Abstract
Background
Multiple myeloma is featured by the proliferation of malignant plasma cell in bone marrow. We aimed to demonstrate the effects of valproic acid combined with GANT61 on multiple myeloma cell proliferation and clarify its mechanism.
Material/Methods
Multiple myeloma cells were exposed to valproic acid, GANT61, or the combination of valproic acid and GANT61, respectively. MTT assay was performed to detect the cell viability. Quantitative reverse transcriptase polymerase chain reaction and western blotting were used to detect mRNA and expression levels of proteins in Hedgehog signaling pathway. The Q-value of the combination regime was calculated to evaluate the drug combination effect.
Results
Both valproic acid and GANT61 alone inhibited multiple myeloma cell proliferation in a dose-dependent manner compared to the control. In the presence of GANT61 or not, valproic acid inhibited multiple myeloma cell proliferation in a time-dependent manner. These 2 drugs had a synergistic effect at valproic acid concentration of ≥4 mM. Expression analysis showed that valproic acid significantly inhibited the expression levels of PTCH1, GLI1, and HES-1. GANT61 enhanced the inhibition of Hedgehog signaling pathway mediated by valproic acid.
Conclusions
GANT61 and valproic acid inhibited multiple myeloma cell proliferation synergistically by inhibiting the Hedgehog signaling pathway. The present study may provide a combination regime for the therapy of multiple myeloma.
MeSH Keywords: Hedgehog Proteins, Multiple Myeloma, Valproic Acid
Background
Multiple myeloma (MM) is a neoplastic plasma-cell disorder and characterized by the clonal proliferation of malignant plasma cell in bone marrow [1]. It accounts for approximately 13% of hematologic malignancies [2]. Over the last decade, the 5-year relative survival rates of MM have improved obviously due to the introduction of the novel therapies [3]. Although these novel therapies, including proteinase inhibitors, immunomodulators, monoclonal antibodies and autologous hematopoietic stem cell transplantation, have significantly improved the outcomes of MM patients, the major obstacle of MM therapy was the resistance to therapy [4–6].
Hedgehog (HH) signaling pathway plays a major regulating effect in cell differentiation, proliferation and tissue polarity. The target proteins of HH signaling pathway include glioma-associated oncogene homolog 1 (GLI1), patched homolog 1 (PTCH1), patched homolog 2 (PTCH2), B-cell lymphoma 2 (Bcl2), Notch homolog 2 (Notch2), Hes family bHLH transcription factor 1 (HES-1), etc. [7,8]. HH signaling pathway was reported to be highly conserved in normal tissues and cells with low expression. However, the abnormal expressions of the proteins in HH signaling pathway were observed in various malignancies, such as MM, leukemia, B cell lymphoma, and colorectal cancer [9–11]. It was demonstrated that HH signaling pathway plays an important role in the MM drug resistance [12]. In the state of inactivation, the HH receptor PTCH1 inhibits smoothened (SMO), which is a 7-transmembrane protein essential for HH activation [13]. When HH ligands is bound to PTCH1, SMO is released and activated, and then initiates the transcriptional program of GlI1 [14,15]. Additionally, a study in a mouse model suggested that PTCH1 may mediate the interaction between MM cells and bone marrow microenvironment [16]. GLI1 protein is the main effector of the HH signaling, and the deletion of GLI1 led to the HH inhibitory drug-resistant in human bone marrow mesenchymal stem cells (BMSCs). More importantly, it was reported that sonic HH ligands can support the survival and proliferation of human plasma cells [16]. Therefore, the inhibition of HH signaling may be important for exploring the therapeutic target of MM therapy.
Recently, histone deacetylase inhibitors (HDACis) as the emerging anti-cancer agents have been incorporated into the National Comprehensive Cancer Network Guidelines for MM [2]. Among them, valproic acid (VPA) is a well-established anti-convulsant drug and has been safely applied for 3 decades [17]. The bioavailability of these oral dosage forms approaches 95% to 100% and is well tolerated by patients [3,18,19]. In recent years, VPA was also suggested to exert its anti-cancer effects by suppressing histone deacetylase [20]. Multiple clinic trials of VPA have performed to evaluate its anti-cancer effects in various cancers, such as leukemia, advanced solid tumors, melanoma [21–23]. However, its anti-cancer effects in MM has not been well illuminated. Our previous study demonstrated that VPA inhibit the proliferation of MM cell lines by inhibiting the Notch pathway in cells [24,25]. GANT61, a cell-permeable hexa-hydro-pyrimidine compound, is an inhibitor of Gli-mediated gene transactivation, which was proved to have anti-cell growth and anti-cancer stem cell activities in tumor cells [18,26]. Because Gli-mediated transcription is the final step in HH signaling pathway, GANT61 could halt HH pathway. Single agent treatment has limited viability on cancer management, while combination therapy was emerging the norm in many tumors therapy. Thus, our study aimed to evaluate whether GANT61 and VPA could synergistically inhibit the cell viability of MM cells and to explore the molecular mechanism of inhibitory effects in MM cells.
