ABSTRACT
Background: Hemoptysis is an alarming and common symptom leading to thorough diagnostic evaluation with computed tomography and fiberoptic bronchoscopy. Increasing evidence suggests that bronchoscopy is not necessary in diagnosing lung cancer in hemoptysis patients because of high sensitivity of computed tomography. However, less attention has been paid to non-malignant etiologies of hemoptysis.
Objective: We aimed to identify the etiologies established in hemoptysis patients with no malignancy suspected on computed tomography in order to assess the necessity of bronchoscopy in these patients.
Design: We retrospectively reviewed clinical records of consecutive patients referred to evaluation for hemoptysis with no malignancy suspected on computed tomography at Aalborg University Hospital, Denmark, in an eleven-year period from 2006 to 2016.
Results: One thousand one hundred and eighty-five patients (mean age 57.5 ± 15.44 years, 61.3% male) were included in the study. Bronchoscopy was performed in 91.9% of cases. Most patients (83.5%) had cryptogenic hemoptysis, while the most frequently identified etiologies were respiratory tract infection (12.6%) and bronchiectasis (2.2%). No patients had malignant disease as their etiology.
Conclusions: The vast majority of hemoptysis cases with no malignancy suspected on computed tomography were cryptogenic and all were benign. Bronchoscopy may be reserved for patients with specific conditions suspected and those with persistent symptomatology.
KEYWORDS: Hemoptysis, cryptogenic hemoptysis, bronchoscopy, computed tomography of the thorax, histology, cytology, microbiology
Introduction
Hemoptysis, defined as the coughing or spitting of blood from the lower airways, is an alarming symptom for the patient and has a wide variety of etiologies ranging from benign conditions to malignancies of the tracheobronchial tree and lung parenchyma [1–3].
In previous literature, the etiologies of hemoptysis vary greatly. Lung cancer prevalences between 4 and 30% have been reported in hemoptysis patients in later years [4–13], the lowest of these in Danish populations. Bronchiectasis has been reported as the etiology of hemoptysis in 6–33% of cases [4–12] and pneumonia in 4–16% [4,6–9,12,14]. Cryptogenic hemoptysis, in which diagnostic evaluation with thoracic imaging and fiberoptic bronchoscopy does not result in a diagnosis, has been reported as the etiology of hemoptysis in 5–50% of cases [4,6–13]. Other common etiologies include tuberculosis, fungal infections, and pulmonary embolisms [1].
Current guidelines from the Danish Society of Respiratory Medicine for the diagnostic evaluation of hemoptysis include computed tomography (CT) in all patients aged ≥40 years, smokers, or experiencing symptoms suspicious of malignancy. Bronchoscopy is recommended with continuous suspicion of malignancy, severe or persistent hemoptysis or abnormal CT [15]. The literature agrees on the use of chest x-ray, CT, and bronchoscopy in the evaluation of hemoptysis, but no international scientific consensus seems to have been reached with regard to the priority and distribution of those investigations [4]. Increasing evidence suggests that bronchoscopy is not necessary in hemoptysis patients with no pathology on CT [4,11]. However, these reports primarily focus on the ability to diagnose cancer correctly and less attention has been paid to the majority of etiologies comprising non-malignant diagnoses. In daily practice, a large number of bronchoscopies are performed which is unpleasant and not without risk for the patient while expensive and time-consuming procedures for the health-care system. To our knowledge, no larger studies have addressed the necessity of bronchoscopy in patients not suspected of malignancy.
We hypothesize that the etiologies identified in hemoptysis patients with no malignancy suspected on chest CT are indeed non-malignant etiologies. Hence, we aim to evaluate the frequencies of different etiologies in this patient group. What are the etiologies established in hemoptysis patients with no suspicion of malignancy on CT, and how does fiberoptic bronchoscopy contribute to the diagnosis? Also, we aim to compare the rate of non-cryptogenic hemoptysis between patients with no pathology on CT and those with any non-malignant pathology on CT and identify possible risk factors for the various etiologies.
Materials and methods
Setup and study population
This retrospective study reviewed clinical records of patients referred to the Department of Respiratory Diseases, Aalborg University Hospital, Denmark for evaluation of hemoptysis, identified by the ICD-10 diagnosis DR042 from January 1,, 2006, to December 31, 2016.
Exclusion criteria were: under 18 years of age; previous evaluation for hemoptysis; evaluation cancelled for any reason; bleeding not originating from the lower airways as assessed on first visit; no chest CT or no CT description; and CT suspicious of pulmonary malignancy.
Part of the population studied here are also presented in another study from our group [16].
