Keywords: immunofluorescence, MAP2, microRNA, miR-124, Neuro-2A cells, neurite outgrowth, neuronal differentiation, overexpression, real-time PCR, retinoic acid
Abstract
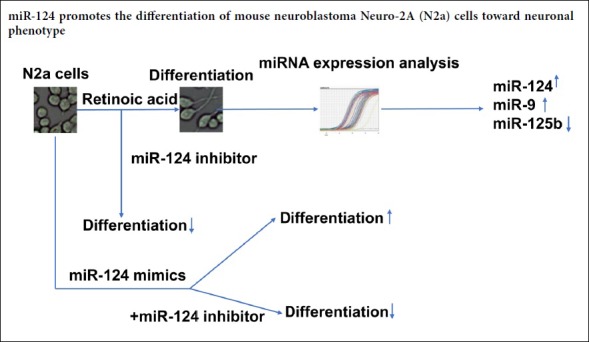
Retinoic acid can cause many types of cells, including mouse neuroblastoma Neuro-2A cells, to differentiate into neurons. However, it is still unknown whether microRNAs (miRNAs) play a role in this neuronal differentiation. To address this issue, real-time polymerase chain reaction assays were used to detect the expression of several differentiation-related miRNAs during the differentiation of retinoic acid-treated Neuro-2A cells. The results revealed that miR-124 and miR-9 were upregulated, while miR-125b was downregulated in retinoic acid-treated Neuro-2A cells. To identify the miRNA that may play a key role, miR-124 expression was regulated by transfection of miRNA mimics or inhibitors. Morphological analysis results showed that inhibition of miR-124 expression reversed the effects of retinoic acid on neurite outgrowth. Moreover, miR-124 overexpression alone caused Neuro-2A cells to differentiate into neurons, and its inhibitor could block this effect. These results suggest that miR-124 plays an important role in retinoic acid-induced differentiation of Neuro-2A cells.
Chinese Library Classification No. R453; R364; R363
Introduction
Retinoic acid, a metabolite of vitamin A, is a common inducer of cell differentiation and can cause many types of cells to differentiate into neurons (Zeng and Zhou, 2008; Eda et al., 2009; Walker et al., 2018; Das et al., 2019; Zhang et al., 2019). Retinoic acid has been shown to induce neuronal differentiation mainly via two mechanisms. First, retinoic acid enters the nucleus, binds to retinoic acid receptors and alters the transcription of genes with promoters containing retinoic acid binding elements (Chambon, 1996; Kanungo, 2017). Second, retinoic acid treatment leads to phosphorylation of Erk1/2 and the downstream cAMP responsive element binding protein and upregulation of several immediate-early genes (Canon, 2004; Pasquali et al., 2005). Other studies have shown that retinoic acid-induced neuronal differentiation is associated with neuronal growth factor (Scheibe and Wagner, 1992), cyclophilin A (Song, 2004), tissue-specific proteins, receptors and ion channels (Guan et al., 2001). Although an increasing number of studies have identified the mechanism of action of retinoic acid, the genes and pathways downstream of retinoic acid that mediate cell cycle exit are still unknown (Janesick et al., 2015).
MicroRNAs (miRNAs) are small non-coding RNA molecules that are 21–25 bp in length and universally expressed in many organisms (Ambros, 2001). MiRNAs have been shown to participate in organism growth, development and pathogenesis by downregulation of their target genes (He and Hannon, 2004). A previous study indicated that miRNAs play important roles in nervous system development and neural cell differentiation (Krichevsky et al., 2003). MiRNA expression profiling results of retinoic acid and brain derived neurotrophic factor-treated SH-SY5Y cells revealed that miR-199, miR-199*, miR-124, miR-125b, miR-214 and miR-7 were upregulated after neuronal differentiation (Le et al., 2009). Reduction of miR-23 has also been found during retinoic acid-induced neuronal differentiation of human NT2 cells (Kawasaki and Taira, 2003). MiR-124 is expressed mainly in the central neural system of both embryos and adults (Lagos-Quintana et al., 2002; Kim et al., 2004; Aboobaker et al., 2005; Wienholds et al., 2005). It is the most abundant miRNA expressed in the brain (Lagos-Quintana et al., 2002) and is upregulated when neural precursor cells differentiate into neurons (Deo et al., 2006), indicating a function in neuronal differentiation. MiR-9 has been demonstrated to be involved in the regulation of Cajal–Retzius cell differentiation by inhibiting Foxg1 expression (Shibata et al., 2008). MiR-221 and miR-222 expression levels have been found to increase during PC12 cell differentiation induced by nerve growth factor (Shibata et al., 2008). MiR-124 has been demonstrated to affect neuronal differentiation by altering the splicing of brain-specific proteins (Makeyev et al., 2007). Additionally, miR-124 and let-7a are associated with P19 cell differentiation (Yu et al., 2008; Eda et al., 2009). This evidence indicates that miRNAs might be general regulators of neuronal differentiation. However, it is unclear whether miRNAs are involved in the regulation of retinoic acid-induced Neuro-2A (N2a) cell differentiation and which miRNAs might play a role in this process.
