg + PPA treatment. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Spontaneous locomotion
PPA-treated rats showed a significant elevation in locomotor activity as compared to normal and FSK only-treated rats (P < 0.05). No significant change in spontaneous locomotion was observed in FSK only-treated rats than in normal rats (P > 0.05) on days 1, 8, 15, and 22. 10, 20, and 30 mg/kg FSK-treated rats showed significant reduction in elevated locomotion counts than PPA-treated rats on days 8, 15, and 22 (P < 0.05; Figure 4).
Figure 4.
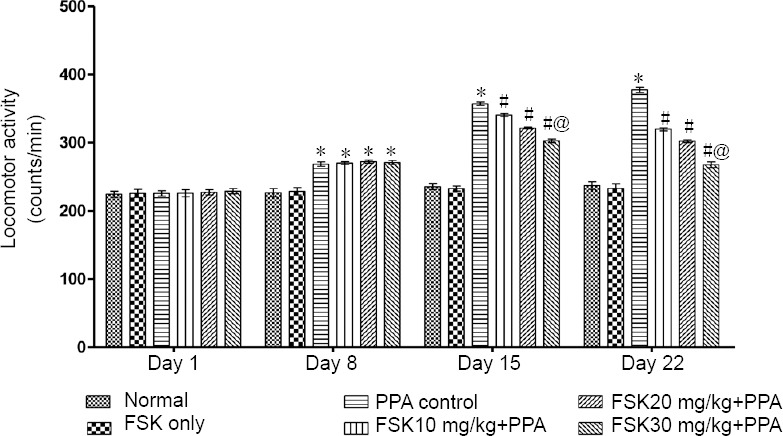
Effect of FSK on spontaneous locomotion in rats with autism induced by intracerebroventricular injection of PPA as determined by actophotometer.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK 10 mg/kg + PPA and FSK 20 mg/kg + PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Grip strength
On day 1, change in grip strength was insignificant in all groups compared with before treatment (P > 0.05). On days 9, 15 and 22, significant loss in grip strength was observed in FSK-treated rats compared with normal rats (P < 0.05). Grip strength was significantly improved in 10, 20, and 30 mg/kg FSK treated rats than in normal rats (P < 0.05; Figure 5).
Figure 5.
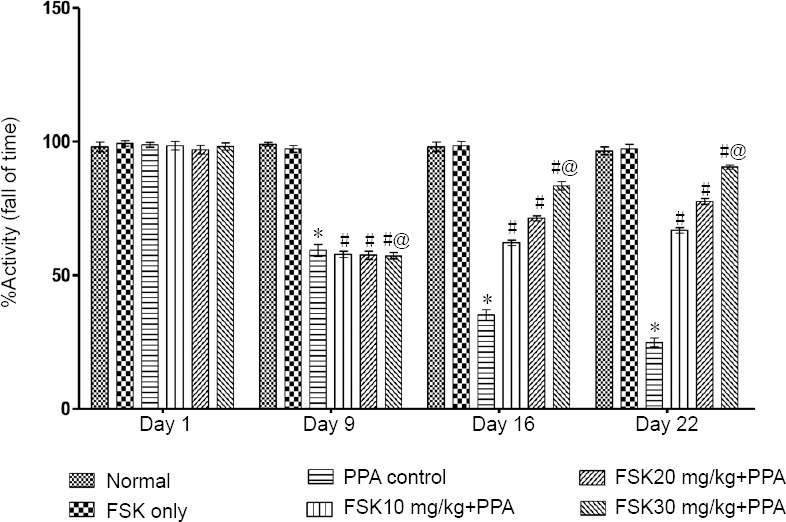
Effect of FSK on grip strength in rats with autism induced by intracerebroventricular injection of PPA as determined by string test.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK 10 mg/kg + PPA and FSK 20 mg/kg +PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Memory retention (working memory)
Percent memory retention was recorded on day 21 of protocol schedule to investigate working memory. Transfer latency was recorded on day 22 to evaluate learning and memory. On day 22, PPA-treated rats showed marked increase in transfer latency as compared to normal and FSK-administered rats (P < 0.05). There was no significant difference in memory retention between FSK-treated and normal rats. Transfer latency was obviously decreased in 10, 20 and 30 mg/kg FSK treated rats than in PPA-treated rats (P < 0.05; Figure 6).
Figure 6.
