Abstract
Background and Aims:
Vedolizumab, a novel monoclonal antibody to the α4β7 integrin, is approved for Crohn’s disease (CD) and ulcerative colitis (UC). The clinical trials leading to this approval excluded concomitant therapy with the calcineurin inhibitors, tacrolimus or cyclosporin. Therefore, the success and safety of using calcineurin inhibitors as induction agents in combination with vedolizumab is unknown.
Methods:
We performed a sub-analysis of patients receiving vedolizumab and concomitant calcineurin inhibitors for the treatment of UC or CD from our prospective vedolizumab database. Patients had clinical activity scores and inflammatory markers prospectively measured at baseline, weeks 14, 30 and 52 of vedolizumab treatment. Clinical remission was defined as HBI≤4 or SCCAI≤2 and steroid-free clinical remission as clinical remission without concomitant corticosteroids.
Results:
20 patients (9CD; 11UC) received combination therapy with a calcineurin inhibitor and vedolizumab as a bridge to vedolizumab monotherapy of which 44% of CD and 55% of UC patients achieved steroid-free clinical remission by week 14. At one-year, 33% of CD and 45% of UC patients were in steroid-free clinical remission. A further seven patients (2UC; 5CD) received salvage therapy with a calcineurin inhibitor after primary non-response to vedolizumab; one of two UC patients and two of five CD patients were off calcineurin inhibitors and achieved steroid-free remission at week 52. In total, 59%(n=16) of patients remained on vedolizumab at one year and serious adverse events secondary to calcineurin inhibitors were uncommon.
Conclusions:
Combination therapy of vedolizumab with either cyclosporin or tacrolimus is effective and safe at inducing and maintaining clinical remission in IBD patients with up to 52 weeks of follow-up. Larger studies using calcineurin inhibitors to induce IBD remission in patients on vedolizumab are warranted.
Keywords: Alpha-4 integrin inhibitors, calcineurin inhibitors, Response to Therapy, vedolizumab
Introduction
A significant number of patients with inflammatory bowel disease (IBD) fail conventional therapies and are either steroid-resistant or steroid-dependent. These patients require additional treatment strategies. Available options in such cases involve treatment with biological agents including several anti-TNF therapies or the anti-integrin therapy, vedolizumab. However, it is still unclear which agent to use and, even when cycling through these agents, there is a significant gap in information regarding primary response and secondary loss of response.1–4 Potential reasons for this medically resistant disease include mechanistic challenges or inadequate exposure, either due to under-dosing or secondary to loss of serum protein, which may include monoclonal antibody therapies, through an inflamed and “leaky” gut.5 Novel treatment strategies that overcome these challenges are, therefore, needed.
The calcineurin inhibitors, tacrolimus and cyclosporin, have demonstrated short-term efficacy in CD and UC, are fast-acting and may be an option in treatment refractory patients.6, 7 In UC, cyclosporin has been successfully used as a rapidly-acting bridge to the slower acting immunomodulators in immunomodulatory-naïve patients with short-term response rates for patients with acute severe UC of greater than 80%.6 In CD, reports of a rapid response to IV and oral cyclosporin have also been reported in luminal CD with response rates up to 59%.8,9 Tacrolimus also demonstrates excellent short-term efficacy in UC and CD with short-term response rates of 61–96% in UC10 and partial and complete response rates of 38.8% and 28.6% in CD on systematic review.11 However, despite this evidence for their short-term efficacy, protracted use of calcineurin inhibitors is limited by adverse events including infection, nephrotoxicity, hypercholesterolemia and hypertension and long term prognosis is poor with high relapse rates on cessation of therapy.8, 10, 12 As a result, their use has traditionally been limited in IBD due to the lack of an appropriate maintenance therapy demonstrating the requirement for a novel maintenance therapy if calcineurin inhibitors are to be used as induction agents in IBD.
Vedolizumab is a selective humanized immunoglobulin G1 monoclonal antibody to α4β7 integrin that blocks lymphocyte trafficking to gut mucosa.13 It is approved for use in moderate to severe CD and UC and has demonstrated efficacy in inducing and maintaining remission in both when compared to placebo in large trials.14, 15 While vedolizumab has been demonstrated to be efficacious and safe, improvement in clinical symptoms may be slow, with rising response and remission rates demonstrated at least over the course of the first 10 weeks.14–16 Accordingly, the prescribing guidelines in the Unites States suggest a minimum 14-weeks therapy before clinicians evaluate its efficacy. In addition, similar to other monoclonal antibodies, vedolizumab treatment in patients with severe disease is theoretically associated with increased drug loss and lower drug levels due to protein loss via a leaky gut and hence may be associated with reduced response in patients with more severe disease.5
Patients with severe disease are at increased risk of failing biological therapy.17 A strategy used to improve clinical response and remission rates in IBD is to use combination therapy in patients commenced on biologic therapies. This is most commonly achieved utilizing immunomodulator and anti-TNF therapy and is associated with increased remission rates, increased anti-TNF drug levels and reduced loss of response.18, 19 Evidence for other combination strategies with biologic agents is limited. Both calcineurin inhibitors and anti-TNF therapies are individually effective for treatment of IBD and there are reports of patients tolerating concomitant anti-TNF and low doses of tacrolimus use in patients following a liver transplant for PSC who require biologic therapy for active IBD.20 However, as the combined immunosuppressive properties at therapeutic doses of these two agents can lead to adverse events including death, concomitant administration to induce disease remission has been discouraged.21
With this in mind, the concept of utilizing a combination of tacrolimus or cyclosporin to rapidly induce remission followed by a maintenance phase of vedolizumab is appealing in both UC and CD. Since vedolizumab has impressive safety data, we hypothesized that combination therapy with a calcineurin inhibitor would not pose the same risks as combination therapy with a calcineurin inhibitor and a (systemically active) anti-TNF agent. However, patients with exposure to calcineurin inhibitors were excluded from the vedolizumab clinical trials. Hence, the success and safety of inducing remission and bridging from a calcineurin inhibitor to vedolizumab in IBD remain unknown.