N2a cells are a mouse neural crest-derived cell line that has been extensively used to study neuronal differentiation and neurite growth (LePage et al., 2005). These cells respond quickly to serum deprivation and other factors in the environment that induce neuronal differentiation and neurite growth (Evangelopoulos et al., 2005; Tremblay et al., 2010). Retinoic acid can induce differentiation of these cells into neurons, and the process involves mTOR signaling and functional decreases in P2X7 receptors (Zeng and Zhou, 2008; Wu et al., 2009). However, it is still unknown whether miRNAs play a role in differentiation. The present study determined whether specific differentiation-related miRNAs were involved in retinoic acid-induced N2a cell differentiation.
Materials and Methods
Cell culture and differentiation conditions
Mouse neuroblastoma N2a cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Inc., Carlsbad, CA, USA) supplemented with 1% glutamine (Gibco Inc.), 10% fetal bovine serum (PAA Laboratories Inc., Alberta, Canada) and penicillin (10,000 IU/mL)/streptomycin (10,000 µg/mL) (PAA Laboratories Inc.), pH 7.2. Cells were placed in a humidified 5% CO2 water-jacketed incubator (Thermo Forma Inc., Waltham, MA, USA). In differentiation studies, 4 × 104 N2a cells were plated in 24-well plates for 24 hours. The medium was replaced by DMEM + 2% fetal bovine serum with 10 µM or 20 µM retinoic acid in dimethyl sulfoxide (DMSO) (Sigma, St Louis, MO, USA). The medium was replaced every day for 5 days. An equal amount of DMSO in DMEM + 2% fetal bovine serum was used in control cells (DMSO concentration, 1%). The retinoic acid-treated cells and control cells were imaged every day. Five fields were chosen to photograph at random, and cell counts were performed. Cells with neurites at least twice the length of the cell body diameter were considered differentiated cells (Zeng and Zhou, 2008). At least 800 cells were analyzed every day, and the percent of differentiated cells was quantified.
Transfection of miRNA mimics and antisense oligonucleotides
Retinoic acid-treated N2a cells or normal N2a cells were transfected with miR-124 mimics, inhibitors or a negative control to examine the role of miR-124 in N2a cell differentiation. The miR-124 mimics, negative control (NC), miR-124 inhibitor (miR-124i) and inhibitor negative control (iNC) (Table 1) were synthesized by GenePharma Company (Shanghai, China). N2a cells were seeded at 80,000 cells/well in a 24-well plate. On the next day, the cells were transfected for 5 hours with 1 µL of one of these RNA oligonucleotides per well using Lipofectamine 2000 reagent (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s instructions. The transfection medium was replaced with fresh growth medium either with or without 10 µM retinoic acid. The cells were observed with a microscope every other 24-hour period.
Table 1.
Sequence of miR-124 mimics and antisense oligonucleotides
| Name | Sequence (5′–3′) |
|---|---|
| miR-124 | UAA GGC ACG CGG UGA AUG CCA A |
| CAU UCA CCG CGU GCC UUA UU | |
| miR-124i | TTG GCA UUC ACC GCG UGC CUU A |
| NC | UUC UCC GAA CGU GUC ACG UTT |
| ACG UGA CAG GUU CGG AGA ATT | |
| iNC | CAG UAC UUU UGU GUA GUA CAA |
miR-124i: miR-124 inhibitor; NC: negative control; iNC: inhibitor negative control.
Immunofluorescence staining
Retinoic acid-treated N2a cells and N2a cells transfected with miR-124, NC, miR-124 + miR-124i or miR-124 + iNC were subjected to immunofluorescence staining. Cells that had been treated for the indicated times were washed gently with 0.01 M phosphate-buffered saline (PBS) twice. After 4% paraformaldehyde / PBS fixation for 30 minutes at room temperature, the cells were gently rinsed with 0.3% Triton X-100 in PBS for 20 minutes and incubated with 10% normal goat blocking serum. Then, the cells were incubated with mouse anti-microtubule associated protein 2 (MAP2) monoclonal antibody (1:300; Bioworld, Nanjing, China) at 4°C overnight. After washing with PBS with 0.1% Tween (PBST), the cells were incubated with Alexa Fluor 488-labeled goat anti-rabbit IgG (1:500; Beyotime, Nanjing, China) or Cy3-labeled goat anti-mouse IgG (1:500; Millipore, Bedford, MA, USA) for 1 hour at room temperature. The cells were then washed three times with PBST and incubated with 0.5 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime) for 5 minutes. A Leica DW4000BTL fluorescent microscope (Leica, Wetzlar, Germany) was used for imaging.
