Abstract
Adrenal vein sampling (AVS) is required to distinguish unilateral (U) from bilateral (B) aldosterone sources in primary aldosteronism (PA), and cortisol is used for AVS data interpretation, but cortisol has several pitfalls. In this study, we present the utility of several other steroids in PA subtyping, both during AVS, as well as in peripheral serum. We included PA patients who underwent AVS at University of Michigan between 2012–2018. We used mass spectrometry to simultaneously quantify 17 steroids in adrenal veins (AV) and periphery, both at baseline and after cosyntropin administration. PA was classified as unilateral (U) or bilateral (B) based on a lateralization index (LI) ≥ or < 4, respectively, separately for baseline and post-cosyntropin administration. Of 131 participants, AV catheterizations was deemed failed in 28 (21 %) patients (36 AVs) at baseline. Eight steroids demonstrated higher AV/periphery ratios than cortisol (p < 0.01 for all); 11β-hydroxyandrostenedione (11OHA4), 11-deoxycortisol (11dF) and corticosterone rescued most failed baseline catheterizations. Lateralization was generally consistent when using these alternative steroids. Based on pre- and post-cosyntropin data, the remaining 103 patients were classified as: U/U, 37; B/B, 32; U/B, 20; B/U, 14. Discriminant analysis of multi-steroid panels from peripheral serum showed distinct profiles across the four groups, with highest aldosterone, 18-oxocortisol and 11-deoxycorticosterone in U/U patients. In conclusion, 11OHA4 and 11dF are superior to cortisol for AVS data interpretation. Single-assay multi-steroid panels measured in peripheral serum are helpful in stratified PA subtyping and have the potential to circumvent AVS in a subset of PA patients.
Keywords: primary aldosteronism, adrenal vein sampling, hypertension, renin, aldosterone
Graphical Abstract
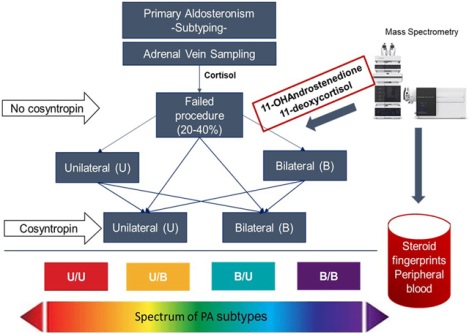
Introduction
Primary aldosteronism (PA) is traditionally subtyped into unilateral forms, most commonly aldosterone producing adenoma (APA), or bilateral hyperaldosteronism (BHA).1 Accurate PA subtyping is essential for guiding clinical management. Unilateral PA can be cured or improved by unilateral adrenalectomy, while BHA requires life-long medical therapy.1 Adrenal vein sampling (AVS) is recommended by expert guidelines for PA subtyping.1,2 AVS protocols and criteria for data interpretation have varied between referral centers, leading to heterogeneity in selecting PA surgical candidates.3,4
Cortisol is used in all steps of AVS results interpretation. Disadvantages of using cortisol include longer half-life relative to aldosterone and fluctuations during the procedure, particularly important in the absence of cosyntropin stimulation. Furthermore, mild autonomous cortisol excess is relatively common in patients with PA,5,6 which can lead to cortisol suppression in the contralateral adrenal gland and alter AVS results. Recent studies have proposed alternative biomarkers for AVS data interpretation, including metanephrines, androstenedione (A4), dehydroepiandrosterone (DHEA), 17α-hydroxyprogesterone (17OHP), and 11-deoxycortisol (11dF).7–11 A4, DHEA and 17OHP, however, are also produced by the gonads, and the latter has cyclical variations in reproductive age women. In contrast, 11β-hydroxyandrostenedione (11OHA4) is produced primarily and abundantly in the adrenal glands,12,13 and we therefore hypothesized that 11OHA4 could be a valuable biomarker for adrenal vein catheterization.
Beyond the variability in protocols and data interpretation among expert centers, additional AVS drawbacks, including its high cost, scarce availability and technical challenges, have recently driven efforts to develop non-invasive PA subtyping methods, such as steroid biomarkers measured in peripheral serum.8,14 Peripheral 18-oxocortisol (18oxoF) and 18-hydroxycortisol (18OHF) have been shown to perform well in identifying APAs in Asian patients, who have a high prevalence of KCNJ5 mutations.14 The utility of these hybrid steroids was, however, poor when used alone in Europeans,8 who display a variety of somatic aldosterone-driver mutations.15 In such populations, multi-steroid panels hold more promise in PA subtyping based on peripheral blood tests.8 Herein, we present the performance of a broad spectrum of steroid biomarkers in AVS data interpretation and PA subtyping.
Patients and Methods
Study Participants
We included patients who underwent AVS at the University of Michigan for PA subtyping between April, 2012 and July, 2018. The diagnosis of PA was ascertained in accordance with the Endocrine Society guidelines.1 Additional inclusion criteria comprised: written informed consent from participants, and available serum left after completion of clinical assays. The study was conducted with University of Michigan Institutional Review Boards approval. The data that support the findings of this study are available from the corresponding author upon reasonable request.
AVS- Protocol and Data Interpretation
Simultaneous AVS was performed prior to and after 0.25 mg cosyntropin administration, as previously described.16 Samples were first stored at 4 °C for clinical assays and subsequently frozen at −80 °C until used for liquid chromatography-tandem mass spectrometry (LC-MS/MS) steroid measurement. Catheterization was considered successful when the selectivity index (SI=AV/IVC cortisol concentrations) was ≥ 2 prior to and ≥ 5 after cosyntropin administration, respectively. PA was classified as unilateral (U) or bilateral (B) based on a lateralization index (LI = aldosterone/cortisol ratio between the dominant and contralateral AVs) was ≥ or < 4, respectively, separately for pre- and post-cosyntropin administration. A contralateral index (CI), defined as (aldosterone/cortisol)non-dominant AV / (aldosterone/cortisol)IVC < 1 defined contralateral suppression. Clinical laboratory cortisol, aldosterone and plasma renin activity were measured with immunoassays, as previously reported.16
Steroid Quantification by Liquid Chromatography-Tandem Mass Spectrometry
We used LC-MS/MS to quantify 17 Δ4 steroids in a single assay, including: aldosterone, 18OHF, 18oxoF, 11-deoxycorticosterone (DOC), corticosterone, cortisol, cortisone, 11dF, A4, 11OHA4, 11-ketoandrostenedione (11KA4), testosterone (T), 11β-hydroxytestosterone (11OHT), 11-ketotestosterone (11KT), progesterone (Prog), 17OHP, and 16α-hydroxyprogesterone (16OHP). Steroid extraction and LC-MS/MS quantitation were performed as recently decribed.17 The assay performance for four steroids not previously published (aldosterone, 18oxoF, 18OHF, and 16OHP) are presented in Supplemental Table S1. For steroid quantitation in AV serum, 5–10 μL aliquots were used (dilution 10 and 20-fold for pre- and post-cosyntropin, respectively); and 100 μL aliquots were used to measure steroids in peripheral serum (no dilution, both pre- and post-cosyntropin).
