Abstract
Background
Interleukin-36 has been demonstrated to be involved in inflammatory responses. Inflammatory responses due to ischemia-reperfusion injury following cardiopulmonary bypass (CPB) can cause heart dysfunction or damage.
Material/Methods
The CPB models were constructed in IL-36R−/−, IL-36RN−/−, and wild-type SD rats. Ultrasonic cardiography and ELISA were used to evaluate the cardiac function and measuring myocardial biomarker levels in different groups. TUNEL assay was used to evaluate apoptosis. Western blot assays and RT-PCR were performed to measure the expression of chemokines and secondary inflammatory cytokines in the heart. Oxidative stress in tissue and cultured cells was assessed using a DCFH-DA fluorescence probe and quantification of superoxide dismutase activity.
Results
Improved systolic function and decreased serum levels of myocardial damage biomarkers were found in IL-36R−/− rats compared to WT rats, while worse cardiac function and cardiomyocyte IR injury were observed in IL-36RN−/− rats compared to WT rats. TUNEL staining and Western blot analyses found that cardiomyocyte apoptosis and inflammation were significantly lower in the hearts of IL-36R−/− rats compared with that of WT rats. Oxidative stress was significantly lower in IL-36R−/− rats compared to WT rats. iNOS expression was significantly reduced, while eNOS expression was increased in the hearts of IL-36R−/− rats. Silencing of IL-36R expression in vitro activated SIRT1/FOXO1/p53 signaling in cardiomyocytes.
Conclusions
IL-36R deficiency in cardiomyocytes repressed infiltration of bone marrow-derived inflammatory cells and oxidative stress dependent on SIRT1-FOXO1 signaling, thus protecting cardiomyocytes and improving cardiac function in CPB model rats.
MeSH Keywords: Interleukin-11 Receptor alpha Subunit, Interleukin-3 Receptor alpha Subunit, Interleukin-4 Receptor alpha Subunit
Background
Administration of anesthesia during cardiopulmonary bypass initiates myocardial ischemia-reperfusion (I/R) injury [1,2]. Reperfusion following persistent organ ischemia causes pro-inflammatory response and oxidative stress, which are crucial pathophysiological characteristics of ischemia/reperfusion injury [3,4]. I/R injury is commonly seen in many clinically pathological conditions, such as revascularization following myocardial infarction, septic shock, cardiopulmonary bypass, and thrombolysis therapy following cerebral infarction, which lead to cellular apoptosis and organ dysfunction [1,2]. Activation of inflamed signaling upregulates the expression of tissue chemokines and adhesion molecules in vascular endothelial cells, which facilitate infiltration and recruitment of bone marrow-derived myeloid cells (BMDMs) [5,6]. The inflammatory cells then produce excessive cytokines, such as matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), interleukin 1β (IL-1β), and MCP1, which lead to tissue damage in target organs [6]. An imbalance between antioxidants and oxidants following reperfusion induces oxidative stress followed by mitochondrial dysfunction, because rapid oxygen accumulation following reperfusion causes excessive production of reactive oxygen species [7]. Next, cardiomyocyte death is induced by the mitochondrial pathway [8,9]. Increased permeability of the mitochondrial inner membrane leads to release of cytochrome c and other activating factors, which induce apoptosis by promoting activation of caspase. It has been proved that permeability transition, which occurs during the IR process, is induced by increased ROS production, insufficiency of antioxidants, changes in pyridine nucleotide ratios, and calcium overload [8,9]. Clinically, the incidence of systemic inflammatory response syndrome is 2–10% because cardiopulmonary bypass induces a systemic inflammatory response due to contact between peripheral blood and artificial tube material used for the operation, which can aggravate the local inflammatory response in myocardial IRI [10,11]. Numerous studies have found that oxidative stress promotes the inflammatory process in a variety of models of inflammatory diseases [7]. The inflammatory response is characterized by recruitment of BMDMs and oxidative stress, as mitochondrial dysfunction coordinates the induction of IRI in the CPB model [7,12].
Interleukin-36 is composed of 3 IL-36 receptor agonists, including IL-36a, IL-36b, and IL-36r, derived from the IL-1 cytokine family [13,14], and this has been shown to be associated with a variety of autoimmune diseases. An antagonist of IL-36 improved the skin inflammation phenotype in psoriasis [14,15]. IL-36 expression is seen in various tissues and cells, including epithelial cells and immune cells. The production of IL-36 affects keratinocytes that express IL-36 receptors through the paracrine or autocrine pathways. IL-36 receptor activation can activate pro-inflammatory cytokines (IL-1β) and promote the release of TNF-α, IL-6, and IL-8. The association between IL-36 in immune cells and IL-36R in keratinocytes has been demonstrated. Induction of IL-36r, an isoform of IL-36R agonists, enhanced the expression of cytokines and induced nitric oxide synthase. These findings demonstrate that IL-36 can exert a potential effect, which is not inflammatory, on the oxidative stress process.
SIRT1 is the most evolutionarily conserved mammal sirtuin, regulating stress response and promoting cell survival. The activity of SIRT1 deacetylase is significantly increased during stress. Deacetylation of SIRT1 downregulated transcriptional function of p53 to induce cell survival [16,17]. An increase in SIRT1 deacetylase activity can inhibit stress-induced cell apoptosis and oxidative stress injury via activation of SIRT1-FOXO1/p53 signaling [18]. Activation of SIRT1 under stress may be an important mechanism for avoiding I/R injury characterized by oxidative stress. In skeletal muscle injury induced by I/R, oxidative stress injury can be aggravated by downregulation of the SIRT1-FOXO1/p53 signaling pathway. Activation of SIRT1-FOXO1/p53 inhibits oxidative stress and apoptosis in skeletal muscle I/R models.
Here, we revealed that the blocking IL-36/IL-36R signaling pathway could be utilized to reduce IRI injury in CPB model rats, which might be due to the inhibition of SIRT1/FOXO1/p53 signaling in cardiomyocytes. The inhibition of IL-36 signaling may be a potential therapeutic strategy for controlling I/R injury in patients who receive CPB.
