Abstract
Objectives:
The aim of this study was to determine the evaluation and management of dysphagia in amyotrophic lateral sclerosis (ALS) patients by speech-language pathologists (SLPs).
Methods:
A 15-question web-based survey sent to SLPs in general clinical practice.
Results:
Forty-nine SLPs responded. Although only 8 (17.0%) of the SLPs worked in ALS clinics, 46 (93.9%) had worked with ALS patients. A variety of dysphagia evaluation protocols were used by 43 (97.7%) SLPs. Most SLPs, 40 (88.9%), recommended instrumental assessments, but timing and indication varied greatly: 19 (42.2%) SLPs recommended this at baseline even without bulbar symptoms, whereas others recommended this based on symptoms and/or clinical assessments.
Conclusions:
There is currently no uniform approach as to the indication, timing, and specific methods to use in the evaluation of dysphagia in ALS patients among SLPs. There is need for further research to assist in the development of definitive guideline recommendations for this population.
Key Words: amyotrophic lateral sclerosis, dysphagia, speech-language pathologists, instrumental evaluation of swallowing, clinical swallowing assessment
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting the upper and lower motor neurons innervating limb, trunk, respiratory, and bulbar muscles. In approximately one third of the patients, the bulbar muscles are affected first and the initial symptoms consist of dysarthria and dysphagia. Two thirds of the patients have spinal onset ALS in which limb muscle weakness is the initial symptom. Respiratory muscle weakness can rarely be the initial presenting symptom.1 Most patients die of respiratory failure on average 2–4 years after symptom onset. Bronchopneumonia followed by aspiration pneumonia are the leading causes of death.2,3
Swallowing is a complex act that involves oral, pharyngeal, and esophageal stages and requires multiple elements to be effective; ALS patients may have a combination of spastic and flaccid weakness affecting their swallowing caused by degeneration of cortical motor neurons, corticobulbar tracts, and brainstem nuclei.4 The loss of supranuclear innervation to the brainstem nuclei results in spasticity of the jaw, facial, palatal, pharyngeal, laryngeal, and tongue muscles whereas degeneration of the brainstem nuclei causes flaccid paralysis in these muscles. The characteristic symptoms resulting from the combination of upper and lower motor neuron dysfunction include fatigue when chewing, leakage of food or liquid out of the mouth, nasal regurgitation, prolonged duration while swallowing, inability to clear the oral cavity, and coughing or choking when swallowing.5–7 In a typical ALS patient, both mechanisms are likely contributing the dysphagia and dysarthria.
Dysphagia is very frequent in ALS, affecting approximately 85% of patients.8 Bulbar onset ALS is more common in older individuals compared with younger individuals diagnosed with ALS.9 It may lead to complications such as malnutrition, dehydration, aspiration pneumonia, respiratory failure, and reduced quality of life and social isolation.4,10 Dysphagia and aspiration are confirmed by instrumental assessments such as modified barium swallow (MBS) and fiberoptic endoscopic evaluation of swallowing (FEES).4 Guidelines recommend for speech-language pathologists (SLPs) to evaluate and manage bulbar symptoms in ALS patients; and studies have shown that earlier intervention and monitoring improve dysphagia outcomes in ALS patients whose care is provided by a multidisciplinary team that includes a neurologist and SLP.2,11,12 To effectively diagnose and treat swallowing dysfunction in an ALS patient in a multidisciplinary team, a neurologist has to understand the current clinical practices of SLPs.