Statistical analysis
Comparison of continuous variables between the dominant and contralateral AV was done with paired t test. Kruskal-Wallis with Dwass-Steel pair-wise comparison were performed for comparison of continuous variables across multiple groups. Linear correlation between continuous variables were assessed by the Spearman correlation test. Predictions of binary responses using a panel of steroids were done using regularized (penalized) logistic regression analysis with elastic nets penalties. The penalty parameters were chosen by cross-validation to achieve the best prediction. Receiver-operating characteristics were used to characterize the prediction performance. Linear and quadratic discriminant analysis was used on multi-class classification problems. Analyses were performed using SAS 9.4. (Cary, NC) and R-3.4.3 (Foundation for Statistical Computing, Vienna, Austria). Statistical significance was determined atαlevel of 0.05 for two-tailed tests.
Results
During the study period, 131 patients (81 men, median age 57 years) meeting all entrance criteria were recruited. Demographic and clinical characteristics of study participants are presented in Table 1. In total, 54 patients underwent unilateral adrenalectomy within our institution. Based on the primary aldosteronism surgical outcomes (PASO ) criteria,18 clinical benefit was achieved in 87% of patients: cure in 16 (30 %) and partial clinical success in 31 (57%) patients; clinical benefit was absent in 7 (13%) patients. Biochemical follow up was available in 47 (87 %) patients. Of these, biochemical cure was achieved in 43 (91.5%) patients; one patient had partial biochemical benefit, and three patients had no biochemical benefit following adrenalectomy.
Table 1.
Characteristics of study participants
| PATIENT CHARACTERSTIC | UNILATERAL N=37 | BILATERAL N=32 | DISCORDANT N=34 | TOTAL N=131 | p |
|---|---|---|---|---|---|
| Sex (M/%) | 21 (57%) | 19 (59%) | 25 (74%) | 81 (62%) | 0.30 |
| Age | 52 [45–58] | 50 [45–61] | 50 [46–60] | 57 [42–65] | 0.98 |
| sBP (mmHg) | 150 [139–161] | 160 [139–189] | 156 [137–171] | 156 [140–174] | 0.15 |
| dBP (mmHg) | 90 [78–99] | 89 [79–94] | 86 [80–93] | 88 [79–96] | 0.50 |
| ATC/DDD index | 4.3 [2.3–5.6] | 5.2 [1.7–7.1] | 6.2 [3.4–8.4] | 5 [2.7–7.1] | 0.05 |
| H/o hypokalemia (%) | 97% | 84% | 94% | 89% | 0.12 |
| PAC (ng/dL) | 36 [25–61] | 24* [18–33] | 26* [22–40] | 28 [22–40] | 0.0007 |
| PRA (ng/mL/hr) | 0.2 [0.1–0.6] | 0.3 [0.1–0.7] | 0.4 [0.1–0.6] | 0.3 [0.1–0.6] | 0.86 |
| ARR | 105 [53–371] | 86 [33–185] | 74 [51–219] | 89 [45–245] | 0.24 |
Data are expressed as medians [interquartile range]. sBP, systolic blood pressure; dBP, diastolic blood pressure; ATC/DDD index, standardized daily defined dose of antihypertensive medications; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, PAC/PRA ratio; H/o, history of.
p < 0.05, compared with unilateral
Performance of steroids as indicators of AV catheterization
Based on clinical data, 28 (21 %) patients had an SI < 2 prior to using cosyntropin in at least one AV, including 8 patients with bilateral SI < 2 (Fig. 1). Of the 17 steroids measured, 8 steroids demonstrated significantly higher SI than cortisol, both before and after cosyntropin: 11OHA4, 11dF, corticosterone, 16OHP, 17OHP, A4, DOC and Prog (p < 0.01 for all, compared to cortisol; Fig. 2). Prior to cosyntropin administration, 11dF, corticosterone and 11OHA4 demonstrated the highest SI (medians of 23, 18.7 and 18.6, respectively), while the median baseline cortisol SI was 4.1 (Fig. 2). Importantly, 30/36 (83%) AV samples with a clinical pre-cosyntropin SI < 2 were above the threshold of 2 when using 11OHA4 and corticosterone, and in 29/36 (80.6%) AV samples SI was minimum 2 when using 11dF. After cosyntropin administration, SI was amplified in all of these 8 steroids, and achieved the highest values for 16OHP, Prog, DOC, and 17OHP, all with median SI > 200, while the median SI for cortisol was 49 (Fig. 2).
Figure 1. Aldosterone lateralization of study participants.