Material and Methods
Materials and reagents
RPMI 8226 and U266 cell lines were originally obtained from BioHermes Bio & Medical Technology Co. (Wuxi, Jiangsu, China). VPA and GANT61 were purchased from Sigma Chemicals Co., Ltd. (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from BioInd (Kibbutz Beit Haemek, Israel).
Cells culture
RPMI 8226 and U266 cell lines were grown in RPMI-1640 medium, containing with 10% FBS, 100 U/mL penicillin and 100 g/mL streptomycin (Solarbio, Beijing, China) in humidified air containing 5% CO2 at 37°C.
Cell viability
The effects of drugs on cell proliferation were evaluated by cell viability. Cell viability was measured by tetrazolium (MTT) assay. Briefly, cells were inoculated into the 96-well culture plates (8×103 cells/well). Then, cells were treated with corresponding drugs for different exposure durations according to the study design. Cells were exposed to different concentrations of GANT61 (2.5, 5.0, 10.0, 20.0, and 30.0 μmol/L) or VPA (2, 4, 6, 8, and 10 mmol/L; with or without 5.0 μmol/L GANT61) for 18 hours, 24 hours, and 36 hours, respectively. Cells in the control group were treated with equivalent RPMI-1640 complete medium (supplemented with 10% FBS, 100 U/mL penicillin, and 100 g/mL streptomycin). After incubation, MTT (0.5 mg/mL, Sigma, USA) was added and then the plate was incubated for another 4 hours at 37°C. Subsequently, medium was removed, and the formazan was solubilized in 100 μL Tryple lysate. All samples were transferred to a 96-well plate and absorbance was measured at 490 nm used an absorbance microplate reader (Bio-Rad, Hercules, USA). The inhibition rate was calculated using the following formula: Growth inhibition rate (%)=[1-(absorbance of drug-treated cells/absorbance of control cells)]×100%.
Evaluation of synergistic effect
Interactions between the GANT61 and VPA were determined using the following formula: Q=E(a+b)/(Ea+Eb–Ea×Eb), where Ea, Eb and E(a+b) were defined as the inhibition rate of GANT61, VPA, and the GANT61 combined with VPA, respectively [27]. When Q value was less than 0.85, the combination of the 2 drugs had an antagonistic effect; when Q was between 0.85 and 1.15, the combination of the 2 drugs had a simple additive effect; and when Q was more than 1.15, the combination of the 2 drugs had a synergistic effect.
Western blotting
Cell lysates were isolated in ice-cold RIPA buffer with complete EDTA-free Protease Inhibitor Cocktail (Roche, USA). The soluble fractions from lysates were collected by centrifugation at 17 000 g for 10 minutes at 4°C. The isolated protein was then separated on 4% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the ethanol-pretreated polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST). Subsequently, the PVDF membrane was probed with rabbit anti-Ptch1 (1: 1000 dilution, AP06278PU-N, Origene, USA), rabbit anti-GlI1 (1: 1000 dilution, ab49314, Abcam, USA), rabbit anti-Hes-1 (1: 1000 dilution, ab71559, Abcam, USA) and anti-β-actin (1: 1000 dilution, sc-517582, Santa Cruz, USA) antibody in 5% bovine serum albumin in TBST at 4°C overnight. The PVDF membrane was then washed with TBST (3 times; 5 minutes each) and incubated with the secondary antibodies (goat anti-rabbit 1: 3000, ab6721, Abcam, USA) in 5% milk for 1 hour at room temperature. Membranes were then washed 3 times with TBST before being visualized using ECL Western Blotting Substrate Kit (Solarbio, Beijing, China). The β-actin was used as an internal control.
Real-time quantitative reverse transcriptase polymerase chain reaction (RT-qPCR)
Total RNA was isolated by using a RNeasy Mini kit (Qiagen, Dusseldorf, Germany). The total RNA was subjected to reverse transcriptase reaction. RT-qPCR was performed in triplicates with an iQ5 iCycler and iQ SYBR Green Supermix (Bio-Rad, USA) according to manufacturer’s protocol. Following primers were used:
PTCH1, forward 5′-CTGCTGGTATGCTCGGGACTC-3′,
reverse 5′-TAAATCGCTGGGAGTTTCTGG-3′;
GLI1, forward 5′-TGTGTATGAAACTGACTGCCC-3′ and
reverse 5′-CCCAGTGGCACACGAACTC-3′;
HES-1, forward 5′-ATCACACAGGCTGGGGTAGC-3′ and
reverse 5′-TGACACTGGCTGGGGTAGC-3′;
β-actin, forward 5′-CATGTACGTTGCTATCCAGGC-3′ and
reverse 5′-CTCCTTAATGTCACGCACGAT-3′.
qPCR was conducted according to the following cycles: 95°C for 30 seconds, 95°C for 10 seconds, 62°C for 30 seconds, and 72°C for 30 seconds.