Real-time PCR
Real-time polymerase chain reaction (PCR) was used to assess miRNA expression at different time points after retinoic acid induction. N2a cells were plated on five 10-cm dishes at a concentration of 4 × 105 cells per dish. After incubation at 37°C for 24 hours, one dish was subjected to RNA extraction. Retinoic acid was added to the other dishes to induce cell differentiation. Dishes were used to extract RNA after 48, 72, 96 and 120 hours of treatment. Total miRNA was extracted using an miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Reverse transcription of miRNAs and real-time PCR were performed using stem-loop primers as previously described (Chen, 2005). The sequences of miR-124, miR-125b, miR-9 and let-7a were downloaded from miRBase. MiRNA-specific primers for reverse transcription and real-time PCR were designed accordingly (Table 2) and synthesized by Sangon Company (Shanghai, China). Then, miRNA-specific cDNA was synthesized using an M-MLV cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA) and subjected to an Evergreen real-time PCR assay using miRNA-specific primers or internal control U6-specific primers. The miRNA expression level was calculated using the 2–ΔΔCt method and expressed as the relative ratio to U6 (Chen, 2005).
Table 2.
Sequence of miRNA-specific primers for reverse transcription (RT) and real-time polymerase chain reaction
| miRNA ID | Primer | Sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| miR-124 | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACG GCA TTC TTG GCA | 65 |
| Forward | CCG CTA AGG CAC GCG GTG AA | ||
| Reverse | GTG CAG GGT CCG AGG T | ||
| miR-125b | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT CAC AA | 67 |
| Forward | CGT GTC CCT GAG ACC CTA AC | ||
| Reverse | GTG CAG GGT CCG AGG T | ||
| miR-9 | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT GTC TC | 71 |
| Forward | GCG GCG GTC TTT GGT TAT CTA GCT | ||
| Reverse | GTG CAG GGT CCG AGG T | ||
| let-7a | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA ACT A | 68 |
| Forward | GCC GCT GAG GTA GTA GGT TGT A | ||
| Reverse | GTG CAG GGT CCG AGG T | ||
| U6 | RT | CGC TTC ACG AAT TTG CGT GTC AT | 89 |
| Forward | GCT TCG GCA GCA CAT ATA CTA AAA T | ||
| Reverse | CGC TTC ACG AAT TTG CGT GTC AT |
MiRNA target prediction
Online software programs including miRDB (www.mirdb.org/) (Wong and Wang, 2015), TargetMiner (www.isical.ac.in/~bioinfo_miu/tools.html) (Bandyopadhyay and Mitra, 2009) and TargetScan (www.targetscan.org/vert_72/) (Lewis et al., 2005) were used to predict the targets of miR-124. The common targets predicted by the three software programs were subjected to Gene Ontology (GO) analysis. Genes with a GO_0030182 (neuronal differentiation) term were considered possible targets of miR-124 in retinoic acid-induced neuronal differentiation.
Statistical analysis
Data are shown as the mean ± SEM. Plots were created using GraphPad Prism 7.0 software (GraphPad, San Diego, CA, USA). Statistical analysis was performed using two-way analysis of variance with Tukey’s multiple comparisons test or Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results
Retinoic acid induces differentiation of N2a cells into neurons
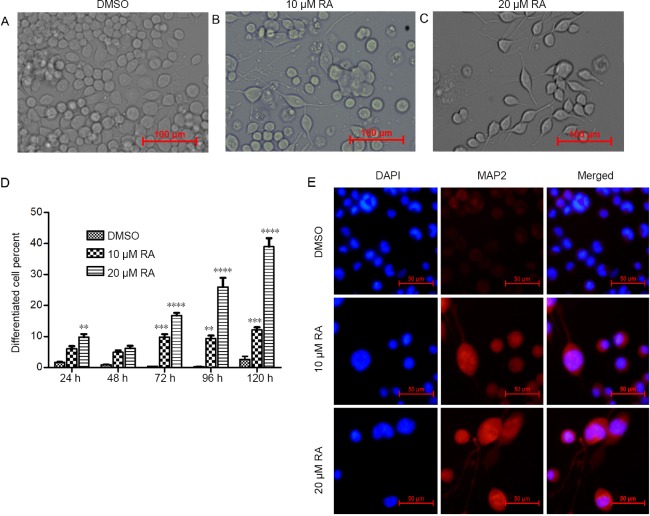
To study the role of miRNAs in retinoic acid-induced N2a cell differentiation, N2a cells were treated with 10 µM or 20 µM retinoic acid or the vehicle, DMSO. Neuron-like morphological changes were observed after retinoic acid treatment. Most of the treated cells had apparent neurite outgrowth, and some had neurite lengths five times or greater than the cell diameter at 5 days after administration (Figure 1A–C). Differentiated cells were defined as cells with neurites longer than twice the cell body diameter. Twenty micromolar retinoic acid induced approximately 40% differentiation of N2a cells (Figure 1D; P < 0.05). Conversely, most DMSO-treated cells proliferated rather than undergoing differentiation (Figure 1A and D). Furthermore, MAP2 immunostaining showed that retinoic acid treatment enhanced MAP2 expression and caused neurite outgrowth and obvious cell body enlargement, suggesting the differentiation of N2a cells into neurons (Figure 1E). Meanwhile, increased cell death was observed in the 20 µM retinoic acid-treated wells (Figure 1A). Thus, 10 µM retinoic acid was used in the subsequent experiments.