In this study, we report the short and long-term response, remission, steroid-free remission and adverse event rates in patients treated concomitantly with a calcineurin inhibitor and vedolizumab.
Methods:
Study Design
Patients with an established diagnosis of IBD who were commenced on vedolizumab at The University of Chicago Medicine Inflammatory Bowel Disease Center were invited to be included in the prospective University of Chicago Vedolizumab Database, part of the larger University of Chicago IBD Research Database. Among consenting patients, baseline patient and disease characteristics were recorded and patient outcomes were evaluated prospectively at weeks 14, 30 and 52 of vedolizumab treatment. Clinical remission and response rates were assessed with Harvey-Bradshaw Index (HBI)22 for CD patients and Simple Clinical Colitis Activity Index (SCCAI) for UC patients.23
We performed a sub-analysis on patients included in the prospective database who were commenced on vedolizumab between its U.S. FDA approval (May 20, 2014) and March 30, 2015 who received concomitant calcineurin inhibitors during the first twelve months of vedolizumab therapy. Patients were eligible if they had confirmed clinical, endoscopic or histological diagnosis of CD or UC and had at least 6 months follow-up after commencement of concurrent calcineurin inhibitor and vedolizumab therapy.
Treatment of patients with calcineurin inhibitors was at the discretion of the primary treating physician and was commenced in patients with refractory IBD, who included patients with severely active UC or CD despite high-dose oral or intravenous prednisolone and/or anti-TNF therapy, in those with acute severe UC with the aim of bridging to vedolizumab or in those who required a steroid-sparing agent. The University of Chicago Institutional Review Board approved the study (IRB: 14–1371). Baseline and outcome measures were extracted from the University of Chicago IBD Research Database.
Intervention
Standard protocols for calcineurin inhibitor therapy induction dosing were used. Concomitant therapy with tacrolimus was initiated at 0.05 mg/kg twice daily. Dosage was adjusted according to trough level aiming for blood concentration of 10–15ng/ml, clinical response and side effects. Trough levels, blood counts, renal and liver profiles were measured 48 hours after treatment initiation, then 1–2 weeks later, and every 2–3 weeks thereafter. After reaching steady-state trough levels, these laboratory data were checked monthly.
Cyclosporin was administered intravenously at initial dose 2 mg/kg/day. Serum cyclosporin concentrations were measured every other day and dose adjusted to target level 300–400 ng/ml. Intravenous therapy was continued for 5–7 (up to 14) days with dose adjustments based on CRP, clinical symptoms, blood pressure, and renal function. Transition to oral therapy was performed when patients had improved clinical disease activity. Oral therapy was commenced at double the intravenous dose and adjusted to reach similar trough concentrations as intravenous therapy. Outpatient cyclosporin levels, renal function and liver function were monitored weekly.
All patients initiating calcineurin inhibitors were started on trimethoprim/sulfamethoxazole 800 mg/160 mg three times weekly for prophylaxis against Pneumocystis jirovecii. This was continued whilst patients remained on concomitant corticosteroid and tacrolimus or cyclosporin.
Calcineurin inhibitors were weaned after at least 6 weeks of therapy in patients who achieved clinical remission and, at the discretion of the primary treating physician, in those who had significant clinical improvement. The initial calcineurin inhibitor dose was decreased by 50% for two weeks prior to discontinuation.
All patients received 300 mg of vedolizumab intravenously at weeks 0, 2 and 6 with maintenance dosing at 8-weekly intervals thereafter as per standard-of-care guidelines and according to FDA-approved dosing regimen for IBD. In those commencing a calcineurin inhibitor as induction agent, the first dose of vedolizumab was administered after initiation of calcineurin therapy.
Other immunosuppression use (including steroids) and prior treatment exposure were all recorded throughout study duration. Steroids were weaned at discretion of the physician and baseline immunomodulators were continued throughout the study period.
Outcomes
Clinical remission was defined as HBI≤ 4 or SCCAI≤2. Clinical response was defined as a reduction of ≥3 points in HBI or SCCAI. Steroid-free clinical remission was defined as clinical remission without need for concomitant systemic corticosteroids.
When available, endoscopic response was assessed utilizing the SES-CD24 for CD patients or Mayo endoscopic sub-score25 for UC patients following at least 3 months vedolizumab treatment. Mucosal healing (MH) was defined by SES-CD score <3 or resolution of all ulcers in CD and in UC as Mayo endoscopic sub-score of 0 or 1.
At each visit patients were questioned about adverse events including infections, infusion reactions or other potential adverse events related to vedolizumab. Adverse events were graded as serious if they resulted in antibiotic treatment, discontinuation of vedolizumab or hospitalization.
Statistical Methods
Patients were analyzed on an intent-to-treat basis and cessation of vedolizumab for any reason was considered treatment failure. For patients who withdrew prematurely, the last observation was carried forward. Descriptive statistics were summarized using medians and interquartile ranges (IQR) or mean and standard deviation (SD) and/or standard error of mean (SEM) for continuous variables. Categorical variables were expressed as percentage and number of cohort. Univariate analysis was conducted using chi-square test or Fisher’s exact test for equal proportion. The Wilcoxon rank-sum test was used where appropriate. Pre-treatment and post-treatment clinical activity scores were compared using paired t-test. A two-sided p-value of 0.05 was considered statistically significant. All data analyses were performed using Stata 12.0 (Stata Corp, College Station, TX).
