Abstract
Objective
To investigate whether milk polar lipids (PL) impact human intestinal lipid absorption, metabolism, microbiota and associated markers of cardiometabolic health.
Design
A double-blind, randomised controlled 4-week study involving 58 postmenopausal women was used to assess the chronic effects of milk PL consumption (0, 3 or 5 g-PL/day) on lipid metabolism and gut microbiota. The acute effects of milk PL on intestinal absorption and metabolism of cholesterol were assessed in a randomised controlled crossover study using tracers in ileostomy patients.
Results
Over 4 weeks, milk PL significantly reduced fasting and postprandial plasma concentrations of cholesterol and surrogate lipid markers of cardiovascular disease risk, including total/high-density lipoprotein-cholesterol and apolipoprotein (Apo)B/ApoA1 ratios. The highest PL dose preferentially induced a decreased number of intestine-derived chylomicron particles. Also, milk PL increased faecal loss of coprostanol, a gut-derived metabolite of cholesterol, but major bacterial populations and faecal short-chain fatty acids were not affected by milk PL, regardless of the dose. Acute ingestion of milk PL by ileostomy patients shows that milk PL decreased cholesterol absorption and increased cholesterol-ileal efflux, which can be explained by the observed co-excretion with milk sphingomyelin in the gut.
Conclusion
The present data demonstrate for the first time in humans that milk PL can improve the cardiometabolic health by decreasing several lipid cardiovascular markers, notably through a reduced intestinal cholesterol absorption involving specific interactions in the gut, without disturbing the major bacterial phyla of gut microbiota.
Trial registration number
NCT02099032 and NCT02146339; Results.
Keywords: lipid absorption, lipoprotein-cholesterol, colonic microflora, lipid metabolism, nutrition
Significance of this study.
What is already known on this subject?
Dietary synthetic emulsifiers alter drastically gut microbiota and promote inflammation and metabolic syndrome in rodent models.
Polar lipids are natural emulsifiers widely used in food formulation, mainly from vegetal sources.
Milk fat globules naturally contain polar lipids rich in sphingomyelin, which was shown in preclinical studies to reduce intestinal cholesterol absorption and improve lipid metabolism because of the natural affinity of sphingomyelin to form complexes with cholesterol.
Available clinical studies on dietary supplementation with milk polar lipids were rather inconclusive regarding their beneficial impact on human lipid metabolism, whereas egg polar lipids or fat devoid of polar lipids were reported to increase lipid markers of cardiovascular risk in these studies.
Significance of this study.
What are the new findings?
Four-week supplementation with milk polar lipids decreases significantly several fasting and postprandial lipid markers of cardiometabolic risk in overweight postmenopausal women at risk of cardiovascular disease (CVD).
Mechanisms of action of milk polar lipids in humans are related to their metabolic fate and impact in the gut, by reducing intestine-derived chylomicrons, intestinal cholesterol absorption via co-excretion with the unabsorbed sphingomyelin fraction, and by increasing cholesterol-to-coprostanol conversion by the gut microbiota.
Milk polar lipid consumption alters neither the major bacterial phyla of gut microbiota nor the profile of short-chain fatty acids.
How might it impact on clinical practice in the foreseeable future?
Considering their lowering effect on lipid cardiovascular markers in overweight adults at risk for developing metabolic syndrome, milk polar lipids should be part of new dietary strategies for primary and secondary prevention of CVDs.
The complementary mechanistic study in ileostomy patients suggests the enhancement by milk polar lipids of the transintestinal cholesterol excretion, known as the new target of clinical strategies to improve the cardiometabolic profile.
Present findings demonstrate the need to further investigate the impact of milk polar lipids on inflammatory cardiovascular risk markers and also their potential effect on coprostanoligenic bacteria and their potential role in restoring gut microbiota eubiosis in disease.
Introduction
Dietary synthetic emulsifiers were reported to alter drastically gut microbiota, thereby promoting both systemic and gut inflammation and metabolic syndrome in rodents.1 2 Dietary polar lipids (PL) are widely used as natural emulsifiers and texture-promoters in food formulation and represent 1%–10% of daily lipid intake (about 2–8 g/day),3 mainly provided by soybean lecithin (90% of world PL market). However, dietary lecithin recently raised concerns of sustainable development. On the other hand, milk and dairy products are consumed by >6 billion people worldwide4 and represent another natural source of emulsifiers. Indeed, milk fat globules comprise a core of triacylglycerols (TAG) surrounded by a specific biological membrane called the milk fat globule membrane (MFGM) that is rich in PL and cholesterol.5 MFGM extracts have gained much interest as a potential nutraceutical, notably for their natural PL composition6 with a large proportion of sphingomyelin (SM) (>25% of milk PL)7 compared with other animal sourced PL with <5% of SM or to vegetal PL devoid of SM.3 With longer and more saturated fatty acids than egg or soy PL, milk PL have also been shown to be more efficacious in reducing intestinal absorption of cholesterol in both preclinical and in vitro models.8 9 Up to date, several rodent studies have shown that milk PL impact postprandial lipid metabolism, lower intestinal absorption and hepatic accumulation of cholesterol and increase faecal excretion of cholesterol,10–12 leading to speculate that dietary supplementation with milk PL might be of therapeutic value in humans. However, strong evidence in humans is still lacking since most of the available clinical studies have been performed in healthy subjects with increased energy intake due to PL supplementation and were rather inconclusive regarding the beneficial impact on lipid metabolism.13–16 Considering the intestinal microbial conversion of cholesterol to coprostanol and increasing evidence that the gut microbiota is influenced by dietary factors and may impact host lipid metabolism,17–19 a putative effect of milk PL on human gut microbiome may exist but was so far not investigated. A clinical study shows recently that the efficiency of cholesterol conversion would be related to microbial density, as a coprostanol/cholesterol ratio >15 was associated with high level of coprostanoligenic bacteria (108 cells/g) in postmenopausal women.20 However, no human intervention has yet thoroughly explored the dynamic postprandial response of blood lipid cardiovascular risk factors to milk PL intervention or even their dose-response as part of benefits/risk assessment to foresee their wider use as a natural ingredient or dietary supplement. Furthermore, women are at higher risk of developing cardiovascular disease (CVD) as age advances and the loss of oestrogen during menopause leads to an alteration of the blood lipid profile with an increase of total and low-density lipoprotein (LDL)-cholesterol (LDL-C) levels.21 22 Metabolic syndrome of which dyslipidaemia is a core component is also more prevalent among postmenopausal women than among premenopausal women.23 Menopause is thus a major risk factor for CVD that is the leading cause of morbidity and mortality in postmenopausal women.24
In this context, we set up two first-in-human randomised controlled trials: the first was a 4-week dietary intervention based on daily consumption of PL-enriched cheeses in postmenopausal women at risk for CVD and the second was a proof-of-principle investigation of acute consumption of PL-enriched cheeses in ileostomy patients. To fit with everyday life diet and ensure practical consistency of such clinical results, we used a common type of dairy product, namely cream cheese, enriched with a milk PL-rich fraction derived from buttermilk. The original strategy here was to formulate cheeses with identical total lipid content with partial substitution of TAG by milk PL to avoid increased energy intake. A unique dose-response design up to 5 g/day was made possible by incorporating a buttermilk concentrate from a PL concentration process specifically designed for the present clinical investigations.25 The objective of the intervention trial was to determine: 1) whether dietary milk PL consumption (0, 3 or 5 g-PL/day) was able to impact differentially fasting and postprandial lipid metabolism, including intestine-derived chylomicrons and 2) the extent to which these potential milk PL effects were concomitant with changes in gut microbiota and gut-derived metabolites (coprostanol, short-chain fatty acids (SCFA)). The aim of the complementary study in ileostomy patients was to investigate intestinal mechanisms underpinning the impact of milk PL on cholesterol absorption and metabolism using 2H-cholesterol and 13C-TAG tracers.
Methods
Two clinical studies were performed involving multiple metabolic explorations and analyses; see more details in online supplementary materials and methods.
gutjnl-2018-318155supp001.pdf (422.1KB, pdf)
Clinical studies
The dietary intervention trial (VALOBAB-C study) was a multicentre double-blind randomised trial with a parallel group design in overweight postmenopausal women. The acute milk PL consumption study (VALOBAB-D study) was a multicentre double-blind randomised trial with a crossover design in ileostomy subjects. All participants provided prior informed written consent. Trials were approved by the ethics committee and legal authorities (see online supplementary materials and methods), registered on www.clinicaltrials.gov (NCT02099032, NCT02146339) and conducted at the Human Nutrition Research Centres of Rhône-Alpes (CRNH-RA; Lyon) and of Auvergne (CRNH-A; Clermont-Ferrand, France) according to French law.
Study designs
VALOBAB-C trial
After a 1-week run-in period (100 g control cheese/day), the volunteers were randomly assigned to three different groups for a 4-week intervention period (figure 1A, online supplementary figure S1A) with either cream cheese devoid of PL (control group) or enriched with PL (3 g-PL group or 5 g-PL group) (100 g/day; composition: online supplementary table S1). This increased dietary intake of PL (0.5-fold to 2-fold; SM: 2-fold to 6-fold) versus reported average daily intake in Western countries.3 Cream cheeses were packaged under partial vacuum of −0.4 bar in order to enhance product stability. The PL content of the cream cheeses was measured beyond the 5-month shelf life: PL content=3.05±0.11 g/100 g in 3 g-PL cheeses and PL content=5.02±0.22 g/100 g in 5 g-PL cheeses. The potential appearance of PL degradation products (ie, lyso-phospholipids due to potential phospholipase activity) in PL-enriched cheeses was also evaluated after shelf life, and values of lyso-phosphatidylcholine (a major lipolytic product) were found to be below the analytical detection limit (<1.3 mg/100 g cheese), confirming no major lipolysis in the cream cheese products.