ALS guidelines mostly focus on gastrostomy tube (percutaneous endoscopic gastrostomy) placement,13 and less on the specifics of how to evaluate and manage dysphagia. Although there are ongoing attempts at achieving definitive consensus-based guidelines for the evaluations of bulbar dysfunction in ALS, however, limited data support existing recommendations and many questions remain unanswered, in particular the role, timing, and use of instrumental methods in the evaluation of dysphagia in ALS.14,15 A previous survey of clinicians—primarily neurologists—reported multiple varied approaches to dysphagia evaluation even in a group of specialized ALS centers.16 Currently, the most commonly validated tools used to diagnose or screen for dysphagia in ALS include videofluoroscopic swallowing examination, Eating Assessment Tool-10, Swallow Test, and Voluntary Cough. Unfortunately, the assessments that are often used to track progression are less sensitive to detect mild changes in swallowing dysfunction.17
There is a need to determine the clinical approach and the tools to assess dysphagia used by SLPs active in multiple clinical settings to aid in further guideline development and to improve the care of these patients across a variety of care settings, including community neurology practices and specialized ALS centers. Therefore, the aim of this study was to evaluate the current state of practice in the evaluation and management of dysphagia in ALS patients among a broad group of SLPs active in clinical practice. We hypothesized that there would be significant variation in the dysphagia methods and instrumental assessment used by SLPs.
MATERIAL AND METHODS
This study was approved by the University of Maryland Institutional Review Board. A 15-question anonymous survey was conducted via SurveyMonkey (www.surveymonkey.com) and distributed to 101 SLPs active in clinical practice in the United States from the following groups: (1) SLPs who attended the ALS Association Clinical Conference in Phoenix, AZ, USA, November 2014; (2) SLP members of the American Speech-Language-Hearing Association; and (3) SLP members of the rehabilitation department at hospitals associated with the University of Maryland Medical System. Previous or current work with ALS patients was not required for participation. Respondents were not required to answer every question.
The survey queried each SLP's current clinical practice or perceived approach for the evaluation and management of dysphagia in ALS patients. Questions included binary yes/no answers, short answers, and open-ended answers. The survey was developed by 2 SLPs and a neurologist who had many years of experience treating ALS patients based on the most common techniques used in clinical practice to evaluate and manage patients with dysphagia. Questions included locations of practice and years of experience with ALS patients, dysphagia protocols and clinical evaluations, use of instrumental assessment of dysphagia, and gastrostomy tube placement. Data were collected between April 2016 and July 2017. Descriptive statistics with ratios and percentages were used to report the survey data. An aggregate report was generated, including the ratios and percentages of the answers to the survey's questions and a list of answers for the open-ended questions (4 and 6). The answers to the open-ended questions were grouped by common themes into categories based on what was considered to be the perceived intention of the respondent SLP after discussion among 3 of the authors (D.E., T.T., and M.D.-A.). Decisions on groupings were made by consensus.
RESULTS
Forty-nine SLPs answered the survey; the number of SLPs answering each question ranged from 44 to 49. Participants from 24 states in the United States answered the survey. Table 1 describes all the answers except for questions 4 and 6. Although 8 (17%) of the respondents worked in ALS clinics as their primary work setting, 46 (93.9%) SLPs had worked with ALS patients, and 26 (56.5%) had worked with ALS patients 6 years or longer.
TABLE 1.
Survey questions and answers


A variety of protocols were used in the initial evaluation of dysphagia in patients with ALS by 43 (97.7%) SLPs -question 4. This typically included a clinical evaluation ranging from a screening questionnaire and patient interview, to a simple bedside swallowing screening, to a clinical swallowing assessment [most commonly used, 20 (45.5%)], to instrumental assessments, 11 (25%); and several combinations together or in sequence. Instrumental assessments when specifically mentioned were much more frequently an MBS than an FEES (Fig. 1) (see Text, Supplemental Digital Content 1, http://links.lww.com/JCND/A34, which provides the individual answers from the 44 SLPs respondents to survey question 4). When both clinical and instrumental assessments were performed, the clinical assessments preceded the instrumental assessments.
FIGURE 1.