Data collection and definitions
From the patient record, the following parameters were recorded: gender; age; present somatic diseases at referral; previous somatic diseases (diagnoses no longer applicable to the patient at referral); amount of hemoptysis (mild (<30 ml/24 h); moderate-massive (≥30 ml/24 h)) [10]; episodes of hemoptysis (1–5, >5); anticoagulant treatment at referral; and smoking history (never, ex-smoker (>6 months absence), smoker).
If not noted independently, present somatic diseases were estimated from the medication listed in the patient record. Anticoagulant treatment was grouped as thrombocyte-inhibitors, novel oral anticoagulants (NOACs), coumarins, and anti-thrombin activators. Smoking history in smokers and ex-smokers was quantified in pack-years (≤30, >30) [17].
Results of chest CT were obtained from the radiological description of the scan. Scans older than 2 months at referral were not considered. Any abnormality of the lungs, except for emphysematic shape of the thorax and normal anatomical variations, was considered a positive investigation. Lesions were grouped as bronchiectasis, pneumonia, interstitial disease, suspicion of fungal infection, pulmonary embolism, fibrotic tissue and ‘other’. If a scan was described with more than one lesion, all were recorded.
Bronchoscopy was assessed by the description from the investigating physician and considered macroscopically positive if it identified: the site of bleeding; a tumor or foreign body; a substantially vulnerable mucosa; or sequelae after previous surgery.
Results of histological, cytological, and microbiological samples were obtained from the respective integrated result application of the patient record. Histology and cytology were considered positive if suggestive of dysplasia or malignancy. Metaplasia and inflammation were not considered positive unless the pattern of inflammation suggested interstitial lung disease. As to microbiology, any growth not described as normal flora was considered positive, as was positive polymerase chain reaction (PCR) for mycobacteria.
Etiologies were defined as the diagnoses stated or acted upon by the physician at the end of initial diagnostic workup, regardless of the findings of the diagnostic modalities. Etiologies were grouped into primary lung cancers, metastatic cancer to the lungs, bronchiectasis, mycobacterial infections, fungal infections, respiratory tract infection (including all but mycobacterial and fungal infections) and interstitial lung disease. Other etiologies were recorded individually. In patients with more than one final diagnosis, all were recorded as etiologies. Cases with no established etiology were recorded as cryptogenic, while all other etiologies were grouped as non-cryptogenic etiologies for subanalyses.
Data management, statistics, and approvals
Study data were collected and managed using REDCap electronic data capture tools hosted at Aalborg University Hospital [18].
Descriptive statistics were carried out using the integrated ‘Data Exports, Reports, and Stats’ module of REDCap and IBM SPSS Statistics for Macintosh, Version 25.0. The latter was also used for hypothesis testing, cross tabulations and computing of risk ratios. Normally distributed data was presented as mean and standard deviation (SD), while frequencies were presented as absolute numbers and percentages of cases with relevant data available. Cross tabulations and chi-square tests were applied to compare categorical variables between groups and independent t-test to compare age means between groups. Risk ratios were presented along with confidence intervals. A significance level of 95% applied to all tests.
The study was approved by the Danish Patient Safety Authority with case ID 3-3013-2350/1/and by the Danish Data Protection Agency under the umbrella application for the North Denmark Region (2008-58-0028) with project ID 2017–60.
Results
Study population and baseline characteristics
A total of 1493 patients were referred with hemoptysis during the study period. Of these, 308 met one or more exclusion criteria, leaving 1185 patients to be included in the study. The inclusion process is depicted in Figure 1.
Figure 1.