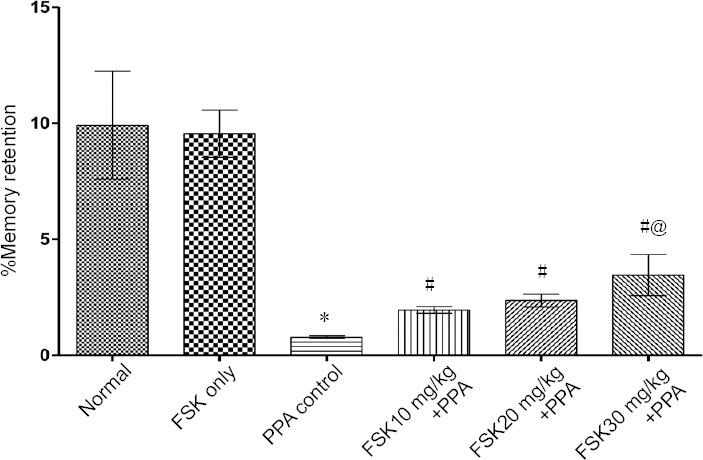
Effect of FSK on transfer latency in rats with autism induced by intracerebroventricular injection of PPA as determined by elevated plus maze task.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA only; @P < 0.05, vs. FSK 10 mg/kg + PPA and FSK 20 mg/kg +PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Neuromuscular coordination
On day 1, change in neuromuscular coordination was insignificant in all groups compared with before treatment (P > 0.05). Neuromuscular coordination was reduced as increased number of slips and walking impairment was alleviated as assessed by beam crossing task on days 9 and 22 in PPA-treated rats than in normal and FSK-treated rats. Neuromuscular coordination was not significantly altered in FSK only-treated rats than in normal rats (P < 0.05). On days 9 and 22, the number of slips was remarkably reduced and the balance and walking were improved in 10, 20, and 30 mg/kg FSK-treated rats than in PPA-treated rats (P < 0.05; Figures 7 and 8). Furthermore, 30 mg/kg was more potent than 10 and 20 mg/kg FSK.
Figure 7.
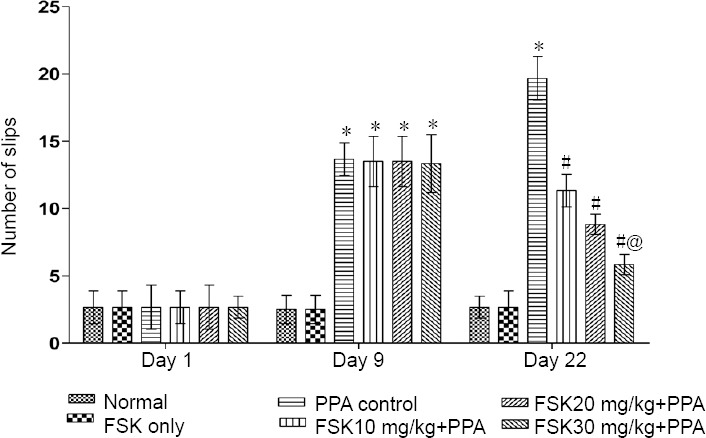
Effect of FSK on neuromuscular coordination (number of slips) in rats with autism induced by intracerebroventricular injection of PPA as determined by beam-crossing task.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK 10 mg/kg + PPA and FSK 20 mg/kg + PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Figure 8.
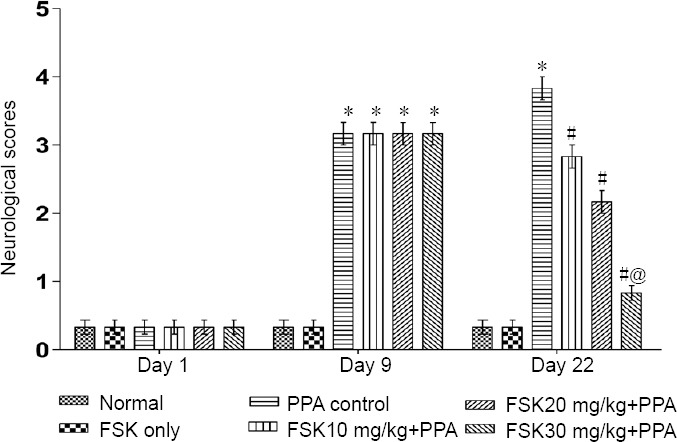
Effect of FSK on neuromuscular coordination (neurological scores) in rats with autism induced by intracerebroventricular injection of PPA as determined by beam-crossing task.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK 10 mg/kg + PPA and FSK 20 mg/kg + PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Stress-like behaviors
On days 9, 16 and 22, a significant increase in immobility was found in PPA-treated rats than in normal rats (P < 0.05). The immobility time was significantly reduced in rats treated with 10, 20 and 30 mg/kg FSK than in PPA-treated rats (P < 0.05; Figure 9).
Figure 9.
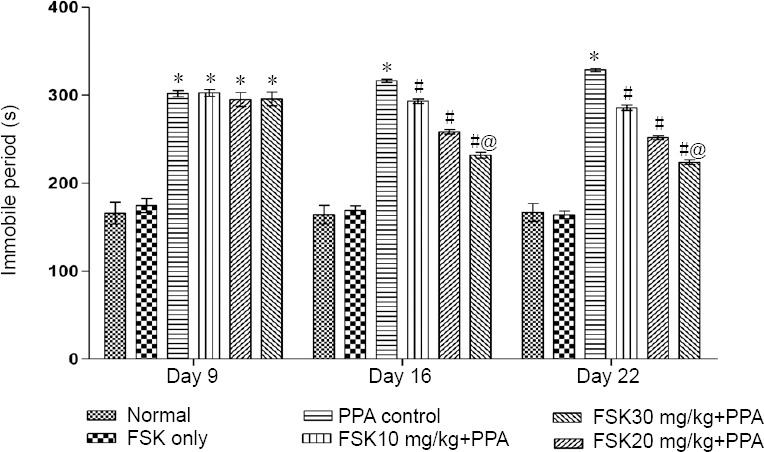
Effect of FSK on stress-like behaviors in rats with autism induced by intracerebroventricular injection of PPA as determined by forced swim test.