Results:
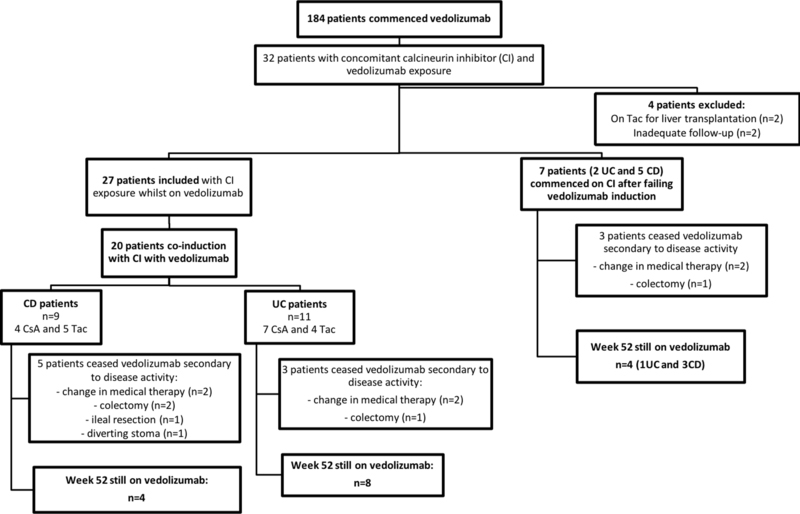
31 patients initiating vedolizumab required concomitant tacrolimus or cyclosporin within the first 12 months of vedolizumab therapy. Of these, 27 patients were included in the final analysis: 2 were excluded as they were on tacrolimus secondary to liver transplant and 2 were excluded due to inadequate documentation/follow-up (Figure 1). The baseline characteristics and indications for vedolizumab are shown in Table 1. All 27 patients were followed for at least one year from their first vedolizumab infusion and 96% (n=26) had failed therapy with at least one anti-TNF agent previously.
Figure 1:
Flow chart of patients included
Table 1:
Baseline Characteristics
| Characteristic | IBD: N=27 | CD: N=14 | UC: N=13 |
|---|---|---|---|
| Age – yr, median (IQR) | 33 (24–39) | 31 (24–36) | 29 (25–39) |
| Male sex – n (%) | 13 (48) | 6 (43) | 7 (54) |
| Current smoker – n (%) | 4 (15%) | 1 (7%) | 3 (23%) |
| Age at diagnosis – yrs, median (IQR) | 21 (16–29) | 18 (15–21) | 29 (21–34) |
| Duration of disease – yr, median (IQR) | 7(4–20) | 5 (2–8) | 18 (6–23) |
| Family History of IBD – n (%) | 10 (37%) | 4 (29%) | 6 (46%) |
| Past surgery for CD – n (%) | 6 (22%) | ||
| Disease Location-Montreal Classification | L1: 1 (7%) L2: 5 (36%) L3: 8 (57%) L4: 1 (8%) P: 2 (17%) |
E1: 1 (2%) E2: 11 (26%) E3: 30 (71%) |
|
| Clinical disease activity at baseline | HBI: <5: 2 (14%) 5–7: 6 (43%) 8–16: 4 (29%) > 16: 2 (14%) |
SCCAI: < 3: 0 (0%) 3–6: 3 (23%) 7–10: 5 (38%) > 10: 5 (38%) |
|
| Concomitant medications with vedolizumab n(%) | |||
| Glucorticoids | |||
| Thiopurines | 21 (78%) | 12 (86%) | 9 (69%) |
| Methotrexate | 7 (26%) | 4 (29%) | 3 (23%) |
| 2 (7%) | 0 (0%) | 2 (15%) | |
| Prior anti-TNF therapy | |||
| Naïve | 1 (4%) | 0 (0%) | 1 (8%) |
| 1 failure | 6 (22%) | 0 (0%) | 6 (46%) |
| >1 failure | 20 (74%) | 14 (100%) | 6 (46%) |
Co-induction with vedolizumab and a calcineurin inhibitor
Twenty patients initiated a calcineurin inhibitor prior to or at the same times as commencing vedolizumab (9CD (5 tacrolimus, 4 cyclosporin); 11UC (4 tacrolimus, 7 cyclosporin)).
15 (9CD;6UC) patients were hospitalized at induction and failed IV steroids requiring salvage therapy with a calcineurin inhibitor. Hospitalized patients received IV cyclosporin (n=10) or oral tacrolimus (n=5). The median duration of the hospital admission was 10 days (IQR 7–12).
On average, patients commenced vedolizumab a median of 30 days (IQR 19–77) days after their first dose of their calcineurin inhibitor. All patients had ceased the calcineurin inhibitor at 12 months. The average duration of combination therapy with the calcineurin inhibitor and vedolizumab was median 64 (IQR 42–87) days.
