Abstract
Background:
The purpose of this study was to evaluate the risk of metastatic disease and orbital recurrence in advanced retinoblastoma treated with systemic chemoreduction versus primary enucleation.
Methods:
A retrospective review of patients with Group D/E retinoblastoma was conducted with data collection from 1995 to 2015. Overall, 345 eyes (294 patients) were included (165 Group D and 180 Group E). Primary outcome measures were orbital recurrence and metastatic disease.
Results:
Of the 345 eyes, 139 were treated with systemic chemoreduction (102 Group D, 37 Group E) and 206 with primary enucleation (63 Group D, 143 Group E). In the chemoreduction group, one patient developed metastasis (0.7%) and one an orbital recurrence (0.7%). In the primary enucleation group, two patients developed metastases (0.9%) and one an orbital recurrence(0.5%). After systemic chemoreduction, 58 of the 139 eyes (30 Group D, 28 Group E) were secondarily enucleated for treatment failure (41.7%). The median time to secondary enucleation from diagnosis was 8.1 months. None of the eyes in the systemic chemoreduction group had high-risk pathologic features. In the primary enucleation group, 56 eyes had high-risk pathology.
Conclusion:
Over a 20-year period, 345 eyes were treated for advanced retinoblastoma at Children’s Hospital Los Angeles. Incidence of orbital recurrence and metastatic disease was <1% and did not vary by treatment modality or group classification. None of the eyes enucleated for treatment failure had high-risk pathology, and none of these patients developed metastatic disease. Globe salvage therapy with systemic chemoreduction and subsequent enucleation for poor response does not increase the risk of metastatic disease or orbital recurrence.
Keywords: chemotherapy, enucleation, metastatic disease, orbital recurrence, retinoblastoma, secondary enucleation, systemic chemoreduction
1 |. INTRODUCTION
In 2005, Murphree proposed the International Intraocular Retinoblastoma Classification (IIRC)1 to allow for prognostication of treatment effect following systemic chemoreduction. This classification system grades eyes from A through E, based on the presence of subretinal and vitreous seeding as well as other ocular findings such as subretinal fluid and neovascular glaucoma. Studies have shown that following treatment with systemic chemotherapy (chemoreduction) and local consolidation, the salvage rate for Group A and B eyes approaches 90%–100%, while the rate for more advanced Group D eyes is only 47%.2,3 Group E eyes are described as eyes that have been destroyed anatomically or functionally by tumor1, and according to the IIRC, primary enucleation was recommended for these eyes. However, in certain clinical situations, such as bilateral advanced disease, salvage may be attempted for Group E eyes.4,5 Furthermore, with the advent of new treatment modalities such as intra-arterial chemotherapy, we now have more options in our armamentarium for the treatment of advanced retinoblastoma eyes. The salvage rate for Group D eyes treated with intra-arterial chemotherapy has been reported to range from 36% to 100%,6 with several large series demonstrating that 78%7 to 94% of the treated eyes avoid enucleation or radiation.8 Successful rates of salvage for Group E eyes following intra-arterial chemotherapy range from 17% to 86%,6 with an average of approximately 30%.8
With the use of globe-savaging therapy and the trend toward treating eyes with more advanced intraocular disease, there is a theoretical risk of increasing the incidence of both metastatic disease and orbital recurrences. A meta-analysis by Yousef et al.9 regarding intra-arterial chemotherapy for retinoblastoma found multiple reports of metastatic disease. A retrospective study by Zhao et al.10 showed a higher risk of metastatic disease and lower disease-specific survival for patients with Group E eyes treated with systemic chemotherapy for more than 3 months versus primary enucleation. Yannuzzi et al.11 recently reported a retrospective analysis of advanced Group D/E retinoblastoma eyes treated with intra-arterial chemotherapy versus primary enucleation. They found a 7.9% risk of metastatic disease and a7.9% risk of orbital recurrence in the cohort treated with primary enucleation versus a 4.2% risk of metastatic disease and a 1.3% risk of orbital recurrence from patients treated with intra-arterial chemotherapy. It was not discussed whether adjuvant chemotherapy was given for higher risk pathologic features in the enucleation group; however, it does state that 9.5% of eyes received subsequent intravenous chemotherapy. While the authors concluded that there was no increased risk of either orbital recurrence or metastatic disease following intra-arterial chemotherapy in their series when compared with primary enucleation, it is possible that adjuvant intravenous chemotherapy may have decreased the higher rate of metastatic disease seen in the primary enucleation cohort.11,12
The aim of this 20-year retrospective review was to determine whether there was an increased incidence of orbital recurrence and metastatic disease following attempted salvage of advanced Group D/E retinoblastoma eyes with systemic chemoreduction when compared with primary enucleation.