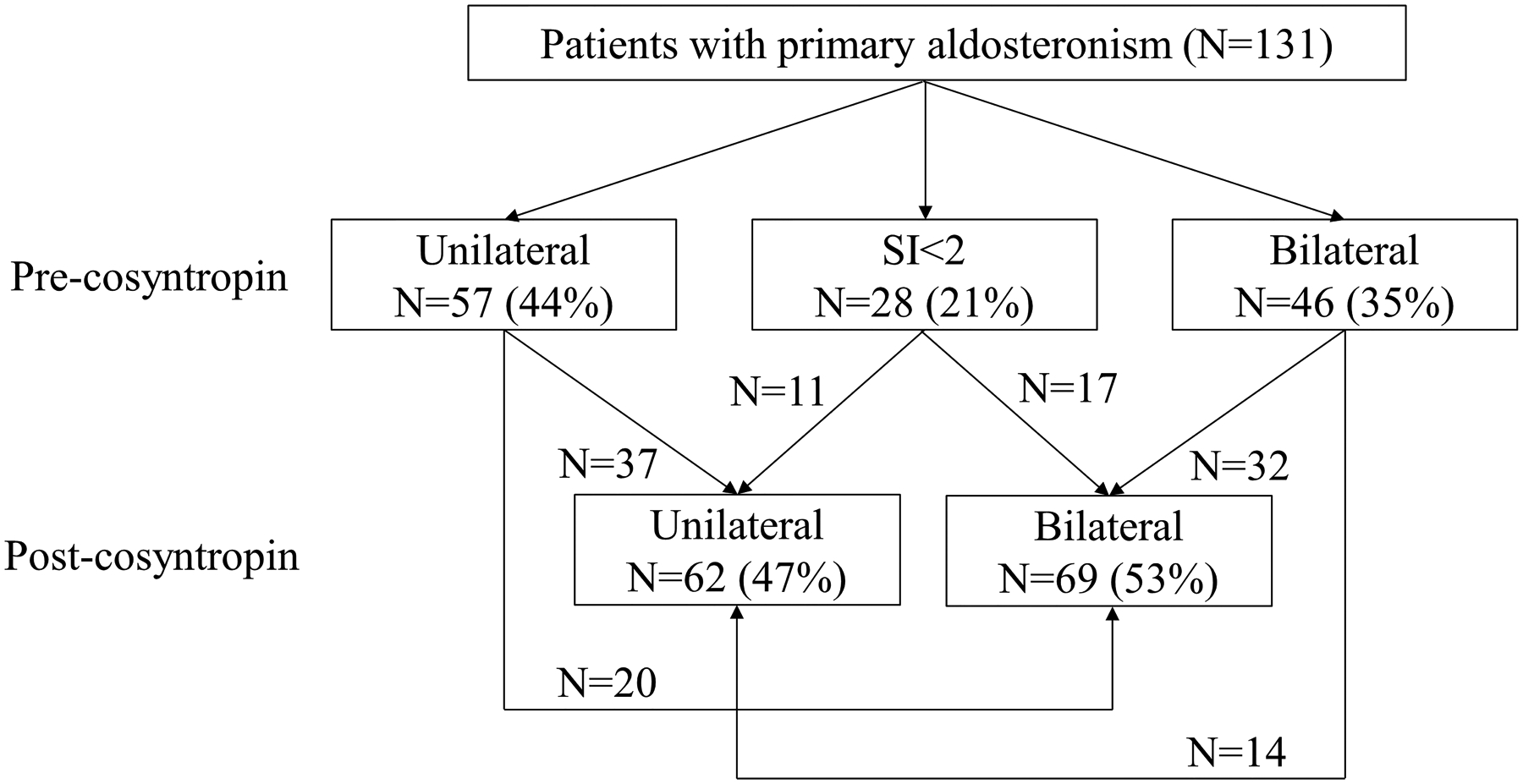
Based on LC-MS/MS lateralization across 6/9 steroids, LI different from clinical LI, as follows: a, 3 patients were classified as bilateral; b, 4 patients were classified as unilateral; c, 6 patients were classified as unilateral.
Figure 2. Comparison of selectivity index (SI) using cortisol and alternative steroids.

Figure illustrates the standard-of-care, cortisol, and all steroids with SI higher than cortisol (p < 0.01 for all). The boxes represent the interquartile range, the horizontal line marks the median, and the whiskers mark the 10–90 percentiles.
11OHA4, 11β-hydroxyandrostenedione; 11dF, 11-deoxycortisol; 17OHP, 17α-hydroxyprogesterone; 16OHP, 16-hydroxyprogesterone; A4, androstenedione; DOC, 11-deoxycorticosterone; Prog, progesterone.
PA Lateralization
Of the 103 patients in whom catheterization was deemed successful both before and after cosyntropin based on clinical laboratory data, lateralization was concordant pre- and post-cosyntropin stimulation in 69 patients (37 U/U and 32 B/B), while 34 patients had discrepant lateralization results (20 U/B and 14 B/U) (Fig. 1). Of the 28 patients with failed catheterization pre-cosyntropin based on clinical laboratory cortisol, 17 patients were diagnosed with bilateral PA and 11 with unilateral PA based on post-cosyntropin clinical laboratory data (Fig. 1). When lateralization was adjudicated based on consistent LI for at least 6 of 9 LC-MS/MS–measured steroids, including cortisol and the 8 steroids with SI performance superior to cortisol, results differed from clinical laboratory data-based lateralization in 12 patients. Of these, a single case with B/B clinical data classification was considered U/U based on steroids measured by mass spectrometry, and this patient did not undergo adrenalectomy. The other 11 cases had partially discordant classification (only pre- or post-cosyntropin) between clinical and LC-MS/MS-derived LI. Three such patients, all with LC-MS/MS unilateral classification (vs. bilateral based on clinical data LI), underwent surgery and all three had complete biochemical resolution of PA.
Overall, clinical laboratory LI correlated tightly with LI based on LC-MS/MS measurements using as denominators cortisol and the 8 steroids with SI superior to cortisol (r2 ≥ 0.75, p < 0.001 for all, and the correlation of cortisol-based LI was tightest with A4, 11OHA4 and 11dF (Fig. 3). Paired comparison of LI across steroids, showed that without cosyntropin, 11OHA4 and A4 were significantly higher than clinical LI (p = 0.02 and 0.04, respectively), while after cosyntropin, LI was overall similar across all steroids used.
Figure 3. Correlation between lateralization indices (LI) based on correction of aldosterone to cortisol vs. alternative steroids.