Figure 1.
RA induces differentiation of Neuro-2A cells into neurons.
Neuro-2A cells were treated with DMSO or 10 µM or 20 µM RA. (A–C) Representative images of Neuro-2A cells at 5 days after treatment under an optical microscope. Scale bars: 100 µm. (D) Statistical results showing the percent of differentiated cells at the indicated time points. Data are shown as the mean ± SEM (two-way analysis of variance followed by Tukey’s post hoc test). The experiments were performed in triplicate. The differentiated cell percent of RA-treated cells was compared with that of DMSO-treated cells using two-way analysis of variance with Tukey’s multiple comparisons test. **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. DMSO group. (E) Representative MAP2 immunostaining of the treated cells under fluorescence microscopy. Scale bars: 50 µm. DAPI: 4′,6-Diamidino-2-phenylindole; DMSO: dimethyl sulfoxide; MAP2: microtubule associated protein 2; RA: retinoic acid.
Expression changes of miRNAs during retinoic acid-induced N2a cell differentiation
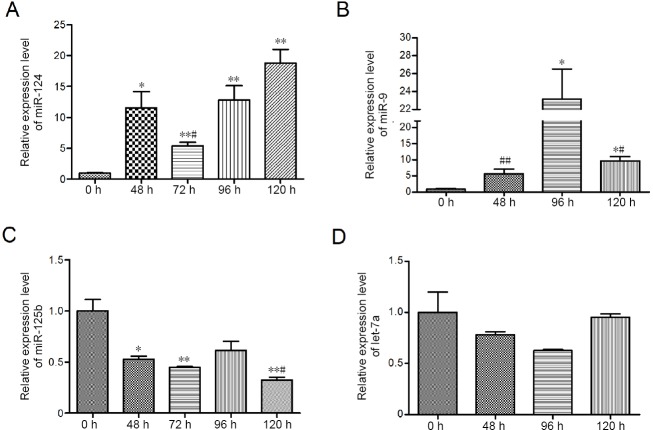
Real-time PCR results revealed that miR-124 and miR-9 expression levels were upregulated after retinoic acid treatment (Figure 2A and B; P < 0.05). Surprisingly, miR-125b expression decreased, while no significant change in let7a expression was observed (Figure 2C, P < 0.05; Figure 2D, P > 0.05).
Figure 2.
Changes in miR-124, miR-9, miR-125b and let-7a expression in Neuro-2A cells after treatment with 20 µM retinoic acid.
(A–D) Real-time polymerase chain reaction analysis of miR-124, miR-9, miR-125b and let-7a levels as indicated by the ratio of the miRNA expression level to the U6 expression level in the same sample. Data are shown as the mean ± SEM (Student’s t-test). The experiments were performed in triplicate. *P < 0.05. **P < 0.01, vs. 0 hour; #P < 0.05; ##P < 0.01, vs. 96 hours. Comparisons between the indicated time points are shown by a line.
Retinoic acid-induced N2a cell differentiation is mediated by miR-124
Our results showed that the expression levels of miR-124, miR-9 and miR-125b were regulated in retinoic acid-treated N2a cells. However, whether expression changes in these miRNAs initiate neuronal differentiation remains to be elucidated. To address this issue, retinoic acid-treated N2a cells were transfected with miR-124i, iNC, miR-124 or NC at 48 hours after retinoic acid treatment. The cells were observed 72 hours later (Figure 3). The miR-124 inhibitor impeded retinoic acid-induced differentiation (Figure 3A), while iNC had no obvious effect (Figure 3B). The differentiation levels of miR-124- and NC-transfected cells were comparable (Figure 3C and D). The results suggested that retinoic acid-induced N2a cell differentiation might be mediated by miR-124. We also examined the effects of an miR-9 inhibitor, but no obvious changes were visible (data not shown).