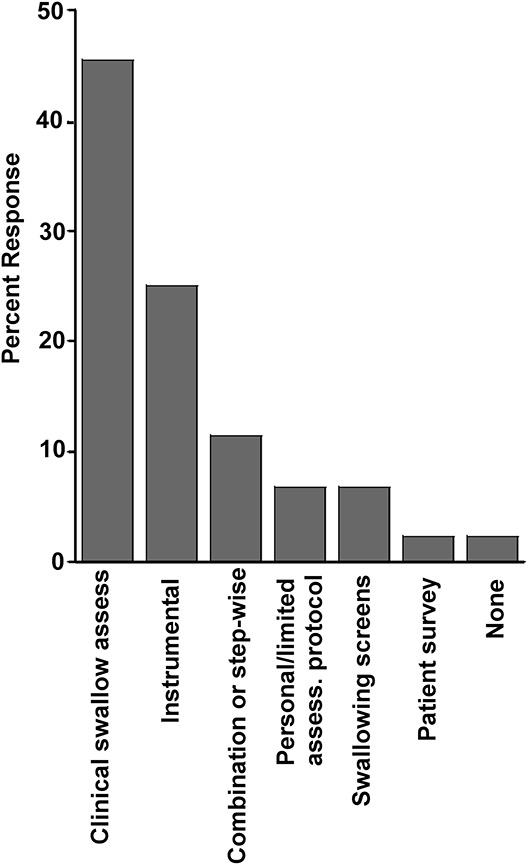
SLPs' answers to question 4, n = 44: What dysphagia protocols do/would you use initially in the assessment of patients with ALS? Clinical swallowing assessment: 20 (45.5%). Instrumental: 11 (25%). Combination or step-wise: 5 (11.4%) personal/limited assessment protocol: 3 (6.8%). Swallowing screens (Yale Swallow Protocol): 3 (6.8%). Patient survey (EAT-10): 1 (2.3%). None: 1 (2.3%). Instrumental assessments presumed to be done in combination with some clinical assessment.
Most SLPs—40 (88.9%)—would perform or recommend instrumental dysphagia assessments, but indication and timing varied widely: 19 (42.2%) of the SLPs would perform an instrumental assessment in ALS patients even without bulbar symptoms at the baseline evaluation. Other SLPs recommended testing as needed, depending on the clinical evaluation and/or symptoms (Fig 2) (see Text, Supplemental Digital Content 2, http://links.lww.com/JCND/A35 which provides the individual answers from the 45 SLPs respondents to survey question 6).
FIGURE 2.
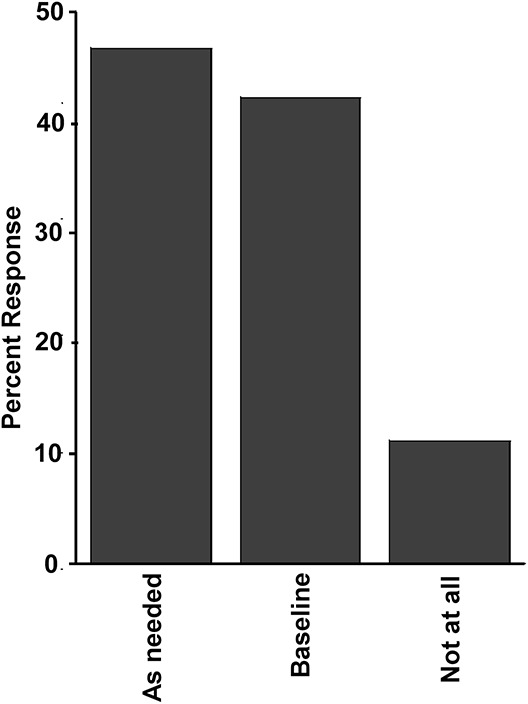
SLPs' answers to question 6, n = 45: How soon do/would you recommend a FEES or MBS after your initial assessment? 45 responses: As needed/varied/clinical findings/symptoms: 21 (46.7%). Baseline: 19 (42.2%). Not at all: 5 (11.1%).