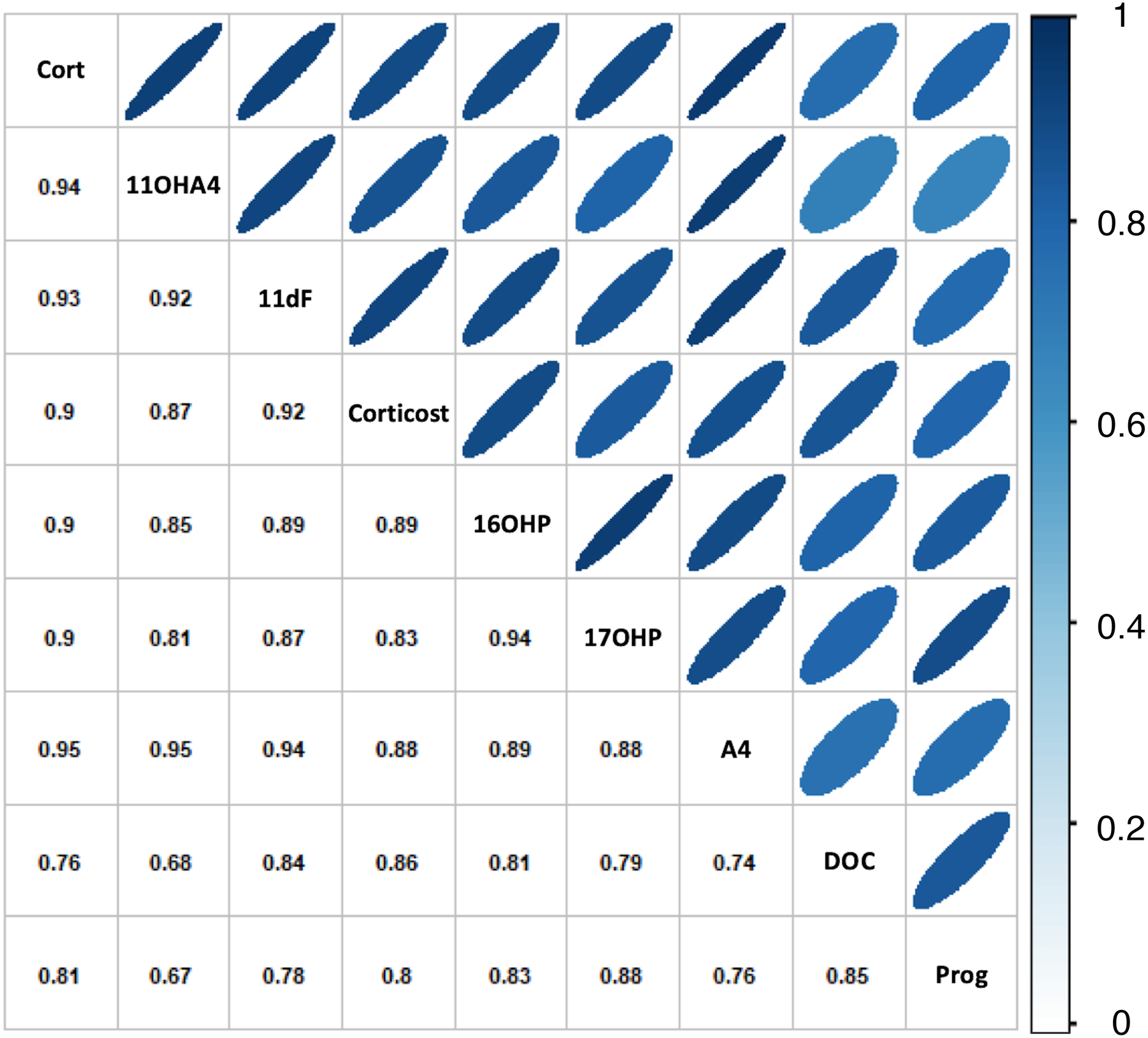
11OHA4, 11β-hydroxyandrostenedione; 11dF, 11-deoxycortisol; corticost, corticosterone; 17OHP, 17α-hydroxyprogesterone; 16OHP, 16-hydroxyprogesterone; A4, androstenedione; DOC, 11-deoxycorticosterone; Prog, progesterone.
Steroid profiles in PA subtypes
Within-group comparison of steroids in the dominant vs. the contralateral AV revealed that in the U/U group not only aldosterone, but also 18oxoF, 18OHF and DOC were higher in the dominant AV both pre- and post-cosyntropin stimulation (Table 2). Conversely, in the U/B, the same four steroids were significantly higher in the dominant AV only at baseline, while in the B/U group, they were significantly higher only after cosyntropin stimulation (Table 2).
Table 2.
Comparison of baseline and cosyntropin-stimulated steroid concentrations in the adrenal veins (AV) and peripheral serum of PA patients across lateralization groups.
| Steroid | Baseline Concentrations, Dominant AV (ng/dL) | Cosyntropin-stimulated Concentrations, Dominant AV (ng/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B/B | B/U | U/B | U/U | p | B/B | B/U | U/B | U/U | p | |
| 18OHF | 5361 [2254–13039] | 3024 [1289–6617] | 4506 [2362–10354] | 11936 [3854–58500] | 0.009 | 73349 [40577–147242] | 69421 [39342–129379] | 63714 [41582–121160] | 84488 [54549–145029] | 0.69 |
| 18oxoF | 61 [19–155] | 40 [13–153] | 91 [44–230] | 294 [74–1732] | 0.0004 | 588 [234–1474] | 1044 [493–3213] | 503 [328–966] | 2235 [1073–4700] | 0.0002 |
| Aldo | 461 [237–1052] | 179 [56–650] | 1141 [485–2719] | 2309 [801–6529] | <.0001 | 4597 [2440–9261] | 8392 [4496–14212] | 4434 [2503–9163] | 18878 [7973–32221] | <.0001 |
| DOC | 52 [29–163] | 32 [22–75] | 132 [56–241] | 259 [65–1285] | 0.0002 | 5727 [2950–10010] | 5371 [3856–8759] | 5751 [3991–10622] | 11682 [5627–17538] | 0.0015 |
| Cort | 25 [19–56] | 18 [13–28] | 27 [18–41] | 28 [18–101] | 0.12 | 947 [528–1264] | 634 [407–1003] | 961 [615–1138] | 719 [525–1059] | 0.38 |
| Cortisone | 1968 [1483–3033] | 1513 [1195–2500] | 1408 [1255–2598] | 2182 [1252–4015] | 0.28 | 30416 [11313–49079] | 19174 [4289–31223] | 25823 [14733–41557] | 17142 [11260–41048] | 0.83 |
| 11OHT | 59 [33–101] | 54 [26–132] | 32 [15–79] | 52 [31–83] | 0.33 | 288 [150–463] | 212 [113–392] | 298 [198–388] | 213 [118–333] | 0.45 |
| 11KT | 45 [30–55] | 37 [23–53] | 27 [18–43] | 36 [27–58] | 0.44 | 97 [59–141] | 75 [39–118] | 101 [60–169] | 73 [56–105] | 0.45 |
| 11OHA4 | 5705 [3019–11709] | 2875 [1278–8972] | 4737 [2613–11089] | 5250 [3090–11840] | 0.28 | 67597 [39870–102234] | 51730 [33216–81854] | 69006 [48205–103691] | 53477 [30143–66649] | 0.17 |
| 11KA4 | 170 [74–343] | 99 [50–181] | 90 [66–164] | 103 [59–126] | 0.04 | 463 [239–1514] | 400 [162–861] | 813 [385–1202] | 317 [179–584] | 0.051 |
| 11dF | 603 [319–1323] | 348 [240–494] | 815 [346–994] | 648 [418–3197] | 0.04 | 27912 [15860–36632] | 21060 [12217–27370] | 25862 [18437–38522] | 25107 [17825–34616] | 0.37 |
| Corticost | 2797 [1285–5884] | 1235 [381–2317] | 3093 [1585–4070] | 2624 [1428–18386] | 0.02 | 244267 [113286–415793] | 192696 [75742–259351] | 223477 [156043–264565] | 211714 [143397–319911] | 0.49 |
| 16OHP | 134 [57–631] | 71 [39–112] | 166 [93–331] | 151 [58–947] | 0.09 | 23582 [11527–40850] | 17458 [9189–28057] | 31107 [16821–46469] | 20499 [12361–32502] | 0.12 |
| 17OHP | 348 [163–991] | 210 [147–257] | 426 [243–907] | 280 [192–1790] | 0.09 | 47590 [22766–81813] | 28405 [19059–55290] | 71899 [43643–108654] | 42390 [22838–61863] | 0.01 |
| T | 203 [68–373] | 252 [178–479] | 170 [46–344] | 166 [91–287] | 0.31 | 500 [323–732] | 489 [345–759] | 464 [204–725] | 436 [289–545] | 0.72 |
| A4 | 565 [419–1420] | 348 [244–675] | 578 [316–1127] | 632 [351–3618] | 0.19 | 14961 [10106–24745] | 12449 [8841–20879] | 11744 [9824–22868] | 13423 [8871–18793] | 0.70 |