Figure 3.
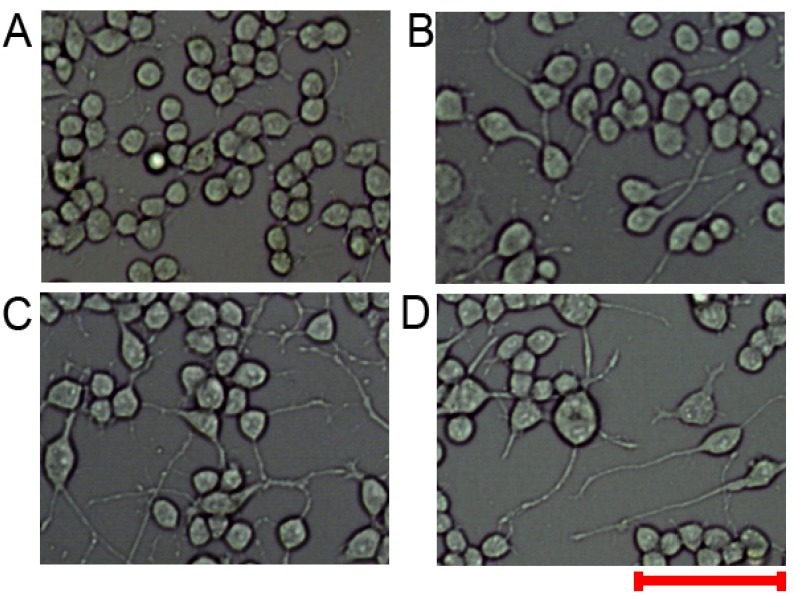
Retinoic acid-induced cell differentiation might be mediated by miR-124.
(A–D) Neuro-2A cells were treated with 20 µM retinoic acid. Two days later, the cells were subjected to treatment with an miR-124 inhibitor, inhibitor negative control, miR-124 or a negative control. Three days later, the cells were observed under an optical microscope. Scale bar: 100 µm.
MiR-124 can regulate N2a cell differentiation
Next, we aimed to determine whether miR-124 alone affected differentiation of N2a cells into neurons. N2a cells were transfected with miR-124 or NC for 2 days. The results revealed that miR-124 transfection induced apparent neurite outgrowth (Figure 4A and B). To confirm the results, miR-124 was co-transfected with miR-124i or iNC. MiR-124i completely blocked the effects of miR-124, while iNC did not alter neurite outgrowth (Figure 4C and D). Meanwhile, we examined the role of miR-124 in neuronal differentiation of N2a cells. MAP2 immunostaining results showed that miR-124 transfection enhanced MAP2 expression and that miR-124i co-transfection could reverse the effects of miR-124 (Figure 5). These results indicated that treatment with miR-124 alone could cause N2a cells to differentiate into neurons.
Figure 4.
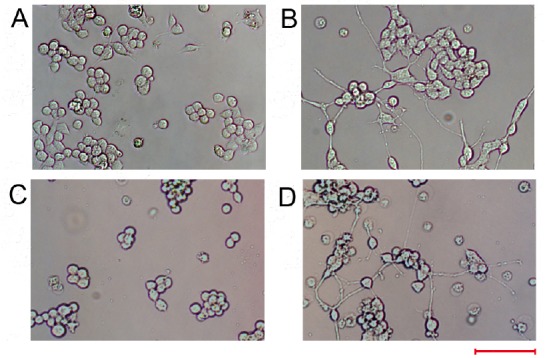
MiR-124 overexpression alone induces neurite outgrowth in Neuro-2A cells.
Neuro-2A cells were transfected with miR-124 alone or in the presence of an miR-124 inhibitor or inhibitor negative control. (A–D) Images under an optical microscope of the morphology of Neuro-2A cells transfected with the negative control (A), miR-124 (B), miR-124 combined with an miR-124 inhibitor (C) or an inhibitor negative control (D) at 48 hours after transfection. Scale bar: 100 µm.
Figure 5.
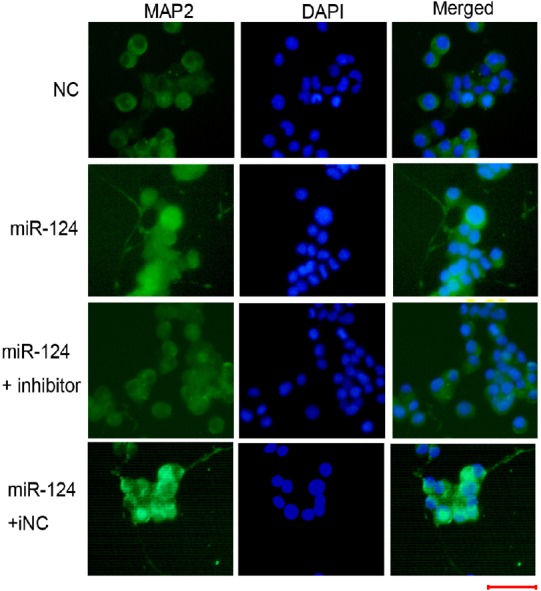
MiR-124 overexpression alone induces neuronal differentiation in Neuro-2A cells.