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK10 mg/kg + PPA and FSK20 mg/kg + PPA. Two-way analysis of variance followed by Bonferroni test. FSK: Forkolin; PPA: propionic acid.
Effect of FSK on biochemical markers in rat brain
Mitochondrial enzyme activity
In PPA intoxicated autistic rats, mitochondrial enzyme activity of complex-I, II, IV and V was remarkably decreased compared with normal and FSK treated rats (P < 0.05; Table 2). Treatment with oral 10, 20, and 30 mg/kg FSK significantly restored PPA mediated reduction in levels of complex-I, II, IV and V when compared with PPA treated autistic rats (P < 0.05). Among the treatment doses of direct adenyl cyclase activator FSK, highest dose of 30 mg/kg (p.o.) was found to be most effective when compared with FSK 10 and 20 mg/kg groups.
Table 2.
Effect of FSK on neuromitochondrial ETC complexes enzymatic assay in the brain homogenate of rats with autism induced by intracerebroventricular injection of PPA
| Treatment | Complex I (%control) | Complex II (nmol/mg protein) | Complex IV (%control) | Complex V (μmol/g tissue) |
|---|---|---|---|---|
| Normal | 100.0±0.0 | 7.34±0.61 | 100.0±0.0 | 607.5±14.52 |
| FSK only | 91.67±1.03 | 7.28±0.67 | 94.20±2.53 | 607.2±16.04 |
| PPA control | 25.83±3.71* | 1.34±0.37* | 32.73±2.34* | 208.5±15.83* |
| FSK 10mg/kg+PPA | 37.50±4.08# | 3.04±0.63# | 47.35±3.71# | 300.2±16.80# |
| FSK 20mg/kg +PPA | 52.50±8.01# | 4.76±0.59# | 66.20±3.92# | 392.2±22.29# |
| FSK30mg/kg +PPA | 74.00±7.48#@ | 6.15±0.42#@ | 81.61±2.59#@ | 485.5±20.74#@ |
Data expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK10 mg/kg + PPA and FSK 20 mg/kg + PPA (one-way analysis of variance followed by Tukey’s post hoc test). FSK: Forkolin; PPA: propionic acid.
LDH and AChE
Lactatedehydrogenase (LDH) and AChE significantly increased in PPA control as compared to normal and FSK groups in rat brain homogenate (P < 0.05). FSK 10, 20 and 30 mg/kg p.o. significantly reduced the LDH as compared with PPA control group (P < 0.05). FSK treatment at a high dose of 30 mg/kg (p.o.) was significantly more effective when compared with low doses (10 and 20 mg/kg, orally) of FSK treated rats (P < 0.05; Table 3).
Table 3.
Effect of FSK on AChE and LDH levels in the brain homogenate of rats with autism induced by intracerebroventricular injection of PPA
| Treatment | LDH (IU/L) | AchE (μmol/mg protein) |
|---|---|---|
| Normal | 393.5±13.98 | 12.12±0.64 |
| FSK only | 394.2±13.32 | 12.20±0.50 |
| PPA control | 1114±26.69* | 39.31±0.82* |
| FSK10mg/kg+PPA | 876.8±22.73# | 33.84±0.63# |
| FSK20mg/kg+PPA | 704.8±15.00# | 27.46±0.87# |
| FSK30mg/kg+PPA | 460.3±18.01#@ | 20.59±0.98#@ |
Data expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK10 mg/kg + PPA and FSK 20 mg/kg + PPA (one-way analysis of variance followed by Tukey’s post hoc test). AChE: Acetyl cholinesterase; FSK: forkolin; LDH: lactate dehydrogenase; PPA: propionic acid.
Oxidative stress markers
MDA and nitrite levels: MDA and nitrite levels in rat brain homogenate were increased in PPA-treated rats than in normal and FSK-treated rats (P < 0.05). There were no obvious differences in MDA and nitrite levels between FSK-treated and normal rats. MDA and nitrite levels were significantly reduced in 10 and 20 mg/kg FSK-treated rats than in PPA-treated rats (P < 0.05). MDA and nitrite levels were significantly reduced in 30 mg/kg FSK-treated rats than in PPA-treated rats or low doses (10 and 20 mg/kg) of FSK-treated rats (P < 0.05; Table 4).
Table 4.
