Abstract
Disease stresses caused by pathogenic microorganisms are increasing, probably because of global warming. Conventional technologies for plant disease control have often revealed their limitations in efficiency, environmental safety, and economic costs. There is high demand for improvements in efficiency and safety. Non-thermal atmospheric-pressure plasma has demonstrated its potential as an alternative tool for efficient and environmentally safe control of plant pathogenic microorganisms in many studies, which are overviewed in this review. Efficient inactivation of phytopathogenic bacterial and fungal cells by various plasma sources under laboratory conditions has been frequently reported. In addition, plasma-treated water shows antimicrobial activity. Plasma and plasma-treated water exhibit a broad spectrum of efficiency in the decontamination and disinfection of plants, fruits, and seeds, indicating that the outcomes of plasma treatment can be significantly influenced by the microenvironments between plasma and plant tissues, such as the surface structures and properties, antioxidant systems, and surface chemistry of plants. More intense studies are required on the efficiency of decontamination and disinfection and underlying mechanisms. Recently, the induction of plant tolerance or resistance to pathogens by plasma (so-called “plasma vaccination”) is emerging as a new area of study, with active research ongoing in this field.
Keywords: atmospheric-pressure plasma, plasma-treated water, decontamination, disinfection, plant pathogens
Introduction
The control of plant diseases is essential for sustainable food crop production. Many plant disease management strategies have played significant roles in reducing the frequency and intensity of diseases. However, 20%–30% loss of crop production still occurs annually because of plant diseases (Oerke, 2006). Particularly, the current global climatic change makes disease control more complicated and difficult. The elevation of temperature and CO2 concentration can change the susceptibility of plants to specific pathogens, leading to the emergence of new diseases (Elad and Pertot, 2014).
Under the current circumstances, traditional disease management strategies have often shown limitations in control efficiency and have multiple drawbacks. Chemical-based methods are most frequently applied for plant disease control; however, the emergence of pathogen resistance and environmental pollution make this strategy less reliable. The induction of plant resistance through species breeding and genetic engineering has been considered as a stable and long-lasting method for plant disease control. Public view on genetically modified organisms is still a barrier for popular use. Biocontrol is the most environmentally friendly method, but the control efficiency in the field needs to be improved.
Disease management in modern agriculture should include considerations from various points of view. Integrative approaches and technologies are one of those considerations for efficient plant disease control strategies. As a potential alternative, atmospheric-pressure non-thermal plasma has recently received attention in managing plant diseases. The antimicrobial activity of atmospheric-pressure non-thermal plasma has been most frequently demonstrated in many studies (for review, Pignata et al., 2017; Sakudo et al., 2019). Owing to its efficient antimicrobial activity, plasma can be usefully applied to eradicate microbes that are pathogenic to plants. Reports on the control of plant diseases by plasma are currently being accumulated (Ito et al., 2017). The majority of studies demonstrate either the efficient inactivation of plant pathogenic microbes in vitro or the disinfection of seeds and plants. In this review, we overviewed the case studies of plant disease control by plasma against plant pathogenic microbes cultured in vitro and those associated with seeds or plants. In addition, the mechanisms of plasma action are discussed. Owing to the limitation of length, literature particularly related to plant pathogenic microbes was included in this review.
Atmospheric-Pressure Non-Thermal Plasma Technology
Plasma is an ionized gas, often referred to as the fourth state of matter (Compton and Langmuir, 1930). When high energy is applied to gas, it transforms into the plasma state. In reality, the plasma state can be produced by applying a high electric voltage to gas. Reactive species, charged species, UV radiation, free electrons, and an electric field are generated in the plasma state, and these components can greatly affect the biological processes. Since non-thermal plasma can be produced at atmospheric pressure, its biological application has been actively studied in the fields of medicine and agriculture (for review, Adamovich et al., 2017; Ito et al., 2017). Plasma has frequently been demonstrated to be effective in microbial inactivation and cancer cell death. In contrast, plasma can also enhance cell differentiation, wound healing, seed germination, and plant growth. These two contrasting effects (inactivation and activation) are often observed as a consequence of the plasma dose applied.
Inactivation of Phytopathogenic Microorganisms in Culture Suspension by Plasma
Numerous studies have demonstrated the antimicrobial activity of non-thermal atmospheric-pressure plasma on plant pathogenic microorganisms, mostly focusing on bacteria and fungi (for review, Ito et al., 2017). In those studies, plasma treatment was performed on microbial culture suspension or contaminated food sources, plant bodies, and seeds. In this section, we first discuss the effect of the plasma treatment on the phytopathogenic microbial cell suspension (Tables 1 and 2).
Table 1.
Control of bacterial pathogens by atmospheric-pressure non-thermal plasma.
| Treated object | Target bacteria | Plasma types | Reference |
|---|---|---|---|
| Suspension culture | Erwinia carotovora | Gliding arc discharge | Moreau et al., 2007 |
| Post-harvest grape and banana | Bacteria | High-field plasma | Liu et al., 2011 |
| Post-harvest almonds |
Salmonella anatum Salmonella enteria serovar Stanley Salmonella enteritidis Escherichia coli |
Atmospheric-pressure plasma jet | Niemira, 2012 |
| Post-harvest corn salad leaves | E. coli K12 | Atmospheric-pressure plasma jet | Baier et al., 2013 |
| Post-harvest lettuce, tomato, carrot | E. coli | Argon atmospheric-pressure cold plasma | Bermudez-Aguirre et al., 2013 |
| Tomato seeds | Ralstonia solanacearum | Helium plasma | Jiang et al., 2014 |
| Post-harvest strawberry (in package) | Mesophilic bacteria | Atmospheric cold plasma | Misra et al., 2014 |
| Suspension culture | Erwinia amylovora | Gliding arc discharge plasma | Mráz et al., 2014 |
| Post-harvest cherry, tomato, and strawberry |
E. coli Salmonella Listeria monocytogenes |
Dielectric barrier discharge (DBD) atmospheric cold plasma | Ziuzina et al., 2014 |
| Post-harvest whole black peppers |
Bacillus subtilis Bacillus atrophaeus Salmonella enterica |
Radio frequency plasma jet Microwave-generated plasma |
Hertwig et al., 2015 |
| Post-harvest cabbage, lettuce, and figs |
Salmonella typhimurium L. monocytogenes |
Microwave-powered cold plasma | Lee et al., 2015 |
| Onion, radish, cress, and alfalfa seeds | E. coli | Atmospheric-pressure volumetric DBD plasma | Butscher et al., 2016a |
| Wheat seeds | Geobacillus stearothermophilus | Atmospheric-pressure DBD plasma | Butscher et al., 2016b |
| Post-harvest romaine lettuce |
E. coli O157:H7 Salmonella L. monocytogenes |
DBD atmospheric cold plasma | Min et al., 2016 |
| Cruciferous seeds | Xanthomonas campestris | Low-pressure plasma | Nishioka et al., 2016 |
| Post-harvest radicchio leaf |
E. coli O157:H7 L. monocytogenes |
DBD atmospheric cold plasma | Pasquali et al., 2016 |
| Hydroponic solution | R. solanacearum | Discharge plasma reactor | Okumura et al., 2016 |
| Post-harvested grape tomato, spinach, and cantaloupe |
E. coli O157:H7 S. typhimurium Listeria innocua |
Cold plasma-activated hydrogen peroxide aerosol | Jiang et al., 2017 |
| Broccoli seeds |
Bacillus cereus E. coli Salmonella spp. Staphylococcus aureus L. monocytogenes |
Corona discharge plasma jet | Kim et al., 2017 |
| Rice seeds | Burkholderia plantarii | Atmospheric-pressure plasma jet | Ochi et al., 2017 |
| Suspension culture | X. campestris pv. campestris | Roller conveyer atmospheric-pressure plasma | Toyokawa et al., 2017 |
| Post-harvest almonds |
E. coli Salmonella spp. Shigella |
Gliding arc non-thermal plasma | Fatemeh et al., 2018 |
| Post-harvest perilla leaves |
E. coli S. aureus |
Cylinder-type DBD plasma with underwater bubbler | Ji et al., 2018 |
| Post-harvest black peppers |
B. subtilis E. coli S. enteritidis |
Diffuse coplanar surface barrier discharge plasma | Mošovská et al., 2018 |
| Suspension culture |
Clavibacter michiganensis subsp. sepedonicus Dickeya solani X. campestris pv. campestris Pectobacterium atrosepticum Pectobacterium carotovorum subsp. carotovorum |
Glow discharge plasma | Motyka et al., 2018 |
| Post-harvest kumquat fruits |
S. aureus Salmonella spp. B. cereus |
Intermittent corona discharge plasma jet | Puligundla et al., 2018b |
| Post-harvest lettuce and sprout |
E. coli K12 Pseudomonas fluorescens Pseudomonas marginalis P. carotovorum L. innocua |
Microwave plasma processed air | Schnabel et al., 2018 |
| Lentil seeds |
E. coli L. monocytogenes S. enterica S. aureus G. stearothermophilus |
Diffuse coplanar surface barrier discharge plasma | Waskow et al., 2018 |
| Post-harvest mung bean sprout | Aerobic bacteria | Plasma-activated water | Xiang et al., 2019 |
Table 2.
Control of fungal pathogens by atmospheric-pressure non-thermal plasma.
