Abstract
Mitochondria are organelles with highly dynamic ultrastructure maintained by flexible fusion and fission rates governed by Guanosine Triphosphatases (GTPases) dependent proteins. Balanced control of mitochondrial quality control is crucial for maintaining cellular energy and metabolic homeostasis; however, dysfunction of the dynamics of fusion and fission causes loss of integrity and functions with the accumulation of damaged mitochondria and mitochondrial deoxyribose nucleic acid (mtDNA) that can halt energy production and induce oxidative stress. Mitochondrial derived reactive oxygen species (ROS) can mediate redox signaling or, in excess, causing activation of inflammatory proteins and further exacerbate mitochondrial deterioration and oxidative stress. ROS have a deleterious effect on many cellular components, including lipids, proteins, both nuclear and mtDNA and cell membrane lipids producing the net result of the accumulation of damage associated molecular pattern (DAMPs) capable of activating pathogen recognition receptors (PRRs) on the surface and in the cytoplasm of immune cells. Chronic inflammation due to oxidative damage is thought to trigger numerous chronic diseases including cardiac, liver and kidney disorders, neurodegenerative diseases (Parkinson’s disease and Alzheimer’s disease), cardiovascular diseases/atherosclerosis, obesity, insulin resistance, and type 2 diabetes mellitus.
Keywords: mitochondria, dynamics, inflammation, non-communicable diseases
Introduction
Mitochondria are one of the cell organelles that are characterized as round, bean-like, seen as an oval shape under the electron microscope.1 They contain a dynamic branched network that constantly fuses and divide under the control of specific fusion and fission machinery2 which is consistent with the endosymbiotic theory of bacterial ancestor evolution.3 By nature, mitochondria are a highly flexible ultrastructure organelle designed to regulate the bioenergetics flux of key molecular elements.2,4 Mitochondrial proteomics depict that around 1200 proteins are encoded in the nuclear genome with only 13 of them being coded in the maternally inherited mitochondrial genome.5 The overall dynamic nature of mitochondria is governed by Guanosine Triphosphatases (GTPases) dependent antagonist activities called fusion and fission. Bidirectional crosstalk between mitochondria and the nucleus is strictly controlled by different signaling pathways and with the dynamic fusion and fission nature of mitochondria.6 Fusion proteins can be found in outer membrane mitofusins (Mfn1 & Mfn2) and inner membrane optic atrophy 1 (Opa 1). Fission proteins (Dynamin related protein 1 (Drp1)) with other proteins mediate the mitochondrial ultrastructure process.7,8 So, balanced control of mitochondrial dynamics is very important which, if not balanced, can lead to mitochondrial dysfunction. Mitochondrial dysfunction is a condition characterized by loss of membrane potential to decrease Adenosine Triphosphate (ATP) production, decrease respiration or oxidative phosphorylation leading to a metabolic shift to the glycolysis dependent ATP generation that takes place outside mitochondria which increases the formation of mitochondrial reactive oxygen species (ROS).4,9,10 Uncontrolled production of ROS can further damage/distract the mitochondrial membrane and its major constitutes like DNA, lipids, and proteins.11 These fragments can initiate mitophagy to promote cell survival or can induce the initiation of the intrinsic pathway of apoptosis.12,13 Initially, this condition can be regulated by mitochondrial fusion/fission activities. Fusion delays the onset of apoptosis by inhibiting mitochondrial fragmentation while fission has a positive role in apoptosis.14,15 However, the failure of such quality control can contribute to the development of degenerative diseases like type 2 diabetes, cancer, cardiovascular disorders, neuropathies such as Parkinson’s and Alzheimer’s disease and age-related disorders.12,16–20 Mitochondrial dysfunctions play a central role in chronic inflammation through activation of signaling pathways, including mitochondrial calcium handling ROS production and activation of nuclear factor kappa B (NF-kB).21 Damaged mitochondria and degraded mtDNA produce an accumulation of Danger Associated Molecular Patterns (DAMPs) which can bind and activate membrane or cytoplasmic pathogen recognition receptors (PRRs) to stimulate inflammatory responses.22,23 Mitochondrial quality control failure with the downregulation of mitophagy results in spontaneous inflammasomes activation as a consequence of mitochondrial ROS burst.24 Oxidative stress due to ROS burst also damages endothelial cells, which are recognized factors for atherosclerosis; decreased nitric oxide (NO) synthesis contributes to hypertension, upregulates the secretion of adhesion molecules and inflammatory cytokines, and is responsible for the oxidation of low-density lipoproteins.25 Muscle cell mitochondrial dysfunctions lead to a reduction in fatty acid oxidation and inhibition of glucose transport, which is an indication of insulin resistance, and further results in obesity.26 Obesity increases the likelihood of various diseases, particularly atheromatous heart disease, type 2 diabetes, breathing difficulties during sleep, certain types of cancer, osteoarthritis and chronic periodontitis.27,28 However, the exact molecular mechanism of mitochondrial dysfunction and its association with this chronic non-communicable disease is not fully addressed. Therefore, this review aims to describe mitochondrial dynamic dysfunctions as the main determinant factors for inflammatory-related non-communicable diseases.
