Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the predominant form of dementia. Since its initial description by Alois Alzheimer in 1906, several advances have been made in our understanding of the progression of the disease and its clinical consequences, yet the underlying etiology remains contentious. Given the stereotyped patterns of cortical and hippocampal neuronal loss and the progressive degeneration of key neurotransmitter pathways, research has traditionally been focused on factors affecting neuronal viability, including the contribution of glial dysfunction to neuronal degeneration. From a clinical perspective, the fruits of this work have been underwhelming. Key pathological markers of the disease, including β-amyloid (Aβ) plaque formation and tau hyperphosphorylation, have yielded no effective therapies, highlighted by the recent discontinuations of several high profile Aβ immunotherapy trials. The few current symptomatic therapies for AD are predicated on the amelioration of cholinergic or glutamatergic dysfunction. Aside from underscoring the inadequacy of current therapeutic approaches, this also points to the importance of alternative contributors to AD pathogenesis. In recent years, there has been a growing appreciation for the multimodal and multifactorial nature of the condition; the case for combinatorial therapies is thus strong.
Vascular dysfunction is a well attested feature of AD, presenting in subtle ways prior to the onset of clinical symptoms and contributing significantly to the disease from the earliest stages (Govindpani et al., 2019). Aside from their role as regulators of neurovascular coupling to meet dynamic energy requirements in the brain, cerebral vascular cells are being increasingly recognized as support cells in their own right. Indeed, the cerebral vasculature represents a key system in the modulation of cerebral function, immunity and homeostasis and its dysfunction has severe consequences for local and global cerebral health. Vascular dysfunction in AD has been linked to the development of classical pathology like Aβ plaques and neurofibrillary tangles, but vascular cells may themselves exert direct effects on their surrounding milieu in disease, releasing neurotoxic factors, altering the local inflammatory environment and contributing to the pathological remodeling of neuronal and glial networks. Vascular alterations in AD were recognized in older studies at the macrostructural level, but current research is highlighting more microstructural, molecular and biochemical disturbances which may provide promising targets for drug development. Several related “vascular hypotheses” of AD have been proposed, seeking to place vascular dysfunction at the centre of the constellation of deficits observed in the disease. The very early onset of vascular dysfunction in the preclinical stage of AD indeed suggests the potential for a causative role rather than a passive contribution to disease pathogenesis. A stronger focus on the vascular component in the development and progression of AD may thus contribute to our understanding of the early pathogenesis of the disease and open a pathway towards disease modifying therapies or therapies that slow disease progression.
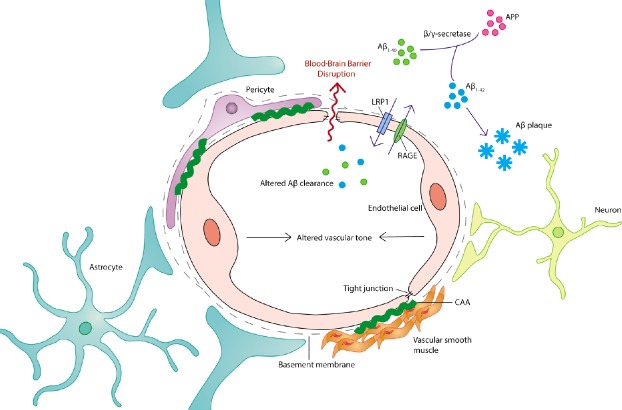
One interesting development in this area has been the discovery of vascular receptor and transport systems that closely regulate Aβ transport and removal in the brain. Therapies attempting to target Aβ deposition directly, including Aβ immunotherapies, have not been successful and are in some cases poorly tolerated by patients. The existence of an endogenous system for vascular Aβ removal raises the potential for therapies that upregulate or co-opt the function of existing systems. Low density lipoprotein receptor-related protein 1 (LRP-1) is a key component of this system, binding free parenchymal Aβ and transporting it into the systemic circulation for removal. The receptor for advanced glycation end-products (RAGE) functions in turn to transport Aβ across the blood-brain barrier (BBB) and into the brain parenchyma (Figure 1). Neuronal LRP-1 and RAGE expression and their alterations in AD have been well characterized, but the contribution of vascular LRP-1 and RAGE to AD pathogenesis has been poorly explored. These systems are known to be present and functional in multiple vascular cell types, including endothelial cells, vascular smooth muscle cells and pericytes. Vascular LRP-1 downregulation has been reported in AD patients and AD mouse models. There is evidence that Aβ at high concentrations can enhance proteasomal LRP-1 degradation and LRP-1 downregulation has been shown to reduce Aβ clearance across the BBB (Deane et al., 2004). In addition, the Aβ1–40 isoform has a higher affinity for LRP-1 than Aβ1–42, potentially resulting in reduced Aβ1–42 clearance and increased parenchymal deposition (Deane et al., 2004). The pharmacological inhibition or genetic knock-down