Crohn’s disease
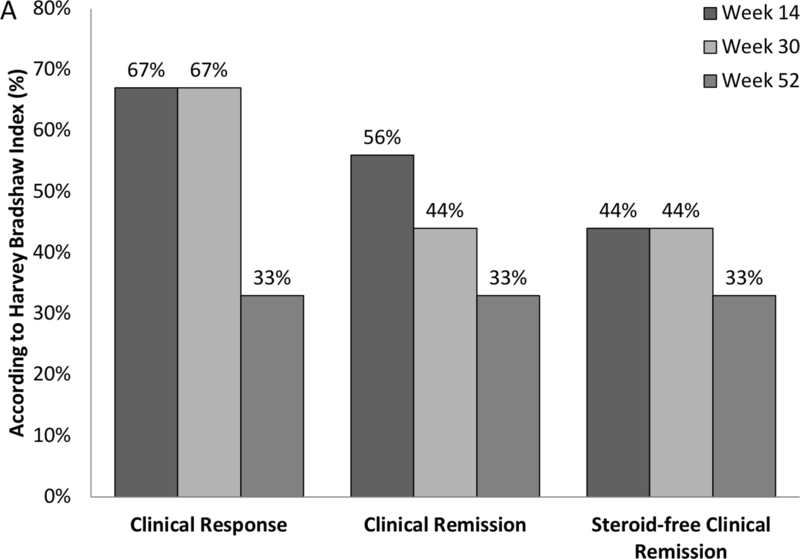
Rates of clinical response, remission and steroid-free remission for patients with CD are shown in Figure 2a. In the 9 CD patients (4 cyclosporin, 5 tacrolimus), 67% (n=6) and 33% (n=3) of patients had clinical response and five of nine, four of nine and three of nine patients achieved clinical remission at week 14, 30 and 52, respectively. 67% (n=6) of patients had weaned from calcineurin inhibitors by week 14 of which 2 achieved calcineurin inhibitor-free clinical remission. Four patients were still on calcineurin inhibitors at week 30, one of whom had recommenced tacrolimus at week 14 after worsening disease activity following its cessation at week 10. This patient eventually proceeded to surgery for treatment refractory disease. By week 52 all patients were off calcineurin inhibitors. Thus, calcineurin inhibitor-free remission was achieved in 33% of patients at week 52.
Figure 2:
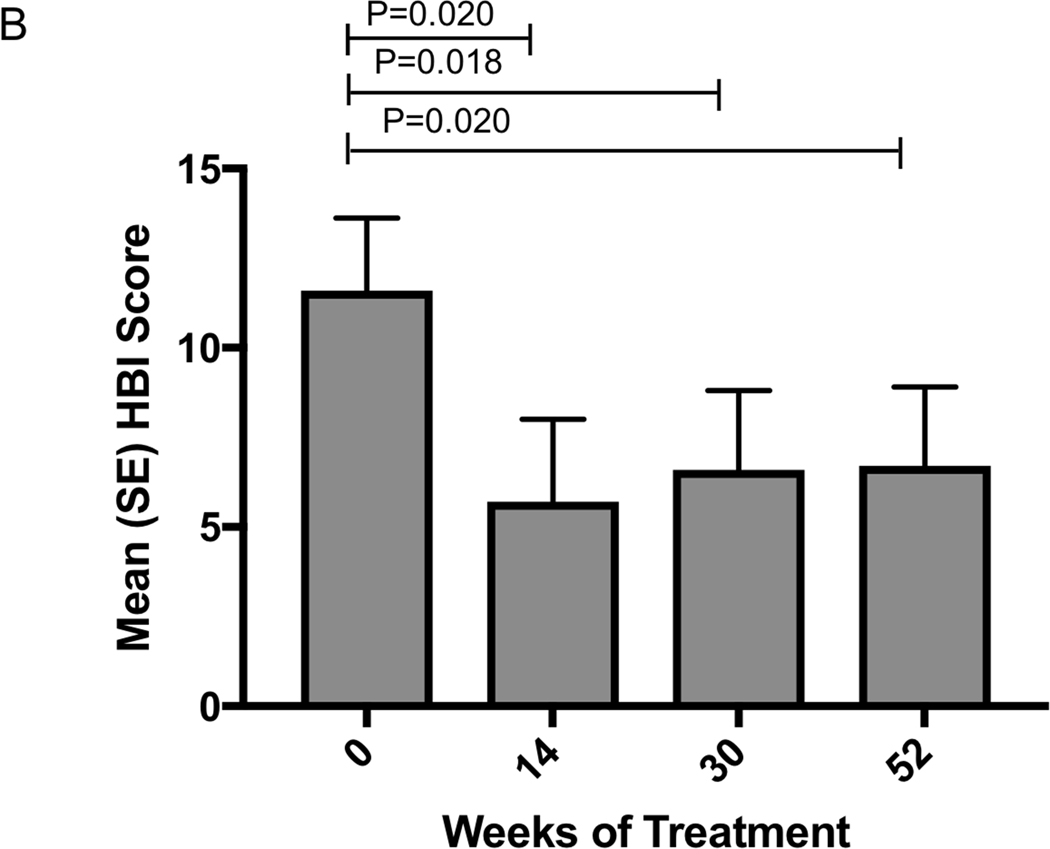
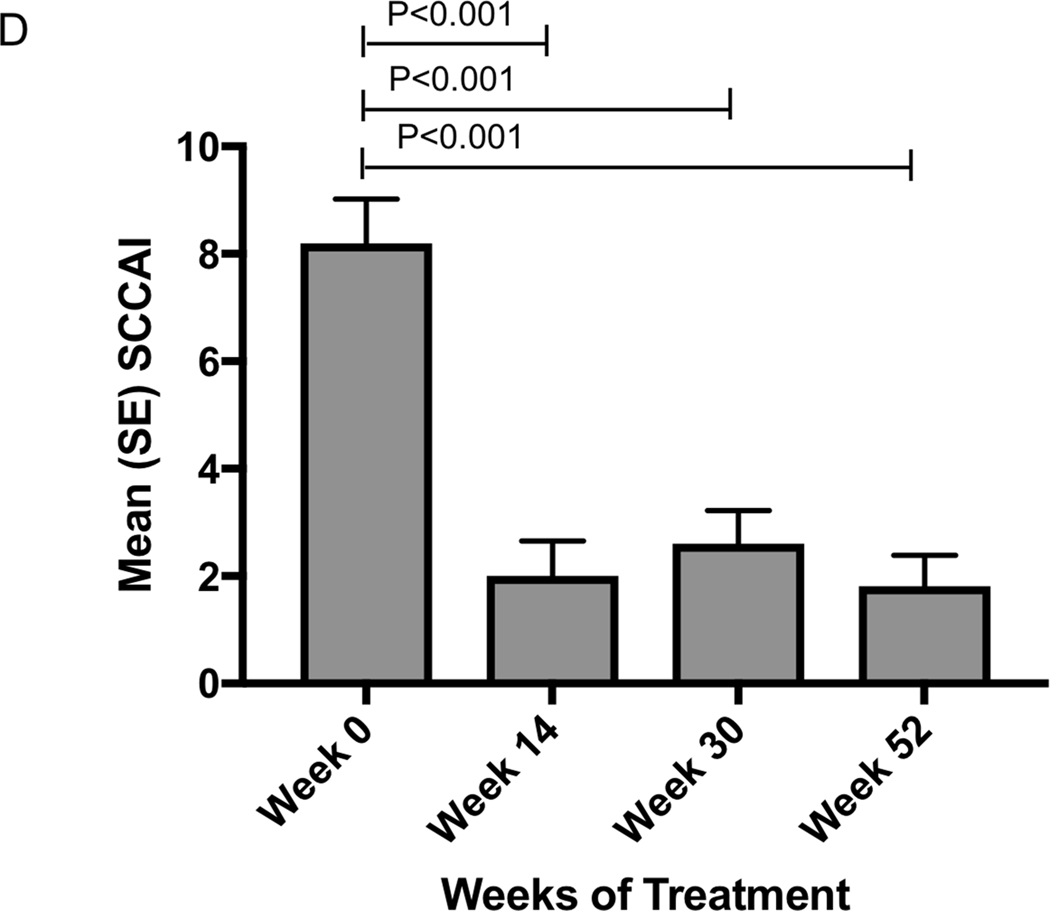
Change in clinical and biochemical markers of disease activity following vedolizumab a) Crohn’s disease clinical response and remission rates b) Mean (SEM) HBI in patients with Crohn’s disease c) Ulcerative colitis clinical response and remission rates d) Mean (SEM) SCCAI in patients with ulcerative colitis