Statistical analysis
Statistical analysis was performed by SPSS 19.0 (SPSS, Inc., Chicago, IL, USA). All experiments were repeated 3 times. The data were expressed as means±standard error (SE) of 3 separate experiments. The significance of differences between 2 groups was determined by unpaired 2-tailed Student’s t-test. A one-way analysis of variance (ANOVA) with a post-hoc least significant difference (LSD) test was applied to perform the comparison of multiple groups. Differences were considered statistically significant when P<0.05.
Results
MM cell lines proliferation was inhibited by GANT61 or VPA
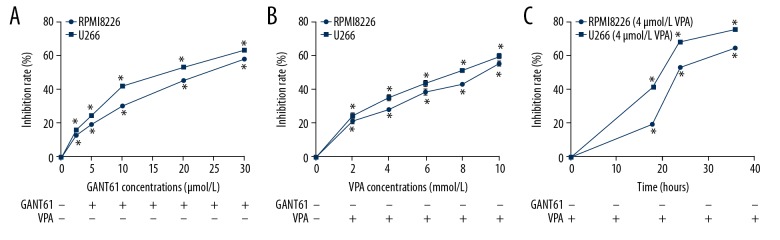
To confirm the effects of 2 drugs on MM cell lines, RPMI 8226 and U266 cells were exposed to various concentrations GANT61 or VPA. As shown in Figure 1A, GANT61 inhibited the cell proliferation of MM cell lines (RPMI 8226 and U266) in a dose-dependent manner (P<0.05). Consistently, VPA also inhibited cell proliferation in a dose-dependent manner (P<0.05, Figure 1B). Besides, to verify the trend of inhibition rate over time, a certain concentration of VPA (4 mmol/L) was applied to treat the MM cell lines (RPMI 8226 and U266 cells). We observed that VPA inhibited the MM cell proliferation in a time-dependent manner (P<0.05, Figure 1C). Overall, we concluded that both GANT61 and VPA alone inhibited the cell proliferation of MM cell lines.
Figure 1.
MM cell lines proliferation was inhibited by GANT61 or VPA. (A) RPMI 8266 and U266 cells were respectively exposed to various concentrations (2.5, 5, 10, 20, and 30 μmol/L) of GANT61 for 24 hours, the cell viability was detected by MTT assay to calculate the inhibition rate. (B) RPMI 8266 and U266 cells were respectively exposed to various concentrations (2, 4, 6, 8 and 10 mmol/L) of VPA for 24 hours, the cell viability was detected by MTT assay to calculate the inhibition rate. (C) RPMI 8266 and U266 cells were respectively treated with 4 mmol/L VPA for 18, 24, and 36 hours. Then cell viability was measured by MTT assay to calculate the inhibition rate. All experiments were performed in triplicate and results are expressed as means±standard error (SE); * P<0.05 versus control. MM – multiple myeloma; VPA – valproic acid.
MM cell proliferation was suppressed by GANT61 and VPA synergistically
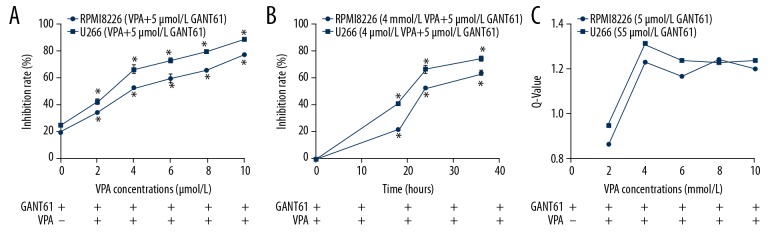
To confirm the synergistic effect of GANT61 and VPA, MM cell lines were exposed to GANT61 and VPA simultaneously. As shown in Figure 2A, when MM cell lines exposed to certain concentration of GANT61 (5 μmol/L), the combination of GANT61 and VPA inhibited cell proliferation in a VPA dose-dependent manner. Notably, the inhibition rates of combination treatment in various VPA concentration were all significantly higher than that of GANT61 alone (P<0.05, Figure 2A), suggesting that the GANT61 and VPA could synergistically inhibit MM cell proliferation. Then, we detected the time dependence of this synergistic effect. The results showed that GANT61 and VPA also inhibited MM cell proliferation in a time-dependent manner (Figure 2B). To evaluate the drug combination effect, we calculated the Q-value of this combination regime. When VAP concentration was 2 mmol/L, 2 drugs had a simple additive effect with a Q-value <1.15. When the VAP concentration was greater than or equal to 4 mmol/L, 2 drugs had a synergistic effect with a Q-value >1.15. Two drugs had the best synergistic effect at the VAP concentration of 4 mmol/L (Figure 2C).
