Abstract
Objective:
An increasing number of studies indicate that autophagy plays an important role in the pathogenesis of spinal cord injury, and that regulating autophagy can enhance recovery from spinal cord injury. However, the effect of regulating autophagy and whether autophagy is detrimental or beneficial after spinal cord injury remain unclear. Therefore, in this study we evaluated the effects of autophagy regulation on spinal cord injury in rats by direct and indirect comparison, in an effort to provide a basis for further research.
Data source:
Relevant literature published from inception to February 1, 2018 were included by searching Wanfang, CNKI, Web of Science, MEDLINE (OvidSP), PubMed and Google Scholar in English and Chinese. The keywords included “autophagy”, “spinal cord injury”, and “rat”.
Data selection:
The literature included in vivo experimental studies on autophagy regulation in the treatment of spinal cord injury (including intervention pre- and post-spinal cord injury). Meta-analyses were conducted at different time points to compare the therapeutic effects of promoting or inhibiting autophagy, and subgroup analyses were also conducted.
Outcome measure:
Basso, Beattie, and Bresnahan scores.
Results:
Of the 622 studies, 33 studies of median quality were included in the analyses. Basso, Beattie, and Bresnahan scores were higher at 1 day (MD = 1.80, 95% CI: 0.81–2.79, P = 0.0004), 3 days (MD = 0.92, 95% CI: 0.72–1.13, P < 0.00001), 1 week (MD = 2.39, 95% CI: 1.85–2.92, P < 0.00001), 2 weeks (MD = 3.26, 95% CI: 2.40–4.13, P < 0.00001), 3 weeks (MD = 3.13, 95% CI: 2.51–3.75, P < 0.00001) and 4 weeks (MD = 3.18, 95% CI: 2.43–3.92, P < 0.00001) after spinal cord injury with upregulation of autophagy compared with the control group (drug solvent control, such as saline group). Basso, Beattie, and Bresnahan scores were higher at 1 day (MD = 6.48, 95% CI: 5.83–7.13, P < 0.00001), 2 weeks (MD = 2.43, 95% CI: 0.79–4.07, P = 0.004), 3 weeks (MD = 2.96, 95% CI: 0.09–5.84, P = 0.04) and 4 weeks (MD = 4.41, 95% CI: 1.08–7.75, P = 0.01) after spinal cord injury with downregulation of autophagy compared with the control group. Indirect comparison of upregulation and downregulation of autophagy showed no differences in Basso, Beattie, and Bresnahan scores at 1 day (MD = −4.68, 95% CI: –5.840 to −3.496, P = 0.94644), 3 days (MD = −0.28, 95% CI: −2.231–1.671, P = 0.99448), 1 week (MD = 1.83, 95% CI: 0.0076–3.584, P = 0.94588), 2 weeks (MD = 0.81, 95% CI: −0.850–2.470, P = 0.93055), 3 weeks (MD = 0.17, 95% CI: −2.771–3.111, P = 0.99546) or 4 weeks (MD = –1.23, 95% CI: −4.647–2.187, P = 0.98264) compared with the control group.
Conclusion:
Regulation of autophagy improves neurological function, whether it is upregulated or downregulated. There was no difference between upregulation and downregulation of autophagy in the treatment of spinal cord injury. The variability in results among the studies may be associated with differences in research methods, the lack of clearly defined autophagy characteristics after spinal cord injury, and the limited autophagy monitoring techniques. Thus, methods should be standardized, and the dynamic regulation of autophagy should be examined in future studies.
Keywords: autophagy; Basso, Beattie, and Bresnahan scores; indirect comparison; meta-analysis; nerve regeneration; neural regeneration; neurological function; rat models; regulation; spinal cord injury; strategy analysis
Chinese Library Classification No. R441; R363; R364
Introduction
Spinal cord injury (SCI) is a debilitating traumatic disease that is a huge burden for families and society, with no effective treatment (Nowrouzi et al., 2017; Yi et al., 2017). The pathophysiologic mechanisms of SCI remain unclear, despite numerous studies (Huang et al., 2018, 2019; Sharma et al., 2019; Wei et al., 2019). Autophagy (mainly referring to macroautophagy) is a generic term for the cellular processes that deliver cytoplasmic materials to the lysosome to degrade and recycle cell contents (Goldshmit et al., 2015; Li et al., 2019).
Autophagy regulates cell death in central nervous system diseases and improves neurological recovery (Galluzzi et al., 2016; Liu et al., 2017c). Although accumulating evidence shows that autophagy plays a critical role in SCI, several controversial views and debates still exist, especially on the overall effect of regulating autophagy and whether autophagy should be induced or inhibited. Some researchers have reported that upregulating autophagy promotes neuronal survival and the recovery of neurological function (Chen et al., 2013; Tang et al., 2014). However, other studies have shown that downregulating autophagy produces better therapeutic results (Fang et al., 2016; Xie et al., 2017a). Therefore, it is necessary to clarify whether upregulation or downregulation of autophagy is the best strategy, to lay the foundation for future studies. Although many tools have been used for evaluating SCI recovery, the Basso, Beattie, and Bresnahan (BBB) score is still the most widely used for neurological functional assessment. A number of studies compared upregulation or downregulation of autophagy with vehicle control, but only a few compared upregulation directly with downregulation. In this study, we performed direct and indirect network meta-analyses of rat models of SCI in which autophagy was upregulated or downregulated by comparing BBB scores to identify the best strategy. Additional systematic review was conducted to help guide future studies of autophagy in SCI.
Data and Methods
Literature search
The following electronic databases were searched from their inception to February 1, 2018: Web of Science, MEDLINE (OvidSP), PubMed, Wanfang, China National Knowledge Infrastructure (CNKI) and Google Scholar. The search was designed and executed by an experienced doctor (Duo Zhang). The search strategy consisted of two main components: spinal cord injury and autophagy, and the results were limited to rat studies. The keywords included “autophagy”, “spinal cord injury”, “rat”, with various combinations of the operators “AND” and “OR” during the literature retrieval.
Study selection, inclusion and exclusion criteria
Two independent reviewers performed the literature selection and included studies if the following criteria were met: (1) the study assessed the therapeutic effect of regulating autophagy in SCI with autophagy level evaluation; (2) the study was performed in rats in vivo; (3) the study contained groups treated with vehicle; (4) the study reported BBB scores.
The exclusion criteria for the title and abstract screen phase included the following: (1) not a primary study; (2) not an intervention of interest (regulation of autophagy); (3) data were not obtained or data were incorrect; (4) did not evaluate autophagy level after intervention.
Study characteristics and data extraction
Data were extracted from the full-text publications. The following items were extracted: author, year, language, species/strain, animal gender, number of animals in control and experimental groups, the method for the establishment of animal models, therapeutic agents, dosage, timing, duration, regulation of autophagy, and BBB scores. Higher BBB scores indicate better recovery of neurological function. BBB scores were extracted as mean ± standard deviation (SD) for the different time points. If BBB scores were not available in the text, but only presented in graph form, the data were obtained by measuring the graphs (Liu et al., 2017a). In brief, the graph was imported into Photoshop software, and we obtained the coordinate value of the scale interval of the Y-axis and the scatter plot or histogram with the SD line. The mean and SD were calculated by converting the coordinate values and scale interval.
Quality assessment of included studies
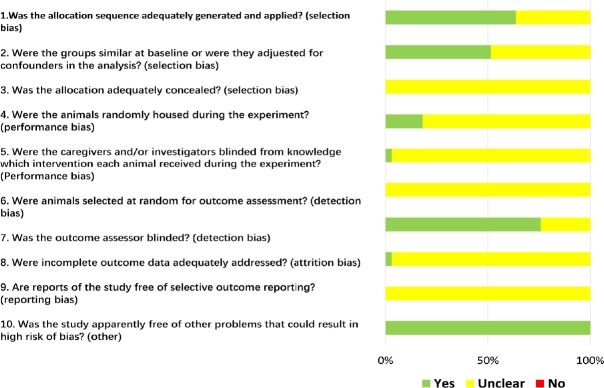
The SYRCLE’s Risk of Bias tool, based on the Cochrane Risk of Bias tool, was used to assess the risk of bias of all included studies, and was performed by two independent investigators (Hooijmans et al., 2014). SYRCLE’s Risk of Bias tool contains 10 entries that are related to selection bias, performance bias, detection bias, reporting bias and other biases. Disagreements were resolved by discussion.
Outcome measure
Locomotor function, evaluated with the BBB scale, was the main evaluation index. Normal function is rated as 21 points; lower scores reflect greater locomotor functional impairment.
Data management and statistical analysis
If studies contained multiple independent paired groups, they were treated as separate experiments; if studies contained multiple experimental groups with the same trends of autophagy regulation and a single control group, they were consolidated; if studies contained a single control group, but multiple experimental groups with different types of autophagy regulation, the control group was split (Higgins, 2011).