All 9 CD patients were on prednisolone at baseline at a median dose of 40 (20–50) mg/day. 67% (n=6), 67% (n=6) and 100% (n=9) were steroid-free and four of nine, four of nine and three of nine were in steroid-free clinical remission at weeks 14, 30 and 52, respectively.
Mean HBI significantly improved from baseline score of 11.6 (SEM 2.0) to 5.7 (2.3) at week 14 (p=0.020) and remained stable at 6.6 (2.2) at week 30 and 6.7 (2.2) at week 52 (p=0.020). (Figure 2b) In those still on vedolizumab, mean HBI was 3.0 (1.29) at 12 months
Ulcerative colitis
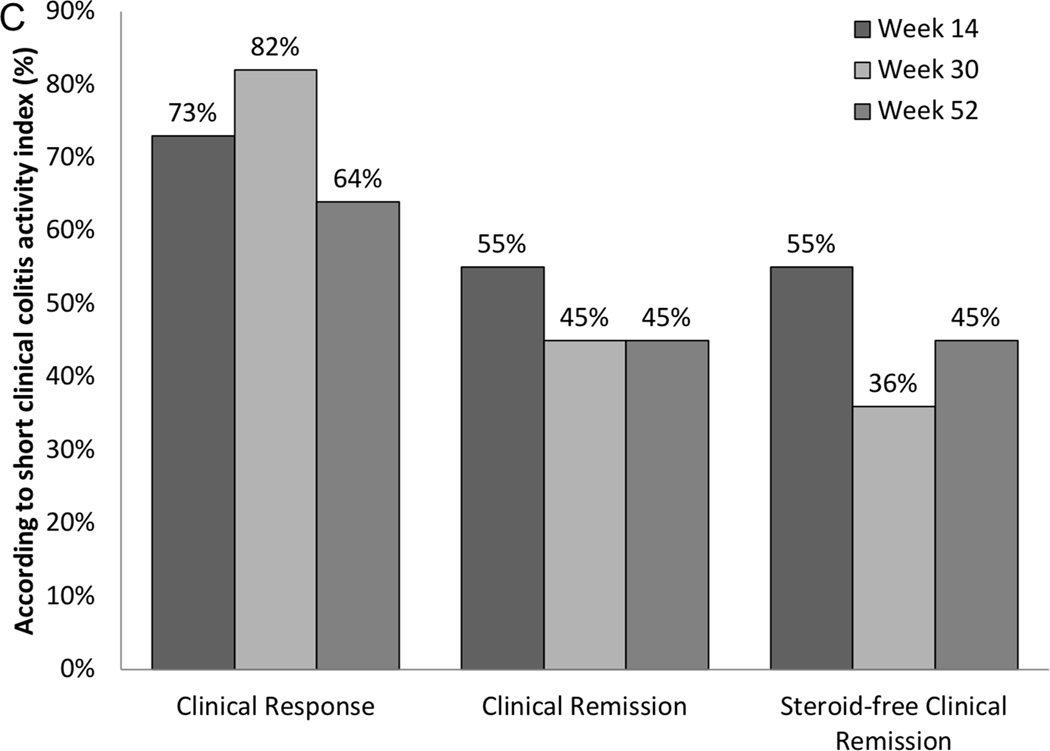
Rates of clinical response, remission and steroid-free remission for UC patients are shown in Figure 2c. Clinical response was achieved in 73% (n=8), 82% (n=9) and 64% (n=7) and clinical remission in 55% (n=6), 45% (n=5) and 45% (n=5) at weeks 14, 30 and 52, respectively. 55%(n=6) of patients weaned from calcineurin inhibitors by week 14. Only one patient remained on a calcineurin inhibitor at week 30 and all patients were off calcineurin inhibitors by week 52. Thus, calcineurin inhibitor-free remission was achieved in 45% at week 52.