| Prog | 35 [20–81] | 20 [16–40] | 73 [46–101] | 87 [29–308] | 0.0007 | 6359 [4102–12525] | 4310 [2648–8723] | 11600 [4876–16463] | 6565 [4410–12737] | 0.11 |
| Baseline Concentrations, Contralateral AV (ng/dL) | Cosyntropin-stimulated Concentrations, Contralateral AV (ng/dL) | |||||||||
| B/B | B/U | U/B | U/U | p | B/B | B/U | U/B | U/U | p | |
| 18OHF | 1879 [1382–7724] | 3084 [968–5401] | 1632 [1131–3842] | 2426 [1784–8629] | 0.19 | 79929 [46433–155073] | 48992 [42991–111783] | 46846 [37231–78585] | 43601 [22112–77795] | 0.036 |
| 18oxoF | 20 [12–53] | 25 [8–63] | 19 [7–31] | 21 [8–51] | 0.54 | 613 [180–1148] | 173 [125–549] | 248 [198–420] | 131 [60–327] | 0.0005 |
| Aldo | 152 [74–455] | 121 [67–272] | 95 [39–248] | 80 [41–283] | 0.26 | 4328 [1460–6752] | 1004 [325–2083] | 1948 [1179–3479] | 635 [236–1648] | <.0001 |
| DOC | 46 [27–77] | 30 [25–81] | 37 [24–50] | 48 [31–92] | 0.44 | 6271 [3580–10194] | 3222 [2488–5505] | 5374 [3545–8268] | 4673 [2707–6541] | 0.062 |
| Cort | 21 [15–46] | 18 [15–32] | 19 [13–27] | 27 [18–97] | 0.11 | 982 [795–1226] | 747 [713–1718] | 727 [507–1099] | 757 [536–1086] | 0.23 |
| Cortisone | 1789 [1328–2490] | 1791 [1158–2692] | 1259 [1045–2632] | 2267 [1508–5628] | 0.045 | 29128 [14027–53349] | 27490 [22668–74159] | 20999 [13781–28681] | 22594 [11919–44734] | 0.33 |
| 11OHT | 41 [20–91] | 79 [28–131] | 35 [18–69] | 53 [29–93] | 0.19 | 269 [189–490] | 321 [216–445] | 268 [115–417] | 214 [144–320] | 0.14 |
| 11KT | 38 [25–55] | 38 [24–65] | 29 [17–42] | 39 [25–56] | 0.24 | 93 [52–176] | 99 [76–140] | 75 [55–134] | 75 [50–113] | 0.28 |
| 11OHA4 | 3634 [2133–10879] | 3751 [2181–9319] | 2855 [2212–11273] | 5819 [2880–17521] | 0.27 | 70963 [44376–119969] | 69827 [54693–108958] | 51296 [38890–102088] | 50179 [32084–81350] | 0.10 |
| 11KA4 | 95 [63–311] | 162 [56–180] | 89 [54–148] | 79 [49–134] | 0.19 | 634 [232–1561] | 490 [280–861] | 479 [312–1111] | 286 [151–627] | 0.03 |
| 11dF | 508 [277–941] | 378 [294–725] | 453 [306–780] | 481 [352–1729] | 0.48 | 27653 [15200–42550] | 18193 [15402–30975] | 24580 [18520–36577] | 19159 [10963–27952] | 0.17 |
| Corticost | 1465 [977–4329] | 1335 [623–3440] | 1027 [668–3346] | 1909 [1040–8286] | 0.14 | 300606 [196666–371580] | 202337 [153672–371142] | 204575 [132741–248671] | 203525 [133218–244159] | 0.04 |
| 16OHP | 129 [62–379] | 80 [45–137] | 107 [54–330] | 129 [55–903] | 0.53 | 27244 [15386–41119] | 20732 [13885–35962] | 29541 [12319–42774] | 18291 [11251–42384] | 0.69 |
| 17OHP | 306 [169–729] | 224 [114–448] | 287 [189–709] | 285 [154–1815] | 0.71 | 45355 [32521–79222] | 30474 [17127–92238] | 60553 [32943–112855] | 35833 [17547–87247] | 0.23 |
| T | 165 [52–362] | 263 [201–465] | 147 [37–326] | 215 [112–392] | 0.09 | 530 [348–787] | 599 [427–1004] | 446 [245–685] | 425 [231–610] | 0.054 |
| A4 | 447 [303–875] | 431 [316–996] | 446 [325–830] | 677 [367–4915] | 0.29 | 16651 [9766–24995] | 15574 [10470–17948] | 12305 [7655–21359] | 12304 [8548–18653] | 0.21 |
| Prog | 28 [16–61] | 24 [16–45] | 28 [19–41] | 29 [17–204] | 0.95 | 8423 [5437–14218] | 6332 [2895–13347] | 9586 [4562–20034] | 5657 [3618–10104] | 0.09 |
| Baseline Concentrations, Peripheral Serum (ng/dL) | Cosyntropin-stimulated Concentrations, Peripheral Serum (ng/dL) | |||||||||
| B/B | B/U | U/B | U/U | p | B/B | B/U | U/B | U/U | p | |
| 18OHF | 372 [236–612] | 418 [234–557] | 298 [105–398] | 421 [202–893] | 0.26 | 552 [314–1151] | 594 [480–1340] | 491 [302–711] | 515 [420–1835] | 0.29 |
| 18oxoF | 2 [1–4] | 2 [1–8] | 2 [1–2] | 5 [2–16] | 0.002 | 5 [3–7] | 7 [2–22] | 4 [3–6] | 10 [7–22] | 0.0005 |
| Aldo | 14 [7–27] | 18 [8–20] | 12 [7–22] | 34 [10–62] | 0.026 | 37 [18–68] | 54 [38–87] | 31 [20–43] | 60 [42–100] | 0.008 |
| DOC | 4 [3–6] | 4 [3–11] | 3 [3–5] | 7 [4–18] | 0.006 | 26 [13–46] | 21 [14–43] | 24 [16–40] | 33 [18–54] | 0.66 |
| Cort | 6 [4–11] | 6 [4–8] | 5 [4–7] | 6 [4–10] | 0.79 | 17 [13–21] | 15 [10–21] | 15 [14–19] | 15 [12–22] | 0.79 |