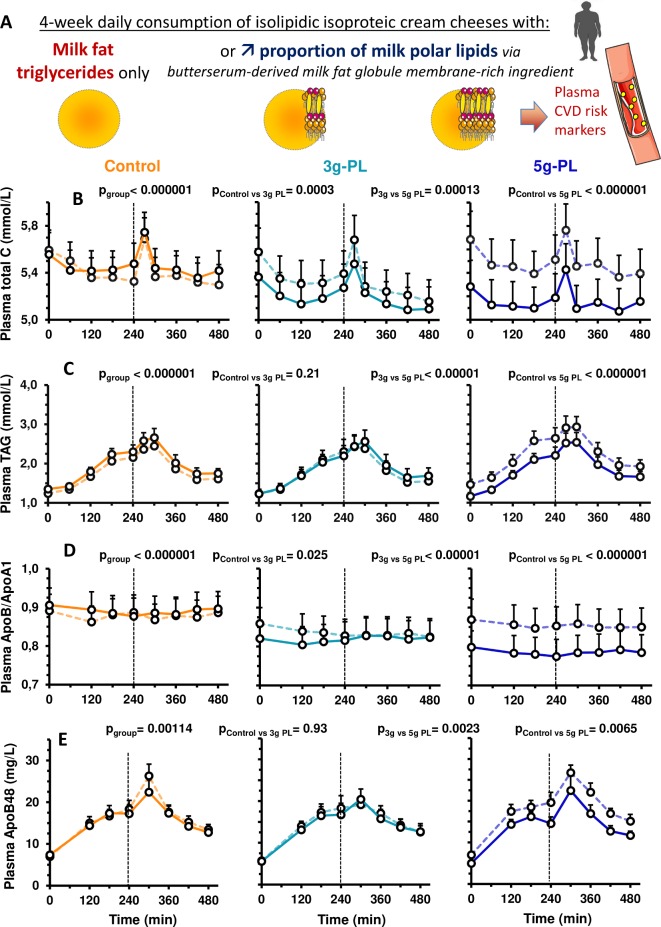
Figure 1.
Impact of 4-week intervention with up to 5 g milk PL in cream cheese on postprandial concentrations of lipid CV risk markers (VALOBAB-C trial). Panel explanation (A) and kinetics of serum total C (B), serum TAG (C), plasma ApoB/ApoA1 ratio (D) and plasma ApoB48 (E) before (V1, dotted line) and after (V2, full line) the daily consumption of 100 g of cheese with or without PL during 4 weeks. Data are represented as mean±SEM. The pgroup and pposthoc are shown for the 8-hour postprandial period (linear mixed model followed by post hoc analyses on ΔV2−V1); ptimexgroup was not significant. Group size: control n=18, 3 g-PL n=18, 5 g-PL n=20. See also online supplementary figure S1 for study design. Apo, apolipoprotein; C, cholesterol; CVD, cardiovascular disease; CV, cardiovascular; PL, polar lipids; TAG, triacylglycerols.
gutjnl-2018-318155supp002.pdf (411.7KB, pdf)
gutjnl-2018-318155supp003.pdf (175.5KB, pdf)
We stratified the group allocation on centre and randomised. Two visits of metabolic explorations (fasting and postprandial) were performed at the beginning (V1) and at the end (V2) of each dietary intervention (meal composition: online supplementary table S2). The primary outcome was the effect over 4 weeks of the daily consumption of milk PL (one control group and two increasing doses) on fasting serum concentration of total cholesterol (C), defined as the difference between V1 and V2. The 4-week period of intervention has been chosen in line with the previous trials reporting the health effects of milk PL or buttermilk14 15 26 and to study adequately the potential changes in gut microbiota composition, which is mostly investigated in dietary interventions >3 weeks.27 28
Hypotheses, sample size and power calculations (based on fasting cholesterol) are presented in online supplementary materials and methods. Regarding 8-hour longitudinal serum concentration of total C, we estimated actual power for a total sample size of 58 individuals to be >0.999 based on the actual mean concentrations and common SD of 0.42 mM. All data were analysed.
VALOBAB-D trial
Volunteers performed three distinct days of metabolic testing separated by a washout period (online supplementary figure S2A). During each visit, a different breakfast was served including one of the three test cheeses (100 g/meal) according to randomised sequences, and stable isotope tracers (13C-triolein, 2H-cholesterol; online supplementary table S2). The primary hypothesis was that milk PL in the meal would increase ileal efflux of SM (primary outcome). The secondary hypothesis was that this would be associated with both increased ileal efflux and decreased absorption of cholesterol (see predefined exploratory hypotheses and sample size calculation in online supplementary materials and methods). Sample size was estimated after reported ileostomy SM concentrations of six subjects,29 assuming unfavourable situation of a parallel group design and the highest variability and expecting crossover design to decrease outcomes’ variability thereby enhancing power analysis.
Study participants
In the VALOBAB-C trial, 58 eligible volunteers were included: 19 in the control group, 19 in the 3 g-PL group and 20 in the 5 g-PL group. They followed the entire trial and their data were analysed (online supplementary figure S1B). In the VALOBAB-D trial, candidates’ prescreening by digestive surgeons and gastroenterologists of local university hospitals allowed to contact 40 individuals. Only four volunteers could ultimately be recruited (recruitment difficulties of this population; non-extendable inclusion period considering PL-ingredient shelf life). Volunteers performed the entire protocol, were followed-up and had their data analysed (online supplementary figure S2B). Online supplementary materials and methods indicate more details (screening, inclusion/non-inclusion criteria).
Nutritional composition of cream cheese
Three full-fat cream cheeses (0, 3 and 5 g-PL) were formulated with identical macronutrient proportions, substituting TAG by milk PL in enriched products (see online supplementary table S1) without modifying the amount of total lipids, proteins and carbohydrates. The milk SM content was 0.8 wt% in 3 g-PL cheese and 1.3 wt% in 5 g-PL cheese. The cholesterol content was higher in milk PL-enriched cheeses because SM is naturally associated with cholesterol within lipid rafts in the MFGM.30 Conversely, the TAG content was higher in the control cheese compared with 3 g-PL and 5 g-PL cheeses due to TAG substitution in enriched cheeses. Because dietary PL (including buttermilk PL)5 contain naturally more monounsaturated fatty acids and polyunsaturated fatty acids (PUFA) and less saturated fatty acids (SFA), the partial TAG substitution by milk PL in cream cheese induced a relative increase of the PUFA amount in milk PL-enriched products associated with a relative decrease of SFA amount (online supplementary table S1).
Metabolic explorations and laboratory analyses
Details are provided in the online supplementary materials and methods about the metabolic explorations performed at each visit (online supplementary figures S1C and S2C) by the VALOBAB-C (before/after intervention) and the VALOBAB-D volunteers; the VALOBAB-C analysis of (i) circulating clinical parameters (total C, LDL-C, high-density lipoprotein (HDL)-cholesterol (HDL-C), TAG and glucose concentrations in serum, apolipoprotein (Apo)B and ApoA1 in plasma, serum insulin, plasma ApoB48 and PCSK9; area under the curve (AUC) calculation), (ii) intestine-derived chylomicron-rich fractions (CMRF) (lipids, hydrodynamic diameter), (iii) stools (faecal lipids, population levels of the main phylogenetic groups and bacterial species of gut microbiota, SCFA in faecal water) and the VALOBAB-D analysis of (i) ileal efflux of SM and cholesterol, (ii) amount and isotopic enrichment of oleic acid in plasma, of CO2 in expired air and of free cholesterol in plasma and CMRF, (iii) exogenous lipid oxidation calculation.
Statistical analyses
In the VALOBAB-C trial, we described continuous variables as mean±SEM (for distribution deviating from normality, median and IQR). The difference between visits (V2−V1) was used as response variable. Our study design planned to measure each parameter longitudinally at each visit (1–10 time points). Apart from single time point parameters that were analysed through general linear model and subsequent post hoc tests following Tukey’s test, we performed mixed linear modelling to account for within-subject repeated measures, seeking for main effects, that is, at least ‘group’ effect, time effect and interaction. Post hoc analyses were performed following Tukey-Kramer test to both detail main effects and control for familywise type I error. In case of residual distribution departing from normality, analyses were performed on ranks. In addition, we performed all these analyses considering global ‘milk PL’ effect as binary factor, that is, lumping together 3 g-PL and 5 g-PL doses in one group versus control. Analyses were performed on SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) with a type I error set at 0.05. Online supplementary table S3 summarises the number of sampled units for each analysis.
In the VALOBAB-D trial, data are presented as means±SEM and were analysed with GraphPad Prism 7 software. For normally distributed data, repeated measures one-way analysis of variance (ANOVA) (according to meal) or two-way ANOVA (according to meal and time) were performed followed by Tukey’s post hoc test. For non-normal data, a Friedman test was performed followed by Dunn’s post hoc test. For each parameter, a Mann-Whitney U test was performed to compare control meal with PL meals lumped together. Nominal two-sided p values are reported.
Results
Subjects’ characteristics
In the VALOBAB-C study, volunteers were overweight postmenopausal women of comparable mean age, without fasting hypertriglyceridemia nor diabetes but at risk for CVD with abdominal obesity, borderline high value of fasting LDL-C and borderline low value of fasting HDL-C (table 1). Volunteers had also a low-grade inflammation with a mean C reactive protein level >3 mg/L, which is considered high risk.31 Participants of the VALOBAB-D study were non-obese and normolipaemic ileostomy patients (online supplementary table S4). This specific population was chosen to collect the residual unabsorbed lipids at the end of the small intestine (ileum), before they reach the colon.
Table 1.
Anthropometric and fasting metabolic characteristics of the VALOBAB-C study population at screening
| Control | 3 g-PL | 5 g-PL | Healthy range/value | |
| (n=19) | (n=19) | (n=20) | ||
| Age (years) | 58±2 | 60±2 | 58±1 | – |
| Body weight (kg) | 78.5±2.5 | 75.3±1.9 | 75.6±1.1 | – |
| BMI (kg/m²) | 30.5±0.8 | 29.3±0.6 | 29.3±0.5 | 20–25* |
| Waist circumference (cm) | 96.5±2.4 | 97.0±1.5 | 97.9±1.4 | ≤80† |
| Hip circumference (cm) | 110.4±1.7 | 106.9±1.8 | 106.5±1.7 | – |
| Waist/hip circumference ratio | 0.87±0.02 | 0.91±0.01 | 0.92±0.01 | ≤0.85* |
| Systolic BP (mm Hg) | 132.1±2.9 | 129.2±3.1 | 133.7±2.6 | <130† |
| Diastolic BP (mm Hg) | 79.8±1.6 | 82.0±1.4 | 80.5±1.8 | <85† |
| Total C (mmol/L) | 6.0±0.2 | 5.7±0.2 | 5.8±0.2 | <5.2‡ |
| LDL-C (mmol/L) | 3.9±0.2 | 3.7±0.2 | 3.8±0.2 | ≤3.3‡ |
| HDL-C (mmol/L) | 1.3±0.0 | 1.4±0.0 | 1.3±0.0 | >1.3† |
| TAG (mmol/L) | 1.6±0.1 | 1.4±0.2 | 1.4±0.1 | <1.7† |
| Glucose (mmol/L) | 4.9±0.1 | 4.9±0.1 | 4.7±0.1 | <5.6† |
| CRP (mg/L) | 5.3±1.0 | 5.3±1.1 | 3.7±0.6 | <1–3§ |
| AST (IU/L) | 21.9±1.0 | 25.2±2.1 | 24.2±1.7 | <34¶ |
| ALT (IU/L) | 27.5±2.4 | 29.2±3.1 | 30.6±3.1 | <55¶ |
Data are presented as mean±SEM. Subjects were randomised therefore no statistical analysis was performed on subject characteristics at screening.