| Treated object | Target fungi | Plasma types | Reference |
|---|---|---|---|
| Fungal spore suspension |
Aspergillus niger Penicillium citrinum |
Microwave-induced argon plasma | Park et al., 2003 |
| Fungal spore suspension |
A. niger Cladosporium cladosporioides P. citrinum Chaetomium sp. |
Microwave-induced argon plasma | Park et al., 2004 |
| Fungal spore suspension |
A. niger P. citrinum |
Hydrogen releasing atmospheric-pressure plasma | Nojima et al., 2007 |
| Post-harvest nuts | Aspergillus parasiticus | Low-pressure cold plasma | Basaran et al., 2008 |
| Grains and legume seeds |
Aspergillus spp. Penicillium spp. |
Low-pressure plasma | Selcuk et al., 2008 |
| Fungal spore suspension |
Ascochyta pinodella Fusarium culmorum |
Atmospheric-pressure dielectric barrier discharge (DBD) plasma | Avramidis et al., 2010 |
| Fungal spore suspension | Penicillium digitatum | High-density non-equilibrium atmospheric-pressure plasma | Iseki et al., 2011 |
| Post-harvest grape and banana | Fungi | High-field plasma | Liu et al., 2011 |
| Fungal spore suspension |
Fusarium graminearum Fusarium oxysporum |
Microwave plasma jet | Na et al., 2013 |
| Post-harvest rice and lemon |
Aspergillus oryzae P. digitatum |
Atmospheric-pressure plasma | Hayashi et al., 2014 |
| Rice seeds | Fusarium fujikuroi | Atmospheric-pressure non-thermal DBD plasma | Jo et al., 2014 |
| Fungal spores and tomato seeds | Cladosporium fulvum | Atmospheric-pressure plasma jet | Lu et al., 2014 |
| Cicer arietinum seeds | Infected microbes | Surface micro-discharge plasma FlatPlaSter 2.0 | Mitra et al., 2014 |
| Brassicaceous seeds | Rhizoctonia solani | Atmospheric- and low-pressure plasma | Nishioka et al., 2014 |
| Plant leaves | Colletotrichum gloeosporioides | Atmospheric-pressure plasma jet | Zhang et al., 2014 |
| Fungal spore suspension | F. oxysporum | Micro DBD plasma | Panngom et al., 2014 |
| Barley and corn seeds | Infected fungi | Glow discharge plasma | Braşoveanu et al., 2015 |
| Fungal spore suspension | P. digitatum | Flux-defined atmospheric-pressure oxygen radical source | Hashizume et al., 2015 |
| Rice seeds | F. fujikuroi | Arc discharge plasma | Kang et al., 2015 |
| Wheat seeds |
Alternaria alternata Alternaria botrytis Alternaria brasiliensis F. culmorum Furasium oxysporum Gibberella avenacea Gibberella intricans Gibberella zaea Penicillium spp. Rhizopus stolonifera Trichoderma spp. Non–spore-forming fungi |
Low-temperature plasma | Kordas et al., 2015 |
| Post-harvest blueberries | Contaminated fungi | Atmospheric-pressure cold plasma | Lacombe et al., 2015 |
| Post-harvest date palm fruit | A. niger | Double atmospheric-pressure cold plasma | Ouf et al., 2015 |
| Post-harvest maize | Aspergillus spp. | Atmospheric-pressure fluidized bed plasma | Dasan et al., 2016a |
| Post-harvest hazelnuts |
Aspergillus flavus A. parasiticus |
Atmospheric-pressure fluidized bed plasma | Dasan et al., 2016b |
| Rice seeds | Infected fungi | Atmospheric hybrid micro corona discharge plasma | Khamsen et al., 2016 |
| Post-harvest pistachio nuts | A. flavus | Cold plasma streamer | Sohbatzadeh et al., 2016 |
| Post-harvest citrus | P. digitatum | Atmospheric-pressure DBD plasma | Yagyu et al., 2016 |
| Wheat seeds |
Fusarium nivale F. culmorum Trichothecium roseum A. flavus Aspergillus clavatus |
Diffuse coplanar surface barrier discharge plasma | Zahoranova et al., 2016 |
| Basil seeds | Infected fungi | Surface DBD plasma | Ambrico et al., 2017 |
| Post-harvest hazelnuts | Aspergillus spp. | Atmospheric-pressure fluidized bed plasma | Dasan et al., 2017 |
| Broccoli seeds | Infected molds and yeasts | Corona discharge plasma jet | Kim et al., 2017 |
| Rice seeds | F. fujikuroi | Atmospheric-pressure plasma | Ochi et al., 2017 |
| Cucumber and pepper seeds |
Didymella bryoniae Didymella licopersici Cladosporium cucumerinum |
Diffuse coplanar surface barrier discharge plasma | Stepanova et al., 2017 |
| Post-harvest mandarin fruit | Penicillium italicum | Atmospheric-pressure cold plasma | Won et al., 2017 |
| Barley and wheat seeds | Penicillium verrucosum | High-voltage DBD plasma | Los et al., 2018 |
| Soybean seeds | Diaporthe/Phomopsis | Atmospheric-pressure DBD plasma | Pérez Pizá et al., 2018 |
| Pak choi seeds | Infected fungi | Corona discharge plasma jet | Puligundla et al., 2018a |
| Post-harvest kumquat fruits | P. digitatum | Intermittent corona discharge plasma jet | Puligundla et al., 2018b |
| Lentil seeds |
Penicillium decumbens A. niger |
Diffuse coplanar surface barrier discharge plasma | Waskow et al., 2018 |
| Pine seeds | Fusarium circinatum | Diffuse coplanar surface barrier discharge plasma | Sera et al., 2019 |
| Fungal spore suspension | C. gloeosporioides | Atmospheric-pressure corona plasma-activated water | Wu et al., 2019 |
| Post-harvest mung bean sprout | Infected fungi | Plasma-activated water | Xiang et al., 2019 |
| Maize seeds |
A. flavus A. alternata F. culmorum |
Diffuse coplanar surface barrier discharge plasma | Zahoranova et al., 2019 |
Bacterial Pathogens
Although causing a smaller number of diseases and relatively less economic damage than fungi or viruses, plant pathogenic bacteria have a great negative impact on the economic condition in many agricultural countries. Approximately 150 species of bacteria have been found to cause plant diseases. Bacterial pathogens are transferred by biological vectors, such as insects and weeds, and physical factors, such as wind and rain, under field conditions (Agrios, 1997). These pathogens invade plants either through natural openings, such as the stomata and lenticels, or by wounding and moving inside the plant through the xylem (Akhavan et al., 2013). Disease symptoms appear in the form of specks, spots, blights, vascular wilts, tumors, rots, and cankers on the leaves, flowers, fruits, stems, roots, and tubers of affected plants (Horst, 2001); a set of pathogenicity genes are responsible for the establishment of a disease (Salmond, 1994; van Baarlen et al., 2007).
Atmospheric-pressure non-thermal plasma has demonstrated antibacterial potential by inhibiting and reducing the growth of bacteria (Mai-Prochnow et al., 2014; Gilmore et al., 2018). The majority of studies have been focused on food-contaminating and -poisoning bacteria, such as Escherichia coli, Staphylococcus aureus, Listeria, and Salmonella (Baier et al., 2013; Sun et al., 2014; Baier et al., 2015; Matan et al., 2015). Compared to that, the treatment of bacteria causing plant diseases has been relatively less explored in the application of plasma (Table 1). Several studies demonstrate that plasma treatment of phytopathogenic bacterial suspension can reduce the number of viable bacteria in a time-dependent manner (Moreau et al., 2007; Mráz et al., 2014; Toyokawa et al., 2017; Motyka et al., 2018). Gliding arc discharge can effectively inactivate the potato pathogen Erwinia carotovora by altering the bacterial membrane (Moreau et al., 2007). Plasma exposure on two plant pathogenic bacteria, Clavibacter michiganensis (Gram-positive) and Erwinia amylovora (Gram-negative), decelerated their growth and reproduction (Mráz et al., 2014). Recently, the direct contact of bacterial suspensions with direct current (DC)-based glow discharge plasma was found to rapidly eradicate phytopathogenic bacteria, such as Clavibacter michiganensis subsp. sepedonicus, Dickeya solani, Xanthomonas campestris pv. campestris, Pectobacterium atrosepticum, and Pectobacterium carotovorum subsp. carotovorum (Motyka et al., 2018). The viability of X. campestris pv. campestris placed on a roller plasma conveyer was decreased, showing significant degradation of lipopolysaccharides and oxidation of genomic DNA (Toyokawa et al., 2017). The pathogenicity and colony forming unit of Ralstonia solanacearum were significantly reduced after the discharge plasma treatment of a hydroponic solution (Okumura et al., 2016). Plasma processed air (PPA) is a novel concept that can be used in the food packaging industry to reduce the load of microbes on packaging material and fresh produce. PPA treatment for 5 min reduced the contamination of PET packaging material with Pseudomonas fluorescens and P. carotovorum by 2 log10 cfu ml−1. A reduction higher than 5 log10 cfu ml−1 was observed for P. carotovorum contamination on sprouts (Schnabel et al., 2018). These studies show no specificity between food and plant pathogenic bacteria with respect to the bactericidal effect of plasma. In addition, plasma treatment can efficiently reduce the plant pathogenic bacterial contamination in water or waste, which may act as a source of plant disease inoculum. However, plasma often induces the sub-lethal state of bacteria to the viable but non-culturable (VBNC) state (Xu et al., 2018); thus, intense research is still needed for complete eradication.
Non-thermal plasma produces many reactive oxygen and nitrogen species (RONS), which are toxic to bacterial pathogens at high concentrations. These RONS oxidize proteins, lipids, and nucleic acids and lead to pathogen destruction. They also drive epigenetic regulation, which might abolish bacterial pathogenicity (Casadesus and Low, 2006). A detailed mechanism of plasma action is discussed later in this review.
Fungal Pathogens
Non-thermal plasma has been frequently applied to inactivate fungal pathogens during food decontamination (for review, Misra et al., 2019). In these studies, the potentiality of plasma is well described in inactivating fungal spores and eradicating mycotoxins. In contrast, the inactivation of plant pathogenic fungi by plasma has been relatively less reported than that of food-spoiling fungi. The plasma-mediated eradication of fungal spores can prevent disease development after germination, inoculum spread, and post-harvest diseases. The efficient inactivation of spores in suspension by plasma has been demonstrated in several phytopathogenic fungi such as Aspergillus, Penicillium, Fusarium, Cladosporium, Phomopsis, Colletotrichum, Ascochyta, Chaetomium, and Rhizoctonia (see Table 2). In these studies, various plasma sources using different combinations of gases were used to eradicate fungal spores submerged in liquid culture media or water under laboratory conditions.
Many studies showed efficient fungal spore eradication by dielectric barrier discharge (DBD)-type plasma (Nojima et al., 2007; Avramidis et al., 2010; Iseki et al., 2011; Panngom et al., 2014; Hashizume et al., 2015). Particularly, Nojima et al. (2007) developed a plasma device releasing atomic hydrogen and showed the deactivation of fungal pathogens and the neutralization of harmful OH radicals in the air by the atomic hydrogen released from plasma. In this study, O and OH radicals generated by plasma were not major players in inactivating fungal spores in the air because of their short lifetime. Atomic hydrogen produced from the plasma device was suggested to be critical for inactivating fungal spores. A hydrogen atom surrounded by water molecules, H+(H2O)m, has a long lifetime (3–5 s in air) and reacts with O2 and O2−(H2O)n in the air, forming HO2 or HO2−, which are very reactive and thus can cause damage to fungal spores. However, the authors also found an additional effect of atomic hydrogen; it alleviates the toxicity of OH radicals in the air to the user. This study was a good example that key plasma factor(s) responsible for antimicrobial activity can vary depending on the condition and plasma sources. The plasma-treated fungal spores often show severe morphological degeneration and seem to undergo necrotic death. However, one study demonstrated the possibility that the fungal spores treated with micro DBD plasma can undergo apoptosis-like death without severe morphological destruction (Panngom et al., 2014). Besides fungal spores, fungal hyphae were occasionally the target of plasma treatment (Avramidis et al., 2010). Plasma can also be produced using microwave power without electrodes, and the microwave-induced plasma can destroy fungal spore cells and inhibit subsequent hyphal growth of Penicillium citricum, Fusarium graminearum, and F. oxysporum (Park et al., 2003; Park et al., 2004; Na et al., 2013).