Figure 2.
MM cell lines proliferation was inhibited by GANT61 and VPA synergistically. (A) In the presence of 5 μmol/L GANT61, RPMI 8266 and U266 cells were exposed to various concentrations (2, 4, 6, 8, and 10 mmol/L) of VPA for 24 hours, cell viability was detected by MTT assay. (B) RPMI 8266 and U266 cells were exposed to both GANT61 (5 μmol/L) and VPA (4 mmol/L) for 24 hours, cell viability was detected by MTT assay. (C) RPMI 8266 and U266 cells were exposed to both GANT61 and VPA for 24 hours. Q-values was calculated to evaluate the synergistic effect of this combination regime. All experiments were performed in triplicate and results are expressed as means±standard error (SE); * P<0.05 versus 0 hour. MM – multiple myeloma; VPA – valproic acid.
VPA inhibits expression of proteins in HH signaling pathway
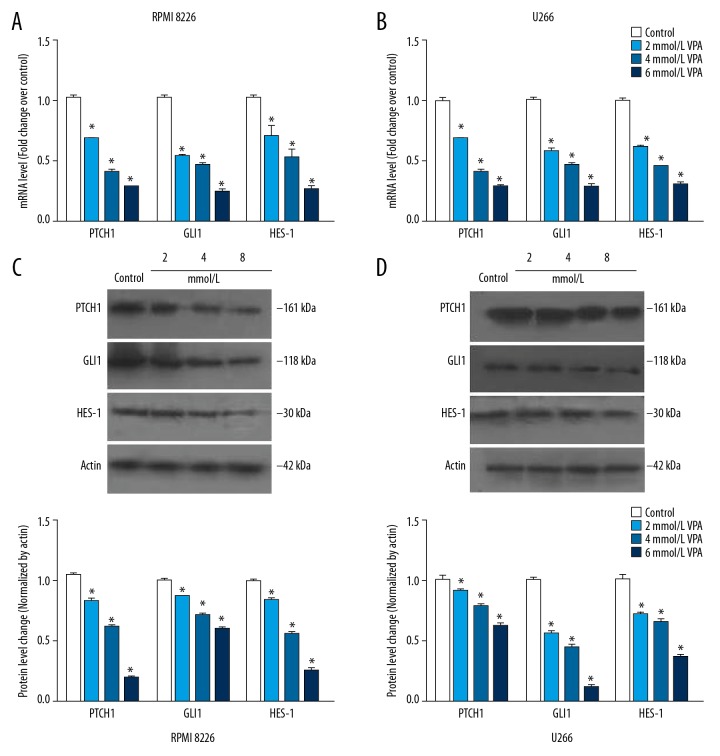
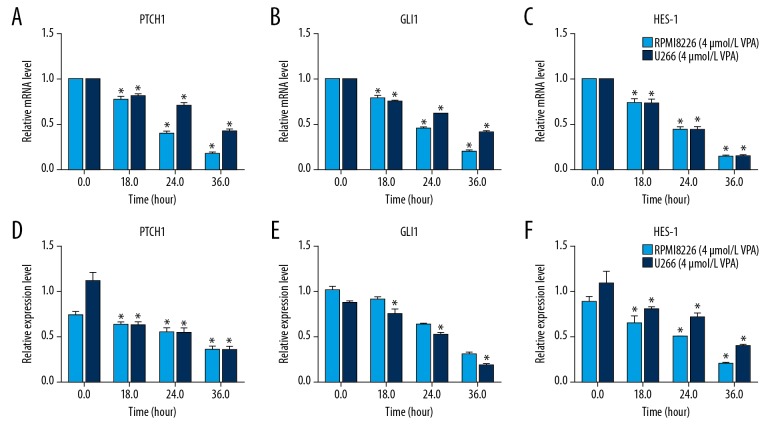
To explore the inhibition mechanism of VPA, qRT-PCR and western blot were performed to measure the expression of proteins in HH signaling pathway. After various concentrations of VPA treatment, mRNA was extracted form RPMI 8226 and U266 cells, respectively. The results of qRT-PCR showed that both in RPMI 8226 and U266 cells, VPA expose significantly suppressed the mRNA expression of PTCH1, GLI1, and HES-1, and in a VPA dose-dependent manner (Figure 3A, 3B). Consistently, a similar result was observed in the western blot analysis (Figure 3C, 3D). Furthermore, MM cells were exposed to VPA for different time durations. As shown in Figure 4, mRNA and protein expression of PTCH1, GLI1, and HES-1 were both significantly decreased in a time-dependent manner. Taken together, these results indicated that VPA inhibits expression levels of proteins in HH signaling pathway, and in a time-dependent manner.