| Cortisone | 1057 [743–1422] | 917 [713–1452] | 877 [744–1177] | 1097 [687–1502] | 0.89 | 1120 [812–1549] | 1082 [732–1739] | 1006 [817–1288] | 1283 [1051–1762] | 0.13 |
| 11OHT | 15 [9–22] | 16 [12–31] | 11 [8–21] | 13 [8–20] | 0.38 | 15 [11–18] | 17 [8–28] | 13 [9–18] | 14 [10–18] | 0.65 |
| 11KT | 21 [13–39] | 22 [12–35] | 19[13–27] | 16 [11–30] | 0.62 | 20 [14–32] | 24 [9–34] | 18 [14–24] | 20 [13–28] | 0.84 |
| 11OHA4 | 211 [150–416] | 186 [142–368] | 187 [150–290] | 158 [119–291] | 0.20 | 476 [323–1090] | 412 [259–633] | 539 [292–783] | 317 [199–500] | 0.027 |
| 11KA4 | 36 [20–68] | 22 [15–34] | 39 [20–59] | 22 [14–33] | 0.005 | 35 [20–120] | 35 [18–59] | 42 [22–66] | 22 [14–30] | 0.042 |
| 11dF | 25 [14–41] | 28 [13–41] | 18 [12–36] | 23 [12–58] | 0.81 | 153 [75–344] | 123 [91–204] | 174 [121–220] | 120 [84–284] | 0.72 |
| Corticost | 91 [52–199] | 71 [36–355] | 69 [38–169] | 128 [58–361] | 0.32 | 1434 [1097–2277] | 1456 [818–2022] | 1291 [934–2057] | 1116 [709–1853] | 0.35 |
| 16OHP | 12 [5–35] | 10 [6–19] | 14 [7–19] | 12 [2–21] | 0.74 | 113 [45–251] | 69 [43–126] | 145 [96–199] | 56 [34–107] | 0.059 |
| 17OHP | 70 [35–131] | 58 [41–118] | 74 [37–137] | 61 [36–89] | 0.82 | 217 [119–393] | 156 [92–267] | 373 [166–547] | 151 [93–305] | 0.06 |
| T | 183 [25–329] | 331 [161–521] | 275 [26–411] | 223 [22–428] | 0.19 | 187 [39–390] | 333 [222–456] | 280 [21–440] | 238 [27–415] | 0.28 |
| A4 | 51 [42–60] | 50 [35–67] | 48 [35–74] | 50 [33–87] | 0.96 | 92 [67–158] | 93 [76–114] | 87 [61–157] | 84 [51–109] | 0.69 |
| Prog | 6 [4–10] | 6 [4–11] | 6 [5–8] | 6 [5–15] | 0.61 | 30 [21–51] | 22 [16–38] | 52 [30–78] | 27 [14–51] | 0.03 |
All steroid concentrations are expressed in ng/dL, as medians [interquartile range].
Across-group comparisons of steroids showed that aldosterone and 18oxoF were consistently highest in the U/U group, not only in the dominant AV (pre- and post-cosyntropin), but also in peripheral serum, both at baseline and after cosyntropin stimulation (Table 2). DOC and 18OHF were also overall highest in the U/U group, reaching statistical significance when compared with the B/B patients in the dominant AV, both before and after cosyntropin stimulation (Table 2). These results suggest a gradient of PA severity across groups, ranging from most severe PA in U/U cases to mildest in the B/B cases. Conversely, in the contralateral AV, cosyntropin-stimulated aldosterone, 18OHF and 18oxoF were highest in the B/B group as compared with the other 3 groups (Table 2), consistent with a mechanism of contralateral suppression for unilateral cases.
Discriminant analysis incorporating all steroids measured in peripheral serum demonstrated specific profiles to the 4 groups, as shown in Fig. 4A. Multi-steroid receiver operating characteristic (ROC) curve analysis showed poor discriminatory power of peripheral steroids measured pre-cosyntropin between patients classified as either U or B based on pre-cosyntropin data alone (AUC = 0.586). When PA subtyping incorporated both pre- and post-cosyntropin AVS data, the discriminatory power between the U/U and B/B groups of the same steroid panel in peripheral serum was improved to an AUC of 0.768 at baseline, and 0.907 after cosyntropin stimulation (Fig. 4B).
Figure 4. Performance of a 17-steroid panel measured in peripheral serum for PA subtyping.

A. Discriminant analysis between four PA subtypes at baseline and after cosyntropin stimulation. B. Receiver-operating characteristics curves for PA subtyping according to baseline and/or cosyntropin stimulated AVS results.
Grey line: baseline peripheral serum, to distinguish U vs. B PA subtyping based on baseline AVS results alone. Blue line: baseline peripheral serum for distinguishing PA subtypes based on both baseline and cosyntropin AVS data (U/U vs. B/B). Red line: cosyntropin-stimulated peripheral serum for distinguishing PA subtypes based on both baseline and cosyntropin AVS data (U/U vs. B/B).