Data were analyzed using RevMan 5.3 software (Cochrane Library,https://community.cochrane.org/help/tools-and-software/revman-5/revman-5-download), ITC software (Canadian Agency for Drugs and Technologies in Health, CADTH,https://www.cadth.ca/resources/itc-user-guide/download-software-win-xp), and mean difference (MD) and corresponding 95% confidence interval (CI) were used as the effect measures. Heterogeneity was quantified using the χ2 test, and the effect measure was I2 (0% ≤ I2 < 25% indicates no heterogeneity, 25% ≤ I2 < 50% indicates low heterogeneity, 50% ≤ I2 < 75% indicates median heterogeneity, and 75% ≤ I2 indicates high heterogeneity). The fixed effects model was used if I2 < 50%, otherwise, the random effects model was used. Subgroup analysis was performed based on the type of SCI model, and was used to analyze the source of heterogeneity. The BBB scores were analyzed according to the time after SCI, from 1 day to 4 weeks. P < 0.05 was considered statistically significant. Our procedures accorded with the PRISMA guidelines for reporting systematic review/meta-analysis (Moher et al., 2010).
Results
Description of the included studies
The comprehensive search strategy on the effect of autophagic regulation in SCI rat models resulted in 622 records (227 from the Web of Science, 45 from Medline (OvidSP), 83 from PubMed, 58 from WanFang data, 64 from CNKI and 145 from Google Scholar). After duplicates were removed, 486 studies were left. After title and abstract screening, 85 studies were screened full-text. Ultimately, 33 studies were included in the meta-analysis shown with the PRISMA flow diagram (Chen et al., 2013, 2014; Hao et al., 2013; Tang et al., 2014; Gao et al., 2015, 2016; Li et al., 2015b, 2016b, 2018a, b; Seo et al., 2015; Yue et al., 2015; Fang et al., 2016, 2017; Sun et al., 2016; Wang et al., 2016, 2017a, b, 2018a, b; Zhou et al., 2016a, b; Bai et al., 2017; Liu et al., 2017b; Xie et al., 2017a, b; Zhang et al., 2017a, b; Zhao et al., 2017a, b; Miao et al., 2018; Tong et al., 2018) (Figure 1). The characteristics of all included studies are described in Table 1.
Figure 1.
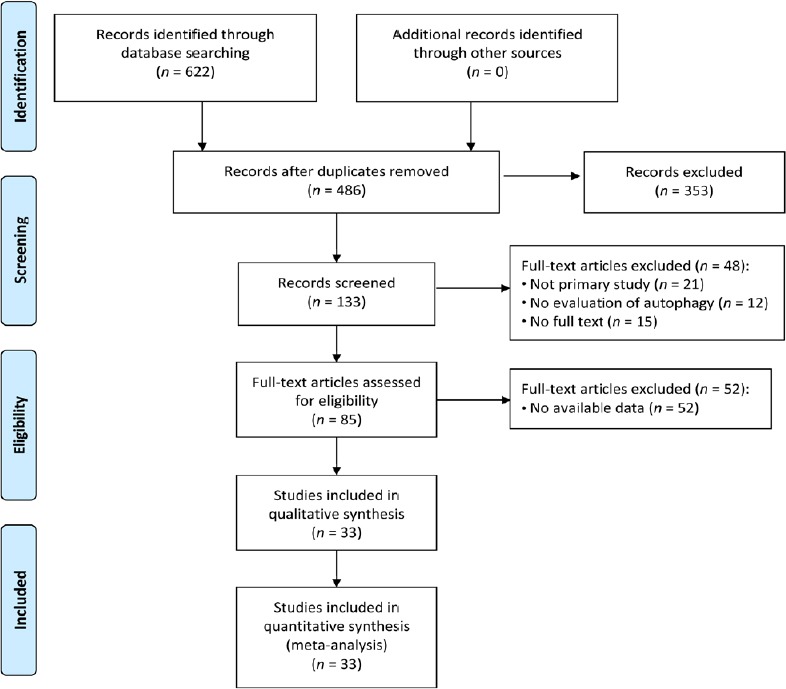
Flow diagram of the literature search and selection results.
Table 1.
Characteristics of the included animal studies
| Study | Spinal injury rat model | Segment | Severity | Intervention | Usage | Timing | Duration | Autophagy regulation | Behavior |
|---|---|---|---|---|---|---|---|---|---|
| Bai et al. (2017) | CTI | T9 | 10 g × 2.0 cm | Netrin-1 | 800 ng/rat | Immediately after SCI | Daily for 2 days | ↑ | ↑ |
| Chen et al. (2013) | CTI | T9–10 | 10 g × 1.25 cm | Rapamycin | 500 mg/kg | After SCI | Daily for 3 days | ↑ | ↑ |
| CTI | T9–10 | 10 g × 1.25 cm | 3-MA | 100 mg/kg | After SCI | Daily for 3 days | ↓ | → | |
| Chen et al. (2014) | CTI | T9–10 | 10 g × 1.25 cm | Vitamin C & E | 240 mg/kg & 200 mg/kg | After SCI | Daily for 7 days | ↑ | ↑ |
| Fang et al. (2016) | I/R | Aorta arch | Clipping for 14 min | Rapamycin | 500 mg/kg | Immediately after SCI | Only once | ↑ | ↑ |
| Rapamycin | 500 mg/kg | 48 h after SCI | Only once | ↑ | ↓ | ||||
| 3-MA | 2.5 mg/kg | Immediately after SCI | Only once | ↓ | ↓ | ||||
| 3-MA | 2.5 mg/kg | 48 h after SCI | Only once | ↓ | ↑ | ||||
| Fang et al. (2017) | I/R | Aortic arch | Clapping for 14 min | EA | 20 min (60 Hz for 1.05 s and 2 Hz for 2.85 s alternately), ≤ 1 mA | Immediately after SCI | Only once | ↑ | ↑ |
| Gao et al. (2015) | CTI | T9 | 10 g × 2.5 cm | Simvastatin | 10 mg/kg | 1 h after SCI | Daily for 2 days | ↑ | ↑ |
| Gao et al. (2016) | CTI | T9–10 | 10 g × 2.5 cm | Atorvastatin | 5 mg/kg | Immediately after SCI | Daily for 2 days | ↑ | ↑ |
| Hao et al. (2013) | CTI | T10 | 10 g × 1.25 cm | Valproic acid | 300 m/kg | Immediately after SCI | Twice daily for 2 weeks | ↓ | ↑ |
| Li et al. (2015b) | I/R | Proximal descending thoracic aorta | Ballon occlusion for 12 h | Hydrogen sulfide (H2S) | 30 μmol/kg | 0.5 h before SCI | Only once | ↑ | ↑ |
| Li et al. (2016a) | CTI | T10 | 25 g × 3.0 cm | MP | 30 mg/kg | After SCI | Daily for 7 days | ↑ | ↑ |
| Li et al. (2016b) | CTI | T9–11 | 10 g × 2.0 cm | Exendin-4 | 2.5 μg/rat 10 μg/rat | Immediately after SCI | Only once | ↑ | ↑ |
| Li et al. (2018a) | CPI | T9 | 15-g force for 1 min | AAG-2 particle of FGF-1 | 1 × 109/TU | Immediately after SCI | Only once | ↑ | ↑ |
| Li et al. (2018b) | CPI | T9–10 | 30-g forces for 1 min | Neuroserpin | 25 μL with 25 μg/mL | After SCI | Only once | ↓ | ↑ |
| Liu et al. (2017b) | TI | T4 | – | Lithium | 50 mg/kg | Immediately after SCI | Daily for 7 days | ↑ | ↑ |
| Miao et al. (2018) | CTI | NR | NR | TUDCA | 100 μg/kg 200 μg/kg | After SCI | NR | ↑ | ↑ |
| Seo et al. (2015) | CTI | T9 | 10 g × 2.5 cm | Hypothermia | 30–32°C for 4 h | Immediately after SCI | Only once | ↓ | ↑ |
| MP | 30 mg/kg then 124.2 mg/kg | After closure and 1 h again | Only once | ↓ | ↑ | ||||
| Sun et al. (2016) | CTI | T9–11 | 5 g × 10 cm | Hyperbaric oxygen | 90–100 min | Within 6 h after SCI | Once daily | ↑ | ↑ |
| Tang et al. (2014) | HI | T9–10 (right) | – | 3-MA | 2.5 mg/kg | 4 h after SCI | Only once | ↓ | ↓ |
| – | Rapamycin | 0.5 mg | 4 h after SCI | Only once | ↑ | ↑ | |||
| Tong et al. (2018) | CPI | T9 | 15-g forces for 1 min | Lithium | 20 mg/kg | Immediately after SCI | Once daily | ↑ | ↑ |
| Wang et al. (2016) | CTI | T9–10 | 10 g × 2.5 cm | Metformin | 100 mg/kg | After SCI | Daily for 3 days | ↑ | ↑ |
| Wang et al. (2017a) | CPI | T9 | Clipping for 1 min | SS31 peptide | 5 mg | Immediately after SCI | Twice daily for 2 weeks | ↑ | ↑ |
| Wang et al. (2017b) | CTI | T9–10 | 10 g × 2.5 cm | Berberine | 10 mg/kg | Immediately after SCI | Daily for 3 days | ↑ | ↑ |
| Wang et al. (2018a) | CTI | T10 | 10 g × 2.5 cm | Resveratrol | 200 mg/kg | Immediately after SCI | Daily for 3 days | ↑ | ↑ |
| Wang et al. (2018b) | CPI | T7–11 | 30-g forces for 1 min | Ginsenoside Rb1 (Rb1) | 20 mg/kg | After SCI | Once daily | ↓ | ↑ |
| Xie et al. (2017a) | I/R | Abdominal aorta | 50-g forces clipping for 60 min | 3-MA | 2.5 mg/kg | Immediately after SCI | Only once | ↓ | ↑ |
| NAC | 300 mg/kg | Immediately after SCI | Only once | ↓ | ↑ | ||||
| Xie et al. (2017b) | I/R | Abdominal aorta | 50-g forces clipping for 60 min | NAD+ | 10 mg/kg 75 mg/kg | Immediately after SCI | Only once | ↓ | ↑ |