Figure 3.
VPA inhibits the expression of proteins in HH signaling pathway in MM cell lines. RPMI 8266 (A) and U266 cells (B) were exposed to various concentrations (2, 4, 6, 8 and 10 mmol/L) of VPA for 24 hours, RT-qPCR was used to measure mRNA expression of PTCH1, GLI1 and HES-1. After treatment, western blot was used to measure protein expression of PTCH1, GLI1 and HES-1 in RPMI 8266 (C) and U266 cells (D). All experiments were performed in triplicate and results are expressed as means±standard error (SE); * P<0.05 versus control. MM – multiple myeloma; VPA – valproic acid; HH – hedgehog; RT-qPCR – real-time quantitative reverse transcriptase polymerase chain reaction.
Figure 4.
VPA inhibits the expression of proteins in HH signaling pathway in MM cell lines in a time-dependent manner. RPMI 8266 and U266 cells were respectively treated with 4 mmol/L VPA for 18, 24, and 36 hours. qRT-PCR and western blot were respectively performed to detect the mRNA (A–C) and proteins (D–F) expression levels in HH signaling pathway. All experiments were performed in triplicate and results are expressed as means ±standard error (SE); * P<0.05 versus 0 hour. MM – multiple myeloma; VPA – valproic acid; HH – hedgehog; RT-qPCR – real-time quantitative reverse transcriptase polymerase chain reaction.
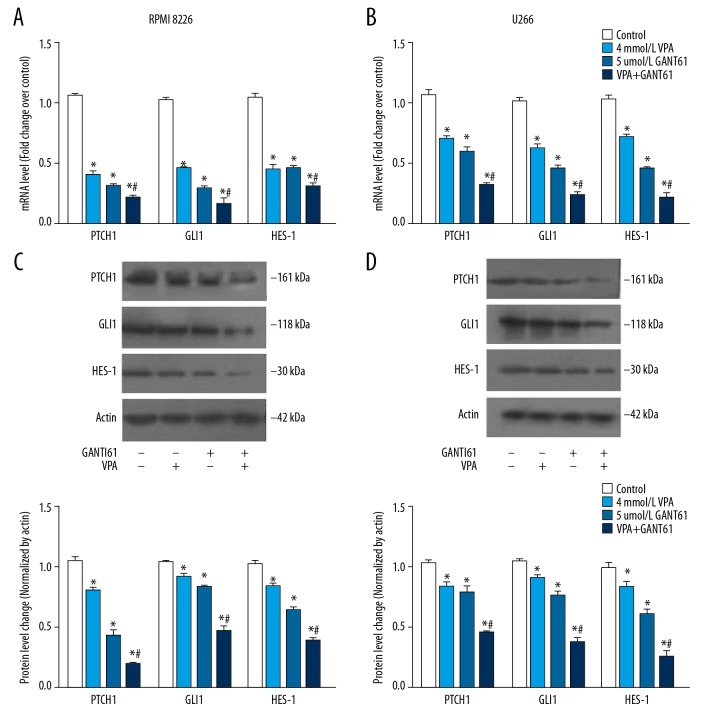
GANT61 and VPA synergistically inhibited expression of proteins in HH signaling pathway in MM cell lines
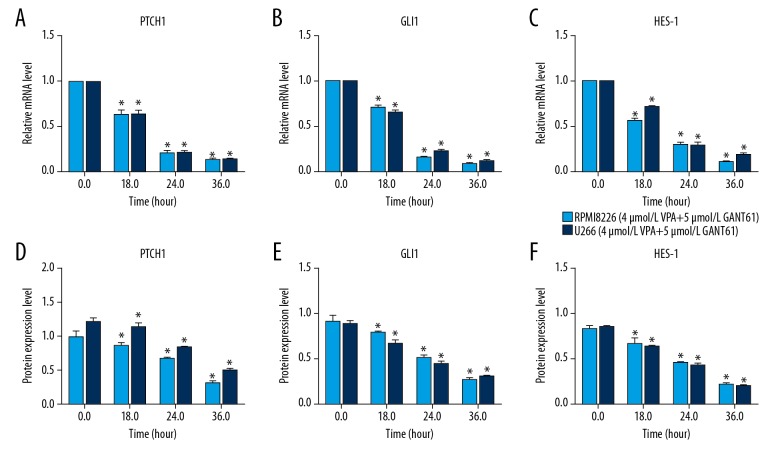
To explore the mechanism of synergistic inhibition effect, the expression of proteins in HH signaling pathway in MM cells exposed to GANT61 combined with VPA were measured. The results showed that GANT61 or VPA alone significantly inhibited the mRNA expression of PTCH1, GLI1, and HES-1 compared to the control, and meanwhile this inhibiting effect was further enhanced by GANT61 and VPA (Figure 5A, 5B). Consistently, western blot analysis showed that the protein expression of PTCH1, GLI1, and HES-1 in MM cells exposed to GANT61 combined with VPA were also inhibited more significantly than that exposed to GANT61 or VPA alone (Figure 5C, 5D). Furthermore, MM cells were treated with VPA and GANT61 for different time durations. As shown in Figure 6, mRNA and protein expression of PTCH1, GLI1, and HES-1 were both significantly inhibited in a time-dependent manner. Taken together, these results indicated that GANT61 and VPA synergistically inhibited the expression of proteins in HH signaling pathway in MM cell lines in a time-dependent manner, in which GANT61 enhanced the inhibition of HH signaling pathway mediated by VPA.