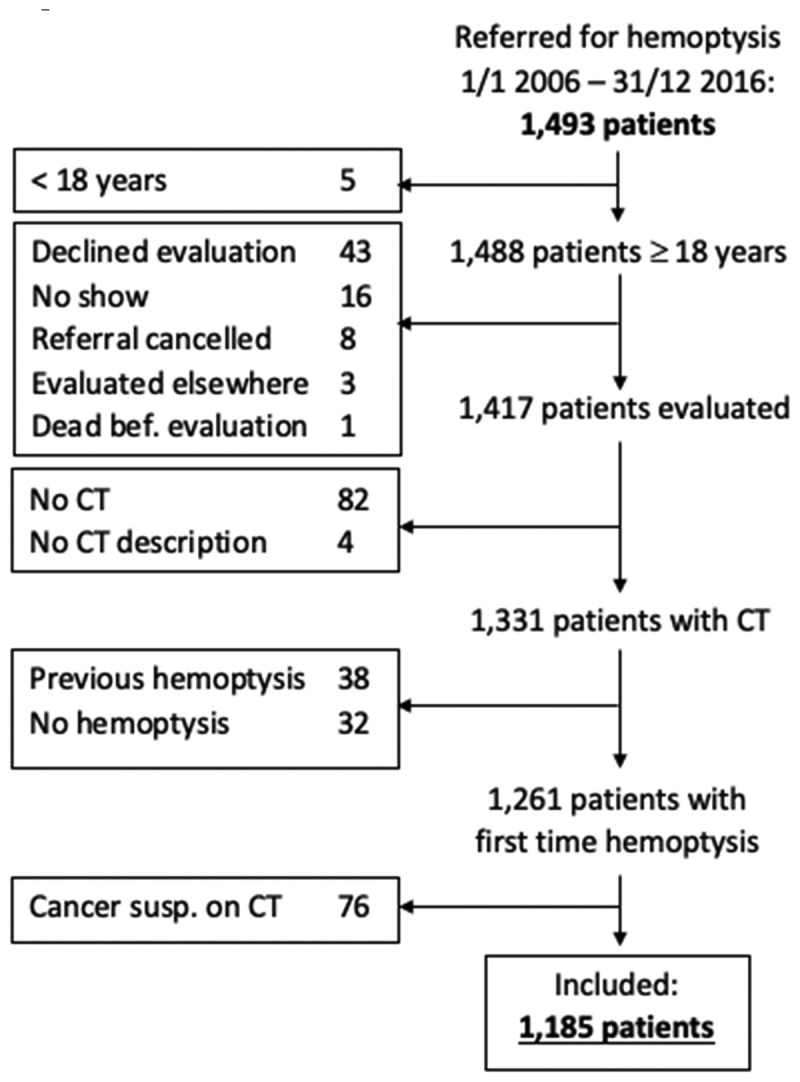
Inclusion process and excluded patients by exclusion criterium
Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics. Absolute numbers (%), where not stated otherwise. Percentages of patients with relevant data available (n)
| Mean age, years (SD) (n = 1,185) | 57.5 (14.55) |
|---|---|
| Male (n = 1,185) | 726 (61.3) |
| Smoking (n = 1,164) | 871 (74.8) |
| Current (n = 1,164) | 476 (40.9) |
| Previous (n = 1,164) | 395 (33.9) |
| Pack years >30 (n = 1,125) | 290 (25.8) |
| Anticoagulant treatment (n = 1,150) | 335 (29.1) |
| Thrombocyte inhibitors (n = 334) | 246 (73.7) |
| Coumarins (n = 334) | 87 (26.0) |
| NOACs1 (n = 334) | 25 (7.5) |
| Antithrombin activators (n = 334) | 6 (1.8) |
| Amount of hemoptysis (n = 761) | |
| Mild | 663 (87.1) |
| Moderate – massive | 98 (12.9) |
| Episodes (n = 1,036) | |
| 1–5 | 565 (54.5) |
| >5 | 471 (45.5) |
| Current/prev. lung disease (n = 1,168) | 291 (24.9) |
| 1: Novel oral anticoagulants |
Present and previous health data are presented in Figure 2. No present somatic disease was known at the time of referral in 42.7% of patients, and 51.1% of patients had no previous somatic diseases. As shown in Figure 2, COPD was known in 12.9% of patients while 1.5% had bronchiectasis and 2.1% were previously treated for lung cancer.
Figure 2.
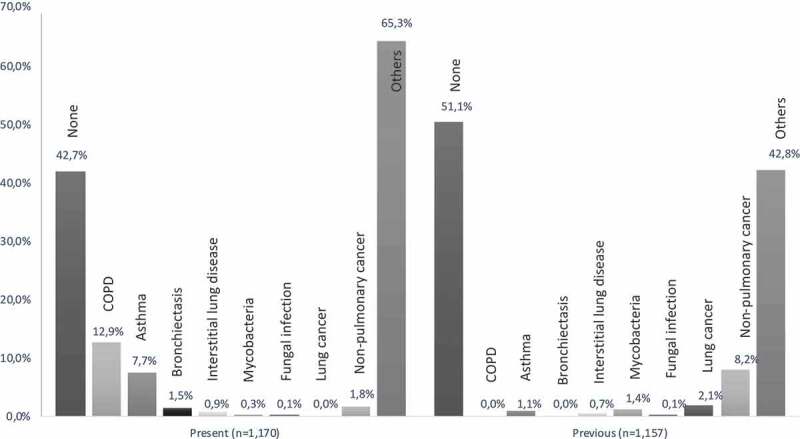
Patients with somatic diagnoses applicable at referral (present) and no longer applicable at referral (previous), as percentage of patients with relevant data available (n)
Results of diagnostic modalities
All 1185 patients had chest CT performed and CT was positive in 71.3% of cases, the most frequent findings being fibrotic tissue (25.8%), bronchiectasis (18.2%) and ‘other’ (56.7%). Bronchoscopy was performed in 91.9% of patients. Microbiological sampling was performed in 59.9% of patients, and 24.4% of these were positive. Proportions of positive findings from the diagnostic modalities are shown in Table 2 along with the proportions of CT lesions.
Table 2.