*According to WHO.66
†Based on the International Diabetes Federation metabolic syndrome worldwide definition.67
‡According to the National Cholesterol Education Program Adult Treatment Panel III.68
§Recommendations of the American Heart Association and Centers for Disease Control and Prevention established levels of cardiovascular risk by assigning CRP values as follows: low risk: <1.0 mg/L; average risk: 1.0–3.0 mg/L; high risk: >3.0 mg/L.31
¶According to reference ranges provided by the hospital laboratory in charge of the measurement of transaminases.
ALT, alanine amino transferase; AST, alanine aspartate transferase; BP, blood pressure; BMI, body mass index; C, cholesterol; CRP, C reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PL, polar lipids, TAG, triacylglycerol.
Compliance and dietary intake in the VALOBAB-C study
The mean compliance to dietary intervention was high (>98%) and similar between groups (p>0.3). Dietary records excluding the study-cream cheese showed that volunteers of the three groups did not differentially modify their energy and macronutrient intakes, nor fibres, alcohol, cholesterol and fatty acid (FA) intakes between V1 and V2 (online supplementary table S5).
Milk PL decrease fasting blood lipids and associated cardiometabolic risk markers
We compared the effects of the 4-week intervention between groups on the change (Δ) of values between visits V2 and V1 (ie, ΔV2−V1). Several fasting markers of cardiometabolic risk were significantly modified by the intervention (table 2). Fasting total C decreased significantly in the 5 g-PL group (pgroup<0.05, pposthoc<0.05 vs control; −0.40 mM, −6.8%; table 2). We also observed concomitant decrease in LDL-C in the 5 g-PL group (pposthoc<0.05 vs control; −0.34 mM, −8.7%) and increase in HDL-C in 5 g-PL vs 3 g-PL group (pposthoc<0.05; +0.06 mM, ie, +5.0%). In the 5 g-PL group, these changes resulted in a decreased ratio total C/HDL-C (pposthoc<0.01 vs control and vs 3 g-PL), a better predictor of CVD than LDL-C.32 Total C/HDL-C ratio in the 5 g-PL group decreased to 4.53, which is close to the target value of 4.4 indicated by the American Heart Association for women to lower CV risk from high to moderate. The 5 g-PL group showed significant decrease in fasting serum TAG versus control and versus 3 g-PL groups (pposthoc<0.05), plasma ApoB (contained in chylomicrons, VLDL and LDL) versus control (pposthoc<0.05) and plasma ApoB48 versus control and versus 3 g-PL (pposthoc<0.01) (table 2). In addition, ApoB/ApoA1 (pposthoc<0.05 vs control; −0.07, –6.8%) and ApoB48/ApoB ratios (pposthoc<0.05 vs control and vs 3 g-PL) were significantly reduced in the 5 g-PL group.
Table 2.
Differences in the effects of 4-week intervention with milk PL in cream cheese on fasting circulating metabolic risk markers in the VALOBAB-C trial
| Control (n=19) | 3 g-PL (n=19) | 5 g-PL (n=20) | pgroup | ||||
| V1 | ΔV2−V1 | V1 | ΔV2−V1 | V1 | ΔV2−V1 | ||
| Body weight (kg) | 77.86±2.36 | 0.03±0.19 | 74.67±1.76 | 0.01±0.22 | 75.22±1.16 | −0.33±0.25 | 0.43 |
| Fat body mass (%) | 43.85±1.03 | 0.03±0.37 | 43.43±0.78 | 0.30±0.55 | 42.48±0.79 | −0.3±0.21 | 0.56 |
| Lean body mass (%) | 56.15±1.03 | −0.03±0.37 | 56.57±0.78 | −0.30±0.55 | 57.52±0.79 | 0.3±0.21 | 0.56 |
| BMI (kg/m²) | 30.22±0.76 | 0.01±0.07 | 29.05±0.58 | −0.02±0.09 | 29.18±0.56 | −0.12±0.1 | 0.53 |
| Systolic BP (mm Hg) | 124.32±2.74 | −4.79±2.28 | 124.68±4.17 | −1.63±2.00 | 124.47±3.94 | −4.21±2.88 | 0.62 |
| Diastolic BP (mm Hg) | 71.68±2.28 | 0.84±1.69 | 76.21±2.07 | −1.84±1.87 | 75.84±1.94 | −2.89±1.24 | 0.25 |
| Total C (mmol/L) | 5.59±0.17 | −0.04±0.1a | 5.58±0.20 | −0.21±0.10a, b | 5.68±0.24 | −0.4±0.09b | 0.04 |
| LDL-C (mmol/L) | 3.70±0.15 | −0.04±0.08a | 3.54±0.17 | −0.17±0.08a, b | 3.63±0.19 | −0.34±0.08b | 0.04 |
| HDL-C (mmol/L) | 1.16±0.05 | −0.02±0.03a, b | 1.23±0.05 | −0.04±0.03a | 1.17±0.06 | 0.06±0.02b | 0.03 |
| Total C/HDL-C ratio | 5.01±0.27 | 0.11±0.16a | 4.67±0.26 | −0.01±0.12a | 5.16±0.36 | −0.63±0.13b | 0.0005† |
| TAG (mmol/L) | 1.24±0.08 | 0.11±0.06a | 1.24±0.11 | −0.01±0.09a | 1.47±0.13 | −0.30±0.10b | 0.003† |
| PCSK9 (ng/mL) | 271.30±27.10 | 12.30±13.50 | 276.00±25.00 | −28.30±17.30 | 259.20±18.40 | −16.60±10.50 | 0.12 |
| ApoA1 (g/L)‡ | 1.16±0.02 | −0.01±0.02 | 1.17±0.02 | 0.00±0.02 | 1.19±0.03 | −0.01±0.02 | 0.86 |
| ApoB (g/L)† | 1.03±0.05 | 0.01±0.02a | 1.01±0.05 | −0.04±0.02a, b | 1.02±0.06 | −0.09±0.03b | 0.03 |
| ApoB48 (10−3 g/L)‡ | 6.98±0.77 | 0.41±0.36a | 5.70±0.53 | −0.13±0.39a | 7.01±0.62 | −2.04±0.50b | 0.0004† |
| ApoB/ApoA1‡ | 0.89±0.04 | 0.01±0.02a | 0.86±0.04 | −0.04±0.02a, b | 0.87±0.05 | −0.07±0.02b | 0.02 |
| ApoB48/ApoB‡ | 6.75±0.48 | 0.38±0.45a | 5.79±0.53 | 0.14±0.47a | 7.48±0.70 | −1.64±0.45b | 0.005† |
| Glucose (mmol/L) | 5.23±0.10 | −0.03±0.07 | 5.11±0.11 | 0.02±0.06 | 5.15±0.1 | −0.11±0.06 | 0.37 |
| Insulin (mIU/L) | 7.29±0.99 | 0.43±0.72 | 7.11±0.72 | −0.06±0.61 | 8.07±1.36 | −0.41±0.90 | 0.74 |
| HOMA-IR | 1.74±0.26 | 0.17±0.18 | 1.67±0.19 | −0.03±0.14 | 1.82±0.28 | −0.10±0.18 | 0.52 |
P values presented in bold highlight significant intervention effect.
Data are presented as mean±SEM. pgroup represents p value associated with group effect as calculated by generalised linear model.
Different superscript letters indicate statistically different intervention effects between groups as calculated by post hoc analyses controlling for familywise type I error (i.e., means sharing a common letter are not significantly different).
†P value remains significant (<0.05) after adjustment for clinical centre, quartiles of volunteer age and waist circumference; the other significant p values only describe a tendency after adjustment (p<0.1).
‡Control n=17, 3 g-PL n=17 and 5 g-PL n=18 due to missing values.
Apo, apolipoprotein; BMI, body mass index; BP, blood pressure; C, cholesterol; CRP, C reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasic model assessment of insulin resistance; LDL, low-density lipoprotein; PCSK9: proprotein convertase subtilisin/kexin type 9; PL, polar lipids; TAG, triacylglycerol; V1, visit 1; V2, visit 2.
Regardless of dose, groups with PL decreased significantly fasting plasma PCSK9 compared with control (binary analysis, pPL=0.047; while pgroup=0.12). In each group with PL, change in total C was positively correlated with ΔApoB (r=0.83 and r=0.80, respectively; p<0.05). Moreover, in the 5 g-PL group only, positive correlations were observed: (i) Δtotal C with ΔTAG (r=0.61, p<0.05) and ΔApoB48 (r=0.50, p<0.05); (ii) ΔTAG with ΔLDL-C (r=0.58, p<0.05) and ΔApoB (r=0.45, p<0.05).
Milk PL lower postprandial lipid cardiovascular risk markers
Dietary intervention (figure 1A) effect was highly significant between groups on the postprandial kinetics of total C, TAG and ApoB/ApoA1 (figure 1B-D; pgroup≤0.001; remaining significant after adjustment). The 4-week intervention with milk PL decreased postprandial cholesterol-related parameters: the higher the dose of milk PL, the greater the reduction in total C and ApoB/ApoA1 ratio (5 g-PL>3 g-PL>0 g-PL, figure 1B, D). Only the 5 g-PL group presented a decrease of postprandial TAG (−10.4%; pposthoc<0.01 vs control and vs 3 g-PL; figure 1C). Consistently, the metabolic effects of milk PL were significant on the postprandial cumulative response (AUC) and peaks of concentration (cmax) of several risk markers. Consumption of 5 g-PL reduced postprandial AUCs of total C by 5.7%, TAG of 10.4% and ApoB/ApoA1 ratio of 7.3% (all p<0.05, V2 vs V1). Compared with control, 5 g-PL induced significant reductions in the change of postprandial AUCs of total C (−156±40 vs 22±46 mM.min), TAG (−170±77 vs 72±42 mM.min) and ApoB/ApoA1 ratio (−33±9 vs 6±9 mM.min) (all pgroup and pposthoc<0.05 for 0–480 min). The cmax of total C and TAG were also decreased after 5 g-PL vs control: −0.34±0.1 mM vs 0.04±0.1 mM for total C (p<0.05), –0.45±0.2 mM vs 0.27±0.1 mM for TAG (p<0.05).