Most SLPs, 41 (85.4%), would not use oral motor (laryngeal) strengthening exercises, and most, 45 (93.8%), would also not use transcutaneous neuromuscular electrical stimulation for dysphagia management in ALS. Most SLPs, 42 (87.5%), also had the opportunity to work collaboratively with a nutritionist and were comfortable recommending gastrostomy tube placement, although 27 (56.3%) would initiate the conversation after first discussing with a neurologist. Almost all respondent SLPs, 47 (97.9%), would provide hand-outs on safe swallowing strategies to the patient with ALS and their families.
DISCUSSION
The major findings of this survey of the clinical practice of SLPs for the evaluation and management of dysphagia in patients with ALS were: (1) although only a minority of SLPs practiced in specialized ALS centers, almost all had treated ALS patients; (2) a wide variety of dysphagia protocols were used by SLPs for the evaluation of dysphagia in ALS; (3) most SLPs recommended evaluation with an MBS or FEES at some point, but timing and indication varied greatly, and (4) most SLPs felt comfortable recommending gastrostomy tube placement to their patients.
Although the goal of this study is not to develop a guideline to assess or manage dysphagia, the lack of uniformity in the approach to evaluate dysphagia emphasizes the importance of a validated dysphagia assessment guideline in ALS. The variation in the dysphagia clinical assessments and instruments used in SLP clinical practices is concerning, given the high prevalence of this symptom in ALS patients, affecting 85% of patients.8 With dysphagia increasing the risk of aspiration and leading to dehydration and malnutrition,4 and with pneumonia and aspiration pneumonia being the leading causes of death in ALS,3 establishing a standardized approach to dysphagia evaluation and management is very important in ALS care across all care settings.
General ALS guidelines address dysphagia with the major focus on the placement of gastrostomy tubes. Although evaluation of dysphagia by an SLP is recommended as part of multidisciplinary ALS care, no specific recommendations on timing and method of evaluation are consistently described across the major general guidelines.2,11,13,18 In addition, there is no consistent approach to the evaluation of dysphagia, even among specialized ALS centers.16 More recently, there have been ongoing attempts at achieving consensus-based recommendations with provisional guidelines reported for the evaluations of bulbar dysfunction in ALS. However, limited data support the recommendations and many questions remain unanswered, in particular the role, timing, and use of instrumental methods in the evaluation of dysphagia in ALS.14,15
The current survey included 49 SLPs. Although only 8 (17.0%) of the respondents worked in ALS clinics, 46 (93.9%) SLPs reported having treated patients with ALS and 26 (56.5%) had worked with ALS patients 6 years or longer, reflecting a common occurrence of ALS patients being treated by SLPs outside multidisciplinary specialized ALS centers. The only previous survey that evaluated practice patterns for bulbar function assessment in ALS included only specialized ALS centers; one clinician per site (n = 38) responded, but only 4 respondents were confirmed as SLPs, with most respondents being neurologists.16
In the current survey, 40 (88.9%) of the SLPs would refer patients for an MBS or FEES; 19 (42.2%) would do a baseline study despite the absence of bulbar symptoms, whereas the rest would do an MBS or FEES depending on symptoms and/or the clinical evaluation findings. Most SLPs, 43 (97.7%), would perform some type of clinical dysphagia evaluation even without bulbar symptoms, although which type of evaluation varied widely. In the previous referenced survey of specialized ALS centers, 44% of respondents never referred patients for MBS, and 45% did not perform a basic clinical swallow test.16 Comprehensive and serial bedside clinical swallowing evaluation has been recommended for ALS patients.7
Clinical and instrumental assessments have use beyond the sole indication of gastrostomy placement, because they also help guide and educate patients and caregivers on aspiration risks and compensatory techniques such as diet modification.7,18–20 The optimal timing of instrumental assessments of dysphagia in ALS has not been determined. In one study, 8% of patients without bulbar symptoms had abnormal FEES, and over time there was increased prevalence and worsening of dysphagia with increased penetration–aspiration scores, arguing for the performance of serial evaluations with FEES in ALS patients.8 Two studies21,22 that performed MBS at the initial visit showed that up to half of ALS patients without bulbar symptoms had impaired bolus transport to the pharynx and postswallow pharyngeal residue, a risk factor for later aspiration events.23 Because of the progressive worsening of dysphagia and increasing aspiration on serial MBS, Higo et al22 recommended that an MBS be performed 6 and 12 months after bulbar symptom onset.