Plasma-treated water also exhibits anti-fungal activity. Wu et al. (2019) demonstrated that the air and oxygen plasma-activated water (PAW) deactivated the spores of C. gloeosporioides. In their study, the air PAW was more efficient than the oxygen PAW, and the long-lived species, such as nitrate and ozone, generated in PAW might play a critical role in this anti-fungal effect (Wu et al., 2019).
Viral Pathogens
Compared to bacterial and fungal phytopathogens, plant viruses have hardly been examined in terms of plasma application. The antiviral effects of plasma have been occasionally demonstrated in human and animal viruses, as well as bacteriophages (Zimmermann et al., 2011; Aboubakr et al., 2015; Guo et al., 2018). The inactivation of plant viruses by plasma was demonstrated in a recent study (Hanbal et al., 2018). In this study, inoculation with plasma irradiated tobacco mosaic virus (TMV) solution did not induce the development of disease in tobacco plants, whereas necrotic local lesions developed on tobacco leaves inoculated with non-irradiated TMV solution (Hanbal et al., 2018). Min et al. (2016) also showed that viral load was significantly reduced when romaine lettuce inoculated with Tulane virus was treated with DBD plasma.
Plasma Application in Disease Control
Seed-Borne Diseases
Seed contamination with pathogenic microorganisms can cause diseases in seedlings, leading to a significant reduction in crop yield. In addition, the pathogens preserved in seeds can be a source of disease propagation. Since many seed-borne diseases are associated with the infection of a flowering part during the pre-harvest period, the pre-harvest control of diseases might be critical for obtaining healthy and high-quality seeds. However, the treatment of post-harvest seeds is also prevalent for controlling seed-borne diseases (Hertwig et al., 2018). Seed treatment for preventing pathogenic diseases focuses on the inactivation of pathogens that are present on the surface and inside of infected seeds. Chemical-based microbicides have been frequently used in the decontamination of seeds, but alternative methods have also been attempted to complement the disadvantages of chemical control tools.
Application of atmospheric-pressure non-thermal plasma to control seed-borne diseases has been mostly performed by exposing the seeds to direct plasma, plasma-generated gas, or plasma-treated water. A significant number of studies have demonstrated that plasma and plasma-originated gas or water can effectively reduce the microbial load in seeds used either as food or for crop production (see Tables 1 and 2). To prevent seed-borne diseases, pathogenic microorganisms contaminating seeds should be inactivated. Many studies demonstrated the plasma-mediated removal of pathogenic microbes from the seed surface (see Tables 1 and 2). In these studies, the direct plasma treatment was most frequently performed on seeds. Plasma-generated gas (Los et al., 2018) and plasma discharged in water (Kang et al., 2015) have also been used to disinfect seeds. In addition, low-pressure plasma treatment showed the efficient removal of pathogenic bacteria from the vegetable seeds (Nishioka et al., 2016).
Despite its efficiency, seed disinfection by plasma demonstrates several issues that need to be solved. First, the standardization of the plasma dose for effective seed disinfection is needed. The plasma treatment unit has recently become a hot topic in plasma bioscience research community (Fridman et al., 2018). Treatment time is not enough to be a standardized unit because plasma sources and parameters vary among research groups. In addition, plasma generates various components, such as reactive species, ions, UV, and electrons, and the level of these components can be greatly affected by surrounding environmental condition. Therefore, the definition of a plasma dose in common terms should be established for fine-tuning plasma application. Second, there is still a great need for experimental evidences on the vitality of the germinated seedlings or plants from plasma-treated seeds. Several studies have reported an improvement in seed germination as well as microbial disinfection after plasma treatment (Mitra et al., 2014; Kordas et al., 2015; Khamsen et al., 2016; Stepanova et al., 2017). This might be a consequence of the removal of pathogenic microbes or activation of the seed germination process by plasma itself (Randeniya and de Groot, 2015). In many studies, plasma treatment demonstrated no adverse effects on seed vitality (such as germination and subsequent growth) at antimicrobial doses (Selcuk et al., 2008; Jo et al., 2014). These results indicate that the plasma treatment is a quite promising technique for seed disinfection.
The mechanisms of seed disinfection by plasma are not yet completely elucidated. Studies have demonstrated the harmful effects of plasma-generated reactive species, charged species, and UV photons on microbial cells (reviewed in Hertwig et al., 2018). Radicals and excited molecules generated from plasma can erode the surface of microbial cells through etching and, thus, lead to their inactivation (Moisan et al., 2001). Plasma-generated reactive species can cause oxidative damage to intracellular macromolecules, such as membrane lipids, proteins, and DNA, and a reduction in intracellular pH from diffusion into the microbial cells disrupting pH homeostasis (Laroussi and Leipold, 2004; Li et al., 2013). Microbial cell membranes can be electrostatically disrupted through the accumulation of charged species on the surface, and UV photons from plasma can induce DNA damage (Laroussi, 2002). Although the plasma-mediated inactivation of microbes on the seed surface is likely caused by the adverse effects of plasma on microbial cells, additional factors, such as the interaction between plasma and the seed surface, should be also considered. The physicochemical changes occurring on the seed surface can cause the damage on microbes (Mitra et al., 2014). More intense studies are needed to elucidate the mechanisms underlying microbial inactivation.
Foliage and Root Diseases
Foliage and root diseases are some of the most serious plant diseases in agriculture because of the difficulties in controlling them. Leaf and root infection with bacterial and fungal pathogens might cause a serious reduction in the quality and yield of plants, particularly of leafy and root vegetables. Compared to seeds, leaves and roots are relatively more difficult to treat with plasma because they are possibly more liable to be damaged and changed by the plasma treatment (Zhang et al., 2014; Seol et al., 2017).
Spores of F. oxysporum, a pathogenic fungus infecting tomato root, can be inactivated after plasma treatment in saline (0.85% NaCl), as demonstrated by Panngom et al. (2014). However, plasma treatment on infected leaves or roots has rarely been reported. Zhang et al. (2014) reported that the plasma jet treatment of fungal infected spots on leaves resulted in the recovery of small-sized infected spots to the normal state. In this study, authors suggested a possible mode of plasma action by which the plasma-generated reactive species could penetrate leaf tissues through the stomata and inactivate fungal cells inside tissues (Zhang et al., 2014).
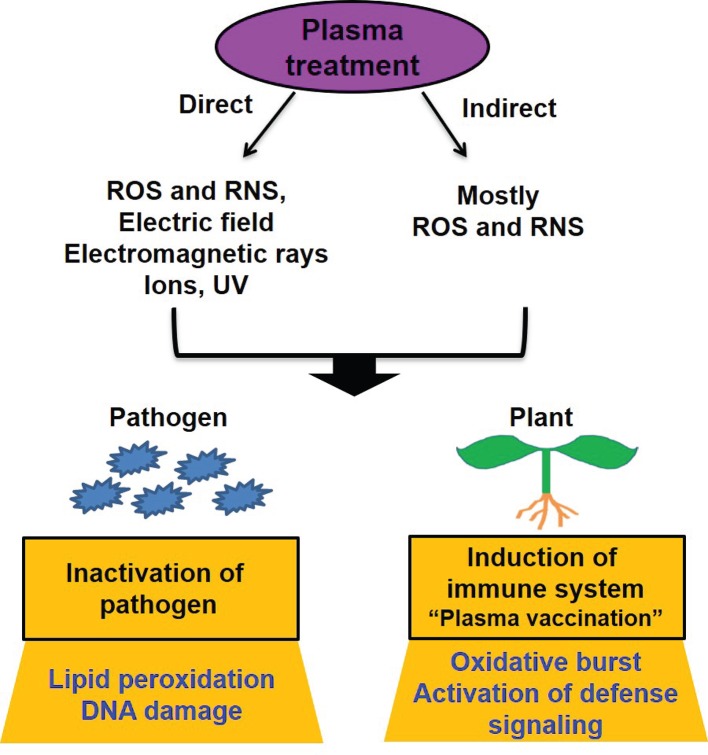
Recently, a new direction has been added to plasma application for the control of foliage and root diseases—the induction of plant resistance by plasma, or so-called “plant vaccination” (Figure 1). The RONS are well-known signaling molecules regulating disease stresses in plants (Simontacchi et al., 2015; Dietz et al., 2016). Since plasma produces reactive species, the induction of disease tolerance and resistance can be possibly triggered by plasma treatment. Several studies have demonstrated experimental evidence supporting this notion. Jiang et al. (2014) observed that tomato seed germination and growth were increased after plasma treatment, and plants became more resistant to bacterial wilt disease in the leaves. After inoculation with a bacterial pathogen, the amount of H2O2 and the activities of resistant enzymes were increased more in plasma-treated plants than in untreated plants (Jiang et al., 2014). The application of PAW reduced the infection rate of Xanthomonas vesicatoria causing leaf spot disease in tomato plants to approximately 83% and increased the expression of disease resistance genes (Bertaccini et al., 2017). In addition, PAW effectively activated the grapevine defense response to phytoplasma (parasitic plant bacteria lacking cell walls) in a vineyard (Bertaccini et al., 2017), indicating the ability of PAW to induce disease resistance. Interestingly, Panngom et al. (2014) observed that the exposure of tomato plant leaves to non-thermal micro DBD plasma also induced an increase in the expression level of pathogenesis-related (PR) genes in the roots without significantly affecting plant growth. This indicates that plasma treatment of plant leaves can induce the development of defense barriers in other plant parts, such as the roots, possibly through defense hormone signaling.
Figure 1.
Models of plant disease control by plasma. Plasma can be applied directly or indirectly (plasma-treated water or media) to plants. Many plasma factors such as ROS, RNS, electric field, electromagnetic rays, active ions, and UV can be involved in disease control in direct plasma treatment whereas ROS and RNS from plasma are major players in indirect plasma treatment. Plasma (direct and indirect treatment) can inactivate pathogens associated with plants and seeds by causing membrane lipid peroxidation and DNA damage. In addition, it can be possible that plasma (direct and indirect treatment) induces plant immune responses by causing oxidative burst and continuously activating defense signaling, leading to the expression of defense genes.
Post-Harvest Diseases
The microbial contamination of post-harvest crop products can occur during pre-harvest, harvest, transportation, storage, and distribution of products to the consumer. Post-harvest diseases lead to the spoilage of plant products used as food, resulting in great economic loss. Surprisingly, more than a third of fruits and vegetables become unavailable to the consumers because of the pathogenic infections (Oerke and Dehne, 2004). Alternative technologies are particularly needed for post-harvest disease control because traditional strategies, such as breeding for disease resistance and the use of chemical agents, have continuously generated problems.