Mitochondrial Dynamics and Functions
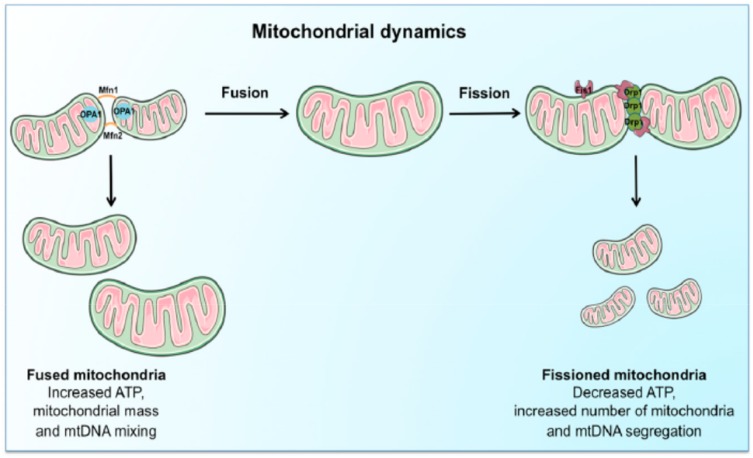
Mitochondria are vital to life. They generate Adenosine Triphosphate (ATP) by the breakdown of fuels (i.e., glucose, amino acids, and fatty acids) through a series of redox reactions performed by a set of five electron transport chain (ETC) enzyme complexes of the mammalian OXPHOS system.29,30 To control the required maintenance of mitochondrial morphology in a dynamic environment, mitochondria continuously undergo tightly regulated and opposite remodeling process called fusion and fission activities.31–33 Fusion and fission activity of the mitochondria is regulated by the coordinated action of the series well-conserved GTPases proteins. These are mitofusins(Mfn1 & Mfn2) transmembrane GTPases embedded in the mitochondrial outer membrane, Optic atrophy1 (OPA) is a dynamin-related GTPases associated with the mitochondrial inner membrane or intermembrane space.34–36 Proper balance of the antagonist activities of fusion and fission is crucial for fundamental mitochondrial integrity and functioning including energy metabolism, ROS generation, and apoptosis regulation.35,37 Fission promotes the removal of damaged mitochondria by mitophagy to maintain proper assembly of electron transport chain complexes. This can allow mitochondria to exchange lipid membranes, and intra-mitochondrial contents while fusion escapes autophagy-mediated destruction to maintain proper mitochondrial ultrastructure and elongation (Figure 1).23,35,38 Fusion allows for mitochondrial interconnection, favoring mtDNA mixing, signal transmission and exchange of metabolites with in-network. On the other hand, fission ensures equal organelle segregation between daughter cells and target defective mitochondria for their subsequent removal through mitophagy.39,40 The dynamic process controls mitochondrial morphology, biogenesis, transportation and localization, quality control and degradation, and apoptotic cell deaths.35,41,42
Figure 1.
Schematic representation of the regulation and coordination of mitochondrial dynamics.
Notes: The coordination of fusion and fission machinery, involving mitofusins (Mfn) 1 and 2, optic atrophy protein 1 (OPA1), dynamin-related protein 1 (Drp1). Mitochondrial fusion, by interconnecting organelles, promotes mtDNA mixing and enhances bioenergetics efficiency while fission removes damaged mitochondria via mitophagy. Reproduced from Picca A, Lezza AMS, Leeuwenburgh C, et al. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci. 2017;18(5):933.23
Mitochondria are integral to normal cellular function as they are responsible for energy production through oxidative phosphorylation; they synthesize key molecules including the phospholipids and Heme, calcium homeostasis, apoptotic activation, and cell death.43–45 Mitochondria have a unique feature of semi-autonomous in which exactly 13 proteins used in ATP production through oxidative phosphorylation are coded by its mtDNA. The remaining 1200–1500 mitochondrial proteins are nuclear gene products that are imported into the organelle.5,46,47 The maintenance of mtDNA is important for normal and efficient functioning as it codes proteins for oxidative phosphorylation of ATP production from oxidation of sugar, fats, and proteins, as well as tRNAs and ribosomal RNAs that are needed for their translation in the mitochondrial matrix.48 As the principal functions of mitochondria are to synthesize ATP from the oxidation of sugar, fat, and proteins through the process of OXPHOS via endosymbiotic principle. Electrons from reduced equivalents are transported along the respiratory chain protein complex to generate electrochemical proton gradient or membrane potential (ΔΨm) across the inner mitochondrial membrane producing ATP.10,27,48 Under normal conditions, 1–2% of electrons can leak from electron transport chain and reduced to superoxide radical there by producing reactive oxygen species (ROS), which will be detoxified by the action of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase.49,50 However, when the production of ROS overrides the capability of antioxidants, oxidative stress will damage cellular macromolecules (i.e. DNA, lipids and proteins). This is linked to multiple pathological conditions such as: neurodegenerative diseases; diabetes; cancer; and premature aging.29,51 The damaged mitochondria with cellular stress are removed by selective mitochondrial autophagy called mitophagy otherwise damaged mitochondria accumulated and induce mitochondrion mediated cell death.52 Fusion and fission regulate mitochondrial damage and repair antagonistically; fusion enriches damaged mitochondria with normal genome and proteins to escape mitochondria from damage while fission, in contrast, destined damaged mitochondria for destruction by mitophagy.31,53,54 Alteration of normal mitochondrial function results in signaling disturbance, energy-dependent disturbance, and genetic defects of the mitochondrial genome.