| Yue et al. (2015) | CTI | T10 | 10 g × 3.0 cm | Berberine | 5 mg/100 g | 2 h after SCI | Only once | ↑ | ↑ |
| Zhang et al. (2017a) | CTI | T9–10 | 10 g × 5.0 cm | Diosgenin | 100 mg/kg 200 mg/kg | After SCI | Daily for 21 days | ↑ | ↑ |
| Zhang et al. (2017b) | CPI | T9 | 30-g forces for 1min | Metformin | 50 mg/kg | Immediately after SCI | Once daily | ↑ | ↑ |
| Zhao et al. (2017a) | CTI | T9–10 | 10 g × 3.0 cm | Resveratrol | 100 mg/kg | Immediately after SCI | Only once | ↑ | ↑ |
| Zhao et al. (2017b) | CPI | T9 | 30-g forces for 2 min | GDNF | 6 μg/rat | Immediately after SCI | Only once | ↓ | ↑ |
| Zhou et al. (2016a) | CPI | T9 | 15-g force for 1 min | Calcitriol | 2 μg/kg | 2 h after SCI | Daily for 7 days | ↑ | ↑ |
| Zhou et al. (2016b) | CPI | T9 | 30-g forces clipping for 2 min | Retinoic acid | 15 mg/kg | Immediately after SCI | Daily for 2 weeks | ↑ | ↑ |
The symbol “↑” stands for upregulation or increase, and “↓” stands for downregulation or decrease and “→” stands for no obvious change. Autophagy upregulation includes higher expression of LC3, LC3b, LCII or Beclin 1 or value of LC II/LC I, while downregulation includes lower expression of LC3, LC3b, LCII or Beclin 1 or value of LC II/LC I. Behavioral test means BBB scores evaluation. Severity means the severity of SCI when model was built. For CTI model, the severity was evaluated as the measurement of the contusion mass multiplied height. NR: Not referred; CTI: contusion injury; CPI: compression injury, I/R: ischemia/reperfusion, TI: transection injury; HI: hemisection injury; SCI: spinal cord injury; 3-MA: 3-methyladenine, MP: methylprednisolone; EA: electroacupuncture; NAC: N-acetyl-cysteine; NAD+: nicotinamide adenine dinucleotide; TUDCA: tauroursodeoxycholic acid; SS31: GDNF: glial cell-derived neurotrophic factor; s: seconds; min: minute(s); h: hour(s).
Risk of bias and quality of the included studies
Based on risk of bias assessment, 21 studies (64%) stated that the allocation was randomized, while no studies described the method of randomization or whether the allocation was adequately concealed. It was probably because the background of the animals was homogenous (Figure 2). Furthermore, many items were scored as “unclear”, as were items 3, 5, 6, 8 and 9, indicating that these animal studies could have been better.
Figure 2.
Graph of the risk of bias for the included studies.
Yes: Low risk of bias; No: high risk of bias; Unclear: unclear risk of bias.
Overall and subgroup meta-analyses of the effects of upregulation of autophagy on BBB scores
Because assessments of BBB scores were performed at different time points, meta-analyses were also performed at the following different time points: 1 day, 3 days, 1 week, 2 weeks ± 1 day, 3 weeks ± 1 day, and 4 weeks ± 1 day after SCI (termed 1 day, 3 days, 1 week, 2 weeks, 3 weeks and 4 weeks).
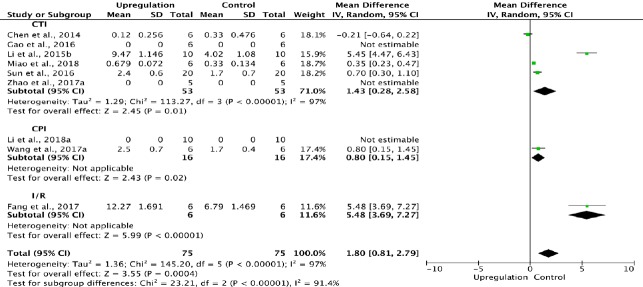
Eight of the 33 studies examining upregulation of autophagy reported BBB scores at 1 day after SCI (Chen et al., 2014; Li et al., 2015b; Gao et al., 2016; Sun et al., 2016; Fang et al., 2017; Wang et al., 2017a; Zhao et al., 2017a; Miao et al., 2018). Meta-analysis of these experiments revealed that upregulation of autophagy significantly elevated BBB scores using the random effects model (MD = 1.80, 95% CI: 0.81–2.79, P = 0.0004). However, heterogeneity was quite high (I2 = 97%). These studies were separated into three subgroups according to the type of SCI model. As shown in Figure 3, upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 1.43, 95% CI: 0.28–2.58, P = 0.01) and the compression injury group (MD = 0.80, 95% CI: 0.15–1.45, P = 0.02) with high inter-subgroup heterogeneity (I2 = 91.4%; Figure 3). The sensitivity analysis showed no heterogeneity (I2 = 0%) between the contusion injury and compression injury groups.
Figure 3.
Effect of upregulation of autophagy on Basso, Beattie, and Bresnahan scores at 1 day after spinal cord injury.
In all three subgroups, upregulation of autophagy significantly improved (increased) Basso, Beattie, and Bresnahan scores. CPI: Compression injury; CTI: contusion injury model; I/R: ischemia/reperfusion.
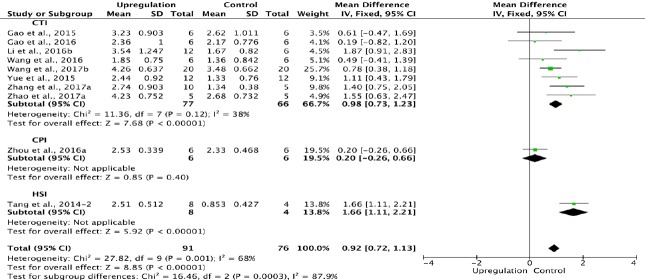
Ten of the 33 studies examining upregulation of autophagy reported BBB scores at 3 days after SCI (Tang et al., 2014; Gao et al., 2015, 2016; Yue et al., 2015; Li et al., 2016b; Wang et al., 2016, 2017b; Zhou et al., 2016a; Zhang et al., 2017a; Zhao et al., 2017a). Meta-analysis of these experiments revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 0.92, 95% CI: 0.72–1.13, P < 0.00001). However, heterogeneity was moderate (I2 = 68%). These studies were separated into three subgroups according to the SCI model. As shown in Figure 4, upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 0.98, 95% CI: 0.73–1.23, P < 0.00001), with low subgroup heterogeneity (I2 = 38%) and high inter-subgroup heterogeneity (I2 = 87.9%; (Figure 4). Sensitivity analysis showed similar heterogeneity levels.
Figure 4.
Effect of upregulation of autophagy on Basso, Beattie, and Bresnahan scores at 3 days after spinal cord injury.
In the CTI subgroup, upregulation of autophagy significantly increased Basso, Beattie, and Bresnahan scores. “–2” indicates one of the two eperimental groups in the same study with a single control group. CTI: Contusion injury; HSI: hemisection injury model.
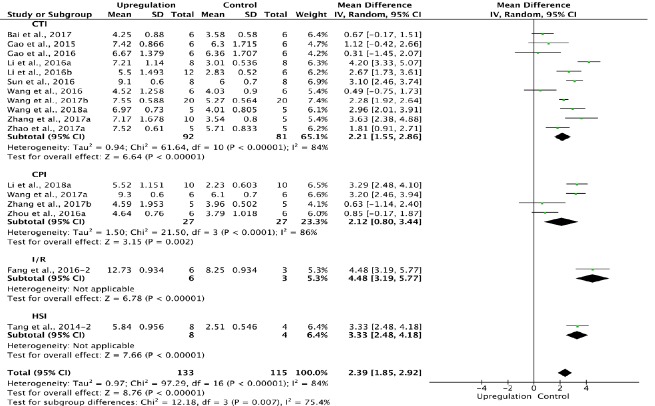
Of the 33 studies examining upregulation of autophagy, 17 reported BBB scores at 1 week after SCI (Tang et al., 2014; Gao et al., 2015, 2016; Fang et al., 2016; Li et al., 2016a, b, 2018a; Sun et al., 2016; Wang et al., 2016, 2017a, b, 2018a; Zhou et al., 2016a; Bai et al., 2017; Zhang et al., 2017a, b; Zhao et al., 2017a). Meta-analysis of these experiments revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 2.39, 95% CI: 1.85–2.92, P < 0.00001). However, heterogeneity was quite high (I2 = 84%). Based on the type of SCI model, these experiments were separated into four subgroups. As shown in Figure 5, upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 2.21, 95% CI: 1.55–2.86, P < 0.00001), with high subgroup heterogeneity (I2 = 84%) and high inter-subgroup heterogeneity (I2 = 75.4%; Figure 5). Sensitivity analysis showed similar heterogeneity levels.