Application of the non-thermal plasma technology for post-harvest disease control has been more often demonstrated in the inactivation of fungal pathogens contaminating vegetables and fruits (Table 2), probably because fungal infection and contamination are a major threat to post-harvest fruits and vegetables (Petriacq et al., 2018). Moreover, the contamination with fungal mycotoxins is a serious problem for food safety because they are very harmful to human and animal health. Fungal pathogens, such as Alternaria, Aspergillus, Botrytis, Colletotrichum, Fusarium, Penicillium, Rhizopus, and Trichothecium, predominantly contaminate post-harvest fruits and vegetables (Petriacq et al., 2018). As described in the earlier section, seed grains, such as rice, barley, wheat, and corn, can be efficiently disinfected by atmospheric-pressure non-thermal plasma without causing serious harmful effects on seeds. The contamination of hazelnuts, pistachio nuts, peanuts, and maize with A. flavus and A. parasiticus can be reduced through a low- or atmospheric-pressure cold plasma treatment (Basaran et al., 2008; Dasan et al., 2016a; Dasan et al., 2016b; Sohbatzadeh et al., 2016; Dasan et al., 2017). Fungal contamination on harvested fruits can be removed by treating them with plasma, as demonstrated in grape, banana, lemon, blueberries, date palm, and citrus fruits (Liu et al., 2011; Hayashi et al., 2014; Lacombe et al., 2015; Ouf et al., 2015; Yagyu et al., 2016; Won et al., 2017; Puligundla et al., 2018b). In most of these studies, fresh fruits were treated directly with plasma. However, Hayashi et al. (2014) treated lemons with active oxygen species generated by a combination of atmospheric-pressure plasma and UV light in ambient air. Liu et al. (2011) developed a special chamber equipped with a high-field plasma system under temperature and humidity control and demonstrated the longer preservation of grapes and bananas in this chamber without increasing bacterial and fungal infection on the fruit surface. In this system, there were no apparent discharges between electrodes and, therefore, the production of harmful factors, such as ozone, OH radicals, and UV, was suppressed. This study indicates that a high electric field generated from plasma could play a major role in fungal inactivation. Plasma can also be used to remove the fungal contamination on fresh vegetables, such as mung bean sprouts, and hence improve their shelf-life (Xiang et al., 2019). However, the fungal disinfection of fresh vegetables is not reported as frequently as that of fresh fruits.
The efficient sanitation of bacteria-contaminated fresh leafy vegetables by plasma is frequently reported, as illustrated in corn salad leaves, vegetable radicchio leaf, romaine lettuce, perilla, cabbage, spinach, and sprout vegetables (see Table 1). These studies also demonstrated that plant quality characteristics, such as pigmentation and leaf properties, are not significantly affected by the plasma treatment. Furthermore, the plasma treatment showed the efficient disinfection of harvested fruits, such as grape, banana, blueberry, tomato, strawberry, pepper, cantaloupe, and citrus, contaminated with Bacillus, E. coli, Listeria, Salmonella, and Staphylococcus (Liu et al., 2011; Niemira, 2012; Misra et al., 2014; Ziuzina et al., 2014; Hertwig et al., 2015; Jiang et al., 2017; Mošovská et al., 2018; Puligundla et al., 2018b). These studies showed that the efficiency of disinfection by plasma is different among species. Bermudez-Aguirre et al. (2013) compared the efficiency of bacteria removal by plasma among three fruits and vegetables, i.e. lettuce, carrots, and tomatoes, contaminated with E. coli, and found that tomato was disinfected more efficiently than lettuce and carrots. The researchers noted that the higher disinfection efficiency of tomato might be caused by its smooth surface structure (Bermudez-Aguirre et al., 2013). As demonstrated in many studies, the plasma treatment does not seem to damage the color and firmness of fruits and vegetables. Misra et al. (2014) demonstrated a 2-log10 reduction in bacterial load on strawberries inside a sealed package within 24 h after a 5-min treatment with plasma generated inside the package using ambient air (42% relative humidity), with no significant change in the color and firmness of strawberries. However, alterations at the cellular level, such as cell membrane irregularity, were observed (Bermudez-Aguirre et al., 2013). Dried fruits, such as dried figs, contaminated with bacteria were efficiently disinfected by plasma under low pH conditions because bacteria on the fruit surface were more sensitive to plasma treatment under the low pH conditions (Lee et al., 2015). Post-harvest almonds inoculated with E. coli, Salmonella, and Shigella have also been reportedly sanitized by the plasma treatment (Niemira, 2012; Fatemeh et al., 2018).
Possible Mechanism(s) of Plasma Action in Disease Control
Non-thermal atmospheric-pressure plasma is well known for generating different types of RONS, along with UV, charged active species, electric field, and electromagnetic rays (Jha et al., 2017). Among these factors, RONS are most frequently stated as key players in microbial inactivation (Graves, 2012; Schmidt et al., 2017; Julák et al., 2018). Particularly, RONS are major components in plasma treated water or media considered as indirect plasma treatment (Figure 1). Plasma is generally known to generate reactive oxygen species (ROS), such as singlet oxygen, superoxide, ozone, and hydroxyl radical, and reactive nitrogen species, such as nitric oxide and nitric dioxide (Kim and Chung, 2016). Plasma might be used as an antimicrobial tool via the synergistic effect of various RONS and other physical factors. However, the composition of plasma-generated RONS can be varied depending on the used feeder gas (source), plasma device setting, and environmental conditions; thus, the efficiency of microbial inactivation can fluctuate (Girard et al., 2016). Each RONS can exert distinctive effects on microbial growth and plant growth and immunity (Turkan, 2018).
To understand the mechanism(s) of plasma-mediated disease control, the interaction of plasma-generated RONS with plant and microbial cells and their microenvironment should be established. The plasma-generated RONS can directly affect microbial cells or interact with the medium (buffer, growth media, or water) between the plant and microbial cells producing various RONS. The build-up of oxidative stress generated by RONS in the plant cells/pathogen microenvironment might eventually destroy microbial cells rather than plant cells because the threshold level of oxidative stress harmful to plant cells might be higher than that harmful to microbial cells (Dobrynin et al., 2009; Gay-Mimbrera et al., 2016). Although both plants and pathogens have antioxidant systems to alleviate oxidative stress, continuous or long-term exposure to plasma-generated RONS might enhance the level of intercellular RONS, which might overcome the antioxidant activity of microbial cells.
RONS play a differential role in plant–pathogen interactions. Their content upsurges in plants during the invasion of a pathogen. They either inactivate the pathogen growth or elicit the pathogen defense-related gene expression system (Shetty et al., 2008). Higher concentrations of ROS, such as superoxide, hydroxyl ion, and hydrogen peroxide, are toxic to pathogens and destroy them via oxidation of their macromolecules. At lower concentrations, RONS, such as NO and H2O2, act as signaling molecules to activate the defense signaling cascades and production of salicylic and jasmonic acids, inducing the systemic acquired resistance and hypersensitive reaction in infected plant parts (Torres et al., 2006).
Direct Effect of RONS on Pathogens
Plasma-generated RONS are assumed to be the prime factors producing microbicidal effects (Dezest et al., 2017). These RONS, upon direct contact with a pathogen, affect its cell membrane. A short exposure to plasma leads to the loss of its culturability or pathogenicity, without affecting its metabolic activity (Dolezalova and Lukes, 2015). This VBNC state in pathogens might result from the initiation of cell membrane degradation by plasma-generated RONS. A longer plasma exposure or post-treatment incubation inactivates the pathogen up to 100% by disrupting the whole cell membrane (Joshi et al., 2011). Dezest et al. (2017) suggested the mechanism of plasma-induced bacterial inactivation by comparing the plasma treatments using three gases (He, He-N2, and He-O2) and different treatment methods. They observed that the He plasma treatment of PBS caused a greater inactivation of bacteria compared to that in the direct treatment. However, in the case of He-N2 plasma, both direct and indirect treatments of bacteria exerted bactericidal effects. In the case of He-O2 plasma, only direct treatment produced short-lived reactive species, such as 1O2, O2.−, and ·OH, and exerts bactericidal activity. In liquids treated with He and He-N2 gas plasmas, H2O2 and NO are the major active components that produce bactericidal effects. Regarding the fungicidal mechanism of plasma, a study demonstrated that F. oxysporum spores might be inactivated by the peroxynitrite and hydroxyl radicles produced in saline treated with argon plasma (Panngom et al., 2014). Ochi et al. (2017) also showed that seed treatment with cold atmospheric plasma suppressed the progression of bakanae (Fusarium fujikuroi) and blight (Burkholderia plantarii) diseases in rice seedlings, and the ROS-mediated pathogen inactivation and immunity stimulation of rice seedlings might be responsible for disease suppression.
Plasma-generated RONS target proteins (intracellular and membrane proteins) rather than lipids. They modify a protein molecule by attaching to a carbonyl moiety (Weber et al., 2015). Several studies have reported that plasma-emitted RONS, charged particles, and electric waves could disrupt the membrane integrity of cells and penetrate them by causing surface abrasions in their membranes (Deng et al., 2010; Hensel et al., 2015; Wende et al., 2015). In a pathogen, the movement of ROS from environment through cell membrane causes oxidative stress, and the oxidation of cell regulatory macromolecules, such as proteins, nucleic acids, and lipids, ultimately leads to cell death (Winter et al., 2011; Lackmann and Bandow, 2014). Interestingly, Han et al. (2016) proposed the different modes of action of plasma-generated ROS on the inactivation of Gram-positive and Gram-negative bacteria. Gram-negative bacteria were inactivated mainly by cell leakage via cell envelope damage caused by plasma-generated ROS. In Gram-positive bacteria, significantly increased levels of intracellular ROS were observed with little envelope damage after plasma treatment, and high levels of ROS damage intracellular biomolecules, such as protein and nucleic acid, leading to bacterial inactivation. According to this study, the thickness of the peptidoglycan layer is not likely to influence greatly on the level of bacterial damage by plasma-generated ROS because both Gram-negative and Gram-positive bacteria are damaged at similar levels. Lipid peroxidation by plasma-generated ROS on the outer membrane lipopolysaccharides of Gram-negative bacteria resulted in breakage of the cell envelope, whereas the oxidation of peptidoglycan did not. This indicated that different structures of the bacterial cell wall may be critical for the mode—but not the level—of cell damage by plasma-generated ROS.
Role of RNS and ROS in Plant Immunity
The plant–pathogen interaction is a complex and evolved process initiated at the time when pathogens try to invade the plant tissue. In this process, pathogens attempt to invade and proliferate, and plants try to identify them and activate their immune system. A major response of plants against pathogen invasion is to speed up the production of RONS in their cells (Fones and Preston, 2012). The accumulation of RONS inside plant cells (oxidative burst) excites their own antioxidant system and is toxic to the invading pathogens. These RONS not only destroy the pathogen, but also activate signaling cascades related to plant defense gene expression and hypersensitive response (Chris and Richard, 1997; Vanacker et al., 1998).
Plasma-generated RONS can enter the plant either through wounding, mechanical stress (in direct treatment), or small openings, such as the stomata. After entering the plant, they can be sensed by the plant cells, leading to the oscillation of intracellular RONS concentration. Unstable RONS, such as superoxide and singlet oxygen, might react with cellular components at close proximity, whereas stable RONS, such as H2O2 and NO, can move through the cell wall via diffusion. In the apoplast, the intracellular space between plasma membrane and cell wall, H2O2 and NO diffuse freely and interact with the cell membrane receptors. The apoplastic NO and H2O2 can move into the apoplast through aquaporins (Jang et al., 2012). Both NO and H2O2 act as signaling molecules and activate the expression of different defense-related genes, proteins, and hormones.