Mitochondrial Dysfunction and Inflammation
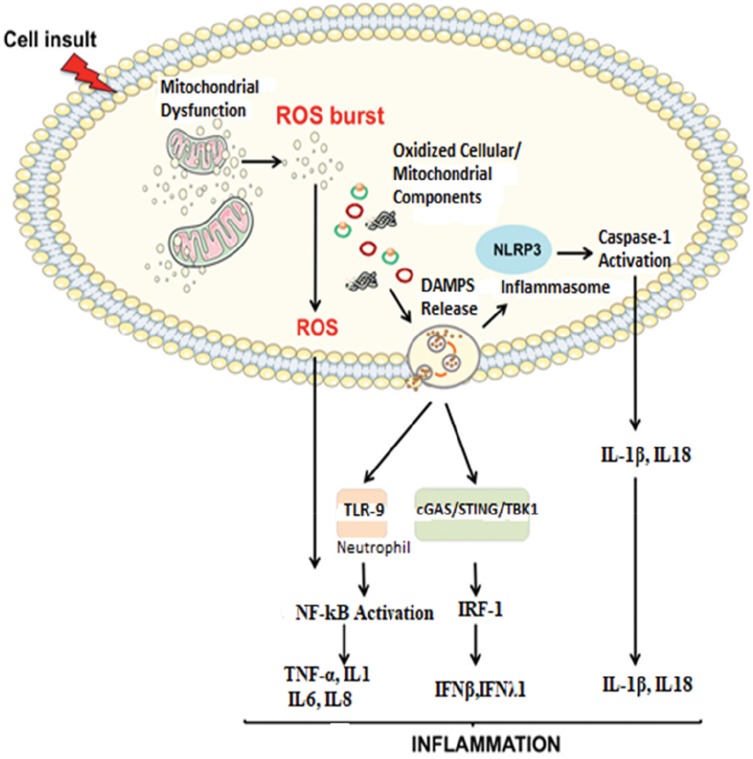
Imbalanced activities of fusion and opposite fission lead to mitochondrial dysfunction. These further results in; mitochondrial fragmentation, loss of oxidative phosphorylation (OXPHOS), mtDNA depletion and ROS production. These are associated with metabolic dysfunction or disease.55 The impairment of mitochondrial quality control to remove damaged and dysfunctional mitochondria leads to the accumulation of damage associated with membrane patterns (DAMPs) released from injured cells, cell-free mtDNA, N-formyl peptides and Cardiolipin.56 Mitochondrial DAMPs can bind and activate membrane or cytosolic pathogen recognition receptors (PRRs) such as toll-like receptors (TLR), nod like receptors (NLRs), like those recognized by pathogen-associated molecular pattern (PAMPs).57 Thus, it activates different early-phase inflammatory mediators like tumor necrosis factor α (TNF-α), interleukins, interferon-gamma (IFN-γ) and ROS/RNS.23 These coexistences of cellular responses to danger of oxidative stress and accumulation of mitochondrial DNA leads to chronic inflammation.23 Mitochondrial respiratory chain, NADPH oxidases, and 5-lipoxygenase are the major cellular sources of ROS production.58 The byproducts of oxidative stress: ROS; and RNS can also further generated because of inflammatory cell activity.59,60 Surprisingly, oxidative stress activates several transcription factors (NF-kB and activated protein 1) leading to the production and activation of pro-inflammatory cytokines, chemokines, and lymphocytes which in turn leads to the production of more ROS and RNS, principally in the form of superoxide, nitric oxide (NO), and peroxynitrite.61–63 This complicated bidirectional self-amplifying and the self-sustaining relationship between the development of chronic oxidative stress and chronic systemic inflammations outcomes to modification of DNA, tertiary protein structure, and lipid peroxidation of the cell membrane. This further results in a net accumulation of DAMPs that can further exacerbate the conditions.64–66 Thus, in turn, it will have a deleterious effect of damaging mitochondria synergistically leading to the depletion of ATP production and promoting a switch to anaerobic glycolysis.67 Therefore, mitochondrial dysfunction with fission upregulation prevents the elimination of damaged mitochondria producing ROS and RNS that exceed the antioxidant activity is likely initiating factors in inflammation, aging, and age-related diseases (Figure 2).44
Figure 2.
Proposed signaling pathways through which damaged-associated molecular patterns (DAMPs) can trigger inflammation.
Notes: The impairment of the mitochondrial quality control process may lead to an accumulation of intracellular oxidized components and their release as DAMPs. Damaged mtDNA molecules, either TFAM-bound (green circles) or unbound (red circles) may be released as DAMPs. These, in turn, can activate an inflammatory response via three distinct signaling pathways by interacting with by interacting with 1) Toll-like receptors (TLRs), 2) nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, and 3) cytosolic cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) DNA-sensing system. Reproduced from Picca A, Lezza AMS, Leeuwenburgh C, et al. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci. 2017;18(5):933.23
Abbreviations: IFN, interferon; IL, interleukin; IRF-1, interferon regulatory factor 1; mtDNA, mitochondrial DNA; NF-kB, nuclear factor-kB; ROS, reactive oxygen species; TBK1, TANK-binding kinase 1; TNF-α, tumor necrosis factor-alpha.
