Activation of myeloid cells by human endogenous retroviral entities: While the exact causes of neurological diseases such as multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS) are still elusive, there is evidence of a new category of pathogenic elements called human endogenous retroviruses (HERVs) which seem to contribute to their evolution and progression by exerting inflammatory and degenerative effects (Küry et al., 2018). HERVs are ancient retroviral elements which account for up to 8% of the human genome and it is known that environmental factors can trigger their (re-)expression (Küry et al., 2018). The resulting production of viral particles and/or proteins, especially from members of the HERV-W and HERV-K family, is strongly correlated with the onset and progression of neurological diseases, such as MS and ALS (Küry et al., 2018).
In ALS, HERV-K expression is mainly found in neurons (Li et al., 2015), whereas in MS increased HERV-W RNA and protein levels have been confirmed for microglia and macrophages (Mameli et al., 2007). In this context, a correlation between induced gliotoxicity, reverse transcriptase activity and HERV-W expression in macrophage cell culture supernatants derived from MS patients had previously been documented (Menard et al., 1997). Subsequent studies were able to detect HERV-W envelope (ENV) protein expression in myeloid cells (i.e., microglia and macrophages) in areas of active demyelination as well as at the rims of chronic active lesions (van Horssen et al., 2016; Kremer et al., 2019). In contrast, only few HERV-W ENV-positive astroglial and lymphoid cells could be detected (van Horssen et al., 2016). Similar to MS, expression of the multicopy HERV-W family in schizophrenia was detected in peripheral blood mononuclear cells (Perron et al., 2012). Against this backdrop, we will focus this perspective on HERV-mediated effects on myeloid cells.
Myeloid cells are part of the innate immune system and can be divided into different subpopulations. On the one hand, there are monocytes circulating in the bloodstream. On the other hand, macrophages are tissue-localized cells, which can be found in virtually every organ. Both monocytes and macrophages originate from hematopoietic stem cells in the bone marrow. In contrast to that, the tissue-localized myeloid cells of the central nervous system, the so-called microglial cells originate from embryonic yolk sac progenitors.
Early functional studies demonstrated that the HERV-W ENV protein can activate the receptors Toll-like receptor 4 (Figure 1) and cluster of differentiation 14 (Figure 1) on human monocytes leading to the production of pro-inflammatory cytokines (Rolland et al., 2006). In addition, the ENV protein was shown to stimulate dendritic cells, a related myeloid cell type, to promote T helper cell type 1 differentiation (Rolland et al., 2006). A similar activation of dendritic cells and a triggering role in experimental autoimmune encephalomyelitis in mice was then corroborated in a follow up in vivo study (Perron et al., 2013). In addition to that, microglial cells also respond strongly to HERV-W ENV protein exposure. As recently demonstrated, ENV induces the expression of pro-inflammatory cytokines and of nitric oxide in these cells while it reduces anti-inflammatory and neuroprotective parameters (Kremer et al., 2019). Furthermore, ENV protein drives microglial cells to physically interact with myelinated axons and to induce leakage of intra-axonal and myelin proteins. These observations suggest an entirely novel axon damage mechanism, which could explain the tight axonal phenotypes of myeloid cells found in chronic active MS lesions (Kremer et al., 2019). In addition, they also provide a biomedical rationale for the anti-degenerative effects observed in a recent phase 2b clinical trial which tested the HERV-W ENV-neutralizing antibody temelimab in MS patients (CHANGE-MS, NCT02782858 followed by ANGEL-MS NCT03239860). In light of these recent developments, it is worth mentioning that a previous study already reported increased nitric oxide production in response to ENV overexpression in a microglial cell line and that cell migration was enhanced (Xiao et al., 2017).
Figure 1.
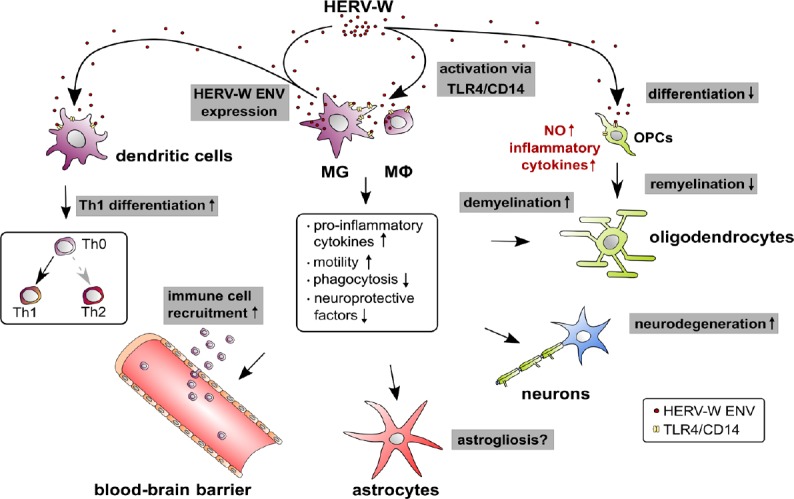
HERV-W mediated effects on myeloid cells.
This illustration summarizes the origin and observed molecular effects of HERV-W on myeloid cells and how it affects neural cells of the central nervous system. Arrow starting points indicate the cellular sources of HERV-W particles or proteins (red dots) while arrowheads point to the influences on different cell types. TLR4/CD14 receptors are marked in yellow. Modulated processes are shown in grey boxes, regulated myeloid molecules and processes are shown in the central panel and regulated molecules in non-myeloid cells are shown in red. Whether microglia and macrophages respond to HERV-W in an auto- and/or paracrine way remains to be shown. CD14: Cluster of differentiation 14; HERV-W: human endogenous retroviruse-W; MG: microglia; Mφ: macrophage; NO: nitric oxide; OPCs: oligodendroglial progenitor cells; Th: T-helper cell; TLR4: Toll-like receptor 4.