As a signaling molecule, hydrogen peroxide (H2O2) mainly targets Ca2+ homeostasis and alters ion channels, transcription factors, kinases, and phosphatases by oxidizing the methionine residues of proteins (Ghesquière et al., 2011; Petrov and Van Breusegem, 2012). The mitogen-activated protein kinase (MAPK) cascade, which is activated during plant–pathogen interactions, is dependent on the H2O2 signal (Zhang et al., 2007; Xing et al., 2008). The MAPK enzyme phosphorylates many transcription factors, such as WRKY and ANAC042. Transcription factors, such as NAC, ZAT, DREB, bZIP, and MYB, are also activated by H2O2 signaling (Petrov and Van Breusegem, 2012). These activated transcription factors can be involved in regulating the expression of defense-related genes. H2O2 also activates the benzoic acid 2-hydroxylase enzyme, which catalyzes the synthesis of salicylic acid from benzoic acid. Salicylic acid triggers the systemically acquired response and induces PR gene expression in cells (Leon et al., 1995).
Another signaling species, nitric oxide, triggers a hypersensitive response, an evolved innate immune response to protect plants from pathogens and herbivores, by regulating jasmonic acid signaling cascade proteins through the nitrosylation and de-nitrosylation of tyrosine residue. Nitric oxide also modifies the conformation of NPR1 (nonexpressor of PR gene 1) by the S-nitrosylation of Cys156 (Tada et al., 2008). Other than activating the plant immune response, nitric oxide also regulates the virulence and developmental stages of fungal pathogens in plants (Arasimowicz-Jelonek and Floryszak-Wieczorek, 2016).
Plasma Technology Transfer to Agricultural Industry
For the past few decades, plasma technology has been frequently used in electronics, surface modification, and the energy saving industry (Weltmann et al., 2019). Moreover, promising research on plasma technology applications in biological science and environmental conservation has gained both public and industry attention. Food security, food packaging, and resources conservation are important parts of the agriculture industry. Plasma technology has been proven to have potential in agricultural applications, such as enhancing seed germination, seed decontamination, physiological plant growth, plant disease control, soil remediation, biological and chemical decontamination, water and gas cleaning, and food packaging and sterilization (Brandenburg et al., 2019). However, only a few of these studies were further processed for transferring the technology to the industrial scale. In Germany, plasma-treated water has been proven to have the ability to decontaminate lettuce on an industrial scale (Andrasch et al., 2017). In Japan, the University of Iwate collaborated with Energy Support Corporation, Japan, to perform a field investigation into delaying fruit ripening during transportation by the decomposition of ethylene gas using DBD plasma (Takahashi et al., 2018). The primary challenges in the transfer of this technology from the lab to industry are the 1) technology efficacies, 2) scale-up and design of the technology according to the industry, 3) toxicology and dose, 4) regulatory approval and validation of the technology, and 5) consumer acceptance (Cullen et al., 2018).
Non-thermal plasma technology has shown greater efficacies in the food industry because it enhances the shelf-life of food products; maintains the quality of food products; enhances the chemical safety of food products; has low energy requirements and low operational and maintenance costs; and is ecofriendly being considered as a green technology. However, scaling up plasma technology for the industrial process without compromising plasma uniformity remains a significant challenge in the food industry. The regulation and validation of the plasma process has met various challenges in different industrial areas because of its multivariate, multi-timescale, time variance, and non-linear operations between different products. New studies on the cytotoxic effects of plasma-activated solutions (Adachi et al., 2015; Chen et al., 2016) raised concerns about product toxicity and the safe treatment dose of plasma. Although no specific legal and regulatory guidance exists for plasma technology, toxicity in food products after plasma treatment needs to be addressed before transferring technology to the industrial level. Another challenge for the use of plasma technology in the agriculture industry is consumer acceptance. Studies have shown that consumers have poor awareness of the plasma food processing technology. Therefore, the acceptance of plasma processed food by consumers depends on only the taste and sensory properties (Cullen et al., 2018).
Pros and Cons of Plasma Technology in Plant Disease Control
Many studies have demonstrated that non-thermal atmospheric-pressure plasma is a potential tool for inactivating bacterial and fungal cells pathogenic to plants. As an alternative disease control tool, plasma has exhibited several advantages and disadvantages compared to traditional control tools. Plasma technology has much less possibility to induce resistance from pathogens, which is a major problem of chemical-based controls. In addition, plasma can efficiently inactive chemical-resistant pathogens as well as non-resistant pathogens because it has no selectivity on types of pathogens. Since many species generated by plasma are unstable reactive species, its impact on the environment is short-lived. Thus, it is considered as an environmentally safer tool. Plasma is relatively safer than ionizing radiations, such as gamma-rays and X-rays, because of its lower energy level. However, plasma also has a limitation that should be improved; the efficiency of plant decontamination and disinfection varies depending on the properties of the plasma device, treatment conditions, plant species, and microenvironment of the plant–pathogen system. Numerous intensive studies are still needed to evaluate the potential of plasma in plant disease control. In addition, the mode of plasma action in plant disinfection and decontamination must be continuously explored.
Future Perspective: Plasma Vaccination
As demonstrated in studies, atmospheric-pressure non-thermal plasma can be efficient for controlling plant diseases by inactivating pathogens or activating plant immune response (Figure 1). Inactivation of phytopathogenic microorganisms by plasma has been actively explored, and enormous information usefully applicable to industry is available. Compared to pathogen inactivation, the activation of plant immune response by plasma, the so-called “plasma vaccination”, is currently gaining attention as an emerging strategy in plant disease control (Figure 1). Vaccination is a phenomenon of activating the immunity of mammalian cells by mild exposure to biological and chemical agents against diseases. Plants also have an innate immune system, which can be activated by exposure to chemical and biological molecules (Gorlach et al., 1996; Nicaise, 2014). Plasma-generated RONS can enter the plant through wounding or small openings and further penetrate plant cells. This may elevate the level of RONS in plant cells, which is a quite similar situation in plant cells undergoing a hypersensitive response. This, in turn, might activate the systemic immune response in plants. A periodic treatment with plasma might reinforce disease resistance in plants.
Extensive experimental data demonstrating the possibility of plasma vaccination have not yet been accumulated. However, there have been occasional reports showing induction of plant disease tolerance by plasma at organismal and molecular level (Jiang et al., 2014; Panngom et al., 2014; Bertaccini et al., 2017). Recently, a study has also demonstrated that the priming of tomato seeds with plasma-treated water has elevated the level of intracellular RONS, defense hormones (salicylic acid and jasmonic acid), and transcripts of PR genes (Adhikari et al., 2019). In these studies, plasma and plasma-treated water generate no adverse effects on plant vitality. This indicates that plasma or plasma-treated water can possibly act as a trigger for inducing plant defense responses with no harm to plant vitality. Although research on plasma vaccination is in its initial stages, the potential of a new control strategy using plasma is quite promising. The pressure of plant diseases on agriculture has recently increased, particularly because of global warming. Thus, the induction of plant tolerance can be an important and environmentally safe strategy that should be intensively studied in the future.
Author Contributions
BA, KP, and GP wrote the main manuscript and MV and SM helped in the literature search and summary. All authors reviewed the manuscript.
Funding
This work was supported by the R&D Program of ‘Plasma Advanced Technology for Agriculture and Food (Plasma Farming)’ through the National Fusion Research Institute of Korea (NFRI), funded by government funds. The work was partially supported by the National Research Foundation of Korea (NRF) (2016R1D1A1B03934922) and by a grant from Kwangwoon University in 2019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for the English language editing.