Milk PL do not induce body weight/fat mass gain but increase total lipid β-oxidation
Indirect calorimetry analysis in one centre revealed a significant effect of intervention on postlunch total lipid β-oxidation (240–480 min, pgroup=0.042; pposthoc<0.075 for each PL group versus control: +1.03±1.18 g in 3 g-PL group and +1.02±0.99 g in 5 g-PL group vs −2.54±1.06 g in control). Regardless of dose, milk PL increased cumulated lipid β-oxidation after lunch (240–480 min; pPL=0.011) and over 8 hours (pPL=0.034) versus control. Changes in participants’ body weight (pgroup=0.43) and fat mass (pgroup=0.56) did not differ among groups (table 2).
Milk PL favourably modulate intestine-derived chylomicrons
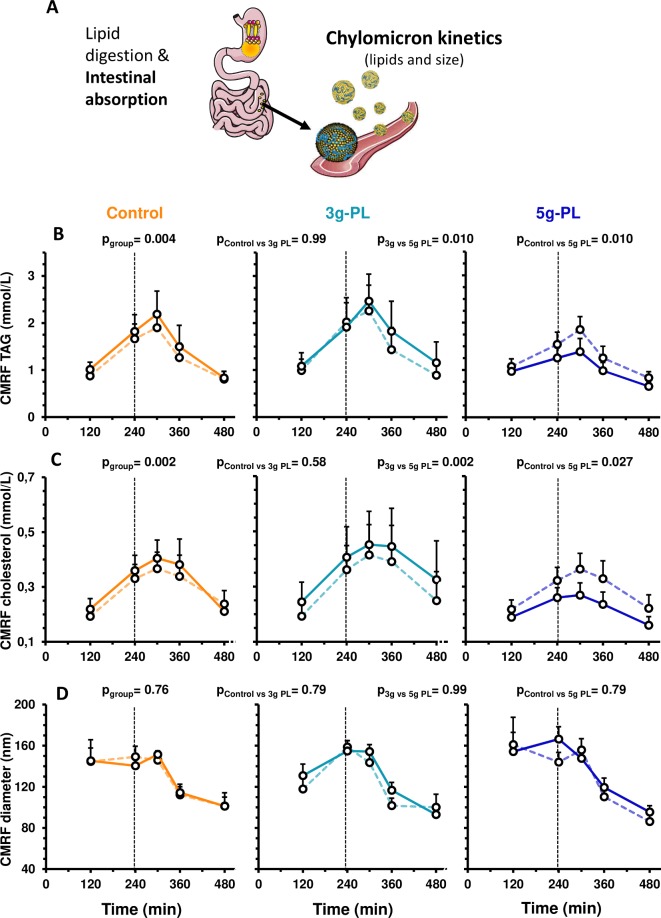
Regarding plasma concentrations of intestinal chylomicron lipids (figure 2A), both cholesterol (CMRF-C) and TAG (CMRF-TAG) of CMRF were decreased in plasma of the 5 g-PL group versus control and 3 g-PL groups (figure 2B, C, pgroup<0.01; remaining significant after adjustment). Postprandial concentrations of plasma ApoB48 were reduced only in the 5 g-PL group (figure 1E; pgroup≤0.001; pposthoc<0.01 vs control and vs 3 g-PL). CMRF particle size was unaffected by intervention (figure 2D).
Figure 2.
Modulation of postprandial chylomicron parameters after 4-week intervention with control, 3 g-PL or 5 g-PL cream cheese (VALOBAB-C trial). Panel explanation (A) and plasma kinetics of CMRF TAG (B), CMRF C (C) and CMRF size (D) before (V1, dotted line) and after (V2, full line) the daily consumption of 100 g of cheese with or without PL during 4 weeks. Data are represented as mean±SEM. pgroup and pposthoc are shown for postprandial period from 120 to 480 min (linear mixed model followed by post hoc analyses on ΔV2−V1). ptimexgroup was not significant. (B, C): after adjustment on quartiles of volunteer age and waist circumference: pgroup<0.05. For technical reason, analyses performed in centre 1 only, sample size: control n=9, 3 g-PL n=9, 5 g-PL n=10. CMRF, chylomicron-rich fraction; PL, polar lipids; TAG, triacylglycerols.
Milk PL increase faecal coprostanol without altering major phylogenetic groups of gut microbiota
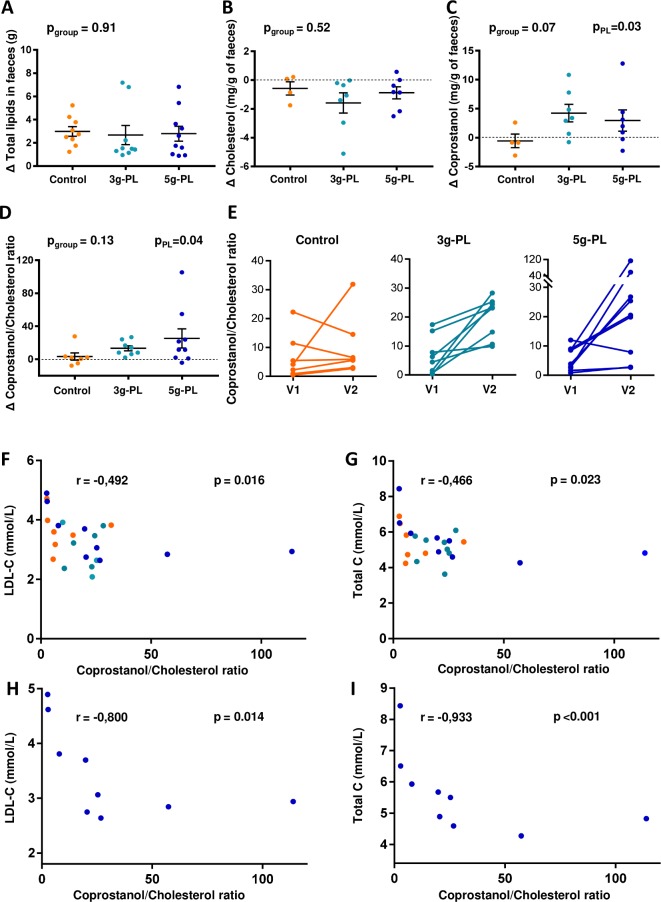
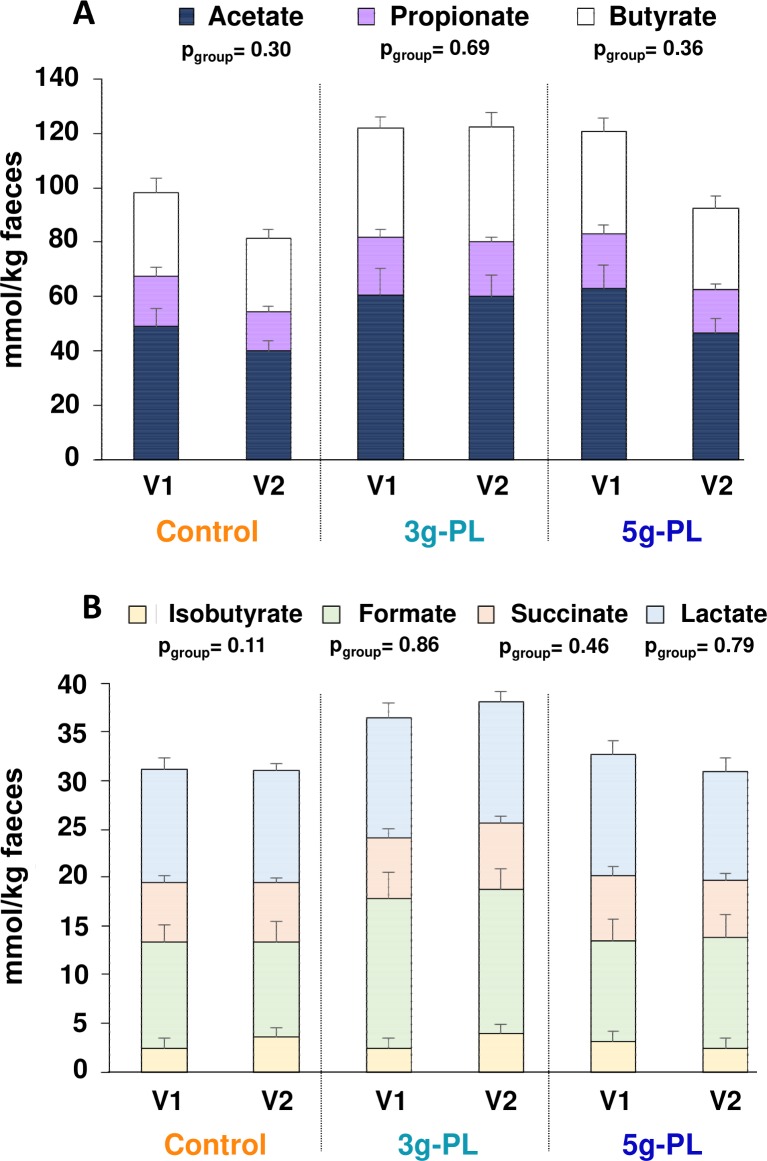
Variations of faecal losses of total lipids and cholesterol were not different among groups (figure 3A, B, pgroup=0.91 and 0.52, respectively). Variation of faecal concentrations of coprostanol tended to differ among groups (figure 3C, pgroup=0.07) and increased significantly after interventions with milk PL regardless of dose versus control (pPL=0.03). Moreover, the faecal coprostanol/cholesterol ratio was significantly increased by milk PL (figure 3D; pPL=0.04). More specifically, around 65% of volunteers in both milk PL groups had a faecal coprostanol/cholesterol ratio exceeding 15 after intervention (ratios from 23 to 28 and 20 to 114 in the 3 g-PL and 5 g-PL subsets, respectively; figure 3E). LDL-C and total cholesterol are negatively correlated with faecal coprostanol/cholesterol ratio after dietary intervention (all groups; figure 3F, G, r=−0.492, p=0.016 and r=−0.466, p=0.023, respectively), while no correlation was observed before intervention. Such correlations are emphasised in the 5 g-PL group after intervention (figure 3H, I). The daily consumption of up to 5 g of milk PL during 4 weeks modulated neither the major phylogenetic groups and bacterial species of gut microbiota (figure 4) nor the measured faecal SCFA profile (figure 5).