Figure 5.
Effect of upregulation of autophagy on Basso, Beattie, and Bresnahan scores at 1 week after spinal cord injury.
In the CTI and CPI subgroups, upregulation of autophagy significantly improved Basso, Beattie, and Bresnahan scores. “–2” indicates one of the two eperimental groups in the same study with a single control group. CPI: Compression injury; CTI: contusion injury; HSI: hemisection injury; I/R: ischemia/reperfusion.
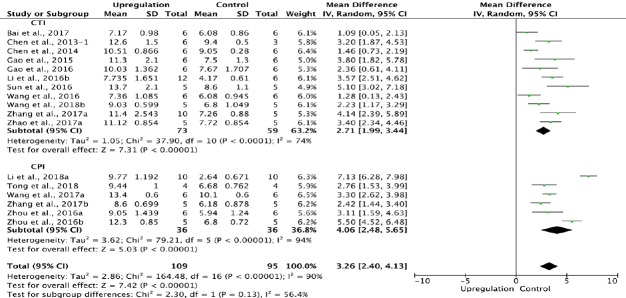
Similarly, 17 of the 33 studies that examined upregulation of autophagy reported BBB scores at 2 weeks after SCI (Chen et al., 2013, 2014; Gao et al., 2015, 2016; Li et al., 2016b, 2018a; Sun et al., 2016; Wang et al., 2016, 2018b; Zhou et al., 2016a, b; Bai et al., 2017; Wang et al., 2017a; Zhang et al., 2017a, b; Zhao et al., 2017a; Tong et al., 2018). Meta-analysis of these experiments revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 3.26, 95% CI: 2.40–4.13, P < 0.00001). However, heterogeneity was quite high (I2 = 90%). Based on the type of SCI model, the experiments were separated into two subgroups. As shown in Figure 6, upregulation of autophagy significantly increased BBB scores in the contusion injury group (MD = 2.71, 95% CI: 1.99–3.44, P < 0.00001), with medium subgroup heterogeneity (I2 = 74%), and in the compression injury group (MD = 4.06, 95% CI: 2.48–5.65, P < 0.00001), with high subgroup heterogeneity (I2 = 94%). The inter-subgroup heterogeneity was moderate (I2 = 56.4%; Figure 6). After two studies (Zhou et al., 2016b; Li et al., 2018a) were excluded, the heterogeneity in the compression injury subgroup and subgroup difference diminished to 0, and the overall heterogeneity decreased from high (I2 = 90%) to moderate (I2 = 68%).
Figure 6.
Effect of upregulation of autophagy on Basso, Beattie, and Bresnahan scores at 2 weeks after spinal cord injury.
In the CTI and CPI subgroups, upregulating autophagy significantly increased Basso, Beattie, and Bresnahan scores. “–1” indicates one of the two eperimental groups in the same study with a single control group. CPI: Compression injury; CTI: contusion injury.
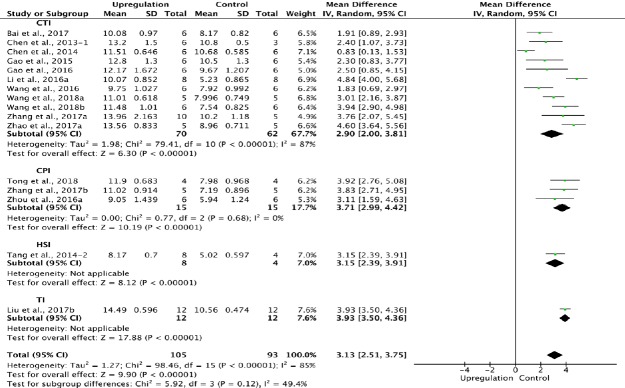
Sixteen of the 33 studies that examined upregulation of autophagy reported BBB scores at 3 weeks after SCI (Chen et al., 2013, 2014; Tang et al., 2014; Gao et al., 2015, 2016; Li et al., 2016a; Wang et al., 2016, 2018a, b; Zhou et al., 2016a; Bai et al., 2017; Liu et al., 2017b; Zhang et al., 2017a, b; Zhao et al., 2017a; Tong et al., 2018). Meta-analysis of these experiments revealed that upregulation of autophagy significantly improved BBB scores using the random effects model (MD = 3.13, 95% CI: 2.51–3.75, P < 0.00001). However, heterogeneity was quite high (I2 = 85%). Based on the type of SCI model, the experiments were separated into four subgroups. As shown in Figure 7, upregulation of autophagy significantly improved BBB scores in the contusion injury group (MD = 2.90, 95% CI: 2.00–3.81, P < 0.00001), with high subgroup heterogeneity (I2 = 87%), and in the compression injury group (MD = 3.71, 95% CI: 2.99–4.42, P < 0.00001), with low subgroup heterogeneity (I2 = 0%). The inter-subgroup heterogeneity was low (I2 = 49.4%; Figure 7). Subgroup heterogeneity decreased (I2 = 6.2%) after the transection injury (TI) subgroup was excluded.
Figure 7.
Effect of upregulation of autophagy on Basso, Beattie, and Bresnahan scores at 3 weeks after spinal cord injury.
In the CTI and CPI subgroups, upregulating autophagy significantly improved Basso, Beattie, and Bresnahan scores. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups. CI: confidence interval; CPI: compression injury; CTI: Contusion injury; HSI: hemisection injury; I/R: ischemia/reperfusion; TI: transection injury.
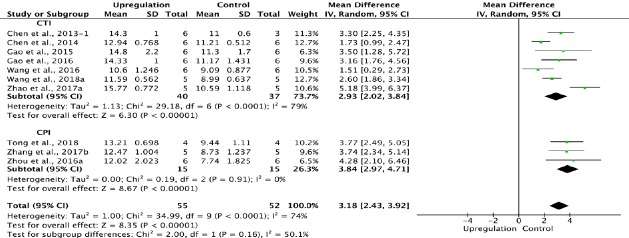
Ten of the 33 studies that examined upregulation of autophagy reported BBB scores at 4 weeks after SCI (Chen et al., 2013, 2014; Gao et al., 2015, 2016; Wang et al., 2016, 2018 a; Zhou et al., 2016a; Zhang et al., 2017b; Zhao et al., 2017a; Tong et al., 2018). Meta-analysis revealed that upregulation of autophagy significantly increased BBB scores using the random effects model (MD = 3.18, 95% CI: 2.43–3.92, P < 0.00001). However, heterogeneity was moderate (I2 = 74%). The experiments were separated into two subgroups according to the type of SCI model used. As shown in Figure 8, upregulating autophagy significantly improved BBB scores in the contusion injury group (MD = 2.93, 95% CI: 2.02–3.84, P < 0.00001), with high subgroup heterogeneity (I2 = 79%), and in the compression injury group (MD = 3.84, 95% CI: 2.97–4.71, P < 0.00001), with low subgroup heterogeneity (I2 = 0%). The inter-subgroup heterogeneity was moderate (I2 = 50.1%; Figure 8). The subgroup heterogeneity decreased (I2 = 0.3%) after one contusion injury study was excluded (Wang et al., 2016).
Figure 8.
Effect of upregulation of autophagy on Basso, Beattie, and Bresnahan scores at 4 weeks after spinal cord injury.
In the CTI and CPI subgroups, upregulating autophagy significantly ameliorated Basso, Beattie, and Bresnahan scores. “–1” indicates one of the two eperimental groups in the same study with a single control group. CPI: Compression injury; CTI: contusion injury;
Overall and subgroup analyses of the effects of downregulation of autophagy on BBB scores
Downregulation of autophagy increased BBB scores at 1 day, and 2, 3 and 4 weeks after SCI, but not at 3 days (MD = 1.20, 95% CI: –0.74–3.14, P = 0.23) or 1 week (MD = 0.56, 95% CI: –1.11–2.23, P = 0.51). Subgroup analysis revealed that downregulating autophagy did not significantly improve BBB scores in the contusion injury group at 1, 3 or 4 weeks, but did so at 2 weeks (MD = 0.88, 95% CI: 0.29–1.48, P = 0.004). In the compression injury group, downregulation of autophagy significantly ameliorated BBB scores at 1, 2, 3 and 4 weeks (Figures 9–14).
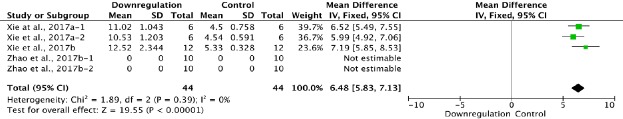
Figure 9.
Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 1 day after spinal cord injury.
Downregulating autophagy significantly increased Basso, Beattie, and Bresnahan scores. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups.
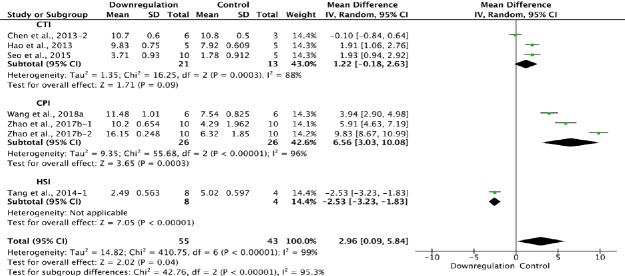
Figure 14.
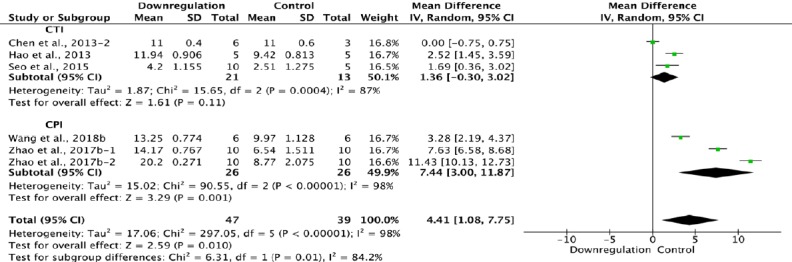
Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 4 weeks after spinal cord injury.
In the CTI subgroup, downregulating autophagy had no effect compared with the control, while in the CPI subgroup, it increased Basso, Beattie, and Bresnahan scores significantly. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups. CPI: Compression injury; CTI: contusion injury.
Three of the 34 studies that examined downregulation of autophagy reported BBB scores at 1 day after SCI (Xie et al., 2017a, b; Zhao et al., 2017b). Meta-analysis of these experiments revealed that downregulation of autophagy significantly increased BBB scores, as assessed with the fixed effects model (MD = 6.48, 95% CI: 5.83–7.13, P < 0.00001). Heterogeneity was quite low (I2 = 0%; Figure 9).
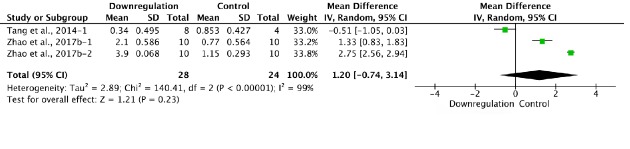
Two of the 34 studies that examined downregulation of autophagy reported BBB scores at 3 days after SCI (Tang et al., 2014; Zhao et al., 2017b). Meta-analysis of these experiments revealed that downregulation of autophagy did not significantly elevate BBB scores using the random effects model (MD = 1.20, 95% CI: –0.74–3.14, P = 0.23). However, heterogeneity was quite high (I2 = 99%; Figure 10). Sensitivity analysis showed similar heterogeneity.
Figure 10.
Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 3 days after spinal cord injury.
There was no difference between downregulation of autophagy and control. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups.
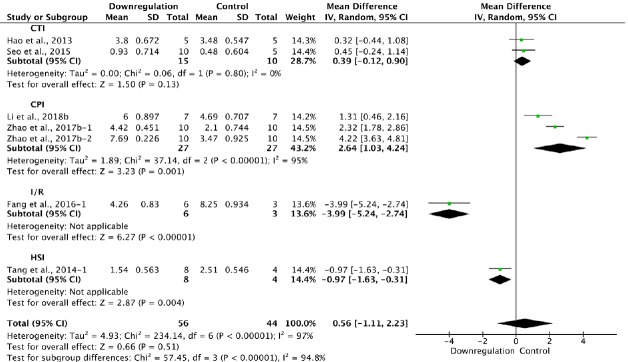
Six of the 33 studies that examined downregulation of autophagy reported BBB scores at 1 week after SCI (Hao et al., 2013; Tang et al., 2014; Seo et al., 2015; Fang et al., 2016; Zhao et al., 2017b; Li et al., 2018b). Meta-analysis of these experiments revealed that downregulation of autophagy did not significantly increase BBB scores using the random effects model (MD = 0.56, 95% CI: –1.11–2.3, P = 0.51). However, heterogeneity was quite high (I2 = 97%). Based on the type of SCI model used, the experiments were separated into four subgroups. As shown in Figure 11, downregulation of autophagy did not improve BBB scores in the contusion injury group (MD = 0.39, 95% CI: –0.12–0.90, P = 0.13), with low heterogeneity (I2 = 0%). In contrast, downregulation of autophagy improved BBB scores in the compression injury group (MD = 2.64, 95% CI: 1.03–4.24, P = 0.001), with high heterogeneity (I2 = 95%). The inter-subgroup heterogeneity was also high (I2 = 94.8%; Figure 11). The heterogeneity was similar when any single study was excluded.
Figure 11.
Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 1 week after spinal cord injury.
In the CPI group, downregulation of autophagy improved neurological functional recovery, while in the CTI group, it made no difference. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups. CPI: Compression injury; CTI: contusion injury model; HSI: hemisection injury; I/R: ischemia/reperfusion.
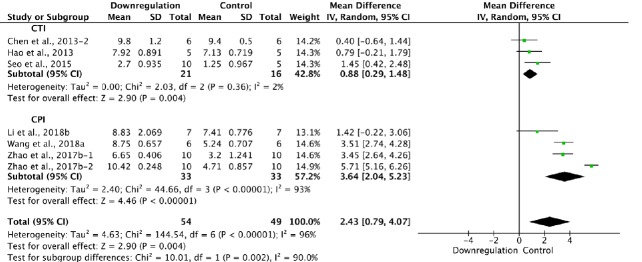
Six of the 33 studies examining downregulation of autophagy reported BBB scores at 2 weeks after SCI (Chen et al., 2013; Hao et al., 2013; Seo et al., 2015; Zhao et al., 2017b; Li et al., 2018b; Wang et al., 2018a). Meta-analysis of these experiments revealed that downregulation of autophagy significantly enhanced BBB scores, as assessed with the random effects model (MD = 2.43, 95% CI: 0.79–4.07, P = 0.004). However, heterogeneity was quite high (I2 = 96%). The experiments were separated into two subgroups according to the SCI model. As shown in Figure 13, downregulation of autophagy significantly improved BBB scores in the contusion injury group (MD = 0.88, 95% CI: 0.29–1.48, P = 0.004), with low heterogeneity (I2 = 2%). Downregulation of autophagy also increased BBB scores in the compression injury group (MD = 3.64, 95% CI: 2.04–5.23, P < 0.00001), but with high heterogeneity (I2 = 93%). The inter-subgroup heterogeneity was high (I2 = 90.0%; Figure 12). The heterogeneity was similar when any single study was excluded.
Figure 13.
Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 3 weeks after spinal cord injury.
Downregulation of autophagy promoted functional recovery in both the CTI and CPI subgroups. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups. CPI: Compression injury; CTI: contusion injury model; HSI: hemisection injury; I/R: ischemia/reperfusion.
Figure 12.
Effect of downregulation of autophagy on Basso, Beattie, and Bresnahan scores at 2 weeks after spinal cord injury.
Downregulation of autophagy promoted functional recovery in both the CTI and CPI subgroups. “–1” and “–2” indicate that the same study contained a single control group but two experimental groups. CPI: compression injury; CTI: Contusion injury.
Six of the 33 studies that examined the effect of downregulating autophagy reported BBB scores at 3 weeks after SCI (Chen et al., 2013; Hao et al., 2013; Tang et al., 2014; Seo et al., 2015; Zhao et al., 2017b; Wang et al., 2018a). Meta-analysis of these experiments revealed that downregulation of autophagy significantly increased BBB scores using the random effects model (MD = 2.96, 95% CI: 0.09–5.84, P = 0.04). However, heterogeneity was quite high (I2 = 99%). Based on the type of SCI model used, the experiments were separated into three subgroups. As shown in Figure 14, downregulation of autophagy did not significantly ameliorate BBB scores in the contusion injury group (MD = 1.22, 95% CI: –0.18–2.63, P = 0.09), with high heterogeneity (I2 = 88%). In contrast, downregulation of autophagy improved BBB scores in the compression injury group (MD = 6.56, 95% CI: 3.03–10.08, P = 0.0003), with high heterogeneity (I2 = 96%). The inter-subgroup heterogeneity was high (I2 = 95.3%; Figure 13). The heterogeneity was similar when any single study was excluded.