References
- Aboubakr H. A., Williams P., Gangal U., Youssef M. M., El-Sohaimy S. A. A., Bruggerman P. J., et al. (2015). Virucidal effect of cold atmospheric gaseous plasma on feline calicivirus, a surrogate for human norovirus. Appl. Environ. Microbiol. 81, 3612–3622. 10.1128/AEM.00054-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Tanaka H., Nonomura S., Hara H., Kondo S., Hori M. (2015). Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 79, 28–44. 10.1016/j.freeradbiomed.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Adamovich I., Baalrud S. D., Bogaerts A., Bruggeman P. J., Cappelli M., Colombo V., et al. (2017). The 2017 plasma roadmap: low temperature plasma science and technology. J. Phys. D 50, 323001. 10.1088/1361-6463/aa76f5 [DOI] [Google Scholar]
- Adhikari B., Adhikari M., Ghimire B., Park G., Choi E. H. (2019). Activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 9, 16080. 10.1038/s41598-019-52646-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrios G. N. (1997). Plant Pathology (London: Academic Press; ). [Google Scholar]
- Akhavan A., Bahar M., Askarian H., Lak M. R., Nazemi A., Zamani Z. (2013). Bean common bacterial blight: pathogen epiphytic life and effect of irrigation practices. SpringerPlus 2, 41. 10.1186/2193-1801-2-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrico P. F., Simek M., Morano M., Angelini R. M. D., Minafra A., Trotti P., et al. (2017). Reduction of microbial contamination and improvement of germination of sweet basil (Ocimum basilicum L.) seeds via surface dielectric barrier discharge. J. Phys. D 50, 305401. 10.1088/1361-6463/aa77c8 [DOI] [Google Scholar]
- Andrasch M., Stachowiak J., Schlüter O., Schnabel U., Ehlbeck J. (2017). Scale-up to pilot plant dimensions of plasma processed water generation for fresh-cut lettuce treatment. Food Packag. Shelf Life 14, 40–45. 10.1016/j.fpsl.2017.08.007 [DOI] [Google Scholar]
- Arasimowicz-Jelonek M., Floryszak-Wieczorek J. (2016). Nitric oxide in the offensive strategy of fungal and oomycete plant pathogens. Front. Plant Sci. 7, 252. 10.3389/fpls.2016.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramidis G., Stuwe B., Wascher R., Bellmann M., Wieneke S., von Tiedemann A. (2010). Fungicidal effects of an atmospheric pressure gas discharge and degradation mechanisms. Surf. Coat. Technol. 205, S405–S408. 10.1016/j.surfcoat.2010.08.141 [DOI] [Google Scholar]
- Baier M., Foerster J., Schnabel U., Knorr D., Ehlbeck J., Herppich W. B., et al. (2013). Direct non-thermal plasma treatment for the sanitation of fresh corn salad leaves: evaluation of physical and physiological effects and antimicrobial efficacy. Postharv. Biol. Technol. 84, 81–87. 10.1016/j.postharvbio.2013.03.022 [DOI] [Google Scholar]
- Baier M., Janssen T., Wieler L. H., Ehlbeck J., Knorr D., Schlüter O. (2015). Inactivation of Shiga toxin-producing Escherichia coli O104:H4 using cold atmospheric pressure plasma. J. Biosci. Bioeng. 120, 275–279. 10.1016/j.jbiosc.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Basaran P., Basaran-Akgul N., Oksuz L. (2008). Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 25, 626–632. 10.1016/j.fm.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Bermudez-Aguirre D., Wemlinger E., Pedrow P., Barbosa-Canovas G., Garcia-Perez M. (2013). Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food Control 34, 149–157. 10.1016/j.foodcont.2013.04.022 [DOI] [Google Scholar]
- Bertaccini A., Biondi E., Canel A., Colombo V., Contaldo N., Gherardi M., et al. (2017). “Plasma activated water to enhance plant defenses,” in Proceedings of the 23rd International Symposium on Plasma Chemistry, Montreal, Canada, 206. [Google Scholar]
- Braşoveanu M., Nemţanu M., Carmen S.-B., Karaca G., Erper İ. (2015). Effect of glow discharge plasma on germination and fungal load of some cereal seeds. Rom. Rep. Phys. 67, 617–624. [Google Scholar]
- Brandenburg R., Bogaerts A., Bongers W., Fridman A., Fridman G., Locke B. R., et al. (2019). White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process Polym. 16, e1700238. 10.1002/ppap.201700238 [DOI] [Google Scholar]
- Butscher D., Van Loon H., Waskow A., von Rohr P. R., Schuppler M. (2016. a). Plasma inactivation of microorganisms on sprout seeds in a dielectric barrier discharge. Int. J. Food Microbiol. 238, 222–232. 10.1016/j.ijfoodmicro.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Butscher D., Zimmermann D., Schuppler M., von Rohr P. R. (2016. b). Plasma inactivation of bacterial endospores on wheat grains and polymeric model substrates in a dielectric barrier discharge. Food Control 60, 636–645. 10.1016/j.foodcont.2015.09.003 [DOI] [Google Scholar]
- Casadesus J., Low D. (2006). Epigenetic gene regulation in the bacterial world. Microbial. Mol. Biol. Rev. 70, 830–856. 10.1128/MMBR.00016-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Lin L., Cheng X., Gjika E., Keidar M. (2016). Treatment of gastric cancer cells with nonthermal atmospheric plasma generated in water. Biointerphases 11, 031010. 10.1116/1.4962130 [DOI] [PubMed] [Google Scholar]
- Chris L., Richard A. D. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. 10.1146/annurev.arplant.48.1.251 [DOI] [PubMed] [Google Scholar]
- Compton K. T., Langmuir I. (1930). Electrical discharges in gases. I. Survey of fundamental processes. Rev. Mod. Phys. 2, 123. 10.1103/RevModPhys.2.123 [DOI] [Google Scholar]
- Cullen P. J., Lalor J., Scally L., Boehm D., Milosavljević V., Bourke P., et al. (2018). Translation of plasma technology from the lab to the food industry. Plasma Process Polym. 15, e1700085. 10.1002/ppap.201700085 [DOI] [Google Scholar]
- Dasan B. G., Boyaci I. H., Mutlu M. (2016. a). Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control. 70, 1–8. 10.1016/j.foodcont.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Dasan B. G., Mutlu M., Boyaci I. H. (2016. b). Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int. J. Food Microbiol. 216, 50–59. 10.1016/j.ijfoodmicro.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Dasan B. G., Boyaci I. H., Mutlu M. (2017). Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 196, 139–149. 10.1016/j.jfoodeng.2016.09.028 [DOI] [Google Scholar]
- Deng S., Cheng C., Ni G., Meng Y., Chen H. (2010). Bacillus subtilis devitalization mechanism of atmosphere pressure plasma jet. Curr. Appl. Phys. 10, 1164–1168. 10.1016/j.cap.2010.02.004 [DOI] [Google Scholar]
- Dezest M., Bulteau A.-L., Quinton D., Chavatte L., Le Bechec M., Cambus J. P., et al. (2017). Oxidative modification and electrochemical inactivation of Escherichia coli upon cold atmospheric pressure plasma exposure. PloS One 12, e0173618. 10.1371/journal.pone.0173618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Mittler R., Noctor G. (2016). Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 171, 1535–1539. 10.1104/pp.16.00938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrynin D., Fridman G., Friedman G., Fridman A. (2009). Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 11, 115020. 10.1088/1367-2630/11/11/115020 [DOI] [Google Scholar]
- Dolezalova E., Lukes P. (2015). Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 103, 7–14. 10.1016/j.bioelechem.2014.08.018 [DOI] [PubMed] [Google Scholar]
- Elad Y., Pertot I. (2014). Climate change impact on plant pathogens and plant diseases. J. Crop Improv. 28, 99–139. 10.1080/15427528.2014.865412 [DOI] [Google Scholar]
- Fatemeh K., Babak S., Mohammad-Reza K., Mohammad H., Farzaneh Z., Atousa A. (2018). A study of the effect of gliding arc non-thermal plasma on almonds decontamination. AIP Adv. 8, 105024. 10.1063/1.5044476 [DOI] [Google Scholar]
- Filatova I., Azharonok V., Lushkevich V., Zhukovsky A., Gaszhieva G., Spasic K., et al. (2013). “Plasma seeds treatment as a promising technique for seed germination improvement,” in Proceedings of the 31st International Conference on Phenomena in Ionized Gases (ICPIG), Granada, Spain. [Google Scholar]
- Fones H., Preston G. M. (2012). Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol. Lett. 327, 1–8. 10.1111/j.1574-6968.2011.02449.x [DOI] [PubMed] [Google Scholar]
- Fridman A., Lin A., Miller V., Bekeschus S., Wende K., Weltmann K. D. (2018). The plasma treatment unit: an attempt to standardize cold plasma treatment for defined biological effects. Plasma Med. 8, 195–201. 10.1615/PlasmaMed.2018026881 [DOI] [Google Scholar]
- Gay-Mimbrera J., García M. C., Isla-Tejera B., Rodero-Serrano A., García-Nieto A. V., Ruano J. (2016). Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv. Ther. 33, 894–909. 10.1007/s12325-016-0338-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghesquière B., Jonckheere V., Colaert N., Van Durme J., Timmerman E., Goethals M., et al. (2011). Redox proteomics of protein-bound methionine oxidation. Mol. Cell. Proteomics 10, M110.006866. 10.1074/mcp.M110.006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. F., Flynn P. B., O'Brien S., Hickok N., Freeman T., Bourke P. (2018). Cold plasmas for biofilm control: opportunities and challenges. Trends Biotechnol. 36, 627–638. 10.1016/j.tibtech.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Girard P.-M., Arbabian A., Fleury M., Bauville G., Puech V., Dutreix M., et al. (2016). Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 6, 29098. 10.1038/srep29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach J., Volrath S., Knauf-Beiter G., Hengy G., Beckhove U., Kogel K. H., et al. (1996). Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 8, 629–643. 10.1105/tpc.8.4.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. B. (2012). The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D 45, 263001. 10.1088/0022-3727/45/26/263001 [DOI] [Google Scholar]
- Guo L., Xu R., Gou L., Liu Z., Zhao Y., Liu D., et al. (2018). Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl. Environ. Microbiol. 84, e00726–e00718. 10.1128/AEM.00726-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Patil S., Boehm D., Milosavljević V., Cullen P. J., Bourke P. (2016). Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 82, 450–458. 10.1128/AEM.02660-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbal S. E., Takashima K., Miyashita S., Ando S., Ito K., Elsharkawy M. M., et al. (2018). Atmospheric-pressure plasma irradiation can disrupt tobacco mosaic virus particles and RNAs to inactivate their infectivity. Arch. Virol. 163, 2835–2840. 10.1007/s00705-018-3909-4 [DOI] [PubMed] [Google Scholar]
- Hashizume H., Ohta T., Takeda K., Ishikawa K., Hori M., Ito M. (2015). Quantitative clarification of inactivation mechanism of Penicillium digitatum spores treated with neutral oxygen radicals. Jpn. J. Appl. Phys. 54, 01AG05. 10.7567/JJAP.54.01AG05 [DOI] [Google Scholar]
- Hayashi N., Yagyu Y., Yonesu A., Shiratani M. (2014). Sterilization characteristics of the surfaces of agricultural products using active oxygen species generated by atmospheric plasma and UV light. Jpn. J. Appl. Phys. 53, 05FR03. 10.7567/JJAP.53.05FR03 [DOI] [Google Scholar]
- Hensel K., Kučerová K., Tarabová B., Janda M., Machala Z., Sano K., et al. (2015). Effects of air transient spark discharge and helium plasma jet on water, bacteria, cells, and biomolecules. Biointerphases 10, 029515. 10.1116/1.4919559 [DOI] [PubMed] [Google Scholar]