Material and Methods
Cell culture and anoxia-reoxygenation treatment
AC16 cell line cells (purchased from ATCC) were inoculated at a density of 104/cm2 and cultured in DMEM medium containing antibiotics and 10% fetal bovine serum (FBS). Cells were then washed with phosphate-buffered saline (PBS) and placed in serum-free DMEM for 24 h. To simulate in vitro I/R injury, H9C2 cells were incubated in glucose-free medium (in an environment with 95% N2 and 5% CO2 conditions for 6 h at 37°C) containing 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 10% FBS. Subsequently, cells were washed with PBS, re-suspended in fresh DMEM, and moved to 95% O2/5% CO2 conditions for reoxygenation. Cells were collected for analysis 16 h after reoxygenation.
Synthesis and selection of SiRNA for IL-36R
The small interference RNA (siRNA) of IL1RL2 was designed by Xuntong Bio Company (Shanghai, China). The primer sequences used were as follows:
5′-ACUGUCGUAGCAUCAGGGCGAUCUUU-3′ (sense),
5′-UAGUGUACGUAACGUGAUCUUCACUG-3′ (antisense).
H9C2 myocardial cells were transfected with siRNA duplexes via Lipofectamine RNAi-Max according to manufacturer’s instructions. Inhibition of IL-36R was evaluated by qRT-PCR 48 h after siRNA transfection.
Animal models
Every procedure was approved by the Animal Care and Use Committee of the First Affiliated Hospital of Guangxi Medical University (30 April 2015). Male Sprague-Dawley (SD) rats (350–450 g), IL-36Rf/f allele rats, IL-36RNf/f allele rats, and the myh6::Cre transgenic rat strain were purchased from the Nanjing University Model Animal Center. The myh6::Cre transgenic rat strain expresses Cre recombinase during early embryonic development. This strain was bred with a floxed rat strain to create tissue-specific gene knockdown rats. We bred rats containing the IL-36Rf/f and IL-36RNf/f allele with myh6::Cre transgenic rats, which resulted in an IL-36R and IL-36RN knock-out in cardiomyocytes and generated rats with cardiac-specific IL-36R or IL-36RN deficiency. Rats containing this specific IL-36R or IL-36RN knock-out in the heart were bred. CPB was prepared by i.p. administration of ketamine (60 mg/kg) and xylazine (5 mg/kg). After intubation, mechanical ventilation was carried out with a small ventilator (respiratory parameters were set as follows: 60 times/min respiratory rate, 2.5 ml/kg tidal volume, and 1: 2 inspiratory-expiratory ratio). The cardiopulmonary bypass was performed as previously described [19]. Catheterization of the caudal vein was used to construct the liquid channel. The right femoral artery was perfused through the catheter, and the right internal jugular vein was catheterized into the right atrium to pump blood from the heart. The cardiopulmonary bypass device consists of a venous reservoir, a rat membrane oxygenator, and a peristaltic pump. The solution filling the flow tube contained 2 mL of mannitol, 10 mL of hydroxyethyl starch solution, 100 IU/heparin, and 1 mL of fresh allogenic blood. The total duration of CPB was 90 min. The relative physiological parameter monitored during CPB was set according to a previously described method [19,20].
Measurement of oxidative stress in heart tissue and cardiomyocytes
For the evaluation of oxidative stress in tissue, superoxide dismutase (SOD) activity and malondialdehyde (MDA) were measured. SOD activity and MDA level in tissues were determined by spectrophotometry using a Superoxide Dismutase Activity and Lipid Peroxidation Assay Kit according to the standard instructions. To evaluate ROS production in vitro, a DCFH-DA probe coupled to confocal fluorescence microscopy was used to detect and analyze the level of ROS in myocardial cells.
ELISA
The serum levels of myoglobin, troponin, and lactic dehydrogenase were detected using the Rat Myoglobin ELISA Kit, Superoxide Dismutase Activity Assay Kit (Colorimetric); (Abcam, Cambridge, UK), and Rat Lactic Dehydrogenase ELISA Kit (Lianke, Shanghai, China) according to the standard instructions.
Evaluation of cardiac function
Cardiac systolic function was evaluated 3 h after reperfusion. The left ventricular pressure (LVP) was measured by microcatheter insertion into left ventricle via the right carotid artery. A hemodynamic analyzing system was used to record the physiological parameters related to myocardial systolic and diastolic function, such as heart rate, ejection fraction, and LVP, to further analyze the instant systolic characterization. Computer algorithms were used to deduce LVSP and the instantaneous first derivation of LVP.
qRT-PCR
TRIzol reagent (Invitrogen) was applied to separate total RNA in the heart, and reverse transcriptase (Takara) was used to convert RNA into cDNA. The ABI 7500 Real-Time system was used to perform the quantitative real-time PCR reactions. β-actin was used for the control. Quantitative analysis was performed via the −2ΔΔCt method.
Western blot
Protein expression in myocardial cells and tissues was determined using Western blot. Immunoblotting was performed with anti-eNOS, anti-iNOS, anti-p53, anti-SIRT1, anti-FOXO3, anti-caspase-3, and anti-caspase8 probes. All primary antibodies for Western blot were obtained from Cell Signaling Technology. Protein bands were incubated with primary antibody at 4°C overnight and with secondary antibody at room temperature for 1 h. ECL-Plus reagent was used for visualization.
Evaluation of apoptosis
TUNEL was used to evaluate the apoptosis according to manufacturer’s instructions. Samples were observed via fluorescence microscopy. The degree of apoptosis was expressed according to the apoptotic index (the ratio of TUNEL-positive cells to total cells).
Immunohistochemical analysis
After the rats were sacrificed, their hearts were removed, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned to a thickness of 5 μm. Immunohistochemical (IHC) staining was performed with an IHC staining kit. Macrophage-specific primary antibody (anti-CD68, Abcam) was used for selective detection of macrophages.
Statistical analysis
Statistical analysis was carried out using SPSS 23.0 software. All values are expressed as mean±standard deviation. Unpaired and 2-tailed t tests or two-way ANOVA was used to assess significant differences.