All SLPs in the current survey would provide consultative information for ALS patients even if no dysphagia was present, and almost all would provide hand-outs on safe swallowing strategies. This likely reflects the high awareness of SLPs of the potential for development of dysphagia in ALS. Most SLPs in the current survey—42 (87.5%)—had the opportunity to work collaboratively with a nutritionist, as recommended in ALS guidelines.2,11,13,18
Almost all SLPs—45 (93.8%)—felt comfortable recommending a gastrostomy tube to their patients, although most would discuss first with a neurologist before initiating the discussion with the patient. This emphasizes the importance of a close collaborative relationship between neurologists and SLPs for the optimal care of ALS patients. Enteral nutrition via gastrostomy improves nutrition and may improve survival and should be considered with impaired oral intake to stabilize weight.18,24 In addition, continued weight loss also has the potential to interfere with tolerance of noninvasive ventilation as changes in face contour can lead to improper mask fitting and increased mask air leakage.25
Of some concern is that guidelines on their own may sometimes not be enough to lead to consistent improvements in clinical practice, begetting the need for continued discussion and dissemination of information across multiple clinical practice settings. One such example is that despite several guidelines across many years addressing gastrostomy indications,2,11,18 gastrostomy is underused in ALS,13 and large variations of gastrostomy insertion rates in ALS patients persist among different centers within individual countries and between different countries: Japan (29%–58%), United States (8%–43%), and European Union (6%–45%).23 This variation in practice may be related to multiple factors, one of them possibly clinician preference.26 Neurologists have a unique role in guiding the evaluation and management of dysphagia through interactions with SLPs by identifying the mechanism of dysphagia. This can in turn inform implementation of appropriate diagnostic studies and treatment in a multidisciplinary context to determine the diagnosis and prognosis of the bulbar dysfunction.27 This study provides more evidence of the importance of close working relationships across multiple disciplines, such as neurologists being aware of the potential variations of care in the evaluation of dysphagia in ALS patients to better coordinate care with SLPs and nutritionists to achieve the best possible outcomes.
In treatment, only 7 (14.6%) of the SLPs would use oral motor (laryngeal) strengthening exercises and only 3 (6.2%) would use transcutaneous neuromuscular electrical stimulation (VitalStim Therapy System, Chattanooga Group, Hixson, TN)28 as part of their dysphagia treatment strategy. Although continuing to swallow—even if limited and with compensatory techniques—is the best exercise to maintain swallowing ability,4 it is unclear whether the above-mentioned techniques would be useful in managing dysphagia in ALS, as intensive exercises may exhaust weak swallowing muscles. This may explain why only a minority of SLPs used these techniques. Further research is needed to support implementation of these techniques in ALS patients.4,7,28
It is not entirely clear why there were significant discrepancies in use of clinical assessments and dysphagia instruments for the evaluations of dysphagia between this survey group and the previous referenced survey,16 with the current survey showing much more frequent use of clinical bedside and instrumental dysphagia assessments. It may be that clinicians who work in specialized ALS centers practice differently given more familiarity with disease progression and outcomes. This may also be because of the availability of complementary measurements in these centers which are not available in other settings, such as the ALS Functional Rating Scale—Revised—which may serve as a very basic bulbar symptom screen because of its bulbar subcomponent-,29 and pulmonary function tests, etc.16 Alternatively, some of the disparate findings may be related to most respondents in the first survey being neurologists as opposed to SLPs in this survey.