Mitochondrial Dysfunction and Signaling Pathway in Inflammation
Mitochondria are multifunctional organelles that contain different proteins used for biosynthesis, metabolism and cell death or survival functions.68 The vast majority of proteins are encoded in the nucleus and imported to mitochondria after translated. Translated proteins were shared with end-to-end collision fashion of mitochondrial fusion movement along the cytoskeleton.2,34 Each fusion movement of mitochondria is mediated by outer membrane proteins Mfn 1 and 2 and inner membrane with OPA1 catalyzed by dedicated GTPases.69–71 Since this continuous fusion of individual mitochondria may produce abnormally elongated mitochondria that were non-functional, an opposing process called fission becomes available.32 The fission process prevents abnormal elongation of mitochondria by splitting a single mitochondrion into two separate organelles.72 If the balance favors fission, mitochondria become fragmented, small spherical organelle while if fusion is favored, elongated and banded together mitochondria will be formed.35 Mitochondrial dynamics imbalance affects energy production, apoptosis, mitophagy, mitochondrial movement, mtDNA stability and the tolerance of cells to mtDNA mutations.73–75 The abnormalities of dynamics dysfunction and bidirectional signaling pathway between mitochondria and nucleus are exposed to, constantly alter physiological, environmental and pathological stimuli.2,6 As the center for many cellular functions, especially in energy supply with oxidative metabolism, maintaining mitochondrial quality control is crucial for tissue homeostasis.23,39 Mitochondrial dysfunction with the release of ROS and mitochondrial-derived DAMPs contribute to initiating an inflammatory response by invoking pro-inflammatory cytokines that interact with receptors like those involved in the pathogen-associated response.76 Mitochondria play a central role in sterile inflammation through the activation of several pathways.21
Mitochondrial Ca2+ Handling and ROS Derived Inflammation
Mitochondrial energy generation in the inner membrane through the electron transport chain by using oxygen as an electron acceptor is the major source of ROS generation.77 ROS are produced by one-electron transfer from redox donor NADH and FADH2 to molecular oxygen in mitochondrial ETC especially from complex I and complex III.78 A redox-sensitive inflammatory signaling pathway engages mitochondrial calcium handling, ROS production, and nuclear factor κB (NF-κB) activation.79 The activation of NF-kB provokes the transcription of pro-inflammatory cytokines such as TNF-α, IL1, IL6, and IL8.77 Under calcium overloads, such as burn injury or sepsis, the electron transport chain (ETC) becomes dysfunctional and elevated ROS generation occurs. Such a ROS burst represents a major pro-inflammatory stimulus through the modulation of the expression and activity of NF-κB (Figure 2).23,77 Antioxidants produced by specific nitric oxide synthase in the biological system eliminate ROS that results in overwhelming the cellular repair system.80 Imbalance of two opposite and antagonist forces, production of ROS and antioxidants called oxidative stress are damaging to mitochondrial cellular components like DNA, protein, lipids and other molecules. Oxidative stress in cellular milieu causes mitochondrial dysfunction and accumulation of damaged products which are recognized by pattern recognition receptors initiating sterile inflammations.81 The coexistence of oxidative stress and mitochondrial dysfunction giving a role for the accumulation of DAMPs which proposed to initiate sterile inflammation.23
Mitochondrial- DAMPs Derived Inflammation
Due to the “bacterial ancestor” theory of mitochondria, the releasing of contents due to damage or dysfunction to cytoplasm provokes an immunological response. In addition to mitochondrial dysfunction with oxidative stress, several conditions characterized by an inflammatory response (e.g., trauma, HIV, cancer) are associated with increased levels of circulating mitochondrial DAMPs. DAMPs, in turn, induce caspase-1 activation and the release of pro-inflammatory cytokines like IL1B and IL18 which finally leads to sterile inflammation.58 In particular, mitochondrial DNA activates different inflammatory responses through different receptors, (Figure 2) triggering transcription and translation of inflammatory cytokines. Uncontrolled and excessive release of mitochondrial DAMPs associated with severity and contributes to the dysregulated process observed in numerous inflammatory and autoimmune conditions.44
Mitochondrial Dysfunctions Associated Non-Communicable Diseases