Effect of FSK on oxidative stress markers (MDA, GSH and nitrite) in the brain homogenate of rats with autism induced by intracerebroventricular injection of PPA
| Treatment | MDA (nmol/mg protein) | GSH (µmol/mg protein) | Nitrite (µmol/mg protein) |
|---|---|---|---|
| Normal | 1.20±0.30 | 11.31±0.56 | 1.27±0.14 |
| FSK only | 1.25±0.29 | 11.54±0.50 | 1.25±0.22 |
| PPA control | 11.73±0.90* | 1.96±0.51* | 9.25±0.31* |
| FSK10mg/kg+PPA | 7.1±0.75# | 3.04±0.22# | 5.87±0.14# |
| FSK20mg/kg+PPA | 4.24±0.41# | 4.07±0.24# | 3.54±0.20# |
| FSK30mg/kg+PPA | 2.69±0.38#@ | 5.34±0.24#@ | 2.18±0.19#@ |
Data expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK10 mg/kg + PPA and FSK 20 mg/kg + PPA (one-way analysis of variance followed by Tukey’s post hoc test). FSK: Forkolin; GSH: reduced glutathione; MDA: malondialdehyde; PPA: propionic acid.
GSH levels: Systematic administration of PPA resulted in remarkable reduction of GSH level in the brain homogenate when compared with normal and FSK-treated rats (P < 0.05). There was no significant difference in GSH level between FSK-treated rats and normal rats. Administration of 10 and 20 mg/kg FSK resulted in remarkable improvement in reduced GSH level. Moreover, reduced GSH level was significantly restored in 30 mg/kg FSK-treated rats than in PPA-treated and low doses (10 and 20 mg/kg, orally) of FSK-treated rats (P < 0.05; Table 4).
SOD and catalase activities: SOD and catalase activities were significantly decreased in PPA-treated rats than in normal and FSK-treated rats (P < 0.05). FSK administration did not show any alterations in SOD and catalase activities as compared with normal rats. SOD and catalase activities in rats treated with 10 and 20 mg/kg FSK were significantly restored than those in PPA-treated rats (P < 0.05). Treatment with 30 mg/kg FSK significantly increased SOD and catalase activities when compared with PPA-treated rats (P < 0.05). High dose (30 mg/kg) of FSK was more effective in restoring SOD and catalase activities than low doses (10 and 20 mg/kg) of FSK (P < 0.05; Table 5).
Table 5.
Effect of FSK on oxidative stress markers (SOD and catalase) in the brain homogenate of rats with autism induced by intracerebroventricular injection of PPA
| Treatment | SOD (%control) | Catalase (%control) |
|---|---|---|
| Normal | 100.0±0.0 | 100.0±0.0 |
| FSK only | 69.67±1.86 | 98.33±1.21 |
| PPA control | 19.33±5.31* | 35.17±1.60* |
| FSK10mg/kg+PPA | 39.67±2.50# | 56.67±1.21# |
| FSK20mg/kg+PPA | 55.67±4.03# | 67.50±1.87# |
| FSK30mg/kg+PPA | 83.50±4.59#@ | 82.50±2.07#@ |
Data expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK10 mg/kg + PPA and FSK20 mg/kg + PPA (one-way analysis of variance followed by Tukey’s post hoc test). FSK: Forkolin; PPA: propionic acid; SOD: superoxide dismutase.
Neuroinflammatory markers
TNF-α, IL-1β, and IL-6: TNF-α, IL-1β, and IL-6 levels were remarkably increased in PPA-treated rats than in normal and FSK-treated rats (P < 0.05). There were no significant differences in TNF-α, IL-1β, and IL-6 levels between normal and FSK-treated rats. However, 10, 20 and 30 mg/kg FSK treatment consequentially decreased TNF-α, IL-1β, and IL-6 levels than PPA administration (P < 0.05). High dose (30 mg/kg) of FSK significantly reduced TNF-α, IL-1β, and IL-6 levels than low doses (10 and 20 mg/kg) of FSK (P < 0.05; Table 6).
Table 6.
Effect of FSK on neuroinflammatory markers (IL-1β, TNF-α and IL-10) in the brain homogenate of rats with autism induced by intracerebroventricular injection of PPA
| Treatment | TNF-α (pg/mg protein) | IL-1β (pg/mg protein) | IL-10 (pg/mg protein) | IL-6 (pg/mg protein) |
|---|---|---|---|---|
| Normal | 32.02±2.09 | 66.53±3.64 | 51.31±2.52 | 44.76±3.36 |
| FSK only | 32.74±2.53 | 66.55±3.04 | 52.44±2.47 | 45.67±3.01 |
| PPA control | 371.3±17.67* | 517.9±15.41* | 32.13±1.72* | 250.7±7.15* |
| FSK10mg/kg+PPA | 274.2±12.87# | 408.0±7.46# | 41.11±1.66# | 209.3±7.11# |
| FSK20mg/kg+PPA | 226.9±19.45# | 348.4±10.96# | 48.03±2.00# | 160.2±3.78# |
| FSK30mg/kg+PPA | 168.9±13.22#@ | 245.6±8.70#@ | 52.96±1.56#@ | 122.8±6.04#@ |
Data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. normal and FSK only; #P < 0.05, vs. PPA control; @P < 0.05, vs. FSK10 mg/kg + PPA and FSK20 mg/kg + PPA (one-way analysis of variance followed by Tukey’s post hoc test). FSK: Forkolin; IL: interleukin; PPA: propionic acid; TNF-α: tumor necrosis factor alpha.