of vascular LRP-1 in APP/PS1 mice precipitates the emergence or worsening of amyloid pathology in the parenchyma and an increase in cerebral amyloid angiopathy (Kanekiyo et al., 2012). On the other hand, supplementation with soluble LRP or recombinant LRP fragments on the luminal side is a possible strategy for increasing soluble Aβ efflux across the BBB. LRP-1 upregulation may be achievable in the future through pharmacological modulation or gene therapy, but the viability of this approach remains to be determined. Studies have reported upregulated vascular RAGE expression in the AD microvasculature, particularly in Aβ-rich regions, and RAGE inhibition appears to have beneficial effects in aged APPSW/0 mice, reducing Aβ accumulation, normalising perfusion deficits and improving cognitive function (Deane et al., 2012). This system is relatively unexplored in the context of human AD and could be a promising avenue in the development of combinatorial therapies targeting Aβ accumulation in patients with early AD. Phosphatidylinositol-binding clathrin assembly protein (PICALM) plays an important role in the LRP-1-mediated BBB transcytosis of Aβ, and microvascular PICALM levels are reduced in late-stage AD. In endothelial monolayers from AD brains, this is associated with reduced internalisation and clearance of Aβ, and PICALM knockdown also appears to worsen cerebral Aβ pathology in APPsw/0 mice (Zhao et al., 2015). Compensation for AD-related PICALM deficiency could thus be a valid therapeutic strategy to promote Aβ clearance. The recently-discovered glymphatic (glial + lymphatic) pathway may provide an alternative route for Aβ flushing from the interstitium into the periarterial space, where it may interact with BBB clearance mechanisms to promote removal (Iliff et al., 2012). The interaction between the glymphatic system and vascular Aβ transport may be a promising area for future investigation. Vascular cells also contribute directly to Aβ catabolism, with Aβ-degrading enzymes like neprilysin and insulin-degrading enzyme being expressed by the endothelium. The genetic or epigenetic modulation of neprilysin expression in vascular cells and neurons has been proposed as a potential strategy for Aβ removal. The role of Aβ itself as a vasoactive factor and modulator of BBB integrity lends further support to vascular Aβ accumulation as a potential therapeutic target alongside parenchymal deposition. Indeed, the mechanisms underlying parenchymal and vascular plaque formation may differ and involve alternative Aβ species.
Figure 1.
The neurovascular unit in Alzheimer’s disease.
The neurovascular unit is the primary structural and functional unit for vascular organization in the mammalian brain. It is comprised of several cell types and non-cell components including endothelial cells, perivascular pericytes, vascular smooth muscle cells, basal lamina, and closely associated astrocyte end-feet and interneuron terminals. Cerebral endothelial cells associate via tight junctions to form a selectively permeable BBB. Alzheimer’s disease is associated with dysfunction across the vasculature, including cell morphological changes and mortality, inflammatory activation, altered expression of receptors and transporters involved in Aβ clearance, the deposition of Aβ as vascular plaques (CAA), alterations in vascular tone, dysregulated endothelial junction formation and aberrant increases in BBB permeability. Therefore, there are several potential vascular targets for therapeutic intervention in Alzheimer’s disease. Aβ: β-Amyloid; APP: amyloid precusor protein; BBB: blood-brain barrier; CAA: cerebral amyloid angiopathy; LRP-1: lipoprotein-related protein-1; RAGE: glycation end-products.
In addition to their role in Aβ clearance, vascular cells may themselves be vulnerable to Aβ-induced dysfunction. Several studies have demonstrated the potential for direct Aβ toxicity in vascular cells. In addition to this, Aβ has the potential to interact with receptor systems on several cell types, including vascular cells, to initiate deleterious responses. Aβ has recently been shown to inhibit the activity and reduce the expression of ATP-binding cassette transporters in cerebral endothelial cells. These transporters are involved in the active transport of several compounds across the endothelial membrane, including lipids, cytokines, glucocorticoids and even Aβ1–42 itself. Thus, the Aβ-induced downregulation of ATP-binding cassette transporters in AD could contribute to both deficits in Aβ clearance and BBB dysfunction (Shubbar and Penny, 2018). Aβ1–42 oligomers have also been shown to bind to calcium-sensing receptors (CaSR) on astrocytes, microglia and oligodendrocytes, stimulating the synthesis and release of nitric oxide, vascular endothelial growth factor (VEGF), pro-inflammatory cytokines and more Aβ1–42 in a cascade that may contribute significantly to a neurotoxic and possibly vasculotoxic environment (Chiarini et al., 2016).