Non-communicable diseases (NCDs) are increasingly becoming the leading public causes of morbidity and mortality globally.82 The leading NCDs encompass a cluster of illness including cardiovascular diseases (CVDs), diabetes mellitus type 2, chronic obstructive lung disease, and cancer. These NCDs have common and key modifiable behavioral risk factors like unhealthy diet, lack of physical activity, the harmful use of alcohol, tobacco use and which in turn leads to overweight and obesity, raised blood pressure, and raised cholesterol, and ultimately disease.83 Cardiovascular diseases accounted for most NCD deaths (17.5 million NCD deaths), followed by cancers (8.2million NCD deaths), respiratory diseases (4.0 million NCD deaths) and diabetes mellitus (1.5million NCD deaths).84 Globally, CVDs being the largest contributor to global mortality, accounting for nearly half of 36 million annual NCDs deaths and produces immense health and economic burdens.85
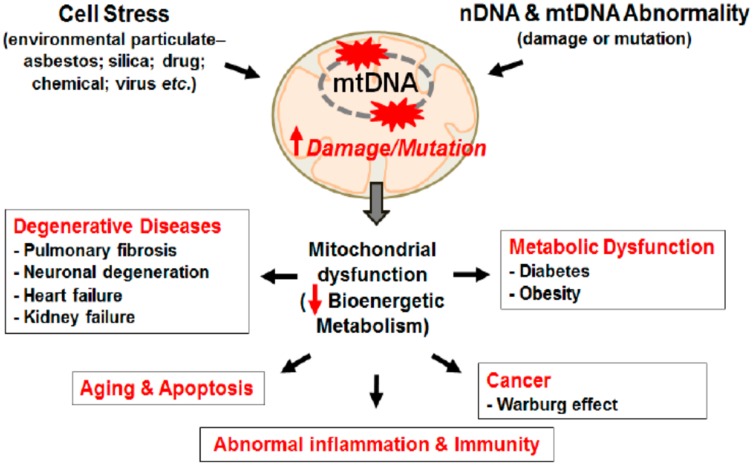
A common feature of all NCDs is excessive fatigue which results from mitochondrial function impairment; the crucial organelle responsible for cellular energy production.86 As stated above, defects in mitochondrial gene results mutation of proteins that involve mitochondrial dynamic activities then proceed to its physiological activity impairment.34,35 The mitochondrial genome is susceptible to oxidative damage as a result of its residence close to the electron transport system, mtDNA lacks histone protein (protects from damage), and repair mechanism rather it Induces to apoptosis once it becomes damaged.87,88 Thus, lack of repair mechanism of damaged mtDNA means cells cannot copy mtDNA accurately resulting in errors of transcription, deletions and mutations and also oxidation from ROS results in a series of cellular abuse; loss of membranes integrity, diminishing proton gradient causing less ATP production, unfolding and loss of cellular protein affinity for their respective enzymes, and releasing cytochromes C into the cytosol stimulating apoptosis, all in a continuous feed-forward cycle of cellular, tissue and organ dysfunction to cause chronic diseases.87 Additionally, high ROS concentration permits histone acetylation to predominate, which accelerates faulty nuclear transcription and thus replication. This initiates the release of NF-kB into the nucleus (a significant pro-inflammatory cytokine which also damages nDNA). Simultaneously, cell differentiation and apoptosis signals are silenced with histone acetylation, eventually resulting in over-replication favoring tumorigenesis.89,90 As the mitochondrial inner membrane is the main site of electron transport chain, dwelling different enzymes and proteins which are also becomes the main source of oxidative stress. Enzymes and proteins (i.e. cytochrome c), and low-molecular-weight redox intermediates (i.e. coenzymes such as ubiquinone or coenzyme Q) that transport reducing equivalents, in the form of hydrogen atoms or just their electrons, down the redox potential from respiratory substrates to oxygen: an oxidative pathway composed of four multiple-subunit complexes in the mitochondria which leaks 0.2–2% electrons to molecular oxygen producing super oxide or hydrogen peroxide.27 These enzymes and proteins are the primary targets of oxidative stress products like ROS and RNS, and it is not surprising that disruption of function and structure of mitochondria are now thought to trigger numerous diseases, including cardiac, liver and kidney disorders, and neurodegenerative diseases (e.g. Parkinson’s disease and Alzheimer’s disease), aging processes and multiple organ failure in septic shock. Cardiovascular disease/atherosclerosis, obesity and type 2 diabetes are closely associated with chronic inflammation characterized by abnormal cytokine production, increased levels of acute-phase reactants and activation of a network of inflammatory signaling pathways.91 Mitochondrial dysfunction because of unbalanced fusion and fission, genetic defects of mtDNA or oxidative damage with ROS and RNS can impair energy metabolism, the central alteration in obesity.8 Cells can manage nutrient supply by increasing mitochondrial contents. However, persistent nutrient leftover devastates the mitochondrial system and causes its dysfunction leading to the accumulation of incompletely oxidized lipid products to cause fat accumulation and oxidative stress which damage endothelial cells.92,93 Obesity is key in the development of major non-communicable diseases (NCDs) including diabetes mellitus, hypertension, metabolic syndrome, non-alcoholic fatty liver disease, cardiovascular disease and several classes of cancer including colorectal, liver, breast, pancreatic endometrial, renal, prostate, lymphoma and myoma.8,27 Dysfunctional mitochondria create a vicious cycle of impaired beta-oxidation giving rise to the production of ceramides, free radicals and inflammatory cytokines which damages the mitochondrial membrane (as shown in Figure 3) and DNA further jeopardizes mitochondrial respiratory capacity.94 Oxygen-free radicals may themselves initiate a chronic inflammatory process in which the pathology is characteristics of obesity and linked to insulin resistance and type 2 diabetes.95
Figure 3.
Mitochondrial damage induces diverse chronic and degenerative diseases.