2 |. METHODS
A retrospective, Institutional Review Board approved, chart review was conducted with data collection from a 20-year period spanning January 1, 1995, to August 1, 2015.
2.1 |. Inclusion and exclusion criteria
Patients diagnosed at Children’s Hospital Los Angeles (CHLA) with advanced retinoblastoma classified as Group D or Group E disease in at least one eye were included. Patients with evidence of extra-ocular disease at the time of diagnosis were treated according to the Children’s Oncology Group protocol for infants with central nervous system disease and were excluded from the study. Patients referred for second opinions and not treated at CHLA at diagnosis were excluded.
2.2 |. Treatment
The treatment protocol for advanced (Group D/E) retinoblastoma eyes at CHLA depends on several clinical features including the age of the patient, the laterality of disease, and the staging of the contralateral eye. Enucleation is offered to parents as a treatment option for advanced unilateral retinoblastoma (Group D or E). Globe salvaging treatment (i.e., chemoreduction) is offered to parents with unilateral Group D disease, or Group E retinoblastoma if the patient has bilateral disease. The CHLA chemoreduction protocol, which has been published previously,2,13,14 consists of systemic chemotherapy (chemoreduction) and local consolidation therapy that includes diode or argon laser therapy (532 or 810 nm laser), and cryotherapy (freeze–thaw cycle ×2) for larger lesions anterior to the equator. Systemic chemoreduction consists of intravenous carboplatin 780 mg/m2 (13 mg/kg for children <36 months) × 2 days, etoposide 150 mg/m2 (5 mg/kg for <36 months) × 2 days, and vincristine 1.5 mg/m2 (0.05 mg/kg for <36 months) × 1 day, for 6 cycles every 28 days (i.e., CEV). Infants less than 6 months of age at diagnosis receive a modified dosing regimen with a 50% decrease in all agents for the first cycle, with subsequent doses modified by clinical response and toxicity.13,14 At our institution, vincristine is omitted for patients less than 2 months of age due to concern for paralytic ileus. Patients were examined at intervals of 4– 6 weeks during treatment with examinations under anesthesia (EUAs).
After completion of the chemoreduction protocol, some patients were referred for external beam radiation therapy (EBRT) if tumor persistence or recurrence was found on EUA that was not amenable to local therapy. Prescribed EBRT was 36 Gy total dose by intensity- modulated radiotherapy (IMRT) at a daily dose of 2 Gy for 18 fractions.
2.3 |. Chart review
At initial evaluation, each patient had a full-staging EUA including B- scan and fluorescein angiography. We classified eyes according to the International Classification System for Retinoblastoma.
A retrospective chart review was conducted to obtain the following information: age at diagnosis in months, sex, race, ethnicity, laterality of retinoblastoma, group classification, family history, primary and subsequent treatment modalities, the development of metastatic disease, orbital recurrence, development of trilateral retinoblastoma, and length of follow-up. Any recorded deaths and their cause were also documented.
2.4 |. Statistical analysis
The data analysis for this paper was generated using SAS/STAT© software, Version 9.2 of the SAS System for Unix (SAS Institute Inc., Cary, NC).
2.5 |. Images
Ocular images were obtained using a wide-angle contact fundus camera (RetCam, Clarity Medical Systems, Inc., Pleasanton, CA) and ultrasound during EUA.