Results
CPB in IL-36R cardiac-specific knockout rats (Myh6-Cre IL1RL2flox/flox) improved systolic cardiac function and decreased levels of serum myocardial injured biomarkers
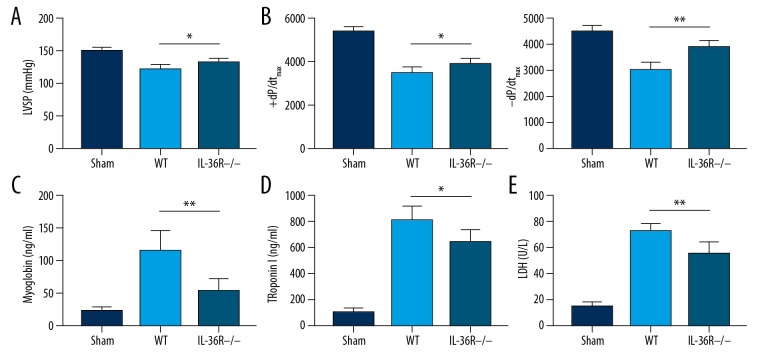
Because IL-36R expressed in cardiomyocytes can interact with IL-36 secreted by immune cells and other epithelial cells in the heart, inhibition of IL-36R in the cardiomyocytes can directly block the biological effect of IL-36 in all cells, and exert other potential effects on endogenous activation of immune cells during CPB-induced IR injury. In rats, IL-36R is normally expressed in immune cells, which results in the pro-inflammatory effect of immune cells, including promotion of Th1 polarization and macrophage differentiation. To verify the effect of IL-36R deficiency in cardiomyocytes, we produced rats with cardiac-specific knockout of IL-36R. We explored the effect of IL-36/IL-36R signaling activation in cardiomyocytes on I/R injury. We found improved left ventricular systolic function, as indicated by left ventricular systolic pressure (LVSP) (left ventricular systolic pressure, WT vs. IL-36R−/− rats, P=0.013) and instantaneous first derivation of LVP (+dp/dtmax) in rat myocardial genetic knockout CPB models (+dp/dtmax, WT vs. IL-36R−/− rats, P=0.020 and −dp/dtmax, WT vs. IL-36R−/− rats, P=0.013) (Figure 1A, 1B). Serum levels of myocardial damage biomarkers, including myoglobin (WT vs. IL-36R−/− rats, P=0.012) (Figure 1C), troponin (WT vs. IL-36R−/− rats, P=0.015) (Figure 1D), and lactic dehydrogenase (LDH) (WT vs. IL-36R−/− rats, P=0.018), were also decreased following reperfusion in rats with myocardial deficiency of IL-36R (Figure 1E). The data demonstrated that myocardial specific knockout of IL-36R improved cardiac systolic function and suppressed myocardial injury induced by IR injury in CPB models.
Figure 1.
IR injury alleviation in the CPB model was characterized by improved systolic cardiac function and decreased levels of myocardial injured biomarkers in the serum of IL-36R−/− rats (Myh6-Cre IL1RL2flox/flox). (A) LVSP was improved in IL-36R−/− rats (Myh6-Cre IL1RL2flox/flox) compared to wild-type rats. (B) Improved systolic function was indicated by instantaneous first derivation of LVP (+dp/dtmax and −dp/dtmax) in rats with IL-36 knockout in CPB models. (C–E) Serum levels of myocardial injury biomarkers, including myoglobin (C), troponin (D), and LDH (E) were decreased in rats with myocardial genetic knockout in CPB models. The t test was used for comparison of 2 groups. All experiments were repeated 3 times. n=8. P<0.05 indicates a significant difference. * P<0.05, ** P<0.01. LDH – lactate dehydrogenase; LVSP – left ventricular systolic pressure; WT – wild-type.
Decreased apoptosis of cardiomyocytes and reduced inflammation in hearts of IL-36R−/− rats
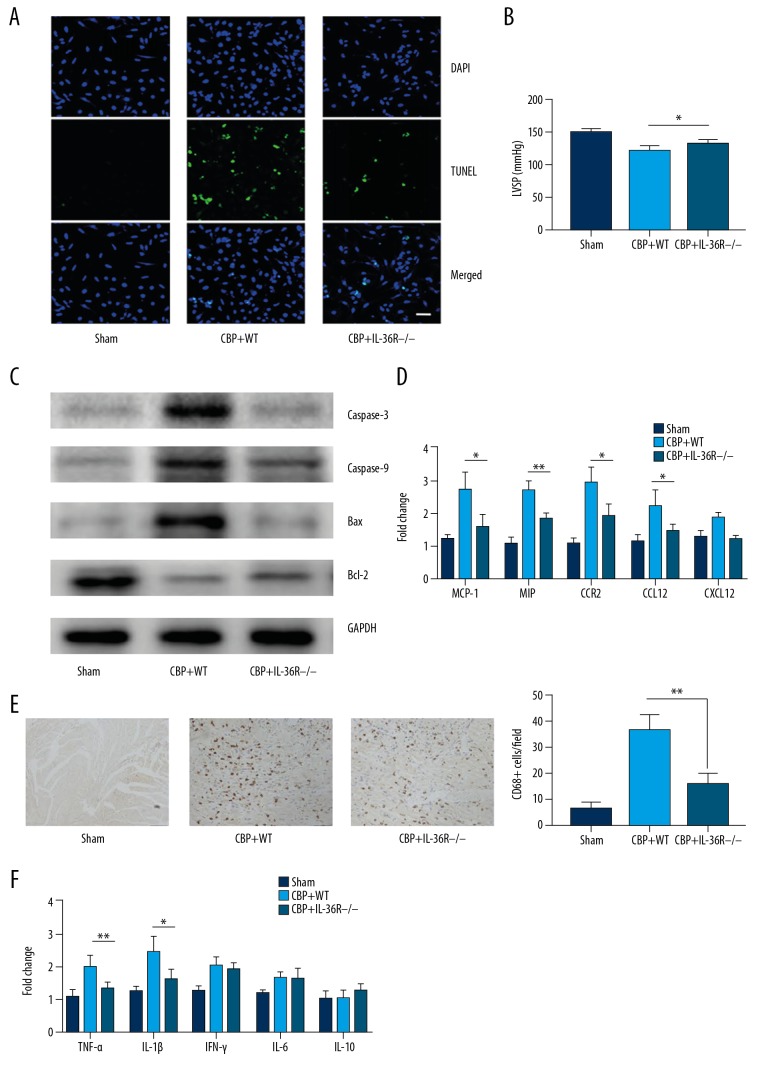
We evaluated cellular apoptosis in cardiomyocytes in the hearts of wild-type (WT) and IL-36R−/− rats using TUNEL staining and Western blot analyses. We found that cardiomyocyte apoptosis was significantly decreased in the hearts of IL-36R−/− rats compared with that of WT rats. The higher apoptotic rates were seen in the hearts of WT rats (Figure 2A, 2B). Expression of apoptotic proteins, including BCL2, Bax, caspase-3, and caspase-8, was measured. We found that the expression of pro-apoptotic genes was increased and Bcl2 expression was decreased in the hearts of WT rats (Figure 