Figure 3.
Impact of 4-week intervention with milk polar lipids (PL)-enriched cream cheese on faecal lipids in the VALOBAB-C trial. Faecal loss variations (ΔV2−V1) of total lipids (A), cholesterol (B), coprostanol (C) and coprostanol/cholesterol ratio (D) after daily consumption of 100 g of cheese with or without PL during 4 weeks. Coprostanol/cholesterol ratio before (V1) and after (V2) the 4-week consumption of 100 g of cheese with or without PL (E). Spearman’s correlation between faecal coprostanol/cholesterol ratio and serum low-density lipoprotein-cholesterol (LDL-C) (F) and total cholesterol (C) (G) after (V2) daily consumption of 100 g of cheese with or without PL during 4 weeks (orange: control group, light blue: 3 g-PL group, dark blue: 5 g-PL group). Spearman’s correlation between faecal coprostanol/cholesterol ratio and serum total C (H) and LDL-C (I) at V2 in the 5 g-PL group. Data are indicated as median with IQR. The pgroup is shown to compare intervention effect between the three groups (analysis on ranks on ΔV2−V1); PPL is shown to compare intervention effect between the control group and both PL groups regardless of dose. For technical reasons, analyses performed in centre 1 only, sample size: (A) n=9 (control) to 10 (PL); (B–C) n=4 (control) to 7 (PL); (D–I) n=7 (control) to 9 (PL). NB: regardless of group and visit, the observed faecal losses of cholesterol (range 0.2–1 mg/g faeces) and coprostanol (range 0.45–5.2 mg/g faeces) were consistent with previous published data (cholesterol: 1.88±0.53 to 5.8±1.56 mg/g dry faeces47; coprostanol: 3–27.4 mg/g lyophilised faeces,48 considering that dry matter is ~25% of total faeces weight).
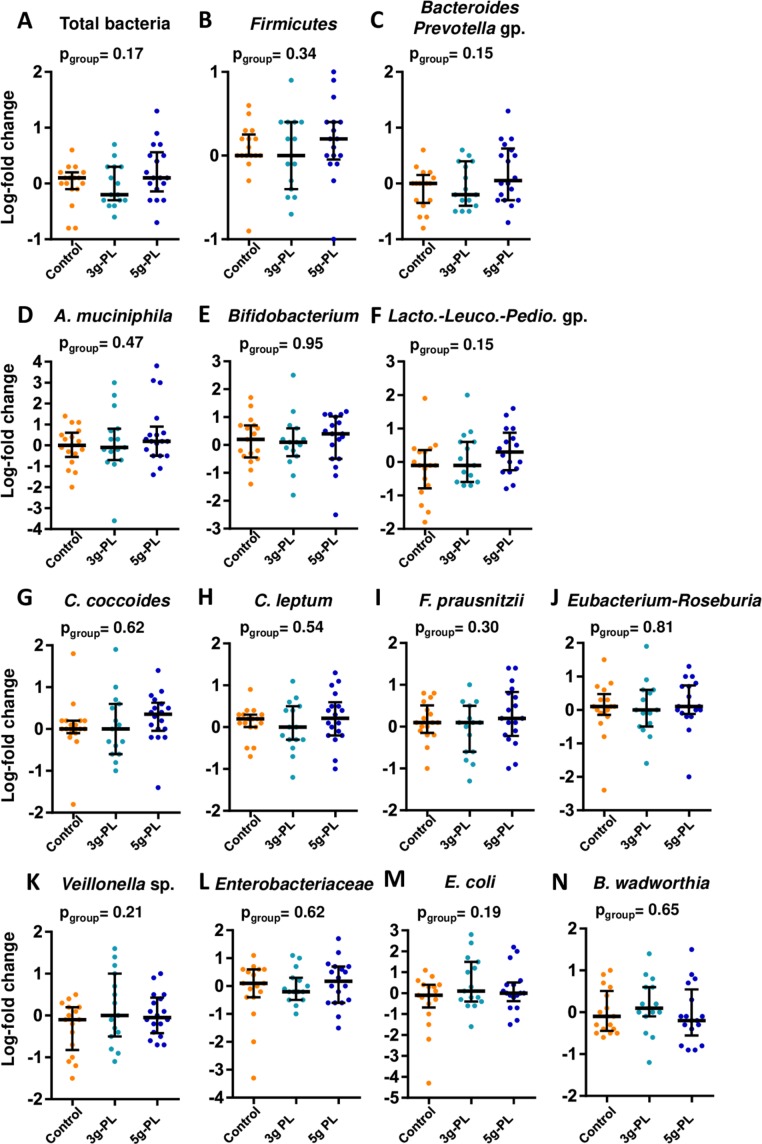
Figure 4.
Major phylogenetic groups and bacterial species of gut microbiota after 4-week intervention with up to 5 g milk polar lipids (PL) in cream cheese in the VALOBAB-C trial. Variations (log-fold change V2/V1) of the abundance of the main bacterial groups and species that were measured as log 16S rDNA gene copies per g of faeces: (A) total bacteria, (B) Firmicutes, (C) Bacteroides-Prevotella group, (D) Akkermancia muciniphila, (E) Bifidobacterium spp, (F) Lactobacillus-Leuconostoc-Pediococcus group, (G) Clostridium coccoides group, (H) Clostridium leptum group, (I) Faecalibacterium prausnitzii, (J) Roseburia-Eubacterium rectale group, (K) Veillonella spp, (L) Enterobacteriaceae family, (M) Escherichia coli and (N) Bilophila wadworthia. Data are indicated as median with IQR; pgroup is shown. Group size: control n=18, 3 g-PL n=18, 5 g-PL n=20.
Figure 5.
Faecal profile of short-chain fatty acids before (V1) and after (V2) 4-week intervention with up to 5 g/day milk polar lipids (PL) in cream cheese in the VALOBAB-C trial. (A) Acetate, propionate and butyrate, (B) isobutyrate, formate, succinate and lactate. Data are represented as mean±SEM, pgroup are shown. NB: regarding the total amount of major short-chain fatty acids in (A), acetate+propionate+butyrate: pgroup=0.35. For technical reasons, analyses performed in centre 2 only, sample size: control n=8, 3 g-PL n=7, 5 g-PL n=8.
Milk PL decrease intestinal cholesterol absorption and increase cholesterol-ileal efflux in ileostomy subjects
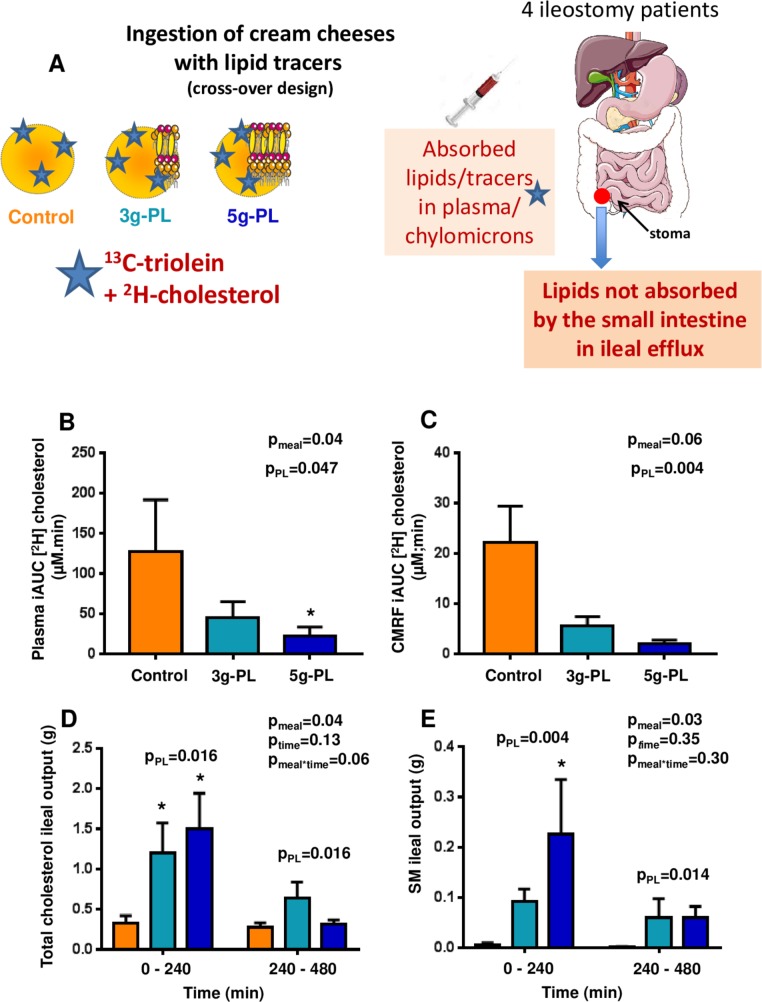
In ileostomy subjects (figure 6A), the incremental AUC (iAUC) over 8 hour of [13C]-oleic acid in plasma lipids and the exogenous FA β-oxidation (13CO2 breath test) were similar between meals (online supplementary figure S3A–F). However, the iAUC of plasma [2H]-cholesterol was significantly lowered by the 5 g-PL versus control meal (pmeal<0.05, figure 6B). Regardless of PL dose, a significantly lower iAUC of [2H]-cholesterol was observed after PL meals in both plasma (pPL=0.047) and chylomicrons (pPL=0.004) (figure 6B, C). Furthermore, each PL meal resulted in higher ileal efflux of total cholesterol versus control during the first 4 hour (pmeal=0.04, pposthoc<0.05, figure 6D; pPL<0.05). Milk PL in the meal increased SM losses in ileal effluent versus control (pmeal=0.03, pPL<0.05 vs control, figure 6E). SM efflux corresponded to 20%–25% of ingested amount over 8 hour, and was composed of typical milk-SM molecular species,7 that is, mostly isoforms with acyl chains over C20 (online supplementary figure S3G).