The current survey complements the findings of the previous survey in several ways: (1) having additional questions and concentrating on dysphagia; (2) focusing only on the clinical practice of SLPs, and (3) including SLPs from a variety of general care settings. Although only a minority of SLPs worked primarily in ALS centers, most had treated ALS patients. This points to the need for broadening the dissemination of future acquired knowledge and guidelines to care settings beyond the confines of the ALS community and specialized multidisciplinary ALS centers, as patients frequently obtain care outside these centers.
There are several limitations to this study. This is a one-time survey based in the United States with a small sample size of respondents from 24/50 states, with a quarter of respondents from one state, and as such findings may not be generalizable to other areas. In addition, it consisted of only 15 questions, which limited the amount of information able to be obtained. Two questions were open-ended, which made precise analysis difficult; however, the wide range of answers provided offered a clear window of how varied were SLPs' use of clinical assessments and instruments for dysphagia evaluation in these patients, and thus, all answers are included in the supplemental digital content for further reference. There are currently wide gaps in the focus of research in multiple areas of dysphagia in ALS, with most of the emphasis on the aspiration–penetration safety aspect and more limited attention on swallowing efficiency and on compensatory and rehabilitation techniques, although research in these later areas has been expanding and evolving over time.30
In conclusion, SLPs treat ALS patients across a variety of care settings and are aware of the need to provide patient education to avoid complications associated with dysphagia. There is agreement among SLPs that clinical assessment/screening for dysphagia in ALS patients is recommended in the absence of bulbar symptoms; however, there is great variation in the type of assessment used. Most SLPs also agree with the need to perform objective instrumental assessments to evaluate dysphagia, but the timing and indication of these instrumental assessments is not uniform. Neurologist can best guide dysphagia management within multidisciplinary models in collaboration with SLPs where professionals can communicate efficiently through enhanced coordination of care. More research is needed to determine the best way to evaluate and manage dysphagia in ALS patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the ALS Association and the MD/DC/VA chapter for the continued support of the University of Maryland ALS Center of Excellence and our patients.
The authors would also like to thank Gregg Davis for assistance with manuscript preparation.
Footnotes
J. W. Russell is supported in part by Department of Veterans Affairs (Biomedical and Laboratory Research Service and Rehabilitation Research and Development, 101RX001030), Baltimore Geriatric Research, Education, and Clinical Center (GRECC), NIH NIDDK 1R01DK107007-01A1.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcnmd.com).
REFERENCES
- 1.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—revised report of an EFNS task force. Eur J Neurol. 2012;19:360–375. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt C, Neuwirth C, Sommacal A, et al. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS One. 2017;12:e0177555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton D, Karam C, Schindler JS. Swallowing and secretion management in neuromuscular disease. Clin Chest Med. 2018;39:449–457. [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer RF. Neurogenic dysphagia. In: Daroff RB, Fenichel GM, Jankovic J, et al., eds. Bradley's Neurology in Clinical Practice. Philadelphia, PA: Elsevier Saunders; 2012:153–163. [Google Scholar]
- 6.Luchesi KF, Kitamura S, Mourão LF. Management of dysphagia in Parkinson's disease and amyotrophic lateral sclerosis. Codas. 2013;25:358–364. [DOI] [PubMed] [Google Scholar]
- 7.Kühnlein P, Gdynia HJ, Sperfeld AD, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4:366–374. [DOI] [PubMed] [Google Scholar]
- 8.Onesti E, Schettino I, Gori MC, et al. Dysphagia in amyotrophic lateral sclerosis: impact on patient behavior, diet adaptation, and riluzole management. Front Neurol. 2017;8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–719. [DOI] [PubMed] [Google Scholar]