Discussion
The first major finding of our study is that several steroids are superior to cortisol for ascertaining AV catheterization. Of these, 11dF, 11OHA4, and corticosterone displayed the highest unstimulated gradients between AV and periphery. These findings are particularly relevant for centers that do not use cosyntropin, as such protocols have been associated with high rates of AV catheterization failure19–21. A report of four PA patients suggested that 11dF might be superior to cortisol in establishing AV catheterization.10 In a larger study, Eisenhofer and colleagues had previously proposed A4 and DHEA as alternatives to cortisol8. In addition to A4, Ceolotto et al also proposed 17OHP, although this study was limited to a small number of patients, and steroids were measured by immunoassays.9 We found that 17OHP and 16OHP achieved particularly dramatic SI values after cosyntropin stimulation, but more modest values at baseline. Other disadvantages of 17OHP are that this steroid also derives from the gonads and increases during the luteal phase in reproductive age women, which might decrease SI. We have previously shown that 11OHA4 has higher AV and peripheral circulation concentrations than A4.12,13 Furthermore, in contrast with its precursor A4, 11OHA4 is produced almost exclusively by the adrenals. Notably, 83% of samples deemed unsuccessful based on clinical laboratory SI < 2 would have been interpretable (SI ≥ 2) if 11OHA4 or corticosterone were used instead of cortisol. Because, unlike corticosterone, 11OHA4 and 11dF are not within the mineralocorticoid pathway and both are adrenal-specific, we suggest these two steroids as the preferred candidates for AV catheter placement ascertainment during AVS, particularly so when cosyntropin is not used.
Overall, lateralization was consistent across clinical and LC-MS/MS assays for the steroids with good SI performance, both in the absence and presence of cosyntropin. A previous multi-center study, which included centers with heterogeneous AVS protocols (one with and two without cosyntropin) found strong positive correlations between LIs obtained from radioimmunoassays and LC-MS/MS assays of cortisol, A4, and DHEA.8 Taken together, these results demonstrate that LI remains reliable when using alternative steroids and suggest that the optimal steroid(s) to normalize aldosterone values during AVS should be guided mainly by their performance and advantages for SI, as discussed above.
The second important finding of our study is the performance of multi-steroid panels measured in peripheral serum for PA subtyping. The hybrid steroids 18oxoF and 18OHF have been previously proposed as biomarkers of APA and showed promising sensitivity and specificity (0.83 and 0.99, respectively) in Japanese PA patients.14 These steroids, however, had rather modest discriminatory power between APA and BHA in a European population.8 It is now recognized that the synthesis of 18oxoF and 18OHF is highest in patients with APAs harboring KCNJ5 mutations,22 due to the co-expression of CYP11B2 and CYP17A1 in such tumors.23 KCNJ5 mutations are highly prevalent in Japanese and East Asian PA patients,24–26 while European and American populations display a wider variety of aldosterone-driver mutations,23,27 explaining the differences in APA/BHA discriminatory power differences among these populations. Expanding to a 12-steroid panel measured in peripheral serum, Eisenhofer and colleagues reported accurate APA vs BHA discrimination is 80% of 216 European PA patients.8 An important caveat of this initial study is the different AVS protocols used in the participating centers, either only without or only with cosyntropin. As discussed above, we and others have found that some patients display discrepant lateralization results during AVS performed with and without cosyntropin.19,28 Our study suggests that PA patients with discrepant pre- and post-cosyntropin lateralization have intermediate disease severity as compared with patients with robust lateralization regardless of the protocol used, and that these groups display distinct steroid profiles in peripheral serum. Furthermore, logistic regression analysis demonstrated poor performance of peripheral serum steroids for distinguishing between cases classified as unilateral or bilateral based on pre-cosyntropin AVS data alone; in contrast, the discrimination was greatly improved for subtypes with consistent AVS lateralization pre- and post-cosyntropin. Notably, the highest discriminatory performance between U/U and B/B groups was achieved by peripheral measurement of cosyntropin-stimulated steroids (AUC of 0.9). Previous studies conducted in China29 and Japan30 utilized cosyntropin stimulation after dexamethasone suppression and demonstrated less overlap of aldosterone concentrations between patients with APA or BHA than that observed at baseline. A small Japanese study subsequently reported that the distinction between APA and BHA based on peripheral serum-aldosterone concentrations alone is better after cosyntropin stimulation, without prior dexamethasone suppression.31 Noninvasive dynamic testing for PA subtyping appears promising but needs to be confirmed in larger, ideally prospective studies. The identification of BHA with a peripheral blood test would circumvent the need for AVS in many PA patients.
In summary, we have developed a multi-steroid panel that could offer two advantages to PA subtyping. First, we propose 11OHA4 and 11dF, two adrenal-specific steroids, as superior to cortisol for reliably establishing AV catheterization during AVS, particularly for centers that do not use cosyntropin and in cases where cortisol-based SI is inadequate. Second, we found that cases with inconsistent lateralization between pre- and post-cosyntropin appear to be overall less severe than those with consistent unilateral aldosterone dominance and display distinct steroid profiles in peripheral serum. While the long-term evolution and surgical benefit for these intermediate subtypes remains to be determined, incorporating both pre- and post-cosyntropin data for PA subtyping is likely to be valuable, and adds yet another need for more reliable SI parameters. The main limitations to our study are its retrospective design, limited number of cases with discrepant lateralization and incomplete post-operative follow up data. Nonetheless, our study is the first to incorporate both pre- and post-cosyntropin AV and peripheral serum datasets, which along with multi-steroid profiling using LC-MS/MS reveals four-tiered rather than binary PA subtypes. Larger, multi-center studies, with prospective follow up of patients, including a large number of PA patients with discordant pre-and post-cosyntropin AVS lateralization are needed to establish the clinical significance of the four-tiered system of PA subtyping and the best management approach for each subtype.
Supplementary Material
Perspectives:
AVS is currently an essential tool for identifying patients with PA who could benefit from unilateral adrenalectomy. Cortisol has been used for AVS data interpretation, but its numerous pitfalls can lead to unreliable results in many patients. Steroid panels measured with highly accurate mass spectrometry assays can serve dual purposes: 1) adrenal-specific steroids that are not normally produced by APAs, such as 11β-hydroxyandrostenedione, could facilitate accurate AVS data interpretation; 2) after validation in larger studies, steroid fingerprints measured in peripheral serum will be able to identify patients who would benefit from adrenalectomy, and as such, eliminate the need for invasive and costly tests in all other PA patients.