In summary, the current available data point to a preferred activation of HERVs in myeloid cells such as monocytes/macrophages and microglial cells leading to endothelial and oligodendroglial stress reactions which may contribute to disease pathology and impaired regeneration (Küry et al., 2018). On the other hand, additional autocrine/paracrine effects of HERVs on the polarization and phenotype of myeloid cells have been reported recently. As there are several specialized macrophage populations present at central nervous system interfaces such as the dura mater, the leptomeninges, the perivascular space and the choroid plexus (Kierdorf et al., 2019), it will be of interest to analyze their specific reactions to ENV production or stimulation. All the more, as HERV-W was initially discovered in a leptomeningeal cell line derived from a MS patient (Perron et al., 1989). Successful treatment of neurodegeneration is still an unmet clinical need particularly in progressive MS, so future studies are required to better understand associated myeloid phenotypes and their functional roles. Ultimately, from a therapeutic standpoint the goal is to identify new means to modulate and control HERV activation and expression.
Research on myelin repair and HERVs in the laboratory of PK was supported by the French societies ARSEP (Fondation pour l’Aide à la Recherche sur la Sclérose en Plaques) and AFM (Association Française Contre les Myopathies), by DMSG Ortsvereinigung Düsseldorf und Umgebung e.V. as well as by Geneuro. JG is a student of the iBrain graduate school and PK and JG are supported by the Stifterverband/Novartisstiftung. DK was funded by the Deutsche Forschungsgemeinschaft (DFG) while carrying research on HERVs at Cleveland Clinic. The MS Center at the Department of Neurology is supported in part by the Walter and Ilse Rose Foundation and the James and Elisabeth Cloppenburg, Peek, and Cloppenburg Düsseldorf Stiftung.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Kierdorf K, Masuda T, Jordao MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20:547–562. doi: 10.1038/s41583-019-0201-x. [DOI] [PubMed] [Google Scholar]
- 2.Kremer D, Gruchot J, Weyers V, Oldemeier L, Göttle P, Healy L, Ho Jang J, Kang TXY, Volsko C, Dutta R, Trapp BD, Perron H, Hartung HP, Küry P. pHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116:15216–15225. doi: 10.1073/pnas.1901283116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Küry P, Nath A, Creange A, Dolei A, Marche P, Gold J, Giovannoni G, Hartung HP, Perron H. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24:379–394. doi: 10.1016/j.molmed.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Lee MH, Henderson L, Tyagi R, Bachani M, Steiner J, Campanac E, Hoffman DA, von Geldern G, Johnson K, Maric D, Morris HD, Lentz M, Pak K, Mammen A, Ostrow L, Rothstein J, Nath A. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med. 2015;7:307ra153. doi: 10.1126/scitranslmed.aac8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mameli G, Astone V, Arru G, Marconi S, Lovato L, Serra C, Sotgiu S, Bonetti B, Dolei A. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not Human herpesvirus 6. J Gen Virol. 2007;88:264–274. doi: 10.1099/vir.0.81890-0. [DOI] [PubMed] [Google Scholar]
- 6.Menard A, Amouri R, Michel M, Marcel F, Brouillet A, Belliveau J, Geny C, Deforges L, Malcus-Vocanson C, Armstrong M, Lyon-Caen O, Mandrand B, Dobransky T, Rieger F, Perron H. Gliotoxicity, reverse transcriptase activity and retroviral RNA in monocyte/macrophage culture supernatants from patients with multiple sclerosis. FEBS Lett. 1997;413:477–485. doi: 10.1016/s0014-5793(97)00889-2. [DOI] [PubMed] [Google Scholar]
- 7.Perron H, Geny C, Laurent A, Mouriquand C, Pellat J, Perret J, Seigneurin JM. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res Virol. 1989;140:551–561. doi: 10.1016/s0923-2516(89)80141-4. [DOI] [PubMed] [Google Scholar]
- 8.Perron H, Dougier-Reynaud HL, Lomparski C, Popa I, Firouzi R, Bertrand JB, Marusic S, Portoukalian J, Jouvin-Marche E, Villiers CL, Touraine JL, Marche PN. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS One. 2013;8:e80128. doi: 10.1371/journal.pone.0080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C, Sarrazin S, LeGuen E, Houenou J, Delavest M, Moins-Teisserenc H, Bengoufa D, Yolken R, Madeira A, Garcia-Montojo M, Gehin N, Burgelin I, Ollagnier G, Bernard C, Dumaine A, et al. Molecular characteristics of Human Endogenous Retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2012;2:e201. doi: 10.1038/tp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006;176:7636–7644. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- 11.van Horssen J, van der Pol S, Nijland P, Amor S, Perron H. Human endogenous retrovirus W in brain lesions: Rationale for targeted therapy in multiple sclerosis. Mult Scler Relat Disord. 2016;8:11–18. doi: 10.1016/j.msard.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Xiao R, Li S, Cao Q, Wang X, Yan Q, Tu X, Zhu Y, Zhu F. Human endogenous retrovirus W env increases nitric oxide production and enhances the migration ability of microglia by regulating the expression of inducible nitric oxide synthase. Virol Sin. 2017;32:216–225. doi: 10.1007/s12250-017-3997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