The contribution of localized perfusion deficits and hypometabolism to early AD pathogenesis has been demonstrated in several studies, and the stereotypical evolution of these deficits could represent a biomarker of disease onset and progression. Interestingly, such changes have been shown to begin preclinically and are potentially measurable with standard medical imaging modalities such as arterial-spin magnetic resonance imaging, fluorodeoxyglucose positron emission tomography and single-photon emission computed tomography (Govindpani et al., 2019). This raises the possibility that more precise screening for AD and related dementias may be achievable in the prodromal or even preclinical stage, where early intervention may be of benefit. Such screening may be of utility in individuals deemed to be at higher risk, for instance those with a family history of AD and positive for the apolipoprotein E4 allele. The finding that apolipoprotein E genotype significantly impacts several indices of vascular function in AD including cerebral perfusion and Aβ clearance suggests that this may be important in the tailoring of vascular-targeted AD treatments.
Focal reductions in blood flow may contribute to AD pathogenesis not just by altering metabolic conditions, but by reducing the vascular-mediated clearance of Aβ and other deleterious agents. Aβ accumulation has consequences not just for parenchymal plaque formation but for the development of cerebral amyloid angiopathy (Figure 1), which further impairs vascular contractility and neurovascular coupling. This suggests vasoactive modulation as a potential means of counteracting such changes at an early stage of disease pathogenesis. Several current symptomatic treatments for AD may involve a vascular modulatory component, but this has not been well studied. Acetylcholinesterase inhibitors have been shown to alter regional blood flow in the AD brain and this may contribute to the observed amelioration of disease symptoms. Other neurotransmitter systems, including the glutamatergic, gamma-aminobutyric acidergic, noradrenergic and serotonergic systems, have been implicated in the complex regulation of neurovascular coupling, and there may be value in investigating the therapeutic potential of these systems in this regard. The dysfunction of these neurotransmitter systems in the AD brain may also have consequences for cerebral perfusion in the disease. Other vasoactive modulators targeting adenosine, histamine, prostaglandin, neuropeptide and purinergic receptors, for example, may also be of utility here, as might the supplementation or modulation of endothelium-released vasoactive agents like nitric oxide, endothelin and arachidonic acid metabolites. Needless to say, this is an area that presents significant scope for therapeutic investigation.
A major paradigm in recent years has been the involvement of the vasculature in neuroinflammatory modulation, with both endothelial cells and pericytes being identified as mediators of neuroinflammation. Not only do cerebral vascular cells respond to several inflammatory cytokines, but they release a repertoire of these agents themselves. There is thus a vascular contribution to local inflammatory environments in the brain, and the local inflammatory state in turn has a significant influence on vascular health and function. The utility of anti-inflammatory drugs in the treatment of AD has been controversial, with the use of non-steroidal anti-inflammatory drugs being variously reported to reduce the risk of AD or to have no measurable effect in patient trials. However, there is evidence that inhibitors of vascular activation such as sunitinib can attenuate the vascular expression of pro-inflammatory mediators, reduce vascular Aβ burden and improve cognitive function in AD transgenic mice as well as protect rat brain endothelial cells from oxidative damage in vitro (Grammas et al., 2014). The therapeutic targeting of vascular inflammatory pathways may thus be a valid adjunct to glial immunomodulation in AD.