Figure 6.
Milk polar lipids (PL) impact on intestinal cholesterol absorption in the VALOBAB-D pilot study in ileostomy volunteers. Panel explanation (A), cumulated enrichment over 0–480 min of [2H]-cholesterol in plasma (B) and chylomicron-rich fraction (CMRF) (C), ileal losses of total cholesterol (D) and sphingomyelin (SM) (E), during the 0–240 min and 240–480 min postprandial periods (see also online supplementary figure S3G for SM composition in ileal efflux). Data are expressed as mean±SEM; n=4 per group. (B) Friedman test (pmeal) followed by Dunn’s post hoc tests for control and 5 g-PL groups. (C) Repeated measures one-way analysis of variance (ANOVA) (pmeal) followed by Tukey’s post hoc tests. (D, E) Repeated measures two-way ANOVA (pmeal, ptime, pmeal×time) followed by Tukey’s post hoc tests. *Pposthoc<0.05 vs control group. The pPL compares the effect of control meal with both PL meals regardless of dose. Sample size: n=4 subjects, crossover design (see online supplementary figure S2). NB: The 8-hour total cholesterol efflux (tracer+tracee in both free and esterified forms) after the control meal (642±57 mg) was consistent with previous reports56; here after 3 g-PL meal the 8-hour total cholesterol efflux was 1510±417 and 1752±354 mg after 5 g-PL meal. NB: The proportion vs ingested milk SM of the 8-hour ileal efflux of milk SM after cheese matrix (20%–25% of ingested dose) was similar with 19% reported after pure milk SM in six ileostomy subjects.29
Discussion
Present results first demonstrate that 4-week dietary intervention based on cream cheese naturally enriched with 3 or 5 g/day of milk PL, as part of a balanced diet, markedly lower an array of lipid markers of CVD risk in postmenopausal women. Particularly, we reveal in humans that 5 g/day milk PL achieves a reduction of 8.7% of LDL-C (−0.34 mM) with an additional reduction of 15.6% of serum TAG. This is a clinically meaningful change considering that a LDL-C reduction of 1 mM is associated with a 19% lower risk of coronary mortality in clinical trial33 and even −52% CV risk in case of early intervention.34 Clinical interest has recently focused on emerging lipid parameters including PCSK9,35 which binds to the LDL-receptor. Here, fasting plasma level of PCSK9 significantly decreased after milk PL intervention regardless of dose, suggesting that the lowering effect of milk PL on serum LDL-C may be mediated at least in part by PCSK9 despite the lack of group effect. Previous studies in healthy men and women reported no reduction of fasting total C, LDL-C, TAG and/or ApoB after 2–12 weeks interventions with 1 g/day of milk SM36 or up to 2.8 g/day supplementation with milk PL/MFGM,15 16 37 38 despite control (other PL or devoid of PL) increased such risk markers. Only one 4-week crossover trial with low-fat buttermilk-based drink showed decreased fasting serum total C (−3.1%), LDL-C (−3.1%) and TAG (−10.7%), although with no effect on HDL-C or ApoB48.26 The present strategy based on triglyceride substitution by milk PL in a full-fat dairy product was successful to reduce lipid markers of CVD risk, probably partly because of products being isolipidic (energy intake not increased) and comprising a butterserum-derived ingredient allowing up to 5 g of milk PL.
We cannot rule out that differences in cheese TAG content may account for triglyceridemia reduction. To the best of our knowledge, there is no available data or model in the literature allowing to estimate the impact of substituting TAG with PL on triglyceridemia. Interestingly, we showed that plasma-TAG, chylomicron-TAG and plasma ApoB48 decreased significantly after 4-week intervention but only in the 5 g-PL group (not in 3 g-PL and control groups). In ileostomy subjects, postprandial accumulations of plasma-TAG and chylomicron-TAG were not different after consumption of the 0, 3 and 5 g-PL enriched cheeses. We showed previously that in mice gavaged with lipid emulsions of equal dietary TAG content but differing in PL type (milk vs soy PL), emulsions with milk PL resulted in faster clearance of postprandial TAG and ApoB48, and lower intestinal APOB gene expression 4 hours after gavage.10 Regarding intestinal lipid absorption, other authors however described that lymphatic TAG were similar after gavage with an emulsion containing 200 mg TAG and an emulsion with 177 mg TAG and 27 mg of milk PL.39 Therefore, preclinical and clinical studies are still needed to elucidate (i) whether a threshold amount of dietary TAG substitution by PL exists in order to impact significantly triglyceridemia and (ii) whether milk PL can impact directly the intestinal chylomicron secretion and/or hepatic clearance, and thereby triglyceridemia. Furthermore, milk PL cheeses contained higher PUFA amounts than the control cheese that could trigger the potential reduction of lipid markers of CV risk, especially LDL-C. We thus examined if the different PUFA contents of cream cheeses could have significantly impacted the observed LDL-C reduction in the VALOBAB-C trial according to the linear predictive model established by Mensink and Katan.40 Assuming the worst effect of SFA and the most beneficial effect of PUFA on LDL-C, the model predicts a LDL-C reduction of −18 µM (ie, −0.5%) in the 5 g-PL group, which remains marginal compared with the observed reduction in the present study (−8.7%). This strengthens our conclusion that the lowering effect of milk PL on lipid markers of CVD risk may be due to mechanisms associated with cholesterol fate in the intestine due to milk SM.
Because people spend the most time in a postprandial state and postprandial lipemia is now recognised as an important independent marker of CVD risk,41 volunteers were submitted to test meal challenges to analyse intestine-derived chylomicrons,42 chylomicron-bound Apo48,32 ApoB/ApoA1 and ApoB48/ApoB ratios during 8 hours. ApoB/ApoA1 ratio reflects the balance between pro-atherogenic LDL particles and anti-atherogenic HDL particles,43 whereas ApoB48/ApoB ratio depicts the contribution of chylomicrons and chylomicron remnants to total atherogenic particles in triglyceride-rich (TRL) lipoprotein fractions.44 The studies in healthy humans performing single postprandial tests showed hitherto no effect of milk PL on postprandial lipids and ApoB/ApoA1 ratio45 or decreased postprandial total C and LDL-C but increased TAG13 versus no PL. The present intervention had a beneficial cardiometabolic impact by reducing significantly postprandial CV risk markers. We also underline the concomitance of sustained changes in postprandial lipids by milk PL. Mechanistically, the 5 g-PL dose specifically reduced the postprandial intestine-derived TRL lipids, consistently with the decreased postprandial TAG. CMRF particle size was unaffected, indicating decreased particle number by the 5 g-PL dose. The collected CMRF includes intestine-derived chylomicrons and other partially delipidated remnant particles. Hence, 5 g/day of milk PL could beneficially limit chylomicron-TAG synthesis/accretion and/or improve hepatic clearance.
Regarding gut-associated mechanisms, we showed in a subgroup that milk PL interventions increased faecal coprostanol, a non-absorbable molecule.46–48 This suggests a contribution of this increased intestinal cholesterol-to-coprostanol conversion in the serum total C-lowering effect of milk PL. As they naturally contained more cholesterol than the control cheese, milk PL-cheeses may have fuelled some specific bacterial strains known to convert cholesterol into coprostanol.46 Gut microbiota is now considered as an ‘essential organ’ in numerous metabolic functions,49 including regulation of lipid homeostasis and cholesterol metabolism,50 and can be altered by synthetic emulsifiers.1 2 Therefore, we quantified in faecal samples the two main phyla (Bacteroides-Prevotella groups (gp.) and Firmicutes) and the dominant bacterial gp., families or genera that may exhibit interesting inflammatory or metabolic functions.51 In high-fat-fed mice, milk SM and milk PL increase the abundance in gut microbiota of Bifidobacterium spp52 53 (associated with benefits regarding metabolic disorders54): this was not observed here in humans. However, milk PL could have modulated other bacterial genera/species, which deserves further studies using high-throughput techniques. More particularly, the recent findings by Cuevas-Tena et al suggest that the efficiency of cholesterol conversion would be related to microbial density, as a coprostanol/cholesterol ratio >15 was associated with high level of coprostanoligenic bacteria (108 cells/g).20 In our VALOBAB-C study, 8.5% of volunteer subset had a coprostanol/cholesterol ratio >15 before intervention vs 50% after intervention, and this observation was related to the milk PL effect. In line with these observations, we cannot rule out that the present dietary intervention with milk PL could have modulated the level of coprostanoligenic bacteria in the gut. Regarding microbiota functionality, we showed in a subgroup of volunteers that faecal SCFA were not differentially impacted by interventions. Thus, the present results suggest that short-term increased intake of milk PL did not have a drastic effect on the main bacterial communities of the gut microbiota, although it does not imply that specific bacterial species not targeted in the present approach could have been modulated by the milk PL supplementation. Moreover, the present negative correlations between the faecal coprostanol/cholesterol ratio and LDL-C and total cholesterol are in line with a recent review describing such an inverse relationship and suggesting that produced coprostanol can modulate cholesterolaemia.55 A high efficiency of cholesterol-to-coprostanol metabolism was also suggested to reduce the risk of CVDs.55 The quantification and functional analysis of coprostanoligenic bacteria thus deserve further investigation for future research in the context of milk PL supplementation.
Considering the lack of human mechanistic studies on dietary PL impact on intestinal cholesterol absorption,3 we performed a pilot postprandial trial on ileostomy subjects using tracers, a gold-standard approach.56 The similar iAUC of serum-lipids and chylomicron-lipids between meals rules out differences in hepatic chylomicron clearance. The lower [2H]-cholesterol in CMRF shows that milk PL can decrease the postprandial incorporation of dietary cholesterol into chylomicrons in humans. Moreover, the 8-hour total ileal cholesterol efflux ((ingested cholesterol fraction remaining unabsorbed by the small intestine)+(endogenous cholesterol that was not reabsorbed)) was significantly increased after milk PL meals, which can explain the lower plasma postprandial concentrations of [2H]-cholesterol with milk PL. Previous rodent and in vitro studies reported that dietary SM could decrease cholesterol absorption by forming unabsorbed complexes with cholesterol.8 9 57 As here 20%–25% of ingested milk SM was found intact in ileal efflux, the lower cholesterol absorption can be partly explained by its interactions with unabsorbed milk SM in the gut. However, total cholesterol ileal efflux after PL meals (~1510–1750 mg) was even twice greater than expected by summing (control cholesterol efflux) +(PL-meal cholesterol content) (~750 mg). ‘Apparent negative absorption’ of cholesterol was also reported after intraduodenal infusion of lecithin (30 g/day) in humans.58 This suggests that (i) the undigested milk SM could also decrease the degree of reabsorption of endogenous intestinal-mucosa cholesterol and (ii) milk PL and/or SM could also enhance the transintestinal cholesterol excretion (TICE), that is, an increased reverse absorption of endogenous cholesterol in the small intestine.59 Under basal conditions in mildly hypercholesterolaemic men, TICE contributes for 35% of faecal neutral sterol excretion.60 Therefore in the overweight women of VALOBAB-C trial, an increased ileal efflux of cholesterol could have contributed to the observed hypocholesterolaemic effects, as supported by VALOBAB-D trial. The hypothesis of the potential involvement of TICE enhancement deserves further mechanistic investigations in humans.