Notes: Mitochondrial damage and mutation can be caused by stress from environment particulate and/or DNA abnormality. DNA mutation/damage causes mitochondrial dysfunction reducing bioenergetics metabolism promoting chronic inflammation and chronic diseases, like cancer, CVD, Diabetes, Obesity, and aging. Kim SJ, Cheresh P, Jablonski RP, Williams DB, Kamp DW. The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis. Int J Mol Sci. 2015;16(9):21486-21519.96
Therefore, preventing chronic sterile inflammation derived with mitochondrial dysfunction resulting in oxidative stress and DAMPs activated inflammasome can significantly diminish non-communicable diseases.97,98 Reducing cellular and mitochondrial membrane and DNA damage and loss of membrane integrity is important in preventing loss of cellular energy and regulating cellular life span.99 Since oxidative stress is the consequence of imbalance between pro- and antioxidants species, supplements containing dietary antioxidants and some accessory molecules, such as zinc and certain vitamins, are important in maintaining free-radical scavenging systems, biosynthetic capacity, membranes, enzymes and DNA.100 Utility of oral mitochondrial replacement supplements, such as replacement glycerol phospholipids, l-carnitine, alpha-Lipoic acid, coenzyme Q10, NADH, pyrroloquinoline quinone and other mitochondrial supplements also improves mitochondrial function.86 Anaerobic and resistance training intervention induced in a mouse model showed an increase in the activation of the signaling pathway involved in mitochondrial biogenesis.101 Preclinical evidence from animal and in vitro studies suggested that regular endurance-based exercise intervention is known to be a potent stimulus for muscle mitochondrial biogenesis.102 It is also shown that endurance-based exercise increases muscle oxidation capacity of mitochondria by increasing the activity of citrate synthase and respiratory chain complexes.103–105
Conclusion
Mitochondria are central to the regulation of energy metabolism and cellular homeostasis due to their principal role in bioenergetics, ROS production, ion homeostasis, apoptosis and signal transduction. This organelle is highly dynamic and can re-program itself depending on various environmental and intracellular signals important for multiple mitochondrial functions, including mtDNA stability, respiratory function, apoptosis, response to cellular stress, and mitochondrial degradation. The dynamic process of mitochondria may not be balanced as a result of proteins required for fusion and fission that decreases the crucial role of mitochondria bioenergetics and the accumulation of damaged mitochondria producing ROS.ROS generated by dysfunctional mitochondria further damage mitochondrial of different organs and tissues that cannot function properly to result in chronic and age-related disorders. Non-communicable diseases that are increasing worldwide in recent years are the consequences of unhealthy diets and physical inactivity, which shares basic mechanisms of mitochondrial defects, systemic inflammation, and oxidative stress. Consequent oxidative stress causing endoplasmic reticulum (ER) and mitochondrial stress leads to excess accumulation of food energy favoring obesity and the development of other metabolic syndrome and related complications. Maintaining the health of mitochondria is very valuable and could aid in the prevention of age-related disorders.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Rongvaux A. Innate immunity and tolerance toward mitochondria. Mitochondrion. 2018;41:14–20. doi: 10.1016/j.mito.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorov DB, Plotnikov EY, Silachev DN, et al. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochemistry (Moscow). 2014;79:1017–1031. doi: 10.1134/S0006297914100046 [DOI] [PubMed] [Google Scholar]
- 4.Willems PHGM, Rossignol R, Dieteren CEJ, Murphy MP, Koopman WJH. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015;22:207–218. doi: 10.1016/j.cmet.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–569. doi: 10.1016/j.cell.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao YL, Meng S, Chen Y, et al. MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature. 2017;542:372–376. doi: 10.1038/nature21077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerdá C, Sánchez C, Climent B, et al. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv Exp Med Biol. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Nagdas S, Kashatus DF. The interplay between oncogenic signaling networks and mitochondrial dynamics. Antioxidants. 2017;6(2):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ježek J, Cooper KF, Strich R. Reactive oxygen species and mitochondrial dynamics: the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants. 2018;7:13. doi: 10.3390/antiox7010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glowacki S, Synowiec E, Blasiak J. The role of mitochondrial DNA damage and repair in the resistance of BCR/ABL-expressing cells to tyrosine kinase inhibitors. Int J Mol Sci. 2013;14:16348–16364. doi: 10.3390/ijms140816348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordi M, Nazio F, Campello S. The close interconnection between mitochondrial dynamics and mitophagy in cancer. Front Oncol. 2017;7. doi: 10.3389/fonc.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Carreres L, Nasrallah A, Fajas L. Cancer: linking powerhouses to suicidal bags. Front Oncol. 2017;7. doi: 10.3389/fonc.2017.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkavan H, Green DR. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018;25:46–55. doi: 10.1038/cdd.2017.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suárez-Rivero J, Villanueva-Paz M, de la Cruz-ojeda P, et al. Mitochondrial dynamics in mitochondrial diseases. Diseases. 2016;5:1. doi: 10.3390/diseases5010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simula L, Nazio F, Campello S. The mitochondrial dynamics in cancer and immune-surveillance. Semin Cancer Biol. 2017;47:29–42. doi: 10.1016/j.semcancer.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Wada J, Nakatsuka A. Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med Okayama. 2016;70(3):151–158. [DOI] [PubMed] [Google Scholar]
- 18.Marín-García J, Akhmedov AT. Mitochondrial dynamics and cell death in heart failure. Heart Fail Rev. 2016;21(2):123–136. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Luo CL, Tao LY. Dynamin-related protein 1 (Drp1) mediating mitophagy contributes to the pathophysiology of nervous system diseases and brain injury. Histol Histopathol. 2017;32(6):551–559. [DOI] [PubMed] [Google Scholar]