55% (n=6) of patients were on corticosteroids at baseline at median dose 40 (25–50) mg/d. Of these, 100% (n=6), 83% (n=5) and 100% (n=6) were steroid-free, but only two of eleven, zero and two of eleven of the patients were in steroid-free clinical remission at week 14, 30 and 52, respectively. Overall steroid-free remission was achieved in 55% (n=6), 36% (n=4) and 45% (n=5) at week 14, 30 and 52 respectively.
Mean SCCAI fell significantly from 8.2(0.82) at baseline to 2.0(0.65) at week 14 (p<0.001) and then remained stable at 2.6(0.62) at week 30 and 1.8(0.58) at week 52 (Figure 2d). In those still on vedolizumab, mean SCCAI was 1.6 (1.4) at 12 months.
Mucosal healing following co-induction:
67% (n=14) of patients (7CD:7UC) had baseline and post-induction endoscopic assessment at median 5 (3–9) months. Six of 7 patients who were treatment failures were included in this analysis.
MH was achieved in one of the seven CD patients and four of the seven UC patients. Week 52 response rates were higher when MH was achieved (100% clinical response rate if MH achieved vs 11% response rate if no MH, p=0.003) and week 52 steroid-free remission rates trended higher when MH was achieved (75% steroid-free remission if MH achieved vs 25% steroid-free remission if no MH, p=0.052). MH was associated with continuation of vedolizumab (100% vedolizumab continuation if MH achieved vs 33% continuation if no MH, p=0.016).
Calcineurin inhibitor use in those failing induction with vedolizumab
Seven patients (5CD, 2UC) commenced calcineurin inhibitor therapy after primary non-response to vedolizumab immunotherapy.
Three patients commenced (2UC; 1CD) a calcineurin inhibitor within three months of commencing vedolizumab. Two UC patients were hospitalized for IV corticosteroids and cyclosporin in the setting of ongoing clinical symptoms despite 40 mg prednisolone and vedolizumab for 3 months. One patient failed induction therapy with cyclosporin and proceeded to total colectomy. The other patient weaned from cyclosporin after 51 days and remained in steroid and calcineurin-free clinical remission at 12 months.
5 CD patients required tacrolimus salvage therapy; one at 3 months of vedolizumab and four at 6 months of vedolizumab therapy. All patients had been steroid-dependent, three had been on 4-weekly vedolizumab with continued disease activity and two required hospitalization for IV steroids with failure to achieve remission. One patient was changed to 4-weekly vedolizumab at the same time as commencing tacrolimus.
The mean duration of tacrolimus was 85 (SD 53) days. Of the five CD patients, two patients achieved steroid and calcineurin inhibitor free remission at 12 months and continued vedolizumab. One patient continued to have disease activity at 12-months despite tacrolimus and proceeded to loop ileostomy with no complications and then continued on vedolizumab monotherapy. Two patients ceased vedolizumab; one who ceased for non-response to tacrolimus went on to surgical resection and the other ceased for inability to then wean from tacrolimus despite response.
Vedolizumab discontinuation and adverse events
41% (7CD, 4UC) of all patients discontinued vedolizumab therapy after median 6 (6–7) months, all due to non-response. Three CD patients and two UC patients were switched to a different therapeutic agent and seven (2UC, CD) proceeded to surgery with four (2UC, 2CD) colectomies, one ileal resection (CD) and one diverting stoma (CD). There were no intra-operative complications. Post-operatively, one patient had delayed perineal healing following total proctocolectomy and one patient developed a mucocutaneous separation of the stoma, which subsequently healed with antibiotics. There were no other surgical complications.
Adverse events are summarized in Table 2. In addition to post-operative complications, there were three serious events in two patients. One patient described an infusion-related reaction with mild swelling of tongue. This patient had severely active disease and also developed a serious infectious complication testing positive for Clostridium difficile and developing CMV colitis with inclusion bodies on colon biopsy. This patient ceased therapy and proceeded to colectomy. One patient developed a viral gastroenteritis that required hospitalization, but recovered with conservative management.