3 |. RESULTS
From January 1, 1995, to August 1, 2015, 294 patients were referred to CHLA and diagnosed with advanced Group D or E retinoblastoma in 345 eyes. Patient demographics are shown in Table 1. Bilateral disease was found in 117 patients (39.8%) with advanced retinoblastoma in at least one eye. Of the advanced eyes, Group D retinoblastoma was diagnosed in 165 eyes (47.8%) and Group E in 180 eyes (52.2%). A modified Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 1.15,16 The mean age at diagnosis was 19.8 months (standard deviation [SD], 17.7 months). The average follow-up time for all patients was 86.3 months (SD, 62.5 months).
Table 1.
Patient demographics
| Characteristic | Mean (SD) |
|---|---|
| Age at diagnosis (months) | 19.8 (17.7) |
| Average length of follow-up (months) | 86.3 (62.5) |
| Sex | Number (%) |
| Male | 151 (51.4) |
| Female | 143 (48.6) |
| Ethnicity | |
| Hispanic | 187 (63.6) |
| Non-Hispanic | 96 (32.7) |
| Unknown | 11 (3.7) |
| Inheritance | |
| Sporadic | 274 (93.2) |
| Inherited | 20 (6.8) |
| Laterality | |
| Unilateral | 177 (60.2) |
| Bilateral | 117(39.8) |
FIGURE 1.
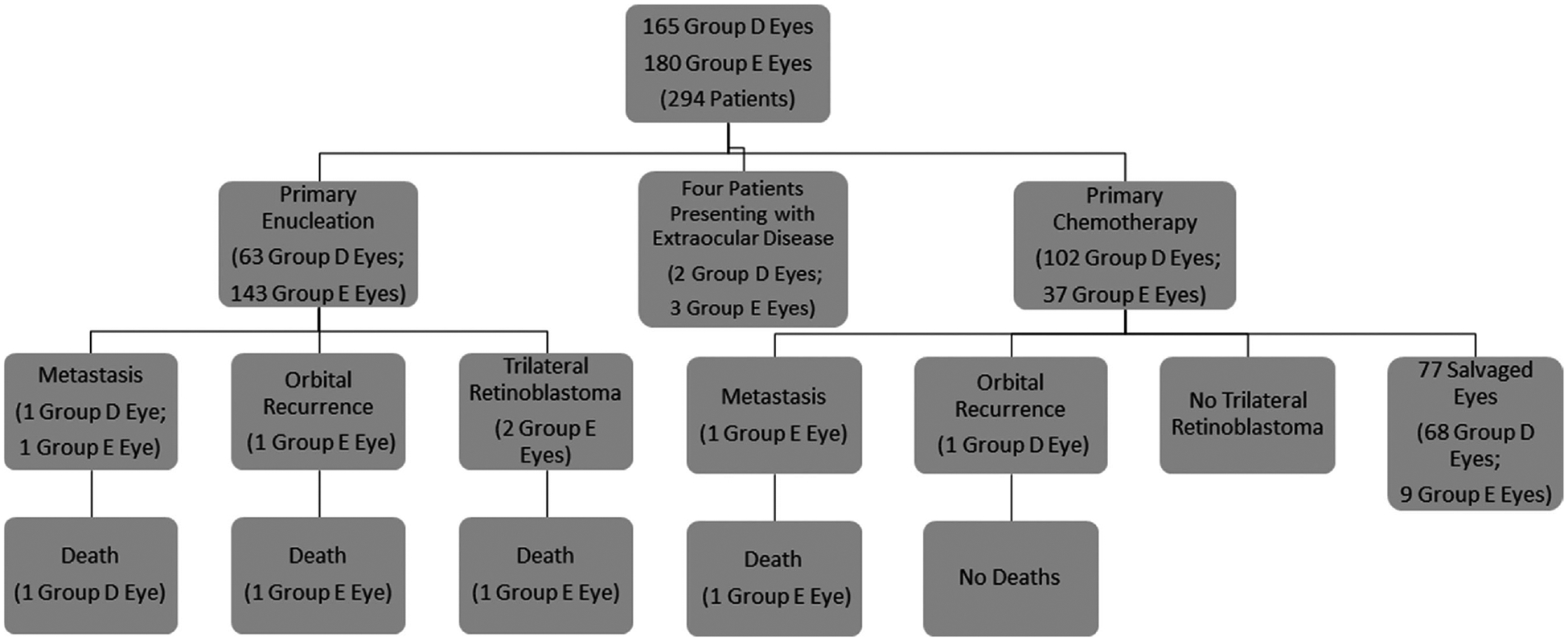
CONSORT diagram showing primary treatment modalities for Group D and E eyes and the subsequent development of metastasis, orbital recurrence, and death
3.1 |. Primary enucleation
Primary enucleation was the treatment modality for 206 (59.7%) advanced retinoblastoma eyes. This included 63 Group D eyes (38.2% of all Group D eyes) and 143 Group E eyes (79.4% of all Group E eyes). Identified high-risk features on pathologic evaluation included retrolaminar optic nerve invasion, massive choroidal invasion (>3 mm), anterior segment invasion, and scleral invasion.17,18 At least one of these features was found in 56 globes (27.2%): 5 with Group D classification (8.9%) and 51 with Group E classification (91.1%). The incidence of high-risk features in the primary enucleation group was 7.9% for Group D eyes (5/63) and 35.7% for Group E eyes (51/143). In the primary enucleation group, there were two cases of metastatic disease(0.9%) and one case (0.5%) of orbital recurrence (Fig. 1). In this group, two patients (0.9%) subsequently developed trilateral retinoblastoma. One of these patients had never received chemotherapy (prior to the diagnosis of trilateral disease) and the other had been treated with three cycles of CEV for Group B disease in the contralateral eye (after enucleation of the Group E eye). In the cohort treated with primary enucleation, there were three deaths (1.46%): two from metastatic disease and one from trilateral retinoblastoma.
3.2 |. Systemic chemoreduction
Systemic chemotherapy with local consolidation (laser, cryotherapy) was attempted in 139 (40.3%) advanced retinoblastoma eyes. There were 102 Group D eyes (61.8% of all Group D eyes) and 37 Group E eyes (20.6% of all Group E eyes) that underwent attempted globe salvage. IMRT was used as a salvage treatment for persistent or recurrent disease in 28.1% of eyes: 17 Group D eyes (16.6%, 17/102) and 22 Group E eyes (59.5%, 22/37). No child was radiated under the age of 12 months.19 Subsequently, 30 Group D eyes and 28 Group E eyes were enucleated for persistent or recurrent disease, for an overall salvage rate of 58.3% (81/139). The median time to enucleation from diagnosis was 8.1 months (mean, 14 months; range, 1–118 months). None of the eyes enucleated after attempted salvage were found to have high-risk pathologic features. In the chemoreduction group, there was one case of metastatic disease (0.7%) and one case (0.7%) of orbital recurrence (Fig. 1). In this group, no patients developed trilateral retinoblastoma. There was one death (0.7%) due to metastatic disease. To date, no child has developed a second nonocular tumor.
3.3 |. Group D versus E eyes
Overall, 165 Group D eyes were included in this study, with 63 (38.2%) being primarily enucleated and 102 (61.8%) treated with systemic chemoreduction. Significantly more Group E eyes underwent primary enucleation: of 180 Group E eyes, 143 (79.4%) were treated with primary enucleation and 37 (20.6%) with systemic chemoreduction. Of those eyes that underwent attempted salvage with chemoreduction, local consolidation, and external beam radiation therapy, 70 of 102 (68.6%) Group D eyes and 10 of 39 (25.6%) Group E eyes were salvaged.