Neuro-2A cells were transfected with miR-124 alone or in the presence of an miR-124 inhibitor or iNC for 48 hours. The cells were subjected to MAP2 immunostaining. A fluorescence microscope was used to observe the results and capture images. Representative images of the indicated cells are shown. Green: MAP2; blue: DAPI. Scale bar: 50 µm. DAPI: 4′,6-Diamidino-2-phenylindole; iNC: inhibitor negative control; MAP2: microtubule associated protein 2; NC: negative control.
The role of miRNAs lies in their inhibition of the expression of their target genes. To identify how miR-124 might be involved in neuronal differentiation, three online software programs and GO analysis were performed to predict the targets of miR-124. In total, 116 possible target genes were related to the GO_0030182 (neuronal differentiation) term. However, further validation is needed to determine which genes are involved in retinoic acid-induced N2a neuronal differentiation.
Discussion
In this report, the roles of several miRNAs that are enriched in neural systems during retinoic acid-induced N2a cell differentiation were studied. The results showed that increased expression of miR-124 and miR-9 and decreased expression of miR-125b were observed after retinoic acid treatment. Moreover, miR-124 might mediate the role of retinoic acid because miR-124i could block retinoic acid-induced neurite outgrowth. Meanwhile, miR-124 overexpression alone can lead to neuronal differentiation of N2a cells, which is consistent with previous studies showing that miR-124 overexpression enhanced differentiation of neural stem cells (Jiang et al., 2016; Jiao et al., 2017, 2018; Wei et al., 2018), human neuroblastoma cells (Gu et al., 2014; Sharif et al., 2017) and mouse embryonal carcinoma cells (Yu et al., 2008). These results suggest that miR-124 plays a key role in N2a cell differentiation.
MiR-124 has long been known to be involved in neuronal differentiation in different neural precursor cells. MiR-124 is also increased in retinoic acid-induced SH-SY5Y neuronal differentiation (Le et al., 2009). MiRNAs act by regulating the expression of their targets. Our online bioinformatics prediction analysis identified 116 possible target genes that are related to neuronal differentiation. However, further validation is needed to determine the actual target genes in N2a cells. Previous results showed that miR-124 inhibited neuron-enriched PTBP1, leading to an increase in correctly spliced PTBP2 mRNA and PTBP2 protein, which might be the mechanism by which N2a cells differentiate into neurons (Makeyev et al., 2007). SCP1, HDAC5, laminin γ1 and integrin β1 have been confirmed as targets of miR-124 in P19 cells and neural progenitors (Cao et al., 2007; Visvanathan et al., 2007; Shi et al., 2016; Gu et al., 2018). Moreover, miR-124 has been shown to participate in neural stem cell differentiation by regulating Dll4 (Jiao et al., 2017), Dact1 (Jiao et al., 2017), Pax3 (Wei et al., 2018), EZH2 (Neo et al., 2014), Ptbp1 and Sox9 (Mokabber et al., 2019). MiR-124 might be involved in N2a cell differentiation by targeting these proteins.
Previous studies showed that other miRNAs, such as miR-125b (Sempere et al., 2004; Le et al., 2009), miR-9 (Shibata et al., 2008; Zhao et al., 2009; Gu et al., 2018), let-7a (Aranha et al., 2010; Song et al., 2016) and miR-29a (Aranha et al., 2010; Shi et al., 2018), were involved in the regulation of neuronal differentiation. Here, our results showed that miR-124 and miR-9 were upregulated, while miR-125b expression was decreased. No significant expression changes in let-7a could be observed. This inconsistency might be caused by differences in the cell context between N2a cells and other neural cells. Nevertheless, the actual reasons and whether the effect is N2a cell-specific require further investigation. Moreover, miR-219 has been demonstrated to be upregulated after retinoic acid treatment and to be involved in retinoic acid-induced neural stem cell differentiation (Hudish et al., 2013; Wu et al., 2017). Whether this miRNA participates in retinoic acid-induced N2a cell differentiation remains unknown.
In conclusion, miR-124 promotes the differentiation of mouse neuroblastoma N2a cells toward the neuronal phenotype. However, the target of this miRNA and the underlying mechanism require further analysis.
Additional file: Open peer review report 1 (109.7KB, pdf) .