Five of the 33 studies that examined the effect of downregulating autophagy reported BBB scores at 4 weeks after SCI (Chen et al., 2013; Hao et al., 2013; Seo et al., 2015; Zhao et al., 2017b; Wang et al., 2018b). Meta-analysis of these experiments revealed that downregulation of autophagy significantly increased BBB scores using the random effects model (MD = 4.41, 95% CI: 1.08–7.75, P = 0.01). However, heterogeneity was quite high (I2 = 98%). Based on the SCI model used, the experiments were separated into two subgroups. As shown in Figure 14, downregulation of autophagy did not significantly increase BBB scores in the contusion injury group (MD = 1.36, 95% CI: –0.30–3.02, P = 0.11), with high heterogeneity (I2 = 87%). In comparison, downregulation of autophagy improved BBB scores in the compression injury group (MD = 7.44, 95% CI: 3.00–11.87, P = 0.001), with high heterogeneity (I2 = 98%). The inter-subgroup heterogeneity is shown in Figure 14(I2 = 84.2%).
Publication bias
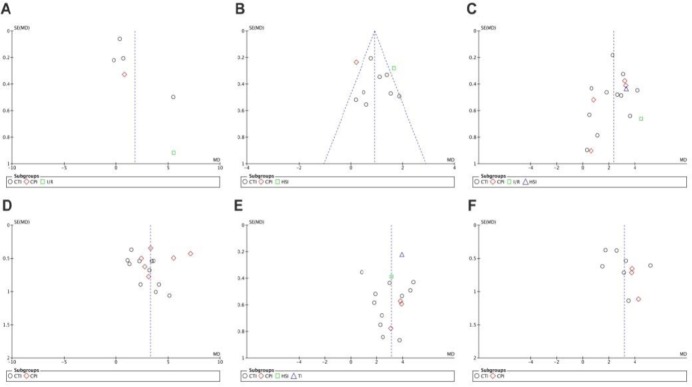
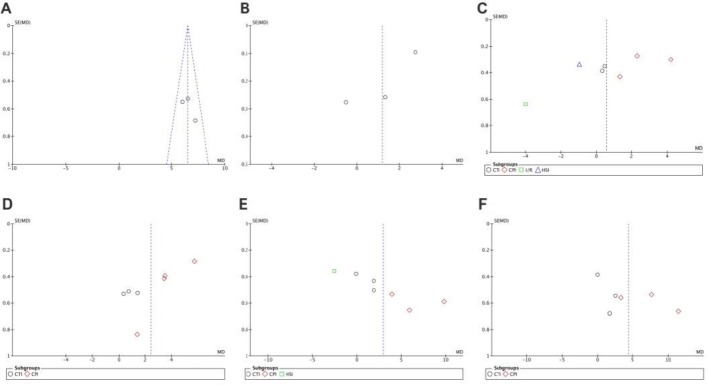
Funnel plots were drawn to evaluate publication bias. The shape of the funnel plots at 3 days of upregulation of autophagy and at 1 day of downregulation of autophagy appeared approximately symmetrical (Figures 15 and 16), suggesting no publication bias. However, publication bias was obvious at other time points.
Figure 15.
Funnel plot for detection of publication bias in meta-analysis of the upregulation of autophagy at different time points.
(A–F) Comparison of upregulation of autophagy at 1 and 3 days, and 1, 2, 3 and 4 weeks. The shape of funnel plots at 3 days of upregulation of autophagy appeared approximately symmetrical. Horizontal ordinate represents mean deviation of Basso, Beattie, and Bresnahan scores; longitudinal ordinate represents standard error of the mean deviation.
Figure 16.
Funnel plot for detection of publication bias in meta-analysis of downregulation of autophagy at different time points.
(A–F) Comparison of downregulation of autophagy at 1 and 3 days, and 1, 2, 3 and 4 weeks. The shape of the funnel plots at 1 day of downregulation of autophagy appeared approximately symmetrical. Horizontal ordinate represents mean deviation of Basso, Beattie, and Bresnahan scores; longitudinal ordinate represents standard error of the mean deviation.
Discussion
The role of autophagy in neurotrauma remains less clear and whether autophagy is good or bad is under debate in experimental models of brain injury or SCI (Wu and Lipinski, 2019). An increasing number of studies have focused on the effects of autophagy in treating SCI (Zhang et al., 2018). Both upregulation and downregulation of autophagy have been shown to have therapeutic effects in SCI, even in the same SCI rat model (Seo et al., 2015; Gao et al., 2016). However, there is lack of studies directly comparing upregulation with downregulation of autophagy for SCI. Therefore, we performed this meta-analysis with direct and indirect comparisons.
Therapeutic effects of autophagic regulation in SCI
In this comprehensive meta-analysis, we analyzed and described the effects of upregulation and downregulation of autophagy on BBB scores in SCI rat models. The neurological function of rats with SCI improved at almost every time point after the enhancement or inhibition of autophagy, expect for downregulation of autophagy at 3 days and 1 week after SCI. Indirect comparison revealed similar therapeutic effects of upregulation and downregulation of autophagy. The results of our current analyses are consistent with our previous publication, indicating that autophagy plays a very important role in SCI (Zhang et al., 2018).
Impact of the SCI model
The subgroup analysis led to the novel finding that the type of SCI model strongly influences autophagy processes after injury. The hemisection injury model, contusion injury model, compression injury model and the ischemia/reperfusion injury model are the four most widely used traumatic SCI models. These different SCI models produce different autophagic patterns after injury. In the hemisection injury model, autophagy levels increase and peak at 3 days after SCI (Kanno et al., 2009, 2011; Hou et al., 2014; Tang et al., 2014). In the contusion injury model, although enhancement of autophagy was observed at 1, 3, 4 and 7 days after SCI among the various studies, the autophagic patterns appear to differ (Sekiguchi et al., 2012; Walker et al., 2012; Chen et al., 2013, 2014; Li et al., 2016b). In some studies, the autophagy level increased and peaked 2 hours after SCI, while in other studies, autophagy peaked at 14 days after SCI (Hao et al., 2013; Wang et al., 2014). Differences in the severity of contusion injury may contribute to the variability. For example, injury in the former study (10 g weight dropped from a height of 25 mm) was more severe than in the latter (8 g weight dropped from a height of 25 mm). In compression injury models, autophagy levels generally increase after SCI. However, few studies have examined the temporal pattern of autophagy (Zhou et al., 2015, 2016b; Zhang et al., 2017b). Wang et al. (2018b) reported that LC-3II/LC3-I increased and peaked at 7 days after SCI. Two peaks of autophagy were seen at 8 and 72 hours after I/R injury with 14-min clipping of the thoracic aortic arch in one study. In another study, the autophagy level increased from 3 hours and peaked at 24 hours after I/R injury with 10-minute balloon occlusion of the thoracic aorta (Fang et al., 2016; Wei et al., 2016).
Our meta-analysis finding that inhibition of autophagy improves BBB scores in rats with compression injury, but not contusion injury, together with previous studies suggests that the type and severity of SCI strongly affects the activation and pattern of autophagy, as well as the response to autophagic regulation (Lipinski et al., 2015). It is difficult to compare severities among the various injury models, given that the mechanisms of primary and secondary injury in the different SCI models are still unclear (Anwar et al., 2016).
Standardizing the SCI model, including the type, severity and injury site, may help clarify the role of autophagy and its regulation in SCI. Contusion injury is the most widely used model in experimental studies, particularly as half of SCI patients have this type of injury (Norenberg et al., 2004). We recommend the contusion injury model produced by the New York University impactor, using a 10-g rod dropped from a height of 25 mm, as the standardized SCI model in rats.
Dual character of autophagy in SCI
An interesting feature of autophagy is its dual character—moderate autophagic activation appears to promote cell survival, while excessive activation promotes cell death (Galluzzi et al., 2016). Autophagic cell death is considered a form of non-apoptotic programmed cell death, but is also considered a type of regulated cell death, indicating that the definition and role of autophagic cell death are still unclear (Clarke, 1990; Berry and Baehrecke, 2007). Additionally, it is unclear whether autophagic cell death is an unsuccessful attempt to avoid programmed cell death (Yonekawa and Thorburn, 2013). Furthermore, there are complex interactions between autophagic and apoptotic signaling pathways and proteins, including p53, Ser/Thr kinase and other effectors. Recent studies suggest that inducing autophagy reduces apoptosis of neural cells, while autophagic flux blockade enhances apoptosis (Fang et al., 2016; Zhou et al., 2016a; Zhang et al., 2017b; Wang et al., 2018a). However, current concepts do not adequately explain all experimental results (Tang et al., 2014; Chen et al., 2017; Jin et al., 2017; Wang et al., 2018b), suggesting that the mechanisms and regulation of autophagy need further investigation.
Other factors that should be considered
As we summarized in Table 1, different drugs affected autophagy, of which 3-MA and rapamycin are most commonly used for inhibiting and inducing autophagy. For the same intervention, there are several factors that could impact the effects of regulating autophagy in treating SCI in rats, such as the timing, dosage, route of administration, and duration of treatment (Zhang et al., 2013; Tang et al., 2014; Zhou et al., 2015; Fang et al., 2016; Zhao et al., 2017b). Upregulation or downregulation of autophagy may have different, or even opposing, effects at different time points, as reported by Fang et al. (2016). These investigators found that BBB scores were worse when autophagy was inhibited at an early stage (immediately) after SCI, while BBB scores were improved when autophagy was stimulated at an early stage after SCI. However, inhibiting autophagy increased BBB scores at the late stage (48 hours) after SCI, while BBB scores were not improved when autophagy was stimulated at this stage. We posit that autophagy is a dynamic process that should be divided into several stages, and that it plays positive or negative effects in the different stages. Further study is needed to clarify the time-dependency of autophagic processes and their regulation.