2C). We then evaluated the expression of chemokines and chemokine receptors, including MCP-1, MIP, CCR2, CCL12, and CXCL2. The expression of some chemokines in WT rats was found to be significantly higher compared to that in Myh6-Cre IL1RL2flox/flox rats (Figure 2D). We performed immunofluorescent staining to assess macrophage infiltration (CD68+) and found significantly larger numbers of CD68+ macrophages in WT rats (Figure 2E). Subsequently, we detected expression of pro-inflammatory cytokines, including TNF-α, IL-1β, IFN-γ, IL-6, and IL-10, and found that expression of TNF-a and IL-1b in the hearts of WT rats was higher compared to that of Myh6-Cre IL1RL2flox/flox rats (Figure 2F). These results illustrated that decreased apoptosis of cardiomyocytes and mitigatory myocardial inflammation was characterized by decreased infiltration of macrophages and production of pro-inflammatory cytokines following CPB.
Figure 2.
Apoptosis and inflammatory infiltration were reduced in the hearts of IL-36R−/− rats (Myh6-Cre IL1RL2flox/flox). (A) Representative figure of TUNEL staining in the hearts of WT and IL-36R−/− rats, respectively. (B) Semi-quantitative analysis of TUNEL staining in the hearts from WT rats and IL-36R−/− rats, respectively, illustrated apoptotic cardiomyocytes were decreased in IL-36R−/− rats. (C) Western blot measurements revealed decreased protein expression relative to apoptosis in hearts of rats with myocardial genetic knockout after reperfusion in CPB models. (D) Decreased chemokine expression in hearts from rats with myocardial genetic knockout after reperfusion in CPB models measured by qPCR. (E) CD68+ macrophage infiltration was reduced in IL-36−/− rat hearts as compared to WT rats. (F) The reduced expression of inflammatory cytokines illustrated by immunohistochemical staining in the hearts of in rats with myocardial genetic knockout after reperfusion in CPB models. The t test was used for comparison of 2 groups. Comparison for multiple groups was performed by ANOVA. All experiments were repeated 3 times, n=8. P<0.05 indicates a significant difference. * P<0.05, ** P<0.01. CPB – cardiopulmonary bypass; WT – wild-type.
Oxidative stress injury was attenuated and iNOS/eNOS ratio was reversed in IL-36R−/− rats
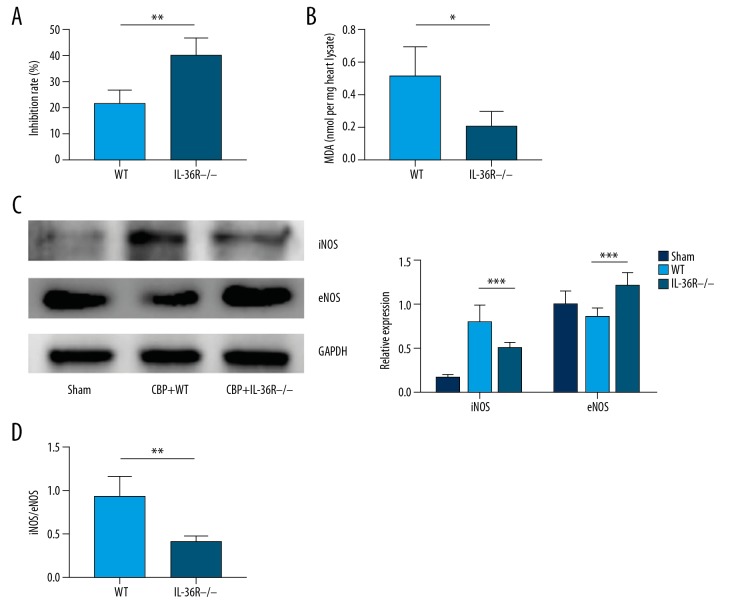
We found SOD and MDA was significantly decreased in Myh6-Cre IL1RL2flox/flox rats compared to those in WT rats (Figure 3A, 3B). Considering that previous studies have demonstrated that IL-36γ can promote the expression of inducible NOS (iNOS), we measured the expression of iNOS and eNOS in the hearts of Myh6-Cre IL1RL2flox/flox rats and WT rats. We found that iNOS expression was significantly reduced, while eNOS expression was increased in the hearts of Myh6-Cre IL1RL2flox/flox rats, as described previously (Figure 3C). These results demonstrated that iNOS inhibition contributes to reduced inflammation and oxidative stress. We also examined the potential downregulating effect of eNOS expression on inflammatory response and oxidative stress, in contrast to inducible NOS. We found an increased eNOS expression and an inverted iNOS/eNOS ratio in the hearts of Myh6-Cre IL1RL2flox/flox rats (Figure 3D). The data demonstrated that IL-36R knockout and blocking of IL-36/IL-36R signaling in cardiomyocytes leads to increased eNOS expression and iNOS inhibition, which attenuates oxidative stress following IR injury in CPB model rats.
Figure 3.