Acknowledgments
We are grateful for Professor Ying-He Hu from Institute of Brain Functional Genomics, East China Normal University, China for his valuable suggestions of designing the experiments.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the Natural Science Foundation of Shanghai of China, No. 16ZR1410500 (to SZD). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jigar Pravinchandra Modi, Florida Atlantic University, USA.
Funding: This work was supported by the Natural Science Foundation of Shanghai of China, No. 16ZR1410500 (to SZD).
P-Reviewer: Modi JP; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Kreiner L, de Souza M, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Aboobaker AA, Tomancak P, Patel N, Rubin GM, Lai EC. Drosophila microRNAs exhibit diverse spatial expression patterns during embryonic development. Proc Natl Acad Sci U S A. 2005;102:18017–18022. doi: 10.1073/pnas.0508823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 3.Aranha MM, Santos DM, Xavier JM, Low WC, Steer CJ, Sola S, Rodrigues CM. Apoptosis-associated microRNAs are modulated in mouse, rat and human neural differentiation. BMC Genomics. 2010;11:514–528. doi: 10.1186/1471-2164-11-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay S, Mitra R. TargetMiner: microRNA target prediction with systematic identification of tissue-specific negative examples. Bioinformatics. 2009;25:2625–2631. doi: 10.1093/bioinformatics/btp503. [DOI] [PubMed] [Google Scholar]
- 5.Canon E. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell. 2004;15:5583–5592. doi: 10.1091/mbc.E04-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao X, Pfaff SL, Gage FH. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 8.Chen C. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179–e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das BC, Dasgupta S, Ray SK. Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer’s disease. Neural Regen Res. 2019;14:1880–1892. doi: 10.4103/1673-5374.259604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deo M, Yu JY, Chung KH, Tippens M, Turner DL. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235:2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- 11.Eda A, Tamura Y, Yoshida M, Hohjoh H. Systematic gene regulation involving miRNAs during neuronal differentiation of mouse P19 embryonic carcinoma cell. Biochem Biophys Res Commun. 2009;388:648–653. doi: 10.1016/j.bbrc.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Evangelopoulos ME, Weis J, Kruttgen A. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene. 2005;24:3309–3318. doi: 10.1038/sj.onc.1208494. [DOI] [PubMed] [Google Scholar]
- 13.Gu X, Meng S, Liu S, Jia C, Fang Y, Li S, Fu C, Song Q, Lin L, Wang X. miR-124 represses ROCK1 expression to promote neurite elongation through activation of the PI3K/Akt signal pathway. J Mol Neurosci. 2014;52:156–165. doi: 10.1007/s12031-013-0190-6. [DOI] [PubMed] [Google Scholar]
- 14.Gu X, Fu C, Lin L, Liu S, Su X, Li A, Wu Q, Jia C, Zhang P, Chen L, Zhu X, Wang X. miR-124 and miR-9 mediated downregulation of HDAC5 promotes neurite development through activating MEF2C-GPM6A pathway. J Cell Physiol. 2018;233:673–687. doi: 10.1002/jcp.25927. [DOI] [PubMed] [Google Scholar]
- 15.Guan K, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- 16.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 17.Hudish LI, Blasky AJ, Appel B. miR-219 regulates neural precursor differentiation by direct inhibition of apical par polarity proteins. Dev Cell. 2013;27:387–398. doi: 10.1016/j.devcel.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janesick A, Wu SC, Blumberg B. Retinoic acid signaling and neuronal differentiation. Cell Mol Life Sci. 2015;72:1559–1576. doi: 10.1007/s00018-014-1815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang D, Du J, Zhang X, Zhou W, Zong L, Dong C, Chen K, Chen Y, Chen X, Jiang H. miR-124 promotes the neuronal differentiation of mouse inner ear neural stem cells. Inter J Mol Med. 2016;38:1367–1376. doi: 10.3892/ijmm.2016.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao S, Liu Y, Yao Y, Teng J. miR-124 promotes proliferation and differentiation of neuronal stem cells through inactivating Notch pathway. Cell Biosci. 2017;7:68. doi: 10.1186/s13578-017-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao S, Liu Y, Yao Y, Teng J. miR-124 promotes proliferation and neural differentiation of neural stem cells through targeting DACT1 and activating Wnt/beta-catenin pathways. Mol Cell Biochem. 2018;449:305–314. doi: 10.1007/s11010-018-3367-z. [DOI] [PubMed] [Google Scholar]