Table 2:
Adverse Events on Vedolizumab
| Event | Patients with inflammatory bowel disease (n=27) |
|---|---|
| Adverse event – Non-infectious | |
| Neurological complaints (n=4) | 4 Total - 1 Paraesthesia - 1 migraine - 2 mild tremor |
| Pruritis | 1 total |
| Rheumatological | 2 Total - 1 new onset arthralgia - 1 leg cramps |
| Infusion related reaction | 1 infusion reaction* |
| Cancer | No cancer documented |
| Constipation | 2 total |
| Perianal disease | 1 Total - 1 worsening perianal fistula |
| Fatigue | 1 total |
| Oro-facial complications | 1 total - 1 gum sensitivity |
| Any serious non-infectious event* | 1 total |
| Adverse event – Infections | |
| Enteric infection | 2 total - 1 viral enteritis* - 1 CMV and C. difficile colitis – colectomy* |
| Sino-pulmonary infections | 1 sinusitis |
| Post-operative complications | 2 total - 1 delayed perineal healing - 1 mucocutaneous separation of stoma |
| Miscellaneous | 1 UTI |
| Any serious infection* | 4 total |
A serious adverse event or infection was defined as any adverse event when leading to treatment interruption, antibiotic therapy, hospitalization, disability or persistent damage, colectomy or death
Side effects possibly attributed to calcineurin inhibitor toxicity were minimal. One with ongoing disease activity discontinued tacrolimus after one month due to gum sensitivity, which resolved on ceasing tacrolimus. Additional side effects that did not result in discontinuation included tremor(n=2), migraine(n=1), paresthesia(n=1), leg cramps(n=1) and fatigue(n=1).
Discussion
The unique gut selectivity and favorable safety profile of vedolizumab has enabled new options for treating IBD patients. In this prospective observational study, we demonstrate the efficacy and safety of a novel treatment approach-use of a calcineurin inhibitor in conjunction with vedolizumab. This approach provides new options for many different patient types including patients failing corticosteroid therapy, patients who have failed or are intolerant to thiopurine therapy, and hospitalized adults with severe IBD with low serum albumin and in whom we are concerned for gut loss of protein-based therapies and inadequate exposure. Calcineurin-based treatment also provides an induction option as a bridge to vedolizumab, in order to overcome the described slower onset of action of this biologic agent.
In this report, we demonstrate that utilizing a combination of tacrolimus or cyclosporin with vedolizumab in patients with active IBD achieves steroid and calcineurin inhibitor-free clinical remission in more than one-third of patients at one year of follow-up. The strategy also was effective when the induction therapy was introduced to patients who had failed vedolizumab monotherapy. Similar to previous reports of calcineurin inhibitor induction for UC and CD,6, 7 high initial response and remission rates at 67% and 56% for CD and 73% and 55% for UC were observed in our study. Although response and remission rates did decrease over the follow-up period, 33% of CD and 45% of UC patients were in steroid-free remission at one year. These rates of remission are similar to those seen in the pivotal vedolizumab trials GEMINI 1 and 2 despite the fact that this patient cohort likely represents a more treatment-resistant group.14, 15 In addition, SCCAI and HBI scores significantly improved by week 14 in both CD and UC, and this improvement was maintained over 52 weeks despite patients ceasing corticosteroids and the calcineurin inhibitor. In fact, in our patient cohort, all patients who continued on vedolizumab were off corticosteroids and calcineurin inhibitors by 12 months. This suggests that calcineurin inhibitor therapy may be utilized as a bridge to vedolizumab in those with moderately to severely active disease as well as a steroid–sparing therapy.
Endoscopically, one of seven CD patients and four of seven UC patients achieved MH. For UC, this is comparable to the results of the pivotal trial, which demonstrated MH rates in initial responders of 54% in maintenance.14 The pivotal trials for CD did not report MH, but a real-world study did demonstrate a higher rate of 30% for MH.26
All patients in our study were treatment refractory and almost all had previously failed anti-TNF therapy. On this basis, it would be anticipated that few patients would have responded to re-induction therapy with standard anti-TNF therapy, but proof that the strategy of calcineurin inhibitors bridging to vedolizumab is more efficacious than re-treatment with a drug that had previously failed can only definitively be addressed by a randomized controlled trial. Whether such a study is ethical is dubious.
In patients who have primary non-response to vedolizumab monotherapy, despite adequate time for onset of action, a short duration of therapy with calcineurin inhibitors successfully salvaged patients who continued vedolizumab. At 6 months, three of seven patients achieved steroid-free and calcineurin inhibitor-free clinical remission. Although follow-up of these patients was short, treatment options are limited in this patient cohort. These results are, therefore, promising and suggest that calcineurin inhibitor salvage may be a strategy to induce remission in this patient cohort although clearly longer-term studies are required to determine durability of this response. The mechanism of this response is not clear, but there may be synergy of the different anti-inflammatory mechanisms of action or simply an additive benefit, followed by more durable response to vedolizumab.
Week 52 steroid-free remission rates trended higher when MH was achieved (p=0.052). In addition, MH was associated with continuation of vedolizumab (p=0.016). These findings support findings from the anti-TNF clinical trials regarding positive prognostic role that MH has post-induction treatment on longer-term outcomes and are similar to the findings reported with standard vedolizumab therapy.27
One hazard of multi-agent immunomodulator therapy in patients with IBD has been infection and other adverse events related to profound immune suppression.12, 28 In fact, combination therapy with a calcineurin inhibitor and anti-TNF therapy has been relatively contraindicated due to the associated severe infection risk and even death that was demonstrated in one case series.21 However, the predominantly gut-selective effect of vedolizumab on immune reactivity and the minimal side effects and infection risk associated with its use as a monotherapy may imply that the addition of a powerful systemically-acting immune suppressing agent like cyclosporin or tacrolimus would not carry infective and other complications greater than that of the individual drugs. Indeed, no significant toxicity was observed in our series despite the fact that many patients were on quadruple immunosuppressive therapy, at least initially.
Although this is a novel treatment mechanism and this is the largest series of patients reported with this treatment strategy, the major shortcoming of our study was the small sample size that makes it more difficult to detect uncommon adverse effects and to define predictors of response to vedolizumab. In addition, despite the prospective nature of the study, some data were missing from patients. Finally, when vedolizumab first received regulatory approval in May 2014, many patients with very complex disease received it as “end of the line” salvage therapy at our referral centre. Therefore, this patient group most likely represents a population with more severe disease than might normally be placed on this therapy in the general community.