Oxidative stress was inhibited and the expression of iNOS/eNOS ratio was significantly decreased in IL-36R−/− compared to WT rats. (A) The inhibition rate of xanthine oxidase by SOD in heart tissue extracts from IL-36−/− rats was increased compared to WT rats, demonstrating the increased activity of SOD in the heart tissue of IL-36−/− rats in IR injury induced by CPB. (B) Significantly decreased MDA levels in the hearts from IL-36R−/− rats compared to WT rats suggests inhibition of lipid peroxidation. (C) Representative figures and semi-quantitative analysis of expression of iNOS and eNOS measured by immunoblot assays in the hearts from IL-36R−/− and WT rats. (D) Ratio of iNOS/eNOS expression in the hearts from IL-36R−/− and WT rats. The t test was used for comparison of 2 groups. Comparison for multiple groups was performed by ANOVA. All experiments were repeated 3 times, n=5. P<0.05 indicates a significant difference. * P<0.05, ** P<0.01. eNOS – endothelial NO synthetase; iNOS – inducible NO synthetase; WT – wild-type.
Silencing of IL-36R expression in vivo and in vitro activated SIRT1/FOXO1/p53 signaling in cardiomyocytes
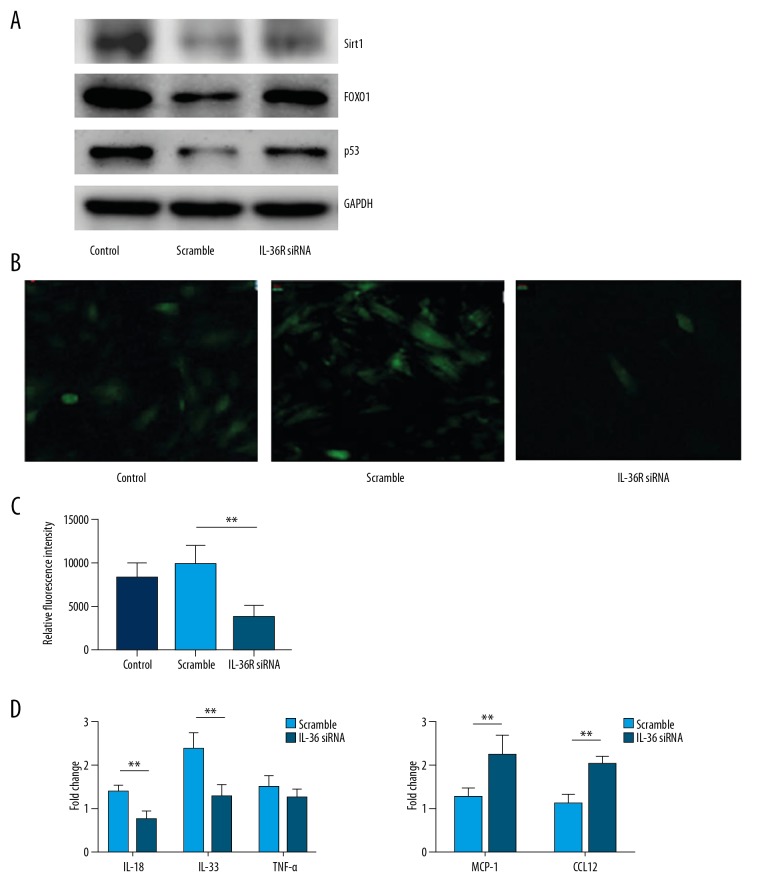
We measured the expression of SIRT1, FOXO1, and p53 in vitro. We found that expression of these proteins in the CM was increased with silencing of IL-36R in the anoxic-reoxygen model (Figure 4A). ROS production in CM was assessed using a DCFA-DH fluorescence probe, and a decrease in ROS production was observed (Figure 4B). Next, we measured the expression of chemokines and pro-inflammatory cytokines. Interestingly there were no significant differences between the expression of pro-inflammatory cytokines, including TNF-α, IL-18, and IL-33. However, an increasing trend in TNF-a expression was observed (Figure 4C). Expression of chemokines, including MCP1 and CCL12, in the CM decreased significantly with IL-36R silencing (Figure 4D). These data demonstrated that IL-36R knockout in CM repressed oxidative stress and recruitment of BMDMs, but did not promote a release of cytokines secreted by CM.
Figure 4.
Inhibition of oxidative stress and expression of inflammatory cytokines was accompanied by activation of SIRT1-FOXO1-p53 signaling. (A) Western blot results of SIRT1, FOXO1, and p53 expression in human cardiomyocytes (AC16) with or without silencing of IL-36R in vitro. (B) Representative figures of ROS production evaluated by DCFA-FH fluorescent probes in the human AC16 cell line with or without silencing of IL-36R in vitro. (C) Quantitative analysis of relative fluorescent intensity of DCFA-FH probes in AC16 with or without silencing of IL-36R in vitro. (D) Expression of inflammatory cytokines and chemokines in AC16 with or without silencing of IL-36R in vitro. The t test was used for comparison of 2 groups. All in vitro experiments were repeated 5 times. P<0.05 indicates a significant difference. * P<0.05, ** P<0.01.