- Hertwig C., Reineke K., Ehlbeck J., Knorr D., Schlüter O. (2015). Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control 55, 221–229. 10.1016/j.foodcont.2015.03.003 [DOI] [Google Scholar]
- Hertwig C., Meneses N., Mathys A. (2018). Cold atmospheric pressure plasma and low energy electron beam as alternative nonthermal decontamination technologies for dry food surfaces: a review. Trends Food Sci. Technol. 77, 131–142. 10.1016/j.tifs.2018.05.011 [DOI] [Google Scholar]
- Horst R. K. (2001). “Plant Diseases and Their Pathogens,” in Westcott"s Plant Disease Handbook. Ed. Horst R. K. (Boston, MA: Springer; ), 86–515. 10.1007/978-1-4684-7682-8_4 [DOI] [Google Scholar]
- Iseki S., Hashizume H., Jia F., Takeda K., Ishikawa K., Ohta T., et al. (2011). Inactivation of Penicillium digitatum spores by a high-density ground-state atomic oxygen-radical source employing an atmospheric-pressure plasma. Appl. Phys. Express 4, 116201. 10.1143/APEX.4.116201 [DOI] [Google Scholar]
- Ito M., Oh J.-S., Ohta T., Shiratani M., Hori M. (2017). Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Processes Polym. 15, 1700073. 10.1002/ppap.201700073 [DOI] [Google Scholar]
- Jang J. Y., Rhee J. Y., Chung G. C., Kang H. (2012). Aquaporin as a membrane transporter of hydrogen peroxide in plant response to stresses. Plant Signal. Behav. 7, 1180–1181. 10.4161/psb.21178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha N., Ryu J. J., Choi E. H., Kaushik N. K. (2017). Generation and role of reactive oxygen and nitrogen species induced by plasma, lasers, chemical agents, and other systems in dentistry. Oxid. Med. Cell. Longev. 2017, 7542540. 10.1155/2017/7542540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S. H., Ki S. H., Ahn J. H., Shin J. H., Hong E. J., Kim Y. J., et al. (2018). Inactivation of Escherichia coli and Staphylococcus aureus on contaminated perilla leaves by dielectric barrier discharge (DBD) plasma treatment. Arch. Biochem. Biophys. 643, 32–41. 10.1016/j.abb.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Jiang J., Lu Y., Li J., Li L., He X., Shao H., et al. (2014). Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt). PloS One 9, e97753. 10.1371/journal.pone.0097753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Sokorai K., Pyrgiotakis G., Demokritou P., Li X., Mukhopadhyay S., et al. (2017). Cold plasma-activated hydrogen peroxide aerosol inactivates Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria innocua and maintains quality of grape tomato, spinach and cantaloupe. Int. J. Food Microbiol. 249, 53–60. 10.1016/j.ijfoodmicro.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Jo Y. K., Cho J., Tsai T. C., Staack D., Kang M. H., Roh J. H., et al. (2014). A non-thermal plasma seed treatment method for management of a seedborne fungal pathogen on rice seed. Crop Sci. 54, 796–803. 10.2135/cropsci2013.05.0331 [DOI] [Google Scholar]
- Joshi S. G., Cooper M., Yost A., Paff M., Ercan U. K., Fridman G., et al. (2011). Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 55, 1053–1062. 10.1128/AAC.01002-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julák J., Hujacová A., Scholtz V., Khun J., Holada K. (2018). Contribution to the chemistry of plasma-activated water. Plasma Phys. Rep. 44, 125–136. 10.1134/S1063780X18010075 [DOI] [Google Scholar]
- Kang M. H., Pengkit A., Choi K., Jeon S. S., Choi H. W., Shin D. B., et al. (2015). Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PloS One 10, e0139263. 10.1371/journal.pone.0139263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamsen N., Onwimol D., Teerakawanich N., Dechanupaprittha S., Kanokbannakorn W., Hongesombut K., et al. (2016). Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl. Mater. Interfaces 8, 19268–19275. 10.1021/acsami.6b04555 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Chung T. H. (2016). Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 6, 20332. 10.1038/srep20332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Puligundla P., Mok C. (2017). Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds. J. Sci. Food Agric. 97, 128–134. 10.1002/jsfa.7698 [DOI] [PubMed] [Google Scholar]
- Kordas L., Pusz W., Czapka T., Kacprzyk R. (2015). The effect of low-temperature plasma on fungus colonization of winter wheat grain and seed quality. Pol. J. Environ. Stud. 24, 433–438. [Google Scholar]
- Lackmann J.-W., Bandow J. E. (2014). Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 98, 6205–6213. 10.1007/s00253-014-5781-9 [DOI] [PubMed] [Google Scholar]
- Lacombe A., Niemira B., Gurtler J., Fan X., Sites J., Boyd G., et al. (2015). Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 46, 479–484. 10.1016/j.fm.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Laroussi M., Leipold F. (2004). Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 233, 81–86. 10.1016/j.ijms.2003.11.016 [DOI] [Google Scholar]
- Laroussi M. (2002). Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review, analysis, and prospects. IEEE Trans. Plasma Sci. 30, 1409–1415. 10.1109/TPS.2002.804220 [DOI] [Google Scholar]
- Lee H., Kim J. E., Chung M.-S., Min S. C. (2015). Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs. Food Microbiol. 51, 74–80. 10.1016/j.fm.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Leon J., Lawton M. A., Raskin I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 108, 1673–1678. 10.1104/pp.108.4.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sakai N., Watanabe M., Hotta E., Wachi M. (2013). Study on plasma agent effect of a direct-current atmospheric pressure oxygen-plasma jet on inactivation of E. coli using bacterial mutants. IEEE Trans. Plasma Sci. 41, 935–941. 10.1109/TPS.2013.2248395 [DOI] [Google Scholar]
- Liu C., Nishida M. Y., Iwasaki K., Ting K. (2011). Prolonged preservation and sterilization of fresh plants in controlled environments using high-field plasma. IEEE Trans. Plasma Sci. 39, 717–724. 10.1109/TPS.2010.2096434 [DOI] [Google Scholar]
- Los A., Ziuzina D., Akkermans S., Boehm D., Cullen P. J., Van Impe J., et al. (2018). Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 106, 509–521. 10.1016/j.foodres.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Lu Q. Q., Liu D. P., Song Y., Zhou R. W., Niu J. H. (2014). Inactivation of the tomato pathogen Cladosporium fulvum by an atmospheric-pressure cold plasma jet. Plasma Process. Polym. 11, 1028–1036. 10.1002/ppap.201400070 [DOI] [Google Scholar]
- Mai-Prochnow A., Murphy A. B., McLean K. M., Kong M. G., Ostrikov K. (2014). Atmospheric pressure plasmas: infection control and bacterial responses. Int. J. Antimicrob. Agents 43, 508–517. 10.1016/j.ijantimicag.2014.01.025 [DOI] [PubMed] [Google Scholar]
- Matan N., Puangjinda K., Phothisuwan S., Nisoa M. (2015). Combined antibacterial activity of green tea extract with atmospheric radio-frequency plasma against pathogens on fresh-cut dragon fruit. Food Control 50, 291–296. 10.1016/j.foodcont.2014.09.005 [DOI] [Google Scholar]
- Min S. C., Roh S. H., Niemira B. A., Sites J. E., Boyd G., Lacombe A. (2016). Dielectric barrier discharge atmospheric cold plasma inhibits Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in Romaine lettuce. Int. J. Food Microbiol. 237, 114–120. 10.1016/j.ijfoodmicro.2016.08.025 [DOI] [PubMed] [Google Scholar]
- Misra N. N., Patil S., Moiseev T., Bourke P., Mosnier J. P., Keener K. M., et al. (2014). In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 125, 131–138. 10.1016/j.jfoodeng.2013.10.023 [DOI] [Google Scholar]
- Misra N. N., Yadav B., Roopesh M. S., Jo C. (2019). Cold plasma for effective fungal and mycotoxin control in foods: mechanisms, inactivation effects, and applications. Compr. Rev. Food Sci. Food Saf. 18, 106–120. 10.1111/1541-4337.12398 [DOI] [PubMed] [Google Scholar]
- Mitra A., Li Y.-F., Klämpfl T. G., Shimizu T., Jeon J., Morfill G. E., et al. (2014). Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol. 7, 645–653. 10.1007/s11947-013-1126-4 [DOI] [Google Scholar]
- Mošovská S., Medvecká V., Halászová N., Ďurina P., Valík Ľ., Mikulajová A., et al. (2018). Cold atmospheric pressure ambient air plasma inhibition of pathogenic bacteria on the surface of black pepper. Food Res. Int. 106, 862–869. 10.1016/j.foodres.2018.01.066 [DOI] [PubMed] [Google Scholar]
- Moisan M., Barbeau J., Moreau S., Pelletier J., Tabrizian M., Yahia L. H. (2001). Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 226, 1–21. 10.1016/S0378-5173(01)00752-9 [DOI] [PubMed] [Google Scholar]
- Moreau M., Feuilloley M. G. J., Veron W., Meylheuc T., Chevalier S., Brisset J. L., et al. (2007). Gliding arc discharge in the potato pathogen Erwinia carotovora subsp. atroseptica: mechanism of lethal action and effect on membrane-associated molecules. Appl. Environ. Microbiol. 73, 5904–5910. 10.1128/AEM.00662-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyka A., Dzimitrowicz A., Jamroz P., Lojkowska E., Sledz W., Pohl P. (2018). Rapid eradication of bacterial phytopathogens by atmospheric pressure glow discharge generated in contact with a flowing liquid cathode. Biotechnol. Bioeng. 115, 1581–1593. 10.1002/bit.26565 [DOI] [PubMed] [Google Scholar]
- Mráz I., Beran P., Šerá B., Gavril B., Hnatiuc E. (2014). Effect of low-temperature plasma treatment on the growth and reproduction rate of some plant pathogenic bacteria. J. Plant Pathol. 96, 63–67. 10.4454/JPP.V96I1.027 [DOI] [Google Scholar]
- Na Y. H., Park G., Choi E. H., Uhm H. S. (2013). Effects of the physical parameters of a microwave plasma jet on the inactivation of fungal spores. Thin Solid Films 547, 125–131. 10.1016/j.tsf.2013.04.055 [DOI] [Google Scholar]
- Nicaise V. (2014). Crop immunity against viruses: outcomes and future challenges. Front. Plant Sci. 5, 660. 10.3389/fpls.2014.00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemira B. A. (2012). Cold plasma reduction of Salmonella and Escherichia coli O157:H7 on almonds using ambient pressure gases. J. Food Sci. 77, M171–M175. 10.1111/j.1750-3841.2011.02594.x [DOI] [PubMed] [Google Scholar]
- Nishioka T., Takai Y., Kawaradani M., Okada K., Tanimoto H., Misawa T., et al. (2014). Seed disinfection effect of atmospheric pressure plasma and low pressure plasma on Rhizoctonia solani. Biocontrol Sci. 19, 99–102. 10.4265/bio.19.99 [DOI] [PubMed] [Google Scholar]
- Nishioka T., Takai Y., Mishima T., Kawaradani M., Tanimoto H., Okada K., et al. (2016). Low-pressure plasma application for the inactivation of the seed-borne pathogen Xanthomonas campestris. Biocontrol Sci. 21, 37–43. 10.4265/bio.21.37 [DOI] [PubMed] [Google Scholar]
- Nojima H., Park R.-E., Kwon J.-H., Suh I., Jeon J., Ha E., et al. (2007). Novel atmospheric pressure plasma device releasing atomic hydrogen: reduction of microbial-contaminants and OH radicals in the air. J. Phys. D 40, 501. 10.1088/0022-3727/40/2/026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Konishi H., Ando S., Sato K., Yokoyama K., Tsushima S., et al. (2017). Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol. 66, 67–76. 10.1111/ppa.12555 [DOI] [Google Scholar]
- Oerke E. C., Dehne H. W. (2004). Safeguarding production - losses in major crops and the role of crop protection. Crop Prot. 23, 275–285. 10.1016/j.cropro.2003.10.001 [DOI] [Google Scholar]