- 20.López-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech Ageing Dev. 2017;162:108–121. doi: 10.1016/j.mad.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 21.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13(2):106–118. doi: 10.1016/j.mito.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Mathew A, Lindsley TA, Sheridan A, et al. Degraded mitochondrial dna is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimer’s Dis. 2012;30:617–627. doi: 10.3233/JAD-2012-120145 [DOI] [PubMed] [Google Scholar]
- 23.Picca A, Lezza AMS, Leeuwenburgh C, et al. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci. 2017;18(5):933. doi: 10.3390/ijms18050933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahira K, Haspel JA, Rathinam VAK, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu TF, Brown CM, El Gazzar M, et al. Fueling the flame: bioenergy couples metabolism and inflammation. J Leukoc Biol. 2012;92:499–507. doi: 10.1189/jlb.0212078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon B, Nedergaard J. Neither brown nor white. Nature. 2012;488:286–287. doi: 10.1038/488286a [DOI] [PubMed] [Google Scholar]
- 27.Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol. 2000;64(1):139–153. [DOI] [PubMed] [Google Scholar]
- 28.Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81:1708–1724. doi: 10.1902/jop.2010.100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567 [DOI] [PubMed] [Google Scholar]
- 30.DiMauro S. Mitochondrial diseases. Biochim Biophys Acta Bioenerg. 2004;1658(1–2):80–88. [DOI] [PubMed] [Google Scholar]
- 31.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013 [DOI] [PubMed] [Google Scholar]
- 32.Bleazard W, McCaffery JM, King EJ, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westrate LM, Drocco JA, Martin KR, Hlavacek WS, MacKeigan JP. Mitochondrial morphological features are associated with fission and fusion events. PLoS One. 2014;9:e95265. doi: 10.1371/journal.pone.0095265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529 [DOI] [PubMed] [Google Scholar]
- 35.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275 [DOI] [PubMed] [Google Scholar]
- 36.Pernas L, Scorrano L. Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011 [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Perry G, Smitha MA, Wang X. Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Alzheimer’s Dis. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Escudero V, Martín-Maestro P, Perry G, Avila J. Deconstructing mitochondrial dysfunction in alzheimer disease. Oxid Med Cell Longev. 2013;2013:1–13. doi: 10.1155/2013/162152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Twig G, Elorza A, Molina AJA, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono T, Isobe K, Nakada K, Hayashi J-I. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–275. doi: 10.1038/90116 [DOI] [PubMed] [Google Scholar]
- 41.Zorzano A, Hernández-Alvarez MI, Sebastián D, Muñoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal. 2015;22:1020–1031. doi: 10.1089/ars.2014.6208 [DOI] [PubMed] [Google Scholar]
- 42.Yin F, Cadenas E. Mitochondria: the cellular hub of the dynamic coordinated network. Antioxid Redox Signal. 2015;22:961–964. doi: 10.1089/ars.2015.6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/tjp.2000.529.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández-Aguilera A, Rull A, Rodríguez-Gallego E, et al. Mitochondrial dysfunction: a basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators Inflamm. 2013;2013:1–13. doi: 10.1155/2013/135698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McInnes J. Mitochondrial-associated metabolic disorders: foundations, pathologies and recent progress. Nutr Metab. 2013;10:63. doi: 10.1186/1743-7075-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. doi: 10.1016/j.molcel.2006.11.024 [DOI] [PubMed] [Google Scholar]
- 47.Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem. 2007;76:701–722. doi: 10.1146/annurev.biochem.76.052305.091720 [DOI] [PubMed] [Google Scholar]
- 48.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenaz G, Baracca A, Barbero G, et al. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta Bioenerg. 2010;1797:633–640. doi: 10.1016/j.bbabio.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 50.Srivastava S. Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clin Transl Med. 2016;5. doi: 10.1186/s40169-016-0104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy CM, Kenny LC. Immunostimulatory role of mitochondrial DAMPs: alarming for pre-eclampsia? Am J Reprod Immunol. 2016;76(5):341–347. doi: 10.1111/aji.2016.76.issue-5 [DOI] [PubMed] [Google Scholar]
- 53.Chauhan A, Vera J, Wolkenhauer O. The systems biology of mitochondrial fission and fusion and implications for disease and aging. Biogerontology. 2014;15:1–12. doi: 10.1007/s10522-013-9474-z [DOI] [PubMed] [Google Scholar]
- 54.Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta Bioenerg. 2012;1817(10):1833–1838. [DOI] [PubMed] [Google Scholar]
- 55.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins LV, Hajizadeh S, Holme E, Jonsson I-M, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328 [DOI] [PubMed] [Google Scholar]
- 58.Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69:2999–3013. doi: 10.1007/s00018-012-0962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer MT, Sharma R, Lim JL, et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135:886–899. doi: 10.1093/brain/aws012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris G, Anderson G, Dean O, et al. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014. [DOI] [PubMed] [Google Scholar]
- 61.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Rad Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prolo C, Álvarez MN, Radi R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. BioFactors. 2014;40:215–225. doi: 10.1002/biof.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaamonde-García C, Riveiro-Naveira RR, Valcárcel-Ares MN, Hermida-Carballo L, Blanco FJ, Lõpez-Armada MJ. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012;64:2927–2936. doi: 10.1002/art.34508 [DOI] [PubMed] [Google Scholar]