Rats with cardiac-specific IL-36RN deficiency suffered from worsened cardiac dysfunction and cardiomyocyte IR injury after cardiopulmonary bypass
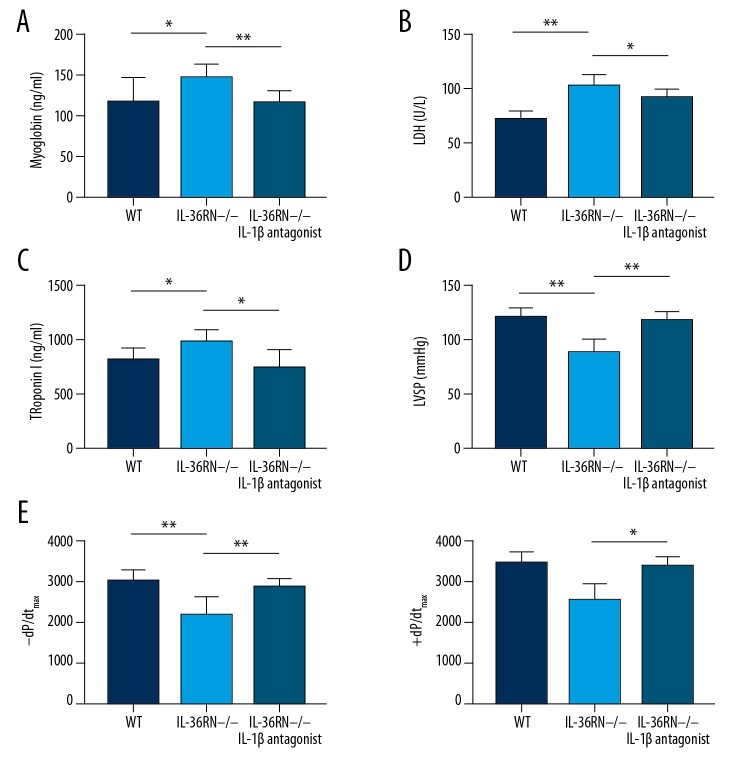
Based on the investigation of the role of IL-36R in the IRI of CPB model, we explored the IRI phenotype in rats with IL-36R antagonist deficiency. We performed CPB on rats with IL-36RN deficiency and WT. We found that WT rats had deteriorating disease phenotypes, characterized by higher levels of myocardial injury biomarkers and worsened systolic function as measured by left ventricular systolic pressure (LVSP) and instantaneous first derivation of LVP. IL-36RN−/− rats had higher serum levels of LDH (WT vs. IL-36RN−/− rats, P<0.001), troponin (WT vs. IL-36RN−/− rats, P=0.031), and myoglobin (WT vs. IL-36RN−/− rats, P=0.042) compared with those of WT rats (Figure 5A−5C). Furthermore, lower left ventricular systolic pressure LVSP (left ventricular systolic pressure, WT vs. IL-36RN−/− rats, P=0.001) and ±dp/dtmax (+dp/dtmax, WT vs. IL-36RN−/− rats, P=0.001 and −dp/dtmax, WT vs. IL-36RN−/− rats, P=0.002) was also found in the IL-36RN−/− rats (Figure 5D, 5E). Interestingly, the administration of recombinant rat IL1R1, an inhibitor of IL-1β, slightly improved cardiac function (Figure 5D, 5E) and appeared to decrease the levels of myocardial biomarkers. These results suggest the potential value of cytokine antagonists originating from IL1 family members for improving IRI phenotypes and cardiac dysfunction following CPB.
Figure 5.
Deficiency of IL-36RN worsened cardiac dysfunction and IR injury after cardiopulmonary bypass. (A–C) Serum levels of myocardial injury biomarkers, including myoglobin (A), LDH (B), and troponin (C), were higher in the IL-36RN−/− rats compared to WT rats. (D) LVSP was worse in the IL-36RN−/− rats compared to WT rats. (E) Systolic function manifested by instantaneous first derivation of LVP (+dp/dtmax and −dp/dtmax) was worse in the IL-36RN−/− rats compared to WT rats. The t test was used for comparison of 2 groups. All experiments were repeated 3 times, n=8. P<0.05 indicates a significant difference. * P<0.05, ** P<0.01. LDH – lactate dehydrogenase; LVSP – left ventricular systolic pressure; WT – wild-type.
Discussion
The present study revealed that IL-36-deficiency rats had improved cardiac function and decreased TUNEL staining in cardiomyocytes following CPB. We evaluated inflammatory response and ROS production due to a higher ratio of eNOS/inducible NOS in the myocardium of rats with IL-36R deficiency. IL-36R knockout attenuated the inflammatory response and decreased ROS production in cardiomyocytes. In conclusion, our investigation revealed that the IL-36/IL-36R signaling pathway could be utilized to reduce IRI injury in CPB models, via induction of aberrant inflammation and oxidative stress. Inhibition of IL-36 signaling may improve IRI in patients who receive CPB.
IRI occurs in patients requiring cardiopulmonary bypass and cardiac surgery. Cardiopulmonary bypass (CPB), which involves open heart surgery, is indispensable for cardiac surgery [3,4,21]. During CPB, improvements must be made during CPB to prevent cardiac arrest and improve the safety of procedures in the surgical field. However, aortic cross-clamping may lead to myocardial ischemia/reperfusion (I/R) injury following reperfusion of blood flow. I/R injury may be the main form of post-operative myocardial injury, and is characterized by elevated serum cardiac injury biomarkers, such as cardiac troponin I (cTnI) and CK-MB [21]. It has been demonstrated that elevated levels of myocardial injury biomarkers are an independent risk factor for adverse reactions following an operation. A previous study indicated that 24 h following cardiac surgery, elevated cTnI was an independent risk factor for death by 30 days, 1 year, and 3 years. Patients with significantly elevated cTnI had a higher risk compared to those with lower cTnl [22]. Another study reported that increased serum CK-MB following cardiac surgery was an independent risk factor for increased mortality after 3 years [23]. Even with improved perioperative myocardial protection methods, increased levels of cardiac injury biomarkers and myocardial stunning due to I/R injury still complicate cardiac surgery and perioperative myocardial protection during CPB.
Recent studies have investigated the potential therapeutic strategy for IR injury during CPB in higher animal models. Adbel et al. found that in the pig CPB model, hypoxic-reoxygenation (H/R) after reperfusion alleviated I/R injury and protected cardiac function following myocardial ischemia [24]. Huang et al. found that, compared with dopamine treatment in cardioplegia, isoflurane addition increased cardiac output more effectively. ST+EI was reported to decrease the release of cTni and CK-MB following CPB [25]. Emulsified isoflurane was combined with cardioplegia to prevent I/R injury. Protection of the integrity of mitochondrial ultrastructure of DNA may mediate this protective effect. CPB induced an increase of myocardial injury biomarkers and apoptosis in rat hearts. Suppressing inflammation by neutralizing IL-6 attenuated myocardial apoptosis and reduced the levels of myocardial biomarkers in CPB rats. Therefore, these studies demonstrated that excessive inflammation and mitochondrial dysfunction due to oxidative stress following reoxygenation contributed to IR injury in the rat CPB model, as demonstrated by increased cardiomyocyte apoptosis and higher myocardial biomarker levels.