4 |. DISCUSSION
This study reports the rates of extraocular relapse for patients with advanced Group D and E retinoblastoma eyes treated at CHLA over an approximate 20-year period. The eyes were grouped according to whether primary treatment was enucleation versus attempted salvage with systemic chemoreduction, and the main outcomes assessed in this study were whether the incidence of orbital recurrence, metastatic disease, and death varied by initial treatment modality. Overall, the incidence of these outcome measures was 1% or less in both groups, which is lower than what has been reported previously in similar retrospective analyses.10–12,20 In our series of 294 patients, metastatic disease developed in three patients for an overall systemic relapse rate of 1%, while orbital recurrence developed in two patients for a rate of0.6%. Given the low incidence of metastatic disease and orbital recurrence in this 20-year retrospective series, a statistical difference in relapse rates between the two groups could not be elucidated. Similarly, we could not correlate the risk of metastatic disease or orbital recurrence with IIRC Group Classification (Group D or E) or patients undergoing secondary enucleation for tumor recurrence. There was a small discrepancy in the death rates between the primary enucleation and systemic chemoreduction cohorts (1.5% vs. 0.7%) as well as a higher rate of development of trilateral retinoblastoma (0.9% vs. 0%) in the primary enucleation group, although these differences were not statistically significant. It has been theorized that treatment with systemic chemoreduction may decrease the incidence of trilateral disease developing in a predisposed population (e.g., those with germline mutations in the retinoblastoma gene).21 One patient in our series was treated with primary enucleation for a Group E eye and three cycles of systemic chemoreduction for the contralateral Group B eye.14 In this single case, the three cycles were not protective against the development of a pinealoblastoma. However, as the overall incidence of events was low in this series, no statistically significant conclusions regarding the effect of systemic chemotherapy could be drawn from this data.
There is a general consensus that microscopic invasions of retinoblastoma into the optic nerve, choroid, or sclera are risk factors for the development of extraocular disease22–24; however, the identification of these features clinically can be difficult.25 Some clinical factors suggestive of higher risk pathologic features include glaucoma, buphthalmos, hyphema, pseudohypopyon, and lag-time to diagnosis.26,27 Imaging is not reliable in this context.28 IIRC Group Classification is also insufficient as a retrospective analysis by Kaliki et al.20 showed the presence of these pathologic features in 17% of Group D and 24% of Group E eyes. In the review by Kaliki et al., there was an 8% overall risk of metastatic disease, in which all patients had high-risk features. Of the five patients who developed orbital recurrence or metastatic disease in this study, only two had high- risk pathologic features (four of five eyes were enucleated). One eye was thought to have developed an orbital recurrence without active intra-ocular disease secondary to aggressive laser therapy. The patient is well without metastatic disease after treatment and was not enucleated.29
In addition, with more effective treatment for intra-ocular retinoblastoma, more eyes with advanced disease are being treated. While some reports of metastatic disease are overall higher than in the specific results from this report,9–12,30 overall most studies show comparable and low rates of metastatic disease whether salvage is attempted with systemic chemoreduction or intra-arterial chemotherapy.9,31 It should be noted however that the majority of these studies are retrospective, the choice of which eyes to treat vary by centers, and most, like ours, advocate enucleation for Group E eyes.
It should also be noted that attempted salvage of advanced eyes theoretically downstages subsequent pathologic findings.10 In our cohort of patients, none of the eyes secondarily enucleated after treatment were found to have high-risk pathologic features in comparison to 27% of primarily enucleated globes. Group E eyes accounted for 91% of these globes, so there is some selection bias for enucleating eyes with higher risk clinical features; however, treatment with subsequent enucleation likely masks histologic risk factors for the development of metastatic disease. Zhao et al., in a cohort of 100 eyes, found that treatment of advanced eyes for more than 3 months before enucleation increased the risk of metastatic disease secondary to this downstaging and subsequent inappropriate management of high-risk disease. In our institution, these results were not corroborated, as there was no increased incidence of metastatic disease in children who were enucleated primarily or secondarily. The median time to enucleation in this cohort was 8.1 months. The one child who did develop metastatic disease after attempted salvage was enucleated 4 months after diagnosis. One difference between our patient cohort and those described by Zhao et al. is that a higher initial dose of carboplatin is used for the treatment of intraocular disease. It is unclear whether or not the higher dose of carboplatin may have been protective in the subsequent development of metastatic disease. A higher dose of carboplatin is frequently used in treating metastatic central nervous system disease; however, often in a four-drug regimen therefore direct comparison cannot be made.32,33 In addition, given the overall low number of metastatic events, a further study is warranted before any clear recommendations can be made.