- 10.Paris G, Martinaud O, Petit A, et al. Oropharyngeal dysphagia in amyotrophic lateral sclerosis alters quality of life. J Oral Rehabil. 2013;40:199–204. [DOI] [PubMed] [Google Scholar]
- 11.National Clinical Guideline Centre (UK). Motor Neurone Disease: Assessment and Management. National Institute for Health and Care Excellence: Clinical Guidelines website; 2016. Available at: https://www.nice.org.uk/guidance/ng42/resources/motor-neurone-disease-assessment-and-management-pdf-1837449470149. Accessed May 10, 2019. [PubMed] [Google Scholar]
- 12.Messing BP, Ward EC, Lazarus C, et al. Establishing a multidisciplinary head and neck clinical pathway: an implementation evaluation and audit of dysphagia-related services and outcomes. Dysphagia. 2019;34:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens AI, Ruytings M, Al-Chalabi A, et al. ; ALS-CARE Consortium. A mapping review of international guidance on the management and care of amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:325–336. [DOI] [PubMed] [Google Scholar]
- 14.Pattee GL, Plowman EK, Brooks BR, et al. ; Contributing Members of the NEALS Bulbar Subcommittee. Best practices protocol for the evaluation of bulbar dysfunction: summary recommendations from the NEALS bulbar subcommittee symposium. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:311–312. [DOI] [PubMed] [Google Scholar]
- 15.Pattee GL, Plowman EK, Focht Garand KL, et al. Contributing Members of the NEALS Bulbar Subcommittee. Provisional best practices guidelines for the evaluation of bulbar dysfunction in amyotrophic lateral sclerosis. Muscle Nerve. 2019;59:531–536. [DOI] [PubMed] [Google Scholar]
- 16.Plowman EK, Tabor LC, Wymer J, et al. The evaluation of bulbar dysfunction in amyotrophic lateral sclerosis: survey of clinical practice patterns in the United States. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yunusova Y, Plowman EK, Green JR, et al. Clinical measures of bulbar dysfunction in ALS. Front Neurol. 2019;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RG, Jackson CE, Kasarskis EJ, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solazzo A, Del Vecchio L, Reginelli A, et al. Search for compensation postures with videofluoromanometric investigation in dysphagic patients affected by amyotrophic lateral sclerosis. Radiol Med. 2011;116:1083–1094. [DOI] [PubMed] [Google Scholar]
- 20.Conde B, Martins N, Rodrigues I, et al. Functional and endoscopic indicators for percutaneous endoscopic gastrostomy (PEG) in amyotrophic lateral sclerosis patients. J Clin Med. 2018;7:pii: E352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murono S, Hamaguchi T, Yoshida H, et al. Evaluation of dysphagia at the initial diagnosis of amyotrophic lateral sclerosis. Auris Nasus Larynx. 2015;42:213–217. [DOI] [PubMed] [Google Scholar]
- 22.Higo R, Tayama N, Nito T. Longitudinal analysis of progression of dysphagia in amyotrophic lateral sclerosis. Auris Nasus Larynx. 2004;31:247–254. [DOI] [PubMed] [Google Scholar]
- 23.Takei K, Tsuda K, Takahashi F, et al. An assessment of treatment guidelines, clinical practices, demographics, and progression of disease among patients with amyotrophic lateral sclerosis in Japan, the United States, and Europe. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:88–97. [DOI] [PubMed] [Google Scholar]
- 24.Katzberg HD, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2011:CD004030. 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Abad M, Brown JE. Use of volume-targeted non-invasive bilevel positive airway pressure ventilation in a patient with amyotrophic lateral sclerosis. J Bras Pneumol. 2014;40:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325:961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes TA, Wiles CM. Neurogenic dysphagia: the role of the neurologist. J Neurol Neurosurg Psychiatry. 1998;64:569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark H, Lazarus C, Arvedson J, et al. Evidence-based systematic review: effects of neuromuscular electrical stimulation on swallowing and neural activation. Am J Speech Lang Pathol. 2009;18:361–375. [DOI] [PubMed] [Google Scholar]
- 29.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 30.Waito AA, Valenzano TJ, Peladeau-Pigeon M, et al. Trends in research literature describing dysphagia in motor neuron diseases (MND): a scoping review. Dysphagia. 2017;32:734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