Limitations
Our meta-analysis has several limitations. First, the number of included studies was limited, and most of these studies focused on the downregulation of autophagy, which may affect the results. The quality of the included studies was also not high, based on evaluation with SYRCLE’s Risk of Bias tool. Many items were assessed as “unclear”, indicating that the methodological quality was unclear, especially because of inadequate blinding, lack of random selection of animals for outcome assessment, or selective outcome reporting. Thus, selection bias, detection bias, attrition bias and reporting bias may be high in these studies. The SYRCLE tool was established in 2014, but many of the studies were published before this date. Methodological quality should be improved by investigators in future studies. High heterogeneity was a widespread feature of the included studies, although it is common in animal studies, and we performed subgroup analysis to clarify potential influencing factors (Li et al., 2015a). There was no commercial funding for any of the studies. Second, different agents, timings, dosages, administration routes and duration of treatment were not consistent among the included studies, and it was difficult to compare the therapeutic effects at the same level of autophagy. Third, we excluded several studies because of the lack of BBB scores. While the BBB score is not the only method to evaluate neurological function, it is the most widely used. Although some studies evaluated neuronal survival or regeneration, the evaluation was not standardized to enable comparison. Moreover, the most important outcome is functional recovery, rather than improved histological findings. Therefore, BBB scores are still currently the best index for comparison. We await a novel method for objective and accurate evaluation.
Implications for clinical practice
Based on the results of our meta-analysis, both upregulation of autophagy and downregulation of autophagy improved long-term neurological recovery in SCI rats. However, the analysis failed to identify major differences between upregulation and downregulation of autophagy. Therefore, much work remains to be done. Research methods need to be improved, such as application of transgenic animals, establishment of standardized protocols, and the development of new noninvasive monitoring techniques for autophagy. At present, there are no autophagy-modulating agents in clinical use. However, autophagy is a potential therapeutic target worth greater attention.
Conclusions
Regulating autophagy promotes the recovery of neurological function. Indirect comparisons between upregulation and downregulation of autophagy and the systematic review underscore the need for standardized research protocols and for a focus on the dynamic regulation of autophagy. Despite the limitations, this meta-analysis provides new insights to help guide the design of high-quality future animal studies with clinical relevancy.
Additional file: open peer review report 1 (99.5KB, pdf) .
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This work was supported by the Beijing Excellent Talent Training Funding of China, No. 2017000021469G215 (to DZhang); the Natural Science Foundation of Capital Medical University of China, No. PYZ2018081 (to DZhang); the Youth Science Foundation of Beijing Tiantan Hospital of China, No. 2016-YQN-14 (to DZhang). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Reporting statement: This study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Beijing Tiantan Hospital, Capital Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study areavailable from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Warin Krityakiarana, Mahidol University-Ratchasuda College, Thailand.
Funding: This work was supported by the Beijing Excellent Talent Training Foundation of China, No. 2017000021469G215 (to DZhang); the Natural Science Foundation of Capital Medical University of China, No. PYZ2018081 (to DZhang); the Youth Science Foundation of Beijing Tiantan Hospital of China, No. 2016-YQN-14 (to DZhang).
P-Reviewer: Krityakiarana W; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Hindle A, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai L, Mei X, Shen Z, Bi Y, Yuan Y, Guo Z, Wang H, Zhao H, Zhou Z, Wang C, Zhu K, Li G, Lv G. Netrin-1 improves functional recovery through autophagy regulation by activating the AMPK/mTOR signaling pathway in rats with spinal cord injury. Sci Rep. 2017;7:42288. doi: 10.1038/srep42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HC, Fong TH, Hsu PW, Chiu WT. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J Surg Res. 2013;179:E203–210. doi: 10.1016/j.jss.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Chen HC, Hsu PW, Tzaan WC, Lee AW. Effects of the combined administration of vitamins C and E on the oxidative stress status and programmed cell death pathways after experimental spinal cord injury. Spinal Cord. 2014;52:24–28. doi: 10.1038/sc.2013.140. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Wang Z, Mao Y, Zheng Z, Chen Y, Khor S, Shi K, He Z, Li J, Gong F, Liu Y, Hu A, Xiao J, Wang X. Liraglutide activates autophagy via GLP-1R to improve functional recovery after spinal cord injury. Oncotarget. 2017;8:85949–85968. doi: 10.18632/oncotarget.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 8.Fang B, Qin M, Li Y, Li X, Tan W, Zhang Y, Ma H. Electroacupuncture preconditioning and postconditioning inhibit apoptosis and neuroinflammation induced by spinal cord ischemia reperfusion injury through enhancing autophagy in rats. Neurosci Lett. 2017;642:136–141. doi: 10.1016/j.neulet.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Fang B, Li XQ, Bao NR, Tan WF, Chen FS, Pi XL, Zhang Y, Ma H. Role of autophagy in the bimodal stage after spinal cord ischemia reperfusion injury in rats. Neuroscience. 2016;328:107–116. doi: 10.1016/j.neuroscience.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G. Autophagy in acute brain injury. Nat Rev Neurosci. 2016;17:467–484. doi: 10.1038/nrn.2016.51. [DOI] [PubMed] [Google Scholar]
- 11.Gao K, Wang G, Wang Y, Han D, Bi J, Yuan Y, Yao T, Wan Z, Li H, Mei X. Neuroprotective effect of simvastatin via inducing the autophagy on spinal cord injury in the rat model. Biomed Res Int. 2015;2015:260161. doi: 10.1155/2015/260161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao S, Zhang ZM, Shen Zl, Gao K, Chang L, Guo Y, Li Z, Wang W, Wang AM. Atorvastatin activates autophagy and promotes neurological function recovery after spinal cord injury. Neural Regen Res. 2016;11:977–982. doi: 10.4103/1673-5374.184498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82–91. doi: 10.1016/j.mcn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Hao HH, Wang L, Guo ZJ, Bai L, Zhang RP, Shuang WB, Jia YJ, Wang J, Li XY, Liu Q. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neurosci Bull. 2013;29:484–492. doi: 10.1007/s12264-013-1355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J. Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0 Cochrane handbook for systematic reviews of interventions Version 510 The Cochrane Collaboration Confidence intervals: Chapter 165. 2011 [Google Scholar]
- 16.Hooijmans C, Rovers M, de Vries R, Leenaars M, Ritskes-Hoitinga M, Langendam M. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou H, Zhang L, Zhang L, Tang P. Acute spinal cord injury in rats should target activated autophagy. J Neurosurg Spine. 2014;20:568–577. doi: 10.3171/2014.1.SPINE13237. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Sharma HS, Chen L, Otom A, Zoubi ZMA, Saberi H, Muresanu DF, He X. Review of clinical neurorestorative strategies for spinal cord injury: exploring history and latest progresses. J Neurorestoratol. 2018;6:171–178. [Google Scholar]
- 19.Huang XQ, Liao XQ, Shi CL, Zhang XL. Methylprednisolone inhibits inflammation mediated by activated microglia after spinal cord injury in rat models. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:3050–3055. [Google Scholar]
- 20.Jin Y, Yang S, Zhang X. Reduction of neuronal damage and promotion of locomotor recovery after spinal cord injury by early administration of methylprednisolone: possible involvement of autophagy pathway. Rsc Adv. 2017;7:2979–2991. doi: 10.1039/d2ra90087d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y, Ding H, Zhou H, Wei Z, Liu L, Pan D, Feng S. Epidemiology of worldwide spinal cord injury: a literature review. J Neurorestoratol. 2017;6:1–9. [Google Scholar]