IL-10 level: IL-10 level was significantly decreased in PPA-treated rats than in normal and FSK only-treated rats (P < 0.05). There was no significant difference in IL-10 level between normal and FSK-treated rats. IL-10 level was significantly increased in rats treated with each FSK dose (10, 20 and 30 mg/kg) than in PPA-treated rats (P < 0.05). 30 mg/kg FSK was more potent in restoring anti-inflammatory maker IL-10 level than 10 and 20 mg/kg FSK (P < 0.05; Table 6).
Discussion
Neurodevelopmental disorders, such as autism, cause reduced social interaction, repetitive and disordered movements (Choudhury et al., 2012). Despite the availability of various treatment strategies, the natural therapies contribute to the effective recovery of neurodegenerative disorders (Velmurugan et al., 2018). Here, in this study, FSK has been utilized for improving mitochondrial dysfunction in rats with autism induced by PPA. The major role of cAMP signaling pathway in neurodegeneration induced by PPA, as well as the new strategies for diagnosing autistic dysfunctions, has been discussed.
PPA, commonly used as food preservative, is a metabolic end-product of enteric gut bacteria and has been reported to retard mitochondrial ETC complex-II and also complex-I activity responsible for neuronal mitochondrial energy dysregulation (Macfabe, 2013). The neuromitochondrial toxin PPA interferes with the conversion of succinate to fumaratein tricarboxylic acid cycle (TCA) cycle, responsible for production of FADH2 mediated complex-II alteration in mitochondrial electron transport chain (ETC) by which it directly inhibits mitochondrial metabolic enzyme succinate dehydrogenase and NADH where it is consumed in complex-I with the help of an enzyme complex-I (NADPH oxidase) as well as involved in the dysregulation of complex-IV (cytochrome-C oxidase). PPA is the final protein complex in the ETC, contributing to contrasting electrochemical potential that the complex-V (ATP synthase) synthesizes ATP. To confirm autism-associated neurochemical and motor alterations, PPA has become a major experimental tool (Figure 10) (Thomas et al., 2014). In rats, PPA leads to increased lactate levels, resulting in neuroexcitation-caused energy depletion, abnormal activation of NMDA receptors and subsequent Ca2+ influx (Nakao et al., 1992).
Figure 10.
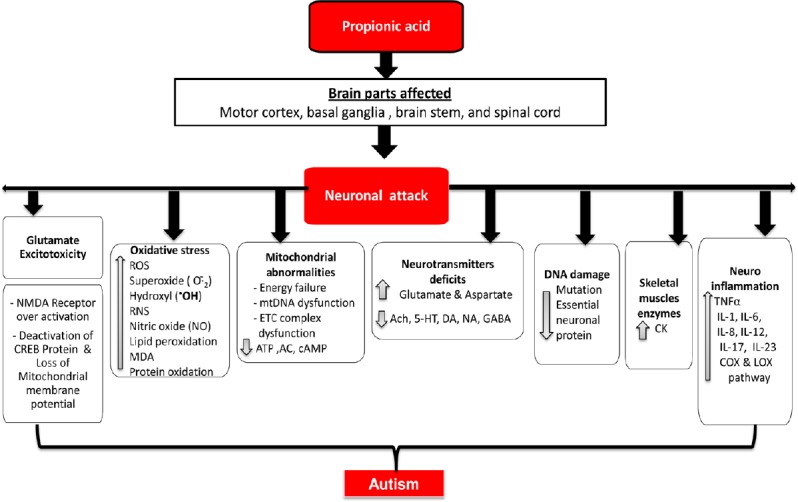
PPA induced neurotoxicity.
5-HT: 5-Hydroxytryptamine; AC: adenyl cyclase; Ach: acetylcholine; ATP: adenosine triphosphate; cAMP: cyclic adenosine monophosphate;cAMP: cyclic adenosine monophosphate; CK: creatine kinase; COX: cyclooxygenase; CREB: cyclic AMP response element binding protein; DA: dopamine; ETC: electron transport chain; GABA: γ-aminobutyric acid; IL: interleukin; LOX: lectin-like oxidized low-density lipoprotein receptor; MDA: malondialdehyde; NA: noradrenaline; NMDA: N-methyl-D-aspartate; PPA: propionic acid; RNS: reactive nitrogen species; TNFα: tumor necrosis factor alpha.
In the present study, marked acquisition and retention latency deficits in MWM and EPM were observed in PPA-treated rats than in normal and FSK only-treated rats. A previous study showed that PPA injection resulted in memory deficits and walking imbalance observed through various behavioural models like EPM, MWM, FST, beam-crossing test and grip strength for autistic rats (Dubey et al., 1981).
Intracerebroventricular administration of PPA in rodents induces renowned biochemical modifications in serotonergic, glutamatergic, GABAergic, cholinergic, and dopaminergic systems (Cannizzaro et al., 2003). The PPA produces behavioral, neurochemical, and pathological alterations in rats. In this study, FSK-mediated increase in ATP is attributable to cAMP/CREB upregulation (Seamon et al., 1981).
It has been reported that autism results in alteration in cholinergic neurotransmission (Minshew et al., 1997). As a result of cholinergic impairment, especially in the brain regions associated with cholinergic neuronal transmission, learning and memory abilities are altered, (Kim et al., 2014). In this study, we noticed significant increase in AchE activity. This increased AchE activity was partially restored by FSK.