- Oerke E. C. (2006). Crop losses to pests. J. Agric. Sci. 144, 31–43. 10.1017/S0021859605005708 [DOI] [Google Scholar]
- Okumura T., Saito Y., Takano K., Takahashi K., Takaki K., Satta N., et al. (2016). Inactivation of bacteria using discharge plasma under liquid fertilizer in a hydroponic culture system. Plasma Med. 6, 247–254. 10.1615/PlasmaMed.2016018683 [DOI] [Google Scholar]
- Ouf S. A., Basher A. H., Mohamed A.-A. H. (2015). Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J. Sci. Food Agric. 95, 3204–3210. 10.1002/jsfa.7060 [DOI] [PubMed] [Google Scholar]
- Panngom K., Lee S. H., Park D. H., Sim G. B., Kim Y. H., Uhm H. S., et al. (2014). Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PloS One 9, e99300. 10.1371/journal.pone.0099300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. J., Lee D. H., Park J. C., Lee I. S., Lee K. Y., Hyun S. O., et al. (2003). Sterilization using a microwave-induced argon plasma system at atmospheric pressure. Phys. Plasma 10, 4539–4544. 10.1063/1.1613655 [DOI] [Google Scholar]
- Park J. C., Park B. J., Han D. W., Lee D. H., Lee I. S., Hyun S. O., et al. (2004). Fungal sterilization using microwave-induced argon plasma at atmospheric pressure. J. Microbiol. Biotechnol. 14, 188–192. [Google Scholar]
- Pasquali F., Stratakos A. C., Koidis A., Berardinelli A., Cevoli C., Ragni L., et al. (2016). Atmospheric cold plasma process for vegetable leaf decontamination: a feasibility study on radicchio (red chicory, Cichorium intybus L.). Food Control 60, 552–559. 10.1016/j.foodcont.2015.08.043 [DOI] [Google Scholar]
- Pérez Pizá M. C., Prevosto L., Zilli C., Cejas E., Kelly H., Balestrasse K. (2018). Effects of non-thermal plasmas on seed-borne Diaporthe/Phomopsis complex and germination parameters of soybean seeds. Innov. Food Sci. Emerg. Technol. 49, 82–91. 10.1016/j.ifset.2018.07.009 [DOI] [Google Scholar]
- Petriacq P., Lopez A., Luna E. (2018). Fruit decay to diseases: can induced resistance and priming help? Plants 7, 77. 10.3390/plants7040077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov V. D., Van Breusegem F. (2012). Hydrogen peroxide—a central hub for information flow in plant cells. AoB Plants 2012, pls014. 10.1093/aobpla/pls014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata C., D'Angelo D., Fea E., Gilli G. (2017). A review on microbiological decontamination of fresh produce with nonthermal plasma. J. Appl. Microbiol. 122, 1438–1455. 10.1111/jam.13412 [DOI] [PubMed] [Google Scholar]
- Puligundla P., Kim J. W., Mok C. (2018. a). Effect of atmospheric pressure plasma treatment on seed decontamination and sprouting of pak choi (Brassica rapa L. subsp. chinensis (L.) Hanelt). Chiang Mai J. Sci. 45, 2679–2690. [Google Scholar]
- Puligundla P., Lee T., Mok C. (2018. b). Effect of intermittent corona discharge plasma treatment for improving microbial quality and shelf life of kumquat (Citrus japonica) fruits. LWT 91, 8–13. 10.1016/j.lwt.2018.01.019 [DOI] [Google Scholar]
- Randeniya L. K., de Groot G. (2015). Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: a review. Plasma Process. Polym. 12, 608–623. 10.1002/ppap.201500042 [DOI] [Google Scholar]
- Sakudo A., Yagyu Y., Onodera T. (2019). Disinfection and sterilization using plasma technology: fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 20, 5216. 10.3390/ijms20205216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond G. P. C. (1994). Secretion of extracellular virulence factors by plant pathogenic bacteria. Annu. Rev. Phytopathol. 32, 181–200. 10.1146/annurev.py.32.090194.001145 [DOI] [Google Scholar]
- Schmidt A., Bekeschus S., Jablonowski H., Barton A., Weltmann K.-D., Wende K. (2017). Role of ambient gas composition on cold physical plasma-elicited cell signaling in Keratinocytes. Biophys. J. 112, 2397–2407. 10.1016/j.bpj.2017.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel U., Schmidt C., Stachowiak J., Bösel A., Andrasch M., Ehlbeck J. (2018). Plasma processed air for biological decontamination of PET and fresh plant tissue. Plasma Process. Polym. 15, 1600057. 10.1002/ppap.201600057 [DOI] [Google Scholar]
- Selcuk M., Oksuz L., Basaran P. (2008). Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 99, 5104–5109. 10.1016/j.biortech.2007.09.076 [DOI] [PubMed] [Google Scholar]
- Seol Y., Kim J., Park S., Chang H. Y. (2017). Atmospheric pressure pulsed plasma induces cell death in photosynthetic organs via intracellularly generated ROS. Sci. Rep. 7, 589. 10.1038/s41598-017-00480-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera B., Zahoranova A., Bujdakova H., Sery M. (2019). Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O'Donnell using non-thermal plasma treatment. Rom. Rep. Phys. 71, 701. [Google Scholar]
- Shetty N. P., Jørgensen H. J. L., Jensen J. D., Collinge D. B., Shetty H. S. (2008). Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 121, 267–280. 10.1007/s10658-008-9302-5 [DOI] [Google Scholar]
- Simontacchi M., Galatro A., Ramos-Artuso F., Santa-Maria G. E. (2015). Plant survival in a changing environment: the role of nitric oxide in plant responses to abiotic stress. Front. Plant Sci. 6, 977. 10.3389/fpls.2015.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohbatzadeh F., Mirzanejhad S., Shokri H., Nikpour M. (2016). Inactivation of Aspergillus flavus spores in a sealed package by cold plasma streamers. J. Theor. Appl. Phys. 10, 99–106. 10.1007/s40094-016-0206-z [DOI] [Google Scholar]
- Stepanova V., Slavicek P., Kelar J., Prasil J., Smekal M., Stupavska M., et al. (2017). Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 15, e1700076. 10.1002/ppap.201700076 [DOI] [Google Scholar]
- Sun S., Anderson N. M., Keller S. (2014). Atmospheric pressure plasma treatment of black peppercorns inoculated with Salmonella and held under controlled storage. J. Food Sci. 79, E2441–E2446. 10.1111/1750-3841.12696 [DOI] [PubMed] [Google Scholar]
- Tada Y., Spoel S. H., Pajerowska-Mukhtar K., Mou Z., Song J., Wang C., et al. (2008). Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321, 952–956. 10.1126/science.1156970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Motodate T., Takaki K., Koide S. (2018). Influence of oxygen concentration on ethylene removal using dielectric barrier discharge. Jpn. J. Appl. Phys. 57, 01AG04. 10.7567/JJAP.57.01AG04 [DOI] [Google Scholar]
- Torres M. A., Jones D. G., Dangl J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378. 10.1104/pp.106.079467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokawa Y., Yagyu Y., Misawa T., Sakudo A. (2017). A new roller conveyer system of non-thermal gas plasma as a potential control measure of plant pathogenic bacteria in primary food production. Food Control 72, 62–72. 10.1016/j.foodcont.2016.07.031 [DOI] [Google Scholar]
- Turkan I. (2018). ROS and RNS: key signalling molecules in plants. J. Exp. Bot. 69, 3313–3315. 10.1093/jxb/ery198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarlen P., van Belkum A., Summerbell R. C., Crous P. W., Thomma B. P. H. J. (2007). Molecular mechanisms of pathogenicity: how do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 31, 239–277. 10.1111/j.1574-6976.2007.00065.x [DOI] [PubMed] [Google Scholar]
- Vanacker H., Carver T. L. W., Foyer C. H. (1998). Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 117, 1103–1114. 10.1104/pp.117.3.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow A., Betschart J., Butscher D., Oberbossel G., Klöti D., Büttner-Mainik A., et al. (2018). Characterization of efficiency and mechanisms of cold atmospheric pressure plasma decontamination of seeds for sprout production. Front. Microbiol. 9, 3164. 10.3389/fmicb.2018.03164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D., Davies M. J., Grune T. (2015). Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol. 5, 367–380. 10.1016/j.redox.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltmann K. D., Kolb J. F., Holub M., Uhrlandt D., Šimek M., Ostrikov K., et al. (2019). The future for plasma science and technology. Plasma Process Polym. 16, e1800118. 10.1002/ppap.201800118 [DOI] [Google Scholar]
- Wende K., Williams P., Dalluge J., Van Gaens W., Aboubakr H., Bischof J., et al. (2015). Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases 10, 029518. 10.1116/1.4919710 [DOI] [PubMed] [Google Scholar]
- Winter T., Winter J., Polak M., Kusch K., Mäder U., Sietmann R., et al. (2011). Characterization of the global impact of low temperature gas plasma on vegetative microorganisms. Proteomics 11, 3518–3530. 10.1002/pmic.201000637 [DOI] [PubMed] [Google Scholar]
- Won M. Y., Lee S. J., Min S. C. (2017). Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 39, 25–32. 10.1016/j.ifset.2016.10.021 [DOI] [Google Scholar]
- Wu M., Liu C., Chiang C., Lin Y., Lin Y., Chang Y., et al. (2019). Inactivation effect of Colletotrichum gloeosporioides by long-lived chemical species using atmospheric-pressure corona plasma-activated water. IEEE Trans. Plasma Sci. 47, 1100–1104. 10.1109/TPS.2018.2871856 [DOI] [Google Scholar]
- Xiang Q., Liu X., Liu S., Ma Y., Xu C., Bai Y. (2019). Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 52, 49–56. 10.1016/j.ifset.2018.11.012 [DOI] [Google Scholar]
- Xing Y., Jia W., Zhang J. (2008). AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 54, 440–451. 10.1111/j.1365-313X.2008.03433.x [DOI] [PubMed] [Google Scholar]
- Xu Z., Cheng C., Shen J., Lan Y., Hu S., Han W., et al. (2018). In vitro antimicrobial effects and mechanisms of direct current air-liquid discharge plasma on planktonic Staphylococcus aureus and Escherichia coli in liquids. Bioelectrochemistry 121, 125–134. 10.1016/j.bioelechem.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Yagyu Y., Hatayama Y., Hayashi N., Mishima T., Nishioka T., Sakudo A., et al. (2016). Direct plasma disinfection of green mold spore on citrus by atmospheric pressure dielectric barrier discharge for agricultural applications. Trans. Mat. Res. Soc Jpn. 41, 127–130. 10.14723/tmrsj.41.127 [DOI] [Google Scholar]
- Zahoranova A., Henselova M., Hudecova D., Kalinakova B., Kovacik D., Medvecka V., et al. (2016). Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 36, 397–414. 10.1007/s11090-015-9684-z [DOI] [Google Scholar]
- Zahoranova A., Hoppanova L., Simoncicova J., Tuekova Z., Medvecka V., Hudecova D., et al. (2019). Effect of cold atmospheric pressure plasma on maize seeds: enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 38, 969–988. 10.1007/s11090-018-9913-3 [DOI] [Google Scholar]
- Zhang A., Jiang M., Zhang J., Ding H., Xu S., Hu X., et al. (2007). Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 175, 36–50. 10.1111/j.1469-8137.2007.02071.x [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu D., Zhou R., Song Y., Sun Y., Zhang Q., et al. (2014). Atmospheric cold plasma jet for plant disease treatment. Appl. Phys. Lett. 104, 043702. 10.1063/1.4863204 [DOI] [Google Scholar]
- Zimmermann J. L., Dumler K., Shimizu T., Morfill G. E., Wolf A., Boxhammer V., et al. (2011). Effects of cold atmospheric plasmas on adenoviruses in solution. J. Phys. D 44, 505201. 10.1088/0022-3727/44/50/505201 [DOI] [Google Scholar]
- Ziuzina D., Patil S., Cullen P. J., Keener K. M., Bourke P. (2014). Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 42, 109–116. 10.1016/j.fm.2014.02.007 [DOI] [PubMed] [Google Scholar]