Figure 5.
GANT61 and VPA synergistically inhibited expression of proteins in HH signaling pathway in MM cell lines. Cells were exposed to both GANT61 (5 μmol/L) and VPA (4 mmol/L) for 24 hours, qRT-PCR was performed to measure mRNA expression of PTCH1, GLI1, and HES-1 in PMI 8266 (A) and U266 cells (B). After treatment, western blot was performed to detect the protein expression of PTCH1, GLI1, and HES-1 in RPMI 8266 (C) and U266 cells (D). All experiments were performed in triplicate and results are expressed as means±standard error (SE); * P<0.05 versus control; # P<0.05 versus GANT61 or VPA alone. MM – multiple myeloma; VPA – valproic acid; HH – hedgehog; RT-qPCR – real-time quantitative reverse transcriptase polymerase chain reaction.
Figure 6.
GANT61 and VPA inhibited the expression of proteins in HH signaling pathway in MM cell lines in a time-dependent manner. RPMI 8266 and U266 cells were respectively treated with GANT61 and VPA for 18, 24, and 36 hours. qRT-PCR and western blot were respectively performed to detect the mRNA (A–C) and proteins (D–F) expression levels in HH signaling pathway. All experiments were performed in triplicate and results are expressed as means±standard error (SE); * P<0.05 versus 0 hour. MM – multiple myeloma; VPA – valproic acid; HH – hedgehog; RT-qPCR – real-time quantitative reverse transcriptase polymerase chain reaction.
Discussion
MM is a malignant proliferative disease that originates from the B cell line and is capable of producing monoclonal immunoglobulins. The clinical manifestations of MM were extensive bone destruction, recurrent infection, anemia, hypercalcemia, hyperviscosity syndrome, renal insufficiency and adverse consequences. There is an urgent need for effective treatment with fewer side effects for MM patients. Few studies have been done in terms of the effectiveness of GANT61 and VPA on the MM cell lines. To our knowledge, this is the first report focused on the effects of GANT61 and VPA on MM cell lines. To investigate the possible mechanism of the synergistic effect of GANT61 and VPA, we used MTT, RT-qPCR, and western blotting to evaluate the efficacy of different drugs on cell proliferation and detect the effect of the 2 drugs on the expression of HH signaling pathway related factors. In the present study, we demonstrated the synergistic inhibitory effect of GANT61 and VPA on MM cell lines. Besides, the present study presented a possible mechanism of this inhibitory effect that GANT61 and VPA inhibited MM cell proliferation synergistically by inhibiting the HH signaling pathway.
VPA has emerged as one of the most promising anti-cancer agents for human cancers [28]. It functions as the HDACi mediating the cell differentiation, apoptosis, and cell cycle arrest [29]. Although trichostatin A (TSA) is considered as a reference for HDACis, a previous study showed that VPA could induce the acetylation of histone proteins, whereas TSA did not [30]. In addition, a higher acetylation level after VPA treatment (1 mmol/L) than TSA (0.02 μmol/L) was observed [30]. To date, no published clinical trials have focused on the TSA in tumors. However, several trials done with VPA has been initiated to various tumors. Notably, despite VPA having a lower potency as a HDACi compared to other HDACis, such as TSA, it has been considered a powerful agent to use as a new therapeutic strategy in cancer therapy. However, in clinical practice, VPA has limited therapeutic effect on MM. Therefore, we investigated whether VPA could be used in combination with another drug to improve treatment outcomes and reduce side effects.
In this study, VPA inhibited the cell proliferation in a time- and dose-dependent manner. Studies have showed that VPA inhibits MM cell proliferation in a time- and dose-dependent manner [24,31]. Our findings were consistent with the previous literatures. At the same time, GANT61 inhibited MM cell proliferation in a time-dependent manner. The combined use of 2 drugs had a synergistic effect when the VPA concentration was greater than or equal to 4 mmol/L. This was consistent with our previous research [24,25]. GANT61 is a specific inhibitor of transcription factor GLI1 in HH signaling pathway [31]. Lauth et al. [26] found that GANT61 can selectively inhibit the gene transcription process mediated by GLI1 and GLI2, and it can inhibit the proliferation of tumor cells. The biological activity of GANT61 was achieved by directly binding to GLI1 and then inhibiting the expression of the downstream target gene of HH signaling pathway [32].