Several nuances have to be made: VALOBAB-C results cannot be extrapolated to individuals with other metabolic disorders/diseases (primary dyslipidaemia, normal weight subjects). Some specific and/or cumbersome analyses have only been carried out on a limited number of subjects. Further studies in men and in premenopausal women and using long-term interventions may now be of particular interest. Parallel to PL, the specific impact of the MFGM proteins, which can generate bioactive peptides in the gut, has not been assessed in the present studies and should be investigated in future research. Finally, characterisation of the gut microbiota in the present work was performed using a targeted metagenomic approach (16S rDNA sequencing) and future mechanistic studies should include shotgun metagenome approaches.
Altogether, these studies provide the first evidence that addition of up to 5 g of milk PL/day can be safely used as part of a balanced diet, with no apparent short-term adverse effect on neither lipid metabolism nor major bacterial phyla of gut microbiota, and with significant favourable effects on cholesterol homeostasis and markers of CVD risk despite a higher cholesterol content in milk PL-enriched cheeses. Moreover, the present dietary strategy is applicable in everyday life as cheese is a widely consumed dairy worldwide.4 Today in addition to lifestyle modifications as first-line therapy,61 many strategies efficiently reduce LDL-C, for example, pharmaceutical treatments (statins, ezetimibe, PCSK9 inhibitors), however costly or with undesirable side effects.62 The present dietary intervention with milk PL reduced LDL-C as effectively as dietary strategy based on phytosterol supplementation (2 g/day inducing −8% to −10% LDL-C but with doubts about undesirable side effects).63 The present results may thus offer a promising alternative for health professionals to improving dietary advice and provides competitive future prospects regarding nutritional formulations. If the present clinical results are further confirmed, they could provide a new open avenue for health claims on labels and new types of enriched foods with milk PL regarding their cholesterol-lowering effects. Moreover, the present findings provide a molecular explanation of possible mechanisms by which MFGM-rich full-fat dairy like cheese are associated with metabolic benefits.64 65 They support a specific role of milk SM that lowers intestinal cholesterol absorption and delivers unabsorbed lipids to the colon. This work thus offers important perspectives for a larger use of milk PL-rich fractions as natural functional food ingredients to substitute the synthetic ones suspected to promote metabolic disorders, representing a potential novel route to improve cardiometabolic health at the population level. Finally, the present findings demonstrate the need to further investigate the impact of milk PL on inflammatory cardiovascular risk markers and also their potential effect on coprostanoligenic bacteria and their potential role in restoring gut microbiota eubiosis in disease.
Acknowledgments
The authors would like to thank the volunteers of VALOBAB-C and VALOBAB-D trials, Dr N Feugier (CRNH-RA) and A Prulière (CRNH-A) for volunteer recruitment/follow-up; C Maitrepierre, J Peyrat, E Bain (CRNH-RA), D Provenchère, H Parrot, N Lyon and N Meunier (CRNH-A) for clinical/technical help; N Lyon-Belgy (CRNH-A) for help in dietary analysis; K Bertrand (ITERG), A Faure, M Cervantes and S Gonin (UNH), P Calmard and J Rivière (HCL) for help in lipid analyses, C Louche-Pélissier (CRNH-RA) for help in tracer analysis; C Cuerq (HCL CBS) for stool lipid analyses; C Jouve (UNH) for technical assistance in blood lipid analysis; S Robert (UCA) for her contribution to the statistical analyses; D Kalnin (Philolao) for help in the management of cream cheese randomisation; Professor Y François (HCL Lyon Sud), Professor D Pezet and B Gillet (Clermont Hospital) for screening ileostomy patients list; Dr S Charrière for useful discussions; N Leconte, JY Gassi and F Gaucheron (STLO, Plateforme Lait) for assistance in transferring PL enrichment process to ENILIA and C Bourlieu (STLO) for contribution in buttermilk PL analysis; V Plattner (HCL) and the clinical research department for trials’ monitoring; C Oudin for her skillful assistance in ANR VALOBAB project coordination. Members of the steering committee of ANR VALOBAB project (partners CarMeN/INRA, UNH/UCA, STLO/INRA, ENILIA-ENSMIC, ITERG, MEDIS, CNIEL) are acknowledged for useful discussions. The authors would like to thank CNIEL (French Dairy Interbranch Organisation) for financial support. C Vors and L Joumard-Cubizolles acknowledge ANR for postdoctoral fellowship. M Lecomte thanks Région Rhône-Alpes ARC1 for PhD grant. The authors acknowledge that part of this work has been presented in congresses (conference abstracts): Nutrition 2018 (Boston, USA, June 2018), EuroFedLipid (Belfast, UK, September 2018), Journées Francophones de Nutrition (Nice, France, November 2018), Nutrition 2019 (Baltimore, USA, June 2019). Figures 1A, 2A and 6A contain pieces of artwork that are derivatives of artworks kindly provided by the Servier Medical Art database licensed under a Creative Commons Attribution 3.0 Unported License.
Footnotes
MLe, EmC and LO contributed equally.
Twitter: @Michalski_MC, @cecile_vors
Contributors: M-CM, CM-B, CV, MLe, BM, MLa, SL-P: conception of the studies. M-CM, CM-B, LO, CV, LJ-C, SL-P, BM, EmCo, JD, FJ: methodology. CV, LJ-C, MLe, LM, FJ, SL-P, JD, ABD: validation of data. LO, CV, LJ-C, EmCo, MLe, LM, FJ, ABD: formal analysis of data. CV, LJ-C, MLe, JD, EB, LM, FJ, SL-P, ABD, CD-F, MS, AC, AB, AW, VS, FM-L, DC, KR, PG: performed clinical and analytical investigations. EdCo, KR, PG, J-PB, GG-G: provided essential resources. CV, LO, EmCo, LJ-C, MLe: performed data curation. CV, LJ-C, M-CM, EmCo, MLe, LO, MLB, CM-B: wrote original draft. All: reviewed and edited the original draft. CV, LJ-C, EmCo, MLe, MLB, M-CM: performed data visualisation. PM, MLa, HV: contributed to results interpretation and revised manuscript. M-CM: coordinated the project and has primary responsibility for final article content. All authors read, revised and approved the final manuscript.
Funding: This work was supported by (i) the Agence Nationale de la Recherche under reference ANR-11-ALID-007-01 (VALOBAB project "sustainable VALOrization of Buttermilk: nutritionAl Benefits, functional value in food products and consumer perception", coordinated by MC Michalski; endorsed by competitiveness cluster VALORIAL), (ii) the regional hospital clinical research programme (Programme Hospitalier de Recherche Clinique Interrégional 2014: VALOBAB, n°14-007) and (iii) the French Dairy Interbranch Organization (CNIEL). The Hospices Civils de Lyon was the study sponsor.
Competing interests: This work was supported in part by a grant from the French Dairy Interbranch Organization (CNIEL). M-CM has received research funding for other research projects from CNIEL, Danone-Nutricia Research, Sodiaal-Candia R&D. M-CM has consultancy activities for food, fats and oils and dairy companies. M-CM is a member of the scientific advisory board of ITERG, the Industrial Technical Centre for the oils and fats business sector. These activities had no link with the present study. BM has received research funding from Sofiprotéol with no link with the present study. PG is an employee of ACTALIA Produits Laitiers, an Agri-Food Technical Institute, with a strong specialisation in dairy research and development, and food safety. KR was an employee of ACTALIA Produits Laitiers and is now employee of Terra Lacta, a dairy cooperative. J-PB: ENILIA had research funding from dairy companies Laiterie des Fayes and Eurial. These activities had no link with the present study. GG-G has received research funding for other research projects from Savencia, Danone, Sodiaal-Candia R&D, Boccard. She has consultancy activities for dairy companies and filtration equipment providers. These activities had no link with the present study. MLa is a member of the Scientific Committee of Roquette and had research collaborations with Mondelez and Bridor. These activities had no link with the present study. The authors have no additional financial interests. Other authors declared no conflict of interest. There are no awarded or filed patents pertaining to the results presented in the paper.
Ethics approval: Scientific Ethics Committee (CPP) of Lyon Sud-Est-IV and ANSM (French Agency for the Safety of Health Products).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: According to French law on the publication of biomedical research/clinical trials, we are not allowed to make the clinical database publicly available on the web, nor send it to third parties, nor to make visible the location of the study associated with the database.
Patient consent for publication: Not required.