In conclusion, this study describes a novel treatment regimen for patients with moderate-severe UC or CD, and one that may be particularly useful in patients who are steroid-refractory or who have already failed an anti-TNF treatment. Cyclosporin with tacrolimus successfully and rapidly induced response and remission in both CD and UC. We propose that this strategy can act as a “bridge” to maintenance vedolizumab treatment when applied to vedolizumab initiation or when there has been primary non-response to vedolizumab. On long-term follow-up, vedolizumab was able to maintain remission in 30–45% of IBD patients without the requirements for steroids or continuing the calcineurin inhibitor. Larger studies using short-term calcineurin inhibitors in conjunction with vedolizumab are warranted and planned.
Acknowledgements:
Britt Christensen receives support through an “Australian Government Research Training Program Scholarship”. Thanks to Dania Saddiqui who helped with patient recruitment and data collection.
Grant Support: Funded in part by Digestive Disease Research Core Center of the University of Chicago (DK42086).
This is an investigator-initiated study, and has no industry funding.
David T. Rubin has received institutional grant support from Abbvie, Janssen and Takeda and served as a consultant for Abbvie, Janssen, Takeda, Amgen, Pfizer and UCB.
Peter R. Gibson has served as consultant or advisory board member for AbbVie, Ferring, Janssen, Merck, Nestle Health Science, Danone, Allergan, Celgene and Takeda. His institution has received speaking honoraria from AbbVie, Janssen, Ferring, Takeda, Mylan and Pfizer. He has received research grants for investigator-driven studies from AbbVie, Janssen, and A2 Milk Company.
Andres Yarur has received research grants from Takeda, served as a consultant for Takeda pharmaceuticals and Prometheus Laboratories and is on the speaker bureau for Abbvie and Prometheus Laboratories
Britt Christensen has received education grants from Takeda and Pfizer.
Abbreviations:
- HBI
Harvey Bradshaw Index
- MH
Mucosal Healing
- SCCAI
Short Clinical Colitis Activity Index
- SE
Standard Error
- SES-CD
Short Endoscopic Score for Crohn’s Disease
Footnotes
Financial disclosures/Relevant conflicts of interest:
Russell D. Cohen has the following conflicts of interest. Speaker’s Bureau: Abbvie, Takeda, Pfizer. Consultant / Advisory/ Scientific Advisory Board: Abbvie, Celgene, Eli Lilly, Hospira, Janssen, Pfizer, Sandoz Biopharmaceuticals, Takeda, UCB Pharma. Clinical Trials (Principal Investigator): Astra-Zeneca, Celgene, Gilead Sciences, Medimmune, Mesoblast Ltd., Osiris Therapeutics, Pfizer, Receptos, RedHill Biopharma, Sanofi-Aventis, UCB Pharma. Disclosures for Spouse: Board of Directors: Protein Sciences, Tokai Pharmaceuticals, Vital Therapies, Inc., CytRx Corporation.
There are no financial disclosures or other relevant conflicts of interest for any other author.
Writing Assistance: None
References
- 1.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65 e1–3. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–1111 e2. [DOI] [PubMed] [Google Scholar]
- 5.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 2015;149:350–5 e2. [DOI] [PubMed] [Google Scholar]
- 6.Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841–5. [DOI] [PubMed] [Google Scholar]
- 7.Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8. [DOI] [PubMed] [Google Scholar]
- 8.Egan LJ, Sandborn WJ, Tremaine WJ. Clinical outcome following treatment of refractory inflammatory and fistulizing Crohn’s disease with intravenous cyclosporine. Am J Gastroenterol 1998;93:442–8. [DOI] [PubMed] [Google Scholar]
- 9.Brynskov J, Freund L, Rasmussen SN, et al. A placebo-controlled, double-blind, randomized trial of cyclosporine therapy in active chronic Crohn’s disease. N Engl J Med 1989;321:845–50. [DOI] [PubMed] [Google Scholar]
- 10.Matsuoka K, Saito E, Fujii T, et al. Tacrolimus for the Treatment of Ulcerative Colitis. Intest Res 2015;13:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McSharry K, Dalzell AM, Leiper K, et al. Systematic review: the role of tacrolimus in the management of Crohn’s disease. Aliment Pharmacol Ther 2011;34:1282–94. [DOI] [PubMed] [Google Scholar]
- 12.Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5. [DOI] [PubMed] [Google Scholar]
- 13.Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009;330:864–75. [DOI] [PubMed] [Google Scholar]
- 14.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 16.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–627 e3. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009;137:1250–60; quiz 1520. [DOI] [PubMed] [Google Scholar]
- 18.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:1248–54. [DOI] [PubMed] [Google Scholar]
- 19.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Loftus EV Jr., Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol 2013;108:1417–25. [DOI] [PubMed] [Google Scholar]
- 21.Maser EA, Deconda D, Lichtiger S, et al. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol 2008;6:1112–6. [DOI] [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 23.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 26.Vivio EE, Kanuri N, Gilbertsen JJ, et al. Vedolizumab Effectiveness and Safety Over the First Year of Use in an IBD Clinical Practice. J Crohns Colitis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen B, Colman RJ, Micic D, et al. Vedolizumab as induction and maintenance for inflammatory bowel disease: 12-month effectivenes and safety. Inflamm Bowel Dis 2018;IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016;15:11–34. [DOI] [PubMed] [Google Scholar]