In mammals, the HH signaling pathway plays a critical function in maintaining homeostasis of tissues, and the occurrence and development of malignant tumors [32]. A previous study has shown that the HH signaling pathway was involved in the pathogenesis of MM, drug resistance, and other processes [33]. Thus, we hypothesized that VPA affect the proliferation of cells by affecting the HH signaling pathway in MM cells. In present study, we chose 3 main target genes PTCH1, GLI1, and HES-1, and detected the expression level of mRNA and protein. VPA inhibited the expression levels of PTCH1, GLI1, and HES-1 significantly in a dose-dependent manner. At the VPA concentration of 4 mmol/L, the expression levels of PTCH1, GLI1, and HES-1 were decreased significantly over time. It suggested that VPA could inhibit the activity of HH signaling pathway in MM cells. What’s more, the combined use of these 2 drugs had a synergistic effect in inhibited the expression levels of PTCH1, GLI1, and HES-1 in a time-dependent manner. Therefore, we considered that GANT61 and VPA could synergistically inhibit MM cell lines via HH signaling pathway. However, the specific mechanism of drug combination is complicated, thus further study and discussion are needed. Further studies on the specific mechanisms of VPA and GANT61 affecting HH signaling pathway may provide new ideas and methods for MM therapy.
Although some findings were reported in the present study, there were several limitations. The mechanism of inhibition effect was not been fully elucidated due to the measurement of limited target proteins. In addition, the present study was performed in vitro rather than in vivo. Research done in vitro may not accurately replicate conditions that occurred in vivo. Thus, our findings needed to be verified by experiments in an animal model, or in a clinical trial in the case of humans.
Conclusions
The present study demonstrated that GANT61 and VPA could synergistically suppress MM cell proliferation by inhibiting the HH signaling pathway. The inhibition effect was in a dose- and time-dependent manner. The present study may provide a combination regime for the MM therapy.
Abbreviations
- MM
multiple myeloma
- BM
bone marrow
- VPA
valproic acid
- HH
Hedgehog
- HDACi
histone deacetylase inhibitor
- RT-qPCR
real-time quantitative reverse transcriptase polymerase chain reaction
- TBST
tris-buffered saline with Tween 20
- SDS
sodium dodecyl sulfate
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PVDF
polyvinylidene fluoride
- PTCH1
patched homolog 1
- GLI1
glioma-associated oncogene homolog 1
- HES-1
hes family BHLH transcription factor 1
- Bcl 2
B-cell lymphoma 2
- Notch 2
Notch homolog 2
- SMO
smoothened
Footnotes
Conflict of Interest
None.
Source of support: This work was supported by the Hebei province natural capital funding projects [NO: H2013406112]
References
- 1.Liu Z, Xu J, He J, et al. A critical role of autocrine sonic hedgehog signaling in human CD138+ myeloma cell survival and drug resistance. Blood. 2014;124:2061–71. doi: 10.1182/blood-2014-03-557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KC, Alsina M, Atanackovic D, et al. NCCN Guidelines insights: Multiple myeloma, Version 3.2016. J Natl Compr Canc Netw. 2016;14:389–400. doi: 10.6004/jnccn.2016.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostrouchova M, Kostrouch Z, Kostrouchova M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol (Praha) 2007;53:37–49. [PubMed] [Google Scholar]
- 4.de la Puente P, Muz B, Azab F, et al. Molecularly targeted therapies in multiple myeloma. Leuk Res Treatment. 2014;2014 doi: 10.1155/2014/976567. 976567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludwig H, Sonneveld P, Davies F, et al. European perspective on multiple myeloma treatment strategies in 2014. Oncologist. 2014;19:829–44. doi: 10.1634/theoncologist.2014-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: A subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99:148–54. doi: 10.3324/haematol.2013.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertrand FE, Angus CW, Partis WJ, Sigounas G. Developmental pathways in colon cancer: crosstalk between WNT, BMP, Hedgehog and Notch. Cell Cycle. 2012;11:4344–51. doi: 10.4161/cc.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sang L, Roberts JM, Coller HA. Hijacking HES1: How tumors co-opt the anti-differentiation strategies of quiescent cells. Trends Mol Med. 2010;16:17–26. doi: 10.1016/j.molmed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katoh Y, Katoh M. Hedgehog target genes: Mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–86. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 10.Tam M, Lin P, Hu P, Lennon PA. Examining Hedgehog pathway genes GLI3, SHH, and PTCH1 and the p53 target GLIPR1/GLIPR1L1/GLIPR1L2 gene cluster using fluorescence in situ hybridization uncovers GLIPR1/GLIPR1L1/GLIPR1L2 deletion in 9% of patients with multiple myeloma. J Assoc Genet Technol. 2010;36:111–14. [PubMed] [Google Scholar]