IL-36 cytokines contain 3 agonists – cytokine IL-36α, IL-36β, and IL-36γ – and the antagonist IL-36Ra. IL-38 (IL-1F10) is an antagonist of IL-36R [26]. Cytokines interact with IL-36R-IL-1RAcP-specific heterodimeric receptors, but IL-36R, a receptor-binding subunit, is specific to IL-36. The antagonism of IL-36Ra was similar to inhibition of IL-1Ra on IL-1. IL-36Ra binding to IL-36R may inhibit the binding of IL-36R to IL-1RAcP and activation of signal complexes. IL-36 is involved in inflammation induction and immune recognition by stimulating innate, adaptive immune responses. A high expression level of IL-36R is present in BMDCs, and BMDCs respond to IL-36 stimulation and produce various inflammatory cytokines [13,14]. IL-36R is expressed in human monocyte-derived DCs (MDCs) and MDCs respond to IL-36 agonists and produce several cytokines [27]. Maturation of DCs is stimulated by IL-36 agonists to enhance the cell surface expression of CD83, CD86, and of MHC class II molecules. The synergistic increase of CD14 expression is induced by the co-culture of IL-36α and IFN-γ, which is related to cell recognition of LPS. It was suggested that stimulation of related inflammatory cytokines (IL-36) may be necessary before expression of CD14 and detection of LPS. T-bet is a key transcript factor in Th1 CD4+ T cell differentiation, and its expression may be upregulated by DCs with overexpression of murine IL-36γ, indicating a positive feedback between T-bet and IL-36γ [28]. IL-36 has strong pro-inflammatory bioactivity and induces Th1 polarization. In vivo studies on the effect of IL-36 in Th1 responses can be performed. Four weeks following injection of M. bovis BCG into IL-36R−/− mice, Th1 responses (including decreases in IFN-γ, TNF-α, and nitric oxide) were decreased by splenocyte stimulation in vitro, suggesting that an endogenous IL-36 signal was required for effective Th1 responses to mycobacteria [29]. In addition, IL-36R was also expressed in the keratinocytes and played a role in the release of inflammatory cytokines in an autocrine or paracrine manner. This effect is not important for survival of the host and clearance of bacteria. In contrast to IL-1R1-deficient mice, especially TNF-α deficient mice, there was no difference in survival following infection with M. bovis BCG in IL-36R-deficient mice as compared with wild-type mice. IL-36R deficient mice showed no susceptibility to M. tuberculosis-induced death. This data demonstrated that IL-36 played a pathological role in inflammatory diseases instead of one of physiological host defense. Therefore, we hypothesized that IL-36 deficiency would enhance inflammation in IR injury during CPB in rats, and indeed found that IL-36R−/− rats had improved post-operative cardiac function, which manifested as reduced serum levels of myocardial injury biomarkers, illustrated by attenuated infiltration of CD68+ macrophages and decreased expression of pro-inflammation.
Excessive oxidative stress is another injury-related factor in the myocardial IR model described in numerous previous studies [30–32]. Downregulation of ROS production in the reperfusion following an ischemia event may attenuate tissue damage and cellular apoptosis. Therefore, we examined malondialdehyde (MPA) activity and superoxide dismutase activity in myocardial tissue, which reflected lipid peroxidation and anti-oxidative abilities. We found SOD and MDA were significantly lower in Myh6-Cre IL1RL2flox/flox rats compared to those in WT rats, which suggested that the knockout of IL-36R attenuated the oxidative stress response following I/R injury in cardiomyocytes. Oxidative stress injury following IR injury also contributes to mitochondrial dysfunction and tissue damage. Sirtuin is a protein family that is highly conserved in bacteria and humans. The deacetylation of SIRT1 and activation of some proteins involved in cell repair and protection, including p53, Fox transcription factors, and PPARγ, can ameliorate pathologic and psychological stress responses [30]. SIRT1 can enhance activation of eNOS and promote vascular relaxation [31]. SIRT1 is also a key cytoprotective agent regulating these proteins in many cell processes, including metabolism, cell survival, and apoptosis [32]. In this study, it was found that SIRT1 expression was increased, while the iNOS/eNOS ratio was decreased following IL-36R knockout. The increased eNOS expression and an inverted iNOS/eNOS ratio in the hearts of Myh6-Cre IL1RL2flox/flox rats were also found in our study, indicating the imbalance of iNOS/eNOS in the cardiomyocytes with I/R injury. It has been reported that SIRT1 is a crucial regulator of vascular homeostasis and that SIRT inhibits endothelial senescence via the eNOS–SIRT1 axis. Another study revealed that an increase in NO can originate due to increased SIRT expression and that SIRT deacetylates eNOS, increasing eNOS activity and production of endothelial nitric oxide (NO).
Therefore, the data suggested that regulation of SIRT1 was associated with protection from oxidative stress-induced tissue injury. SIRT1 promoted the expression of FOXO1 and p53, enhanced activity of eNOS, and increased the production of endothelial NO molecules, which inhibited oxidative stress injury and protected cardiomyocytes from death in I/R injury.
Limitations
Our study has 2 limitations. Firstly, we did not perform in vitro experiments in the primary cardiomyocytes isolated from neonatal rats; we only examined the oxidative stress response in the cell line with or without knockout of IL-36R, and we believe that further investigation in the primary cardiomyocytes should be considered. Secondly, we only established CPB models in healthy animals. However, patients who need to receive CPB have organic heart diseases and even severe cardiac dysfunction. Therefore, CPB models in healthy rats cannot completely mimic the situation in patients receiving CPB.
Conclusions
We revealed that the deficiency of IL-36R in cardiomyocytes plays a protective role in IR injury induced in rat CPB models, via inhibition of oxidative stress and inflammatory response. The findings of the current study may provide a novel therapeutic strategy for IR injury, enabling post-operative myocardial protection in patients who undergo open cardiac surgery and extracorporeal circulation.
Footnotes
Conflict of interests
None.