Disequilibrium occurred as a result of increased production of ROS, reduced levels of free radical scavengers and antioxidants in the autistic brains (Perry et al., 2001). PPA also potentiated the lipid peroxidation and other free radical markers (El-Ansary et al., 2012). This study showed that FSK treatment remarkably restored the anti-oxidant system and profoundly reduced the level of oxidative stress markers and restored complex I, II, IV & V activities.
The reason behind neuroinflammatory action of PPA was the alteration in levels of neuroinflammatory cytokines such as TNF-α, IL-6 and IL-10. In this study, FSK treatments markedly attenuated the levels of inflammatory cytokines such as TNF-α, IL-6, IL-1β and increased IL-10 level in rat brain homogenate. Furthermore, elevation in lactate dehydrogenase level was observed in the brain of PPA-intoxicated rats.
This study signifies that FSK per se resulted in no any significant changes in behavioural and neurochemical parameters. But FSK treatment (10, 20 and 30 mg/kg) led to remarkable modifications in both behavioral and neurochemical assessments, as compared with PPA-treated rats. Results from this study indicate that FSK exhibits anti-oxidant effects through reductions in ROS and providing its potential against oxidative damage induced by PPA. Chronic administration of FSK at 30 mg/kg exhibited highly neuroprotective effects on all behavioral and neurochemical alterations.
There are several limitations to this study. First, we did not measure molecular parameters such as cAMP, PKa, CREB levels in PPA-treated rats. Second, we did not investigate the effects of long-term pharmacological interventions by FSK alone and in combination with other standard clinical drugs used in patients with autism.
To conclude, chronic FSK administration effectively and dose-dependently regulates the toxic neurochemical and behaviroal alterations in PPA-intoxicated rats. FSK, as an adenylate cyclase activator associated with upregulation of cAMP/CREB might be a possible futuristic approach against neurodegenerative diseases like autism (Figure 11).
Figure 11.
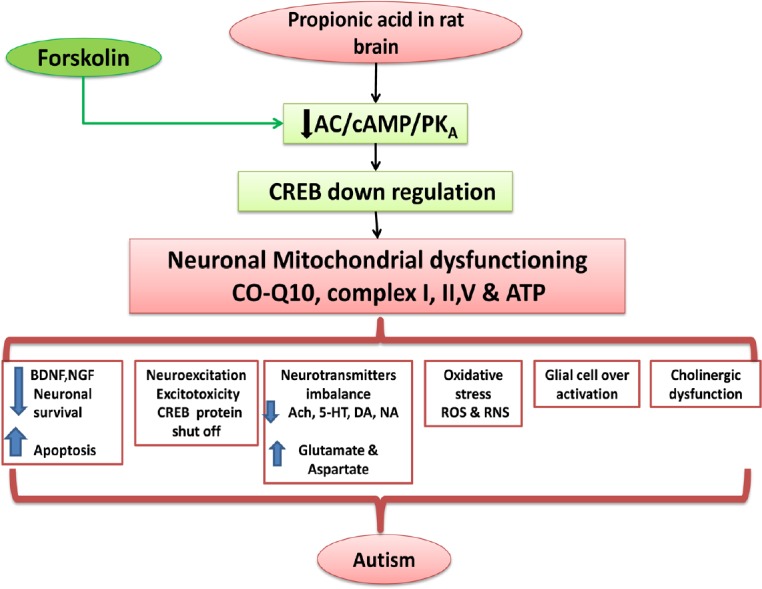
Possible neuroprotective mechanism of FSK in ICV-PPA intoxicated autism.
5-HT: 5-Hydroxytryptamine; AC: adenyl cyclase; Ach: acetylcholine; ATP: adenosine triphosphate; cAMP: cyclic adenosine monophosphate; BDNF: brain-derived neurotrophic factor; cAMP: cyclic adenosine monophosphate; CO-Q10: coenzyme Q10; CREB: cyclic AMP response element binding protein; DA: dopamine; FSK: forskolin; ICV-PPA: intracerebroventricular propionic acid; NA: noradrenaline; NGF: nerve growth factor; PKA: protein kinase A; RNS: reactive nitrogen species; ROS: reactive oxygen species.
Acknowledgments
The authors express their gratitude to M/S BAPEX Pharma, India for ex-gratia sample of Solanesol. They are very thankful to NALWA Laboratories, Haryana, India for their contribution in histopathological and morphological examinations of brain samples. They are also thankful to AccuLab, India for their great support in hematological and ELISA assays. They are really thankful to Dr. Sanjeev Kalra for invaluable financial support and encouragement. They are sincerely thankful to Mr. Parveeen Garg, the chairman, ISF College of Pharmacy, Moga (Punjab), India for their great vision and support.