Novelty and Significance:
1). What Is New
We have used a panel of 17 steroids measured in a single small serum aliquot to assist with subtyping of primary aldosteronism (PA).
We have found that the adrenal specific steroids 11β-hydroxyandrostenedione (11OHA4), 11-deoxycortisol (11dF) and corticosterone are superior to cortisol as indicators of adrenal vein catheterization. Using these three steroids, 80% of cases deemed failed based on the current standard-of-care, cortisol, are valid.
We have found that based on baseline and cosyntropin stimulated adrenal vein sampling results, PA subtyping can be stratified in four groups with distinct steroid profiles, both in adrenal veins, as well as in periphery.
2). What Is Relevant?
11OHA4 and 11dF are the ideal candidates for adrenal vein data interpretation in PA patients.
Multi-steroid profiling can assist in PA subtyping and, in future, will likely circumvent the need for adrenal vein sampling in many PA patients.
Summary
Using mass-spectrometry, we demonstrate the utility of a multi-steroid panel in PA subtyping. Compared to cortisol, 11OHA4 and 11dF have superior adrenal vein/periphery gradients and allow the interpretation of adrenal vein sampling data in most patients. PA subtyping spans a gradient of severities, marked by distinct steroid fingerprints.
Acknowledgements:
We thank the University of Michigan adrenal team members who participated in the care of patients with PA and all study participants.
Sources of funding: This work was supported by grants 1K08DK109116 from NIDDK and 2019087 from the Doris Duke Charitable Foundation, awarded to AFT; grant R01DK106618 to WER; and grant R21DK103183 to WER and RJA, both from NIDDK. ATN was supported by grant 18POST33990227 from American Heart Association.
Footnotes
Conflict of interest: The authors have nothing to disclose.
References
- 1.Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism 2016;101:1889–916. [DOI] [PubMed] [Google Scholar]
- 2.Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014;63:151–60. [DOI] [PubMed] [Google Scholar]
- 3.Monticone S, Viola A, Rossato D, et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. The lancet Diabetes & endocrinology 2015;3:296–303. [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Bertello C, Sukor N, et al. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension 2010;55:667–73. [DOI] [PubMed] [Google Scholar]
- 5.Arlt W, Lang K, Sitch AJ, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich DA, Adolf C, Holler F, et al. Adrenal Insufficiency After Unilateral Adrenalectomy in Primary Aldosteronism: Long-Term Outcome and Clinical Impact. The Journal of clinical endocrinology and metabolism 2019;104:5658–64. [DOI] [PubMed] [Google Scholar]
- 7.Peitzsch M, Dekkers T, Haase M, et al. An LC-MS/MS method for steroid profiling during adrenal venous sampling for investigation of primary aldosteronism. The Journal of steroid biochemistry and molecular biology 2015;145:75–84. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhofer G, Dekkers T, Peitzsch M, et al. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clinical chemistry 2016;62:514–24. [DOI] [PubMed] [Google Scholar]
- 9.Ceolotto G, Antonelli G, Maiolino G, et al. Androstenedione and 17-alpha-Hydroxyprogesterone Are Better Indicators of Adrenal Vein Sampling Selectivity Than Cortisol. Hypertension 2017;70:342–6. [DOI] [PubMed] [Google Scholar]
- 10.Nilubol N, Soldin SJ, Patel D, et al. 11-Deoxycortisol may be superior to cortisol in confirming a successful adrenal vein catheterization without cosyntropin: a pilot study. Int J Endocr Oncol 2017;4:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekkers T, Deinum J, Schultzekool LJ, et al. Plasma metanephrine for assessing the selectivity of adrenal venous sampling. Hypertension 2013;62:1152–7. [DOI] [PubMed] [Google Scholar]
- 12.Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. The Journal of clinical endocrinology and metabolism 2013;98:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turcu AF, Nanba AT, Chomic R, et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. European journal of endocrinology / European Federation of Endocrine Societies 2016;174:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension 2015;65:1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 2014;64:354–61. [DOI] [PubMed] [Google Scholar]
- 16.Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clinical endocrinology 2017;87:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-Oxygenated C19 Steroids Do Not Decline With Age in Women. The Journal of clinical endocrinology and metabolism 2019;104:2615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. The lancet Diabetes & endocrinology 2017;5:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and Post-ACTH Aldosterone and Its Ratios Are Useful During Adrenal Vein Sampling in Primary Aldosteronism. The Journal of clinical endocrinology and metabolism 2016;101:1826–35. [DOI] [PubMed] [Google Scholar]
- 20.Wolley MJ, Ahmed AH, Gordon RD, Stowasser M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clinical endocrinology 2016;85:703–9. [DOI] [PubMed] [Google Scholar]
- 21.Monticone S, Satoh F, Giacchetti G, et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension 2012;59:840–6. [DOI] [PubMed] [Google Scholar]
- 22.Williams TA, Peitzsch M, Dietz AS, et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension 2016;67:139–45. [DOI] [PubMed] [Google Scholar]
- 23.Nanba K, Omata K, Else T, et al. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. The Journal of clinical endocrinology and metabolism 2018;103:3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Li X, Zhang X, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine 2015;94:e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng FF, Zhu LM, Nie AF, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 2015;65:622–8. [DOI] [PubMed] [Google Scholar]
- 26.Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. Journal of atherosclerosis and thrombosis 2015;22:191–200. [DOI] [PubMed] [Google Scholar]
- 27.Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension 2019;73:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clinical endocrinology 2017;87:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Zhang C, Wang W, et al. Diagnostic value of ACTH stimulation test in determining the subtypes of primary aldosteronism. The Journal of clinical endocrinology and metabolism 2015;100:1837–44. [DOI] [PubMed] [Google Scholar]
- 30.Sonoyama T, Sone M, Miyashita K, et al. Significance of adrenocorticotropin stimulation test in the diagnosis of an aldosterone-producing adenoma. The Journal of clinical endocrinology and metabolism 2011;96:2771–8. [DOI] [PubMed] [Google Scholar]
- 31.Inoue K, Omura M, Sugisawa C, Tsurutani Y, Saito J, Nishikawa T. Clinical Utility of the Adrenocorticotropin Stimulation Test with/without Dexamethasone Suppression for Definitive and Subtype Diagnosis of Primary Aldosteronism. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