- 22.Kanungo J. Retinoic acid signaling in P19 stem cell differentiation. Anticancer Agents Med Chem. 2017;17:1184–1198. doi: 10.2174/1871520616666160615065000. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki H, Taira K. Functional analysis of microRNAs during the retinoic acid-induced neuronal differentiation of human NT2 cells. Nucleic Acids Res. 2003;3:243–244. doi: 10.1093/nass/3.1.243. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, Ruvkun G. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 27.Le MTN, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H, Lim B, Lodish HF. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol. 2009;29:5290–5305. doi: 10.1128/MCB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF. On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol. 2005;17:27–50. doi: 10.1615/critrevneurobiol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- 29.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokabber H, Najafzadeh N, Mohammadzadeh Vardin M. miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J Cell Physiol. 2019;234:8941–8950. doi: 10.1002/jcp.27563. [DOI] [PubMed] [Google Scholar]
- 32.Neo WH, Yap K, Lee SH, Looi LS, Khandelia P, Neo SX, Makeyev EV, Su IH. MicroRNA miR-124 controls the choice between neuronal and astrocyte differentiation by fine-tuning Ezh2 expression. J Biol Chem. 2014;289:20788–20801. doi: 10.1074/jbc.M113.525493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquali D, Chieffi P, Deery WJ, Nicoletti G, Bellastella A, Sinisi AA. Differential effects of all-trans-retinoic acid (RA) on Erk1/2 phosphorylation and cAMP accumulation in normal and malignant human prostate epithelial cells: Erk1/2 inhibition restores RA-induced decrease of cell growth in malignant prostate cells. Eur J Endocrinol. 2005;152:663–669. doi: 10.1530/eje.1.01875. [DOI] [PubMed] [Google Scholar]
- 34.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharif S, Ghahremani MH, Soleimani M. Induction of morphological and functional differentiation of human neuroblastoma cells by miR-124. J Biosci. 2017;42:555–563. doi: 10.1007/s12038-017-9714-5. [DOI] [PubMed] [Google Scholar]
- 36.Shi F, Yang Y, Wang T, Kouadir M, Zhao D, Hu S. Cellular prion protein promotes neuronal differentiation of adipose-derived stem cells by upregulating miRNA-124. J Mol Neurosci. 2016;59:48–55. doi: 10.1007/s12031-016-0733-8. [DOI] [PubMed] [Google Scholar]
- 37.Shi Z, Zhou H, Lu L, Pan B, Wei Z, Liu J, Li J, Yuan S, Kang Y, Liu L, Yao X, Kong X, Feng S. MicroRNA-29a regulates neural stem cell neuronal differentiation by targeting PTEN. J Cell Biochem. 2018;119:5813–5820. doi: 10.1002/jcb.26768. [DOI] [PubMed] [Google Scholar]
- 38.Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28:10415–10421. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J. Cyclophilin a is required for retinoic acid-induced neuronal differentiation in p19 cells. J Biol Chem. 2004;279:24414–24419. doi: 10.1074/jbc.M311406200. [DOI] [PubMed] [Google Scholar]
- 40.Song J, Oh Y, Kim JY, Cho KJ, Lee JE. Suppression of MicroRNA let-7a expression by agmatine regulates neural stem cell differentiation. Yonsei Med J. 2016;57:1461–1467. doi: 10.3349/ymj.2016.57.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay RG, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J Neurosci Meth. 2010;186:60–67. doi: 10.1016/j.jneumeth.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker SE, Nottrodt R, Maddalena L, Carter C, Spencer GE, Carlone RL. Retinoid X receptor α downregulation is required for tail and caudal spinal cord regeneration in the adult newt. Neural Regen Res. 2018;13:1036–1045. doi: 10.4103/1673-5374.233447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei C, Ren L, Li K, Lu Z. The regulation of survival and differentiation of neural stem cells by miR-124 via modulating PAX3. Neurosci Lett. 2018;683:19–26. doi: 10.1016/j.neulet.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 45.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen S, Plasterk RH. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 46.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43:D146–D152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu H, Zhao J, Fu B, Yin S, Song C, Zhang J, Zhao S, Zhang Y. Retinoic acid-induced upregulation of miR-219 promotes the differentiation of embryonic stem cells into neural cells. Cell Death Dis. 2017;8:e2953. doi: 10.1038/cddis.2017.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu PY, Lin YC, Chang CL, Lu HT, Chin CH, Hsu TT, Chu D, Sun SH. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signalling. 2009;21:881–891. doi: 10.1016/j.cellsig.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, Chung K, Deo M, Thompson R, Turner D. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314:2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng M, Zhou J-N. Roles of autophagy and mTOR signaling in neuronal differentiation of mouse neuroblastoma cells. Cell Signal. 2008;20:659–665. doi: 10.1016/j.cellsig.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Zhang HN, Guo Y, Ma W, Xue J, Wang WL, Yuan ZW. MGMT is down-regulated independently of promoter DNA methylation in rats with all-trans retinoic acid-induced spina bifida aperta. Neural Regen Res. 2019;14:361–368. doi: 10.4103/1673-5374.244799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.