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Institutional review board statement: The research study protocol was approved by the RITS/IAEC, SIRSA, HARYANA (RITS/IAEC/2014/03/03) on March 3, 2014.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Braz J Psychiatry 35 Suppl. 2013;2:S112–120. doi: 10.1590/1516-4446-2013-1098. [DOI] [PubMed] [Google Scholar]
- 2.Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009;42(10-11):1032–40. doi: 10.1016/j.clinbiochem.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Aliev G, Lipsitt AE, Morales L, Obrenovich ME, Palacios HH, Walrafen B. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int J Biochem Cell Biol. 2009;41:1989–2004. doi: 10.1016/j.biocel.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010;1801:1175–1183. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Alturkistani HA, Tashkandi FM, Mohammedsaleh ZM. Histological stains: a literature review and case study. Glob J Health Sci. 2015;8:72–9. doi: 10.5539/gjhs.v8n3p72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhateja DK, Dhull DK, Gill A A, Sharma S, Sidhu A, Reddy BK. Peroxisome proliferator-activated receptor-α activation attenuates 3-nitropropionic acid induced behavioral and biochemical alterations in rats: possible neuroprotective mechanisms. Eur J Pharmacol. 2012;674:33–43. doi: 10.1016/j.ejphar.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Brock M, Buckel W. On the mechanism of action of the antifungal agent propionate: Propionyl-CoA inhibits glucose metabolism in Aspergillusnidulans. Eur J Biochem. 2004;271:3227–3241. doi: 10.1111/j.1432-1033.2004.04255.x. [DOI] [PubMed] [Google Scholar]
- 8.Cammarota M, Paratcha G, Bevilaqua LR, Levi de Stein M, Lopez M, Pellegrino de Iraldi A, Izquierdo I, Medina JH. Cyclic AMP-responsive element binding protein in brain mitochondria. J Neurochem. 1999;72:2272–2277. doi: 10.1046/j.1471-4159.1999.0722272.x. [DOI] [PubMed] [Google Scholar]
- 9.Cannizzaro C, Monastero R, Vacca M, Martire M. [3H]-DA release evoked by low pH medium and internal H+ accumulation in rat hypothalamic synaptosomes: involvement of calcium ions. Neurochem Int. 2003;43:9–17. doi: 10.1016/s0197-0186(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury PR, Lahiri S, Rajamma U. Glutamate mediated signaling in the pathophysiology of autism spectrum disorders. Pharmacol Biochem Behav. 2012;100:841–849. doi: 10.1016/j.pbb.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 11.de Theije CG, Wu J, da Silva SL, Kamphuis PJ, Garssen J, Korte SM, Kraneveld AD. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol. 2011;668(Suppl 1):S70–80. doi: 10.1016/j.ejphar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Dubey MP, Srimal RC, Nityanand S, Dhawan BN. Pharmacological studies on coleonol, a hypotensive diterpene from Coleus forskohlii. J Ethnopharmacol. 1981;3:1–13. doi: 10.1016/0378-8741(81)90010-6. [DOI] [PubMed] [Google Scholar]
- 13.El-Ansary AK, Ben Bacha A, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation. 2012;9:74. doi: 10.1186/1742-2094-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 16.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Deshpande SB. 3-Nitropropionic acid depresses spinal reflexes involving GABAergic and glycinergic transmission in neonatal rat spinal cord in vitro. Life Sci. 2008;83(21-22):756–760. doi: 10.1016/j.lfs.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Höglinger GU, Carrard G, Michel PP, Medja F, Lombès A, Ruberg M, Friguet B, Hirsch EC. Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J Neurochem. 2003;86:1297–1307. doi: 10.1046/j.1471-4159.2003.01952.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaur R, Parveen S, Mehan S, Khanna D, Kalra S. Neuroprotective effect of ellagic acid against chronically scopolamine induced Alzheimer’s type memory and cognitive dysfunctions: possible behavioural and biochemical evidences. Int J Preven Med Res. 2015;1:45–64. [Google Scholar]
- 20.Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ, Oh HA, Choi CS, Kim KC, Gonzales EL, You JS, Choi DH, Lee J, Han SH, Yang SM, Cheong JH, Shin CY, Bahn GH. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS One. 2014;9:e104927. doi: 10.1371/journal.pone.0104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P, Padi SS, Naidu PS, Kumar A. Cyclooxygenase inhibition attenuates 3-nitropropionic acid-induced neurotoxicity in rats: possible antioxidant mechanisms. Fundam Clin Pharmacol. 2007;21:297–306. doi: 10.1111/j.1472-8206.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 22.Lawler CP, Croen LA, Grether JK, Van de Water J. Identifying environmental contributions to autism: provocative clues and false leads. Ment Retard Dev Disabil Res Rev. 2004;10:292–302. doi: 10.1002/mrdd.20043. [DOI] [PubMed] [Google Scholar]
- 23.Lee LC, Baillie GS, Maurice DH. Targeting protein-protein interactions within the cyclic AMP signaling system as a therapeutic strategy for cardiovascular disease. Future Med Chem. 2013;5:451–64. doi: 10.4155/fmc.12.216. [DOI] [PubMed] [Google Scholar]