- 11.Gonnissen A, Isebaert S, Haustermans K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int J Mol Sci. 2013;14:13979–4007. doi: 10.3390/ijms140713979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies FE, Dring AM, Li C, et al. Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood. 2003;102:4504–11. doi: 10.1182/blood-2003-01-0016. [DOI] [PubMed] [Google Scholar]
- 13.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–97. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 14.Lum L, Beachy PA. The Hedgehog response network: Sensors, switches, and routers. Science. 2004;304:1755–59. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 15.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 16.Blotta S, Jakubikova J, Calimeri T, et al. Canonical and noncanonical Hedgehog pathway in the pathogenesis of multiple myeloma. Blood. 2012;120:5002–13. doi: 10.1182/blood-2011-07-368142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37(Suppl 2):5–16. [PubMed] [Google Scholar]
- 18.Xu Y, Xu D, Zhu SJ, et al. Induction of apoptosis and autophagy in metastatic thyroid cancer cells by valproic acid (VPA) Int J Clin Exp Pathol. 2015;8:8291–97. [PMC free article] [PubMed] [Google Scholar]
- 19.Ye RR, Cao JJ, Tan CP, et al. Valproic acid-functionalized cyclometalated iridium(iii) complexes as mitochondria-targeting anticancer agents. Chemistry. 2017;23:15166–76. doi: 10.1002/chem.201703157. [DOI] [PubMed] [Google Scholar]
- 20.Terranova-Barberio M, Roca MS, et al. Valproic acid potentiates the anticancer activity of capecitabine in vitro and in vivo in breast cancer models via induction of thymidine phosphorylase expression. Oncotarget. 2016;7:7715–31. doi: 10.18632/oncotarget.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daud AI, Dawson J, DeConti RC, et al. Potentiation of a topoisomerase I inhibitor, karenitecin, by the histone deacetylase inhibitor valproic acid in melanoma: Translational and phase I/II clinical trial. Clin Cancer Res. 2009;15:2479–87. doi: 10.1158/1078-0432.CCR-08-1931. [DOI] [PubMed] [Google Scholar]
- 22.Atmaca A, Al-Batran SE, Maurer A, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer. 2007;97:177–82. doi: 10.1038/sj.bjc.6603851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–79. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao L, Yang Y, Zhao RJ, et al. [Effects of VPA on the expression of Notch signaling pathway in multiple myeloma RPMI 8226 cell line]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24:1449–53. doi: 10.7534/j.issn.1009-2137.2016.05.030. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 25.Zhao RJ, Qiao L, Yang Y, et al. [Hematology DO and Hospital A: Effects of histone deacetylase inhibitor on the expression of Notch1 receptor and the ligands of jagged1 and jagged2]. Chinese Journal of Clinical Pharmacology. 2015:861–64. [in Chinese] [Google Scholar]
- 26.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye BG, Lin FA, Shen JZ, Fan LP, Lin CM. [Synergistic effects of VPA and As2O3 on Molt-4 cells in vitro and its possible mechanisms]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:1288–92. [in Chinese] [PubMed] [Google Scholar]
- 28.Sang Z, Sun Y, Ruan H, et al. Anticancer effects of valproic acid on oral squamous cell carcinoma via SUMOylation in vivo and in vitro. Exp Ther Med. 2016;12:3979–87. doi: 10.3892/etm.2016.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalano MG, Fortunati N, Pugliese M, et al. Valproic acid induces apoptosis and cell cycle arrest in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2005;90:1383–89. doi: 10.1210/jc.2004-1355. [DOI] [PubMed] [Google Scholar]
- 30.Aouali N, Palissot V, El-Khoury V, et al. Peroxisome proliferator-activated receptor gamma agonists potentiate the cytotoxic effect of valproic acid in multiple myeloma cells. Br J Haematol. 2009;147:662–71. doi: 10.1111/j.1365-2141.2009.07902.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhu YF, Ye BG, Shen JZ, et al. [Inhibitory effect of VPA on multiple myeloma U266 cell proliferation and regulation of histone acetylation]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18:638–41. [in Chinese] [PubMed] [Google Scholar]
- 32.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–54. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 33.Hu JS, Huang X, Huang YD, et al. [Effect of Hedgehog signaling pathway abnormality on chemotherapeutic resistance of multiple myeloma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:465–70. doi: 10.7534/j.issn.1009-2137.2017.02.028. [in Chinese] [DOI] [PubMed] [Google Scholar]