This retrospective review study suggests that attempted salvage of advanced eyes with systemic chemoreduction using the described protocol does not increase the risk of metastatic disease or orbital recurrence. We found a very low overall incidence of adverse outcomes with both systemic chemoreduction for salvage and primary enucleation, with no significant difference noted with choice of primary therapy. Therefore, treating Group D and Group E eyes with systemic chemotherapy for six cycles, with secondary enucleation for poor tumor response, was found to be as safe as primary enucleation when considering the risk of extraocular relapse. When considering whether to salvage an eye with advanced retinoblastoma, other factors should be considered such as visual potential, unilateral or bilateral presentation, degree of vitreous or subretinal seeding, and the presence of specific ocular findings such as neovascular glaucoma and buphthalmos. It should be noted that the majority of Group E eyes in our cohort, including all unilateral patients, were primarily enucleated. Given that Group E eyes have a significant risk of having high- risk histopathologic features, it is possible that if we had attempted to salvage the majority of these eyes, the risk of extraocular relapse may have increased. Since the overall salvage rate of Group E eyes is low with any modality, we continue to recommend primary enucleation for unilateral Group E cases.
The limitations to this study include the retrospective nature of its data analysis and the low rate of positive events, which limited statistical analysis. Furthermore, there is a selection bias inherent when treatment decisions are not randomized and the majority of Group E eyes were treated with enucleation. On the other hand, the strengths of this study include the relatively large number of patients, the consistency in staging, treatment and follow-up regimen, and the length of follow-up.
This study suggests that attempted salvage of advanced eyes with systemic chemoreduction using the described protocol does not increase the risk of metastatic disease or orbital recurrence. We found a very low overall incidence of adverse outcomes with both systemic chemoreduction and enucleation, with no significant difference with choice of primary therapy. Nonetheless, we did not attempt to salvage the unilateral Group E disease and continue to caution against attempted salvage of these eyes.
ACKNOWLEDGMENTS
The authors would like to Acknowledge Colleen Azen for her statistical support of this manuscript. Her work was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FUNDING INFORMATION
This work was supported by Retinoblastoma International, Inc., The Institute for Families, Inc., and Children’s Hospital Los Angeles. An unrestricted departmental grant was received from Research to Prevent Blindness.
Abbreviations:
- CEV
carboplatin, etoposide, vincristine
- CHLA
Children’s Hospital Los Angeles
- EBRT
external beam radiation therapy
- EUA
examination under anesthesia
- IIRC
International Intraocular Retinoblastoma Classification
- IMRT
intensity-modulated radiotherapy
- SD
standard deviation
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Linn Murphree A Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin N Am. 2005;18:41–53, viii. [DOI] [PubMed] [Google Scholar]
- 2.Berry JL, Jubran R, Kim JW, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013;60:688–693. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–2280. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza PR, Grossniklaus HE. Therapeutic options for retinoblastoma. Cancer Control. 2016;23:99–109. [DOI] [PubMed] [Google Scholar]
- 5.Abramson DH, Shields CL, Munier FL, et al. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;133:1341–1347. [DOI] [PubMed] [Google Scholar]
- 6.Chung CY, Medina CA, Aziz HA, et al. Retinoblastoma: evidence for stage-based chemotherapy. Int Ophthalmol Clin. 2015;55:63–75. [DOI] [PubMed] [Google Scholar]
- 7.Abramson DH, Daniels AB, Marr BP, et al. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for Group D retinoblastoma. PLoS ONE. 2016;11:e0146582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–1460. [DOI] [PubMed] [Google Scholar]