- 24.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 25.Li WP, Ma K, Jiang XY, Yang R, Lu PH, Nie BM, Lu Y. Molecular mechanism of panaxydol on promoting axonal growth in PC12 cells. Neural Regen Res. 2018;13:1927–1936. doi: 10.4103/1673-5374.239439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macfabe D. Autism: metabolism, mitochondria, and the microbiome. Glob Adv Health Med. 2013;2:52–66. doi: 10.7453/gahmj.2013.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacFabe DF, Rodríguez-Capote K, Hoffman JE, Franklin AE, Mohammad-Asef Y, Taylor AR, Boon F, Cain DP, Kavaliers M, Possmayer F, Ossenkopp K. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotechnol. 2008;4:146–166. [Google Scholar]
- 28.McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. J Clin Psychiatry. 2005;66(Suppl 10):9–18. [PubMed] [Google Scholar]
- 29.Mehan S, Kaur G, Dudi R, Rajput M, Kalra S. Restoration of mitochondrial dysfunction in 6-hydroxydopamine induced Parkinson’s disease: a complete review. Open J Park Dis Treat. 2017;1:1–26. [Google Scholar]
- 30.Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: evidence for undersynthesis and increased degradation of brain membranes. Biol Psychiatry. 1993;33(11-12):762–773. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- 31.Minshew NJ, Sweeney JA, Bauman M. Neurological aspects of autism. In: Cohen D, Volkmar F, editors. Handbook of autism and pervasive developmental disorders. New York: John Wiley & Sons; 1997. pp. 344–369. [Google Scholar]
- 32.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 33.Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Myers SM, Johnson CP American Academy of Pediatrics Council on Children With Disabilities. Management of children with autism spectrum disorders. Pediatrics. 2007;120:1162–1182. doi: 10.1542/peds.2007-2362. [DOI] [PubMed] [Google Scholar]
- 36.Nakao S, Fujii A, Niederman R. Alteration of cytoplasmic Ca2+ in resting and stimulated human neutrophils by short-chain carboxylic acids at neutral pH. Infect Immun. 1992;60:5307–5311. doi: 10.1128/iai.60.12.5307-5311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15(5 Pt 2):3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH, Wenk GL. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- 39.Ragini M, Banji O, Banji D, Pratusha NG, Kumar K, Ananthula MR. Biomarkers in autism. Int J Pharm Tech Res. 2011;3:1281–1289. [Google Scholar]
- 40.Ramanathan M, Babu CS, Justin A, Shanthakumari S. Elucidation of neuroprotective role of endogenous GABA and energy metabolites middle cerebral artery occluded model in rats. Indian J Exp Biol. 2012;50:391–397. [PubMed] [Google Scholar]
- 41.Sandhya T, Sowjanya J, Veeresh B. Bacopa monniera (L) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochem Res. 2012;37:1121–1131. doi: 10.1007/s11064-012-0717-1. [DOI] [PubMed] [Google Scholar]
- 42.Schenk PW, Snaar-Jagalska BE. Signal perception and transduction: the role of protein kinases. Biochim Biophys Acta. 1999;1449:1–24. doi: 10.1016/s0167-4889(98)00178-5. [DOI] [PubMed] [Google Scholar]
- 43.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylatecyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Searey RL. Diagnostic Biochemistry McGrane-Hill. New York, NT: 1969. [Google Scholar]
- 45.Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, Cain DP. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Singh S, Jamwal S, Kumar P. Piperine enhances the protective effect of curcumin against 3-NP induced neurotoxicity: possible neurotransmitters modulation mechanism. Neurochem Res. 2015;40:1758–1766. doi: 10.1007/s11064-015-1658-2. [DOI] [PubMed] [Google Scholar]
- 47.Singh S, Kumar P. Neuroprotective activity of curcumin in combination with piperine against quinolinic acid induced neurodegeneration in rats. Pharmacology. 2016;97(3-4):151–160. doi: 10.1159/000443896. [DOI] [PubMed] [Google Scholar]
- 48.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 49.Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria: a biochemical and morphological study. J Cell Biol. 1967;32:415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strömland K, Nordin V, Miller M, Akerström B, Gillberg C. Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol. 1994;36:351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 51.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2014;113:515–529. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 52.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 53.Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier JP, Heinemann U, Friedman A. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Velmurugan BK, Rathinasamy B, Lohanathan BP, Thiyagarajan V, Weng CF. Neuroprotective Role of Phytochemicals. Molecules. 2018;23 doi: 10.3390/molecules23102485. pii: E2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;66:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyse AT, Brusque AM, Silva CG, Streck EL, Wajner M, Wannmacher CM. Inhibition of Na+, K+-ATPase from rat brain cortex by propionic acid. Neuroreport. 1998;9:1719–1721. doi: 10.1097/00001756-199806010-00009. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y, Liu P, Li Y. Impaired development of mitochondria plays a role in the central nervous system defects of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2005;73:83–91. doi: 10.1002/bdra.20110. [DOI] [PubMed] [Google Scholar]
- 58.Zárate G, González S, Chaia AP. Assessing survival of dairy propionibacteria in gastrointestinal conditions and adherence to intestinal epithelia. Methods Mol Biol. 2004;268:423–432. doi: 10.1385/1-59259-766-1:423. [DOI] [PubMed] [Google Scholar]