- 64.Galley HF. Bench-to-bedside review: targeting antioxidants to mitochondria in sepsis. Critical Care. 2010;14. doi: 10.1186/cc9098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, et al. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Devel Immunol. 2013;2013:1–14. doi: 10.1155/2013/708659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucas K, Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Auld D, Lea W, Davis MI, et al..Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science.2013;339:1328–1331. doi: 10.1126/science.1230593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001. [DOI] [PubMed] [Google Scholar]
- 70.Rojo M, Legros F, Chateau D, Lombès A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115(8):1663–1674. [DOI] [PubMed] [Google Scholar]
- 71.Alexander C, Votruba M, Pesch UEA, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944 [DOI] [PubMed] [Google Scholar]
- 72.Richter V, Singh AP, Kvansakul M, Ryan MT, Osellame LD. Splitting up the powerhouse: structural insights into the mechanism of mitochondrial fission. Cell Mol Life Sci. 2015;72:3695–3707. doi: 10.1007/s00018-015-1950-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H, Vermulst M, Wang YE, et al. Mitochondrial fusion is required for mtdna stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- 75.Chan NC, Salazar AM, Pham AH, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quirós PM, Langer T, López-Otín C. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol. 2015;16:345–359. doi: 10.1038/nrm3984 [DOI] [PubMed] [Google Scholar]
- 77.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/embj.1991.10.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang LS, Cobessi D, Tung EY, Berry EA. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J Mol Biol. 2005;351:573–597. doi: 10.1016/j.jmb.2005.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maass DL, White J, Sanders B, Horton JW. Role of cytosolic vs. mitochondrial Ca2+ accumulation in burn injury-related myocardial inflammation and function. Am J Physiol - Hear Circ Physiol. 2005;288:H744–H751. doi: 10.1152/ajpheart.00367.2004 [DOI] [PubMed] [Google Scholar]
- 80.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249 [DOI] [PubMed] [Google Scholar]
- 81.Hu F, Liu F. Mitochondrial stress: a bridge between mitochondrial dysfunction and metabolic diseases? Cell Signal. 2011;23:1528–1533. doi: 10.1016/j.cellsig.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekpenyong CE, Udokang NE, Akpan EE, Samson T. Double burden, non-communicable diseases and risk factors evaluation in Sub-Saharan Africa: the Nigerian experience. Eur J Sustain Devel. 2012;1:249. doi: 10.14207/ejsd.2012.v1n2p249 [DOI] [Google Scholar]
- 83.Riley L, Gouda H, Cowan M. Noncumminicable Diseases Progress Monitor 2017. World Health Organization; 2017:2017. [Google Scholar]
- 84.Mendis S, Armstrong T, Bettcher D, et al. Global Status Report on Noncommunicable Diseases 2014. World Health Organisation; 2014. [DOI] [PubMed] [Google Scholar]
- 85.Benziger CP, Roth GA, Moran AE. The global burden of disease study and the preventable burden of NCD. Glob Heart. 2016;11:393–397. doi: 10.1016/j.gheart.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 86.Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med. 2014. [PMC free article] [PubMed] [Google Scholar]
- 87.Zeviar DD, Gonzalez MJ, Massari JRM, Duconge J, Mikirova N. The role of mitochondria in cancer and other chronic diseases. J Orthomol Med. 2014;29(4):157. [Google Scholar]
- 88.Mason PA. Mismatch repair activity in mammalian mitochondria. Nucleic Acids Res. 2003;31:1052–1058. doi: 10.1093/nar/gkg167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.González MJ, Rosario-Pérez G, Guzmán AM, et al. Mitochondria, energy and cancer: the relationship with ascorbic acid. J Orthomol Med. 2010. [PMC free article] [PubMed] [Google Scholar]
- 90.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:1–13. doi: 10.1155/2012/646354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim S, Cho YM, Park KS, Lee HK. Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann N Y Acad Sci. 2010;1201:166–176. doi: 10.1111/j.1749-6632.2010.05622.x [DOI] [PubMed] [Google Scholar]
- 92.Camps J, Rodríguez-Gallego E, García-Heredia A, et al. Paraoxonases and chemokine (C-C motif) ligand-2 in noncommunicable diseases. Adv Clin Chem. 2014. [DOI] [PubMed] [Google Scholar]
- 93.Chang J-C. Regulatory role of mitochondria in oxidative stress and atherosclerosis. World J Cardiol. 2010;2:150. doi: 10.4330/wjc.v2.i6.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944 [DOI] [PubMed] [Google Scholar]
- 95.Rogge MM. The role of impaired mitochondrial lipid oxidation in obesity. Biol Res Nurs. 2009;10:356–373. doi: 10.1177/1099800408329408 [DOI] [PubMed] [Google Scholar]
- 96.Kim S-J., Cheresh P, Jablonski RP, Williams DB, Kamp DW. The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis. International Journal of Molecular Sciences. 2015;16(9):21486-21519 doi: 10.3390/ijms160921486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophys Res Commun. 2002;294:245–248. doi: 10.1016/S0006-291X(02)00464-3 [DOI] [PubMed] [Google Scholar]
- 98.Conti V, Izzo V, Corbi G, et al. Antioxidant supplementation in the treatment of aging-associated diseases. Front Pharmacol. 2016;7. doi: 10.3389/fphar.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sztretye M, Dienes B, Gönczi M, et al. Review article astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longevity. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicolson GL, Ash ME. Lipid replacement therapy: a natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim Biophys Acta Biomembr. 2014;1838:1657–1679. doi: 10.1016/j.bbamem.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 101.Fiuza-Luces C, Valenzuela PL, Laine-Menéndez S, et al. Physical exercise and mitochondrial disease: insights from a mouse model. Front Neurol. 2019;10. doi: 10.3389/fneur.2019.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 2003;33:783–793. doi: 10.2165/00007256-200333110-00001 [DOI] [PubMed] [Google Scholar]
- 103.Jeppesen TD, Schwartz M, Olsen DB, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129:3402–3412. doi: 10.1093/brain/awl149 [DOI] [PubMed] [Google Scholar]
- 104.Jeppesen TD, Dunø M, Schwartz M, et al. Short- and long-term effects of endurance training in patients with mitochondrial myopathy. Eur J Neurol. 2009;16:1336–1339. doi: 10.1111/j.1468-1331.2009.02660.x [DOI] [PubMed] [Google Scholar]
- 105.Taivassalo T, Shoubridge EA, Chen J, et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol. 2001;50:133–141. doi: 10.1002/(ISSN)1531-8249 [DOI] [PubMed] [Google Scholar]