- 22.Kanno H, Ozawa H, Seldguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143–148. doi: 10.1016/j.nbd.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine. 2011;36:E1427–1434. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Wang G, Han D, Bi J, Li C, Wang H, Liu Z, Gao W, Gao K, Yao T, Wan Z, Li H, Mei X. MP resulting in autophagic cell death of microglia through zinc changes against spinal cord injury. Biomed Res Int. 2016a;2016:6090316. doi: 10.1155/2016/6090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li HT, Zhao XZ, Zhang XR, Li G, Jia ZQ, Sun P, Wang JQ, Fan ZK, Lv G. Exendin-4 enhances motor function recovery via promotion of autophagy and inhibition of neuronal apoptosis after spinal cord injury in rats. Mol Neurobiol. 2016b;53:4073–4082. doi: 10.1007/s12035-015-9327-7. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Hernanda PY, Bramer WM, Peppelenbosch MP, van Luijk J, Pan Q. Anti-tumor effects of metformin in animal models of hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2015a;10:e0127967. doi: 10.1371/journal.pone.0127967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Wang Q, Cai H, He Z, Wang H, Chen J, Zheng Z, Yin J, Liao Z, Xu H, Xiao J, Gong F. FGF1 improves functional recovery through inducing PRDX1 to regulate autophagy and anti-ROS after spinal cord injury. J Cell Mol Med. 2018a;22:2727–2738. doi: 10.1111/jcmm.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Jiang HK, Li YP, Guo YP. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J Biomed Sci. 2015b;22:50. doi: 10.1186/s12929-015-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XG, Du JH, Lu Y, Lin XJ. Neuroprotective effects of rapamycin on spinal cord injury in rats by increasing autophagy and Akt signaling. Neural Regen Res. 2019;14:721–727. doi: 10.4103/1673-5374.247476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Liu F, Zhang L, Cao Y, Shao Y, Wang X, Jiang X, Chen Z. Neuroserpin restores autophagy and promotes functional recovery after acute spinal cord injury in rats. Mol Med Rep. 2018b;17:2957–2963. doi: 10.3892/mmr.2017.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipinski MM, Wu J, Faden AI, Sarkar C. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal. 2015;23:565–577. doi: 10.1089/ars.2015.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Wu H, Yao C, Chen Y, Liu T. Advanced methods of data extraction for continuous outcomes in meta-analysis. Zhongguo Xunzheng Yixue Zazhi. 2017a;17:117–121. [Google Scholar]
- 33.Liu P, Zhang Z, Wang Q, Guo R, Mei W. Lithium chloride facilitates autophagy following spinal cord injury via erk-dependent pathway. Neurotox Res. 2017;32:535–543. doi: 10.1007/s12640-017-9758-1. [DOI] [PubMed] [Google Scholar]
- 34.Miao L, Dong Y, Zhou FB, Chang YL, Suo ZG, Ding HQ. Protective effect of tauroursodeoxycholic acid on the autophagy of nerve cells in rats with acute spinal cord injury. Eur Rev Med Pharmacol Sci. 2018;22:1133–1141. doi: 10.26355/eurrev_201802_14402. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: Defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 37.Nowrouzi B, Assan-Lebbe A, Sharma B, Casole J, Nowrouzi-Kia B. Spinal cord injury: a review of the most-cited publications. Eur Spine J. 2017;26:28–39. doi: 10.1007/s00586-016-4669-z. [DOI] [PubMed] [Google Scholar]
- 38.Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29:946–956. doi: 10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- 39.Seo JY, Kim YH, Kim JW, Kim SI, Ha KY. Effects of therapeutic hypothermia on apoptosis and autophagy after spinal cord injury in rats. Spine. 2015;40:883–890. doi: 10.1097/BRS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 40.Sharma S, Goel SA, Sharma S, Chhabra HS. Polyetheretherketone cages used in anterior cervical discectomy and fusion surgery: a meta-analysis. Clin Trials Orthop Disord. 2019;4:29–33. [Google Scholar]
- 41.Sun Y, Liu D, Su P, Lin F, Tang Q. Changes in autophagy in rats after spinal cord injury and the effect of hyperbaric oxygen on autophagy. Neurosci Lett. 2016;618:139–145. doi: 10.1016/j.neulet.2016.02.054. [DOI] [PubMed] [Google Scholar]
- 42.Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- 43.Tong M, He Z, Lin X, Zhou Y, Wang Q, Zheng Z, Chen J, Xu H, Tian N. Lithium chloride contributes to blood spinal cord barrier integrity and functional recovery from spinal cord injury by stimulating autophagic flux. Biochem Biophys Res Commun. 2018;495:2525–2531. doi: 10.1016/j.bbrc.2017.12.119. [DOI] [PubMed] [Google Scholar]
- 44.Walker CL, Walker MJ, Liu N-K, Risberg EC, Gao X, Chen J, Xu X-M. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:315–326. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Cao Y, Su Z, Li G, Qiu X. Effect and mechanism of SS31 peptide on autophagy after spinal cord injury in rats. Zhonghua Chuangshang Zazhi. 2017a;33:940–944. [Google Scholar]
- 46.Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, Guo Y, Li Z, Yao T, Mei X. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. 2016;477:534–540. doi: 10.1016/j.bbrc.2016.05.148. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Liu C, Mei X, Cao Y, Guo Z, Yuan Y, Zhao Z, Song C, Guo Y, Shen Z. Berberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injury. Oncotarget. 2017b;8:98312–98321. doi: 10.18632/oncotarget.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P, Jiang L, Zhou N, Zhou H, Liu H, Zhao W, Zhang H, Zhang X, Hu Z. Resveratrol ameliorates autophagic flux to promote functional recovery in rats after spinal cord injury. Oncotarget. 2018a;9:8427–8440. doi: 10.18632/oncotarget.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang P, Lin C, Wu S, Huang K, Wang Y, Bao X, Zhang F, Huang Z, Teng H. Inhibition of autophagy is involved in the protective effects of ginsenoside rb1 on spinal cord injury. Cell Mol Neurobiol. 2018b;38:679–690. doi: 10.1007/s10571-017-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang ZY, Liu WG, Muharram A, Wu ZY, Lin JH. Neuroprotective effects of autophagy induced by rapamycin in rat acute spinal cord injury model. Neuroimmunomodulation. 2014;21:257–267. doi: 10.1159/000357382. [DOI] [PubMed] [Google Scholar]
- 51.Wei WB, Zou ZL, Zhou BB, Li BL, Qin JY, Feng ZF, Li JN, Ye LY, Wu RM. Selection of injury segments in a rat model of spinal cord injury: network meta-analysis. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:650–656. [Google Scholar]
- 52.Wei X, Zhou Z, Li L, Gu J, Wang C, Xu F, Dong Q, Zhou X. Intrathecal injection of 3-methyladenine reduces neuronal damage and promotes functional recovery via autophagy attenuation after spinal cord ischemia/reperfusion injury in rats. Biol Pharm Bull. 2016;39:665–673. doi: 10.1248/bpb.b15-00610. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Lipinski MM. Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells. 2019;8:693. doi: 10.3390/cells8070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie L, Yu S, Yang K, Li C, Liang Y. Hydrogen sulfide inhibits autophagic neuronal cell death by reducing oxidative stress in spinal cord ischemia reperfusion injury. Oxid Med Cell Longev. 2017a;2017:8640284. doi: 10.1155/2017/8640284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie L, Yu S, Wang Z, Yang K, Liu Z, Li C, Liang Y. Nicotinamide adenine dinucleotide protects against spinal cord ischemia reperfusion injury-induced apoptosis by blocking autophagy. Oxid Med Cell Longev. 2017b;2017:7063874. doi: 10.1155/2017/7063874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yonekawa T, Thorburn A. Autophagy and cell death. Essays Biochem. 2013;55:105–117. doi: 10.1042/bse0550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yue X, Liu H, Xia B, Ma D, Shen D. Effect of berberine on autophagy and apoptosis after spinal cord injury in rats. Zhonghua Shiyan Waike Zazhi. 2015;32:1516–1518. [Google Scholar]
- 58.Zhang B, Chen Y, Yang B, Jiang F, Zhao J, Ouyang Z. Neuroprotective effects of diosgenin in rats with experimental spinal cord injury via promotion of autophagy. Int J Clin Exp Med. 2017a;10:11655–11663. [Google Scholar]
- 59.Zhang D, Wang F, Zhai X, Li XH, He XJ. Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy. Neural Regen Res. 2018;13:2191–2199. doi: 10.4103/1673-5374.241473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang D, Xuan J, Zheng B, Zhou Y, Lin Y, Wu Y, Zhou Y, Huang Y, Wang Q, Shen L, Mao C, Wu Y, Wang X, Tian N, Xu H, Zhang X. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. 2017b;54:3327–3341. doi: 10.1007/s12035-016-9895-1. [DOI] [PubMed] [Google Scholar]
- 61.Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, Li XK, Xu HZ, Xiao J. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013;48:452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- 62.Zhao H, Chen S, Gao K, Zhou Z, Wang C, Shen Z, Guo Y, Li Z, Wan Z, Liu C, Mei X. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017a;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 63.Zhao YZ, Jiang X, Lin Q, Xu HL, Huang YD, Lu CT, Cai J. Thermosensitive heparin-poloxamer hydrogels enhance the effects of GDNF on neuronal circuit remodeling and neuroprotection after spinal cord injury. J Biomed Mater Res Part A. 2017b;105:2816–2829. doi: 10.1002/jbm.a.36134. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Kl, Zhou YF, Wu K, Tian NF, Wu YS, Wang Yl, Chen DH, Zhou B, Wang XY, Xu HZ, Zhang Xl. Stimulation of autophagy promotes functional recovery in diabetic rats with spinal cord injury. Sci Rep. 2015;5:17130. doi: 10.1038/srep17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou KL, Chen DH, Jin HM, Wu K, Wang XY, Xu HZ, Zhang Xl. Effects of calcitriol on experimental spinal cord injury in rats. Spinal Cord. 2016a;54:510–516. doi: 10.1038/sc.2015.217. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Zheng B, Ye L, Zhang H, Zhu S, Zheng X, Xia Q, He Z, Wang Q, Xiao J, Xu H. Retinoic acid prevents disruption of blood-spinal cord barrier by inducing autophagic flux after spinal cord injury. Neurochem Res. 2016b;41:813–825. doi: 10.1007/s11064-015-1756-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.