- 9.Yousef YA, Soliman SE, Astudillo PP, et al. Intra-arterial chemotherapy for retinoblastoma: a systematic review. JAMA Ophthalmol. 2016. doi: 10.1001/jamaophthalmol.2016.0244. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Dimaras H, Massey C, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29:845–851. [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi NA, Francis JH, Marr BP, et al. Enucleation vs ophthalmic artery chemosurgery for advanced intraocular retinoblastoma: a retrospective analysis. JAMA Ophthalmol. 2015;133:1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yannuzzi NA, Francis JH, Abramson DH. Incidence of orbital recurrence after enucleation or ophthalmic artery chemosurgery for advanced intraocular retinoblastoma-reply. JAMA Ophthalmol. 2016; 134:114–115. [DOI] [PubMed] [Google Scholar]
- 13.Berry JL, Jubran R, Lee TC, Murphree AL, Lee D, Kim JW. Low-dose chemoreduction for infants diagnosed with retinoblastoma before 6 months of age. Ocul Oncol Pathol. 2015;1:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu D, Berry JL, Ediriwickrema L, et al. Long-term outcomes of Group B eyes in patients with retinoblastoma treated with short-course chemoreduction: experience from Children’s Hospital Los Angeles/University of Southern California. Ocul Oncol Pathol. 2016;2:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle RC Jr. High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Arch Pathol Lab Med.2009;133:1203–1209. [DOI] [PubMed] [Google Scholar]
- 18.Honavar SG, Singh AD, Shields CL, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–931. [DOI] [PubMed] [Google Scholar]
- 19.Abramson DH, Ellsworth RM, Kitchin FD, et al. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984;91:1351–1355. [DOI] [PubMed] [Google Scholar]
- 20.Kaliki S, Shields CL, Rojanaporn D, et al. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120:997–1003. [DOI] [PubMed] [Google Scholar]
- 21.de Jong MC, Kors WA, de Graaf P, et al. The incidence of trilateral retinoblastoma: a systematic review and meta-analysis. Am J Ophthalmol. 2015;160:1116–1126, e1115. [DOI] [PubMed] [Google Scholar]
- 22.Gupta R, Vemuganti GK, Reddy VA, et al. Histopathologic risk factors in retinoblastoma in India. Arch Pathol Lab Med. 2009;133:1210–1214. [DOI] [PubMed] [Google Scholar]
- 23.Chantada GL, Dunkel IJ, de Davila MT, et al. Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy? Br J Ophthalmol. 2004;88:1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chantada G, Schaiquevich P. Management of retinoblastoma in children: current status. Paediatr Drugs. 2015;17:185–198. [DOI] [PubMed] [Google Scholar]
- 25.Ghassemi F, Khodabande A. Risk definition and management strategies in retinoblastoma: current perspectives. Clin Ophthalmol. 2015;9:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashyap S, Meel R, Pushker N, et al. Clinical predictors of high riskhistopathology in retinoblastoma. Pediatr Blood Cancer. 2012;58:356–361. [DOI] [PubMed] [Google Scholar]
- 27.Chantada GL, Gonzalez A, Fandino A, et al. Some clinical findings at presentation can predict high-risk pathology features in unilateral retinoblastoma. J Pediatr Hematol Oncol. 2009;31:325–329. [DOI] [PubMed] [Google Scholar]
- 28.de Graaf P, Goricke S, Rodjan F, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol.2012;42:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsen BH, Berry JL, Jubran R, et al. Orbital recurrence following aggressive laser treatment for recurrent retinoblastoma. Ocul Oncol Pathol. 2015;2:76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew AA, Sachdev N, Staffieri SE, et al. Superselective intra-arterial chemotherapy for advanced retinoblastoma complicated by metastatic disease. J AAPOS. 2015;19:72–74. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki S, Yamane T, Mohri M, et al. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis.Ophthalmology. 2011;118:2081–2087. [DOI] [PubMed] [Google Scholar]
- 32.Dunkel IJ, Chan HS, Jubran R, et al. High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4B retinoblastoma. Pediatr Blood Cancer. 2010;55:149–152. [DOI] [PubMed] [Google Scholar]
- 33.Dimaras H, Heon E, Budning A, et al. Retinoblastoma CSF metastasis cured by multimodality chemotherapy without radiation. Ophthalmic Genet. 2009;30:121–126. [DOI] [PubMed] [Google Scholar]