Another intriguing feature of AD is the widespread occurrence of angiogenic remodeling, often involving a combination of pathological vascular regression and aberrant neoangiogenesis. This is evidenced by the appearance of morphological changes in vascular networks, including “wicker-like” networks of new vessels and abnormal sprouting, as well as the formation of string vessels, likely as a result of both sprout regression and vascular degeneration (Govindpani et al., 2019). This could potentially have consequences for the hemodynamic properties of the system as well as the integrity of the BBB, which is significantly impaired in AD. The dysregulation of angiogenic factors has indeed been demonstrated in the human AD brain. In particular, there appears to be a general downregulation in VEGF, an important pro-angiogenic factor, in the capillaries of the brainstem, hippocampus and temporal cortex. VEGF also functions as an important mediator of vascular inflammation. Aβ has been shown to play a direct role in reducing the functional binding of VEGF to VEGF receptor-2 on endothelial cells (Patel et al., 2010). This could point to VEGF supplementation or stimulation as a viable therapeutic strategy in preventing vascular degeneration and promoting re-vascularisation in AD, as well as in addressing AD-associated changes in vascular inflammatory signaling. On the other hand, anti-angiogenic factors like tyrosine kinase inhibitors that inhibit VEGF receptors have been shown to reduce amyloid burden and cognitive deficits in AD mouse models (Grammas et al., 2014). Some studies have reported heightened VEGF levels in perivascular astrocytes and other cell types in AD. This is likely linked to astrocytic CaSR stimulation by Aβ1–42 oligomers, and this VEGF overexpression may have detrimental effects for both neuronal and vascular health (Dal Prà et al., 2014). Hypoxic conditions in the AD brain likely contribute to aberrant angiogenesis by stimulating the upregulation of hypoxia-inducible factor-1 in glial cells, which may also be associated with dysregulated VEGF signaling at the vasculature (Luo et al., 2012). There is a need to better elucidate the pattern of VEGF signaling deficits in AD, but targets modulating VEGF synthesis such as hypoxia-inducible factor-1 and calcium-sensing receptors may represent promising therapeutic targets. Notably, several other angiogenic factors including mesenchyme homeobox 2, matrix metalloproteinase-9 and the transferrin receptor also display dysregulated vascular expression profiles in the AD brain. There are thus several potential targets for the modulation of vascular remodeling in AD and the therapeutic potential of these systems remains to be investigated. This will no doubt require a more thorough understanding of the complex dynamic between inflammation, aberrant angiogenesis, hypoxia and Aβ pathology in the disease, but there is a clear potential for therapeutic development.
In conclusion, there is significant evidence for a vascular contribution to AD, with vascular impairment occurring and worsening at every stage of the disease process. However, little attention has been paid to cerebrovascular pathology as a therapeutically-relevant target for drug design. Indeed, the literature presents an extensive and diverse set of vascular processes affected in the disease that remain relatively unexplored from a therapeutic perspective. Thus, the incorporation of vascular-targeted treatments into combinatorial therapies could be a promising approach in improving or supplementing current AD therapeutic approaches.
Additional file: Open peer review reports 1 (85.8KB, pdf) and 2 (87.3KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Hans-Gert Bernstein, University of Magdeburg, Germany; Ubaldo Armato, University of Verona Medical School, Italy.
P-Reviewers: Bernstein HG, Armato U; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Chiarini A, Armato U, Liu D, Dal Prà I. Calcium-sensing receptors of human neural cells play crucial roles in Alzheimer’s disease. Front Physiol. 2016;7:134–134. doi: 10.3389/fphys.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dal Prà I, Armato U, Chioffi F, Pacchiana R, Whitfield JF, Chakravarthy B, Gui L, Chiarini A. The Aβ peptides-activated calcium-sensing receptor stimulates the production and secretion of vascular endothelial growth factor-A by normoxic adult human cortical astrocytes. Neuromolecular Med. 2014;16:645–657. doi: 10.1007/s12017-014-8315-9. [DOI] [PubMed] [Google Scholar]
- 3.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/Amyloid β-peptide interaction mediates differential brain efflux of aβ isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Govindpani K, McNamara LG, Smith NR, Vinnakota C, Waldvogel HJ, Faull RL, Kwakowsky A. Vascular dysfunction in Alzheimer’s disease: A prelude to the pathological process or a consequence of it ? J Clin Med. 2019 doi: 10.3390/jcm8050651. doi: 103390/jcm8050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grammas P, Martinez J, Sanchez A, Yin X, Riley J, Gay D, Desobry K, Tripathy D, Luo J, Evola M, Young A. A new paradigm for the treatment of Alzheimer’s disease: targeting vascular activation. J Alzheimers Dis. 2014;40:619–630. doi: 10.3233/JAD-2014-132057. [DOI] [PubMed] [Google Scholar]
- 7.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-β. J Neurosci. 2012;32:16458. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J, Martinez J, Yin X, Sanchez A, Tripathy D, Grammas P. Hypoxia induces angiogenic factors in brain microvascular endothelial cells. Microvasc Res. 2012;83:138–145. doi: 10.1016/j.mvr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D. Alzheimer’s β-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J Neurochem. 2010;112:66–76. doi: 10.1111/j.1471-4159.2009.06426.x. [DOI] [PubMed] [Google Scholar]
- 11.Shubbar MH, Penny JI. Effect of amyloid beta on ATP-binding cassette transporter expression and activity in porcine brain microvascular endothelial cells. Biochim Biophys Acta Gen Subj. 2018;1862:2314–2322. doi: 10.1016/j.bbagen.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T7, Sugawara T, et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.