Source of support: This work was supported by the Guangxi Key Research and Development Plan [grant number GuikeAB18126057]
References
- 1.De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29:137–49. doi: 10.1016/j.bpa.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Sirvinskas E, Kinderyte A, Trumbeckaite S, et al. Effects of sevoflurane vs. propofol on mitochondrial functional activity after ischemia-reperfusion injury and the influence on clinical parameters in patients undergoing CABG surgery with cardiopulmonary bypass. Perfusion. 2015;30:590–95. doi: 10.1177/0267659115571174. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/590987. 590987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enc Y, Karaca P, Ayoglu U, et al. The acute cardioprotective effect of glucocorticoid in myocardial ischemia-reperfusion injury occurring during cardiopulmonary bypass. Heart Vessels. 2006;21:152–56. doi: 10.1007/s00380-005-0887-8. [DOI] [PubMed] [Google Scholar]
- 5.Durandy Y. Minimizing systemic inflammation during cardiopulmonary bypass in the pediatric population. Artif Organs. 2014;38:11–18. doi: 10.1111/aor.12195. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood G. Bypass and inflammation. Perfusion. 2017;32:90–91. doi: 10.1177/0267659117691404. [DOI] [PubMed] [Google Scholar]
- 7.Zakkar M, Guida G, Suleiman MS, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/189863. 189863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murase M, Nakayama Y, Sessler DI, et al. Changes in platelet Bax levels contribute to impaired platelet response to thrombin after cardiopulmonary bypass: Prospective observational clinical and laboratory investigations. Br J Anaesth. 2017;119:1118–26. doi: 10.1093/bja/aex349. [DOI] [PubMed] [Google Scholar]
- 9.Kovacevic M, Simic O, Jonjic N, Stifter S. Apoptosis and cardiopulmonary bypass. J Card Surg. 2007;22:129–34. doi: 10.1111/j.1540-8191.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:1243–53. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 11.Sandler N, Kaczmarek E, Itagaki K, et al. Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart Lung Circ. 2018;27:122–29. doi: 10.1016/j.hlc.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Dias AE, Melnikov P, Consolo LZ. Oxidative stress in coronary artery bypass surgery. Rev Bras Cir Cardiovasc. 2015;30:417–24. doi: 10.5935/1678-9741.20150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Todorovic V, Kakavas S, et al. Quantitative ligand and receptor binding studies reveal the mechanism of interleukin-36 (IL-36) pathway activation. J Biol Chem. 2018;293:403–11. doi: 10.1074/jbc.M117.805739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Veerdonk FL, de Graaf DM, Joosten LA, Dinarello CA. Biology of IL-38 and its role in disease. Immunol Rev. 2018;281:191–96. doi: 10.1111/imr.12612. [DOI] [PubMed] [Google Scholar]
- 15.Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5–13. doi: 10.2340/00015555-2808. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Zhang M, Kageyama S, et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J Hepatol. 2017;67:1232–42. doi: 10.1016/j.jhep.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YH, Li SA, Huang CH, et al. Sirt1 activation by post-ischemic treatment with lumbrokinase protects against myocardial ischemia-reperfusion injury. Front Pharmacol. 2018;9:636. doi: 10.3389/fphar.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Di S, Fan C, et al. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis. 2016;21:905–16. doi: 10.1007/s10495-016-1258-x. [DOI] [PubMed] [Google Scholar]
- 19.Madrahimov N, Boyle EC, Gueler F, et al. Novel mouse model of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2018;53:186–93. doi: 10.1093/ejcts/ezx237. [DOI] [PubMed] [Google Scholar]
- 20.Madrahimov N, Natanov R, Boyle EC, et al. Cardiopulmonary bypass in a mouse model: A novel approach. J Vis Exp. 2017;(127) doi: 10.3791/56017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagawa H, Morita K, Nagahori R, et al. Prevention of ischemia/reperfusion-induced pulmonary dysfunction after cardiopulmonary bypass with terminal leukocyte-depleted lung reperfusion. J Thorac Cardiovasc Surg. 2010;139:174–80. doi: 10.1016/j.jtcvs.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Mastro F, Guida P, Scrascia G, et al. Cardiac troponin I and creatine kinase-MB release after different cardiac surgeries. J Cardiovasc Med (Hagerstown) 2015;16:456–64. doi: 10.2459/JCM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 23.Cauliez B, Redonnet M, Darras S, et al. [Cardiac troponin I and CK-MB mass after cardiac surgery with cardiopulmonary bypass]. Ann Biol Clin (Paris) 2004;62:41–46. [in French] [PubMed] [Google Scholar]
- 24.Abdel-Rahman U, Risteski P, Tizi K, et al. Hypoxic reoxygenation during initial reperfusion attenuates cardiac dysfunction and limits ischemia-reperfusion injury after cardioplegic arrest in a porcine model. J Thorac Cardiovasc Surg. 2009;137:978–82. doi: 10.1016/j.jtcvs.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Zhou C, Liu J, et al. Adding emulsified isoflurane to cardioplegia solution produces cardiac protection in a dog cardiopulmonary bypass model. Sci Rep. 2016;6:23572. doi: 10.1038/srep23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutet MA, Bart G, Penhoat M, et al. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol. 2016;184:159–73. doi: 10.1111/cei.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridgewood C, Stacey M, Alase A, et al. IL-36gamma has proinflammatory effects on human endothelial cells. Exp Dermatol. 2017;26:402–8. doi: 10.1111/exd.13228. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa A, Vollmer S, Besgen P, et al. Unopposed IL-36 activity promotes clonal CD4(+) T-cell responses with IL-17A production in generalized pustular psoriasis. J Invest Dermatol. 2018;138:1338–47. doi: 10.1016/j.jid.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Segueni N, Vigne S, Palmer G, et al. Limited contribution of IL-36 versus IL-1 and TNF pathways in host response to mycobacterial infection. PLoS One. 2015;10:e0126058. doi: 10.1371/journal.pone.0126058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han D, Wang J, Ma S, et al. SIRT1 as a promising novel therapeutic target for myocardial ischemia reperfusion injury and cardiometabolic disease. Curr Drug Targets. 2017;18:1746–53. doi: 10.2174/1389450116666150630110529. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Huang Q, Zeng Z, et al. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/7543973. 7543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang JW, Yao H, Caito S, et al. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]