References
- 1. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–6. 10.1038/nature14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017;66:1414–27. 10.1136/gutjnl-2016-313099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohn JS, Kamili A, Wat E, et al. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010;2:116–27. 10.3390/nu2020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. FAO. Food and Agriculture Organization of the United Nations Gateway to dairy production and products: Milk and milk products, 2018. [Google Scholar]
- 5. Bourlieu C, Michalski MC. Structure-function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care 2015;18:118–27. 10.1097/MCO.0000000000000138 [DOI] [PubMed] [Google Scholar]
- 6. Jiménez-Flores R, Brisson G. The milk fat globule membrane as an ingredient: why, how, when? Dairy Science and Technology 2008;88:5–18. 10.1051/dst:2007005 [DOI] [Google Scholar]
- 7. Bourlieu C, Cheillan D, Blot M, et al. Polar lipid composition of bioactive dairy co-products buttermilk and butterserum: Emphasis on sphingolipid and ceramide isoforms. Food Chem 2018;240:67–74. 10.1016/j.foodchem.2017.07.091 [DOI] [PubMed] [Google Scholar]
- 8. Noh SK, Koo SI. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J Nutr 2004;134:2611–6. 10.1093/jn/134.10.2611 [DOI] [PubMed] [Google Scholar]
- 9. Eckhardt ER, Wang DQ, Donovan JM, et al. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 2002;122:948–56. 10.1053/gast.2002.32539 [DOI] [PubMed] [Google Scholar]
- 10. Lecomte M, Bourlieu C, Meugnier E, et al. Milk Polar Lipids Affect In Vitro Digestive Lipolysis and Postprandial Lipid Metabolism in Mice. J Nutr 2015;145:1770–7. 10.3945/jn.115.212068 [DOI] [PubMed] [Google Scholar]
- 11. Wat E, Tandy S, Kapera E, et al. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 2009;205:144–50. 10.1016/j.atherosclerosis.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 12. Kamili A, Wat E, Chung RW, et al. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr Metab 2010;7:90. 10.1186/1743-7075-7-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demmer E, Van Loan MD, Rivera N, et al. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J Nutr Sci 2016;5:5. 10.1017/jns.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keller S, Malarski A, Reuther C, et al. Milk phospholipid and plant sterol-dependent modulation of plasma lipids in healthy volunteers. Eur J Nutr 2013;52:1169–79. 10.1007/s00394-012-0427-0 [DOI] [PubMed] [Google Scholar]
- 15. Ohlsson L, Burling H, Nilsson A. Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis 2009;8:44. 10.1186/1476-511X-8-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiland A, Bub A, Barth SW, et al. Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men - two double-blind, randomised, controlled, clinical trials. J Nutr Sci 2016;5:5. 10.1017/jns.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tindall AM, Petersen KS, Kris-Etherton PM. Dietary Patterns Affect the Gut Microbiome-The Link to Risk of Cardiometabolic Diseases. J Nutr 2018;148:1402–7. 10.1093/jn/nxy141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez-Guryn K, Hubert N, Frazier K, et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018;23:458–69. 10.1016/j.chom.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cuevas-Tena M, Bermúdez JD, Silvestre RLÁ, et al. Impact of colonic fermentation on sterols after the intake of a plant sterol-enriched beverage: a randomized, double-blind crossover trial. Clin Nutr 2018 10.1016/j.clnu.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 21. Stampfer MJ, Colditz GA, Willett WC. Menopause and heart disease. A review. Ann N Y Acad Sci 1990;592:193–203. discussion 57-62. [DOI] [PubMed] [Google Scholar]
- 22. Grundy SM. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993;269:3015–23. 10.1001/jama.1993.03500230097036 [DOI] [PubMed] [Google Scholar]
- 23. Marchi R, Dell’Agnolo CM, Lopes TCR, et al. Prevalence of metabolic syndrome in pre- and postmenopausal women. Arch Endocrinol Metab 2017;61:160–6. 10.1590/2359-3997000000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perk J, De Backer G, Gohlke H, et al. European Association for Cardiovascular Prevention & Rehabilitation (EACPR) ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–701. 10.1093/eurheartj/ehs092 [DOI] [PubMed] [Google Scholar]
- 25. Gassi JY, Blot M, Beaucher E, et al. Preparation and characterisation of a milk polar lipids enriched ingredient from fresh industrial liquid butter serum: Combination of physico-chemical modifications and technological treatments. Int Dairy J 2016;52:26–34. 10.1016/j.idairyj.2015.08.012 [DOI] [Google Scholar]
- 26. Conway V, Couture P, Richard C, et al. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr Metab Cardiovasc Dis 2013;23:1255–62. 10.1016/j.numecd.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 27. Telle-Hansen VH, Holven KB, Ulven SM. Impact of a Healthy Dietary Pattern on Gut Microbiota and Systemic Inflammation in Humans. Nutrients 2018;10:1783. 10.3390/nu10111783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salonen A, de Vos WM. Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol 2014;5:239–62. 10.1146/annurev-food-030212-182554 [DOI] [PubMed] [Google Scholar]
- 29. Ohlsson L, Hertervig E, Jönsson BA, et al. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. Am J Clin Nutr 2010;91:672–8. 10.3945/ajcn.2009.28311 [DOI] [PubMed] [Google Scholar]
- 30. Lopez C, Madec M-N, Jimenez-Flores R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem 2010;120:22–33. 10.1016/j.foodchem.2009.09.065 [DOI] [Google Scholar]
- 31. Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control and Prevention American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 32. McQueen MJ, Hawken S, Wang X, et al. INTERHEART study investigators. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 2008;372:224–33. 10.1016/S0140-6736(08)61076-4 [DOI] [PubMed] [Google Scholar]
- 33. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 34. Ference BA. Causal effect of lipids and lipoproteins on atherosclerosis. Cardiol Clin 2018;36:203–11. 10.1016/j.ccl.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 35. Cariou B, Le May C, Costet P. Clinical aspects of PCSK9. Atherosclerosis 2011;216:258–65. 10.1016/j.atherosclerosis.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 36. Ramprasath VR, Jones PJ, Buckley DD, et al. Effect of dietary sphingomyelin on absorption and fractional synthetic rate of cholesterol and serum lipid profile in humans. Lipids Health Dis 2013;12:125. 10.1186/1476-511X-12-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumgartner S, Kelly ER, van der Made S, et al. The influence of consuming an egg or an egg-yolk buttermilk drink for 12 wk on serum lipids, inflammation, and liver function markers in human volunteers. Nutrition 2013;29:1237–44. 10.1016/j.nut.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 38. Rosqvist F, Smedman A, Lindmark-Månsson H, et al. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am J Clin Nutr 2015;102:20–30. 10.3945/ajcn.115.107045 [DOI] [PubMed] [Google Scholar]
- 39. Morifuji M, Higashi S, Oba C, et al. Milk Phospholipids Enhance Lymphatic Absorption of Dietary Sphingomyelin in Lymph-Cannulated Rats. Lipids 2015;50:987–96. 10.1007/s11745-015-4054-4 [DOI] [PubMed] [Google Scholar]
- 40. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9. 10.1161/01.ATV.12.8.911 [DOI] [PubMed] [Google Scholar]
- 41. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets 2009;10:328–35. 10.2174/138945009787846434 [DOI] [PubMed] [Google Scholar]
- 42. Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–61. 10.1093/eurheartj/ehr112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walldius G, Jungner I, Holme I, et al. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 2001;358:2026–33. 10.1016/S0140-6736(01)07098-2 [DOI] [PubMed] [Google Scholar]
- 44. Tomkin GH, Owens D. The chylomicron: relationship to atherosclerosis. Int J Vasc Med 2012;2012:1–13. 10.1155/2012/784536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohlsson L, Burling H, Duan RD, et al. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur J Clin Nutr 2010;64:1344–9. 10.1038/ejcn.2010.164 [DOI] [PubMed] [Google Scholar]
- 46. Gérard P. Metabolism of Cholesterol and Bile Acids by the Gut Microbiota. Pathogens 2014;3:14–24. 10.3390/pathogens3010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosele JI, Gosalbes MJ, Macià A, et al. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol Nutr Food Res 2015;59:1942–53. 10.1002/mnfr.201500227 [DOI] [PubMed] [Google Scholar]
- 48. Cuevas-Tena M, Alegría A, Lagarda MJ. Determination of Fecal Sterols Following a Diet with and without Plant Sterols. Lipids 2017;52:871–84. 10.1007/s11745-017-4286-6 [DOI] [PubMed] [Google Scholar]
- 49. Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med 2019;216:20–40. 10.1084/jem.20180448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013;54:2325–40. 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lukovac S, Belzer C, Pellis L, et al. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio 2014;5 10.1128/mBio.01438-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Norris GH, Jiang C, Ryan J, et al. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J Nutr Biochem 2016;30:93–101. 10.1016/j.jnutbio.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 53. Milard M, Laugerette F, Durand A, et al. Milk Polar Lipids in a High-Fat Diet Can Prevent Body Weight Gain: Modulated Abundance of Gut Bacteria in Relation with Fecal Loss of Specific Fatty Acids. Mol Nutr Food Res 2019;63:e1801078 10.1002/mnfr.201801078 [DOI] [PubMed] [Google Scholar]
- 54. Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–103. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kriaa A, Bourgin M, Potiron A, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res 2019;60:323–32. 10.1194/jlr.R088989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lia A, Andersson H, Mekki N, et al. Postprandial lipemia in relation to sterol and fat excretion in ileostomy subjects given oat-bran and wheat test meals. Am J Clin Nutr 1997;66:357–65. 10.1093/ajcn/66.2.357 [DOI] [PubMed] [Google Scholar]
- 57. Nyberg L, Duan RD, Nilsson A. A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J Nutr Biochem 2000;11:244–9. 10.1016/S0955-2863(00)00069-3 [DOI] [PubMed] [Google Scholar]
- 58. Beil FU, Grundy SM. Studies on plasma lipoproteins during absorption of exogenous lecithin in man. J Lipid Res 1980;21:525–36. [PubMed] [Google Scholar]
- 59. Le May C, Berger JM, Lespine A, et al. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler Thromb Vasc Biol 2013;33:1484–93. 10.1161/ATVBAHA.112.300263 [DOI] [PubMed] [Google Scholar]
- 60. Jakulj L, van Dijk TH, de Boer JF, et al. Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion. Cell Metab 2016;24:783–94. 10.1016/j.cmet.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 61. Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–333. 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
- 62. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–61. 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 63. EAS. European Atherosclerosis Society: Commentary on Phytosterol-added Foods Focus on lifestyle: EAS Consensus Panel Position Statement on Phytosterol-added Foods, 2015. [DOI] [PubMed] [Google Scholar]
- 64. Drouin-Chartier JP, Côté JA, Labonté MÈ, et al. Comprehensive Review of the Impact of Dairy Foods and Dairy Fat on Cardiometabolic Risk. Adv Nutr 2016;7:1041–51. 10.3945/an.115.011619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thorning TK, Bertram HC, Bonjour JP, et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr 2017;105:1033–45. 10.3945/ajcn.116.151548 [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization W. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. Geneva: WHO, 2008. [Google Scholar]
- 67. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–80. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 68. National Cholesterol Education Program (NCEP). Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2018-318155supp001.pdf (422.1KB, pdf)
gutjnl-2018-318155supp002.pdf (411.7KB, pdf)
gutjnl-2018-318155supp003.pdf (175.5KB, pdf)