Number of investigations performed and percentage of all patients, along with number of positive investigations and percentage of the number performed
| Performed (%) | Positive (%) | |
|---|---|---|
| Computed tomography | 1,185 (100) | 845 (71.3) |
| Fibrotic tissue | 306 (25.8) | |
| Bronchiectasis | 216 (18.2) | |
| Respiratory tract infection | 70 (5.9) | |
| Interstitial lung disease | 9 (0.8) | |
| Fungal infection | 4 (0.3) | |
| Pulmonary embolism | 2 (0.2) | |
| Other | 672 (56.7) | |
| Bronchoscopy or test | 1,089 (91.9) | 445 (40.9) |
| Bronchoscopy | 1,089 (91.9) | 353 (32.4) |
| Microbiology | 710 (59.9) | 173 (24.4) |
| Cytology | 580 (48.9) | 5 (0.9) |
| Histology | 62 (5.2) | 4 (6.5) |
Etiologies and risk factors
Cryptogenic hemoptysis was the final diagnosis in 989 (83.5%) patients. No patients were diagnosed with primary or secondary cancer in the lungs. The most frequent etiology was respiratory tract infection (12.6%), constituting 76% of non-cryptogenic cases, followed by bronchiectasis (2.2%). Seven patients had a double diagnosis of bronchiectasis and respiratory tract infection. Respiratory tract infection was the recorded etiology in 20% of patients with bronchiectasis on CT, while bronchiectasis was the recorded etiology in 11% of patients with bronchiectasis on CT. All etiologies are shown in Figure 3.
Figure 3.

Etiologies of hemoptysis. Absolute numbers (percentages)
In patients with a negative CT an etiology was identified in 28 patients (8%). The etiologies were respiratory tract infection (89.3%), asthma (7.1%) and hemangioma (3.6%).
An etiology was obtained significantly more frequently in patients with a positive CT than in patients with a normal CT (19.9% vs. 8.2%, p < 0.001 – RR 2.414, CI 1.651–3.529) as were the two most frequent etiologies, respiratory tract infection (14.7% vs. 7.4%, p = 0.001 – RR 1.996, CI 1.323–3.010) and bronchiectasis (3.1% vs. 0%, p = 0.001).
Of patients with respiratory tract infection as their etiology, 144 (96.6%) had microbiological sampling performed, and 141 (98%) of these were positive.
Patients with non-cryptogenic hemoptysis were significantly older than those with cryptogenic hemoptysis (59.81 vs. 57.01 years, p = 0.014). Patients aged over 60 had significantly higher rates of non-cryptogenic hemoptysis (19.0% vs. 14.5%, p = 0.036 – RR 1.314, CI 1.017–1.697). Patients with current or previous lung disease also had higher rates of identified etiologies (24.7% vs. 13.9%, p < 0.001 – RR 1.784, CI 1.377–2.310) and higher rates of respiratory tract infections (21.6% vs. 9.6%, p < 0.001 – RR 2.251, CI 1.672–3.028). No significant correlations were found with other final diagnoses in either group, neither were any significant associations found between final diagnoses and gender, pack-years >30, number of events of hemoptysis or volume of hemoptysis. Use of vitamin K antagonists was associated with higher rates of identified etiologies (25.3% vs. 15.8%, p = 0.023 – RR 1.596, CI 1.085–2.348), but no associations were found for other types of anticoagulants.
Discussion
This study supports the initial hypothesis that hemoptysis patients with no malignancy suspected on CT are diagnosed with non-malignant conditions. No malignant diagnoses were established in the study population. The initial diagnostic workup resulted in cryptogenic hemoptysis in 83.5% of patients, and the most prevalent non-cryptogenic etiologies were respiratory tract infection (12.6%) and bronchiectasis (2.2%).
Etiologic distribution in hemoptysis patients
In this study, the proportion of cryptogenic hemoptysis is 83.5% which is considerably higher than those reported in similar studies: Nielsen et al. reported 52.5% to be cryptogenic in a comparable Danish population [4], while studies from the UK, Ireland, and France reported 5.8%, 18%, and 50%, respectively, in populations also similar to ours with regard to gender distribution, age, and smoking history [7,11,13]. In the Danish study, a consensus diagnosis would be agreed on retrospectively if no etiology was established at initial workup, which may explain the lower proportion of undiagnosed patients in this study. Similarly, the low proportion in the Irish study may be due to an included follow-up of at least 6 months, while the French study is limited by being based on diagnostic coding alone, and the UK study found high proportions of acute and chronic bronchitis, an etiology not reported in our study. Much lower proportions of cryptogenic hemoptysis are found in reports from other countries, in which lung cancer and tuberculosis make up substantially larger proportions of hemoptysis cases [8,10,12]. As such, we believe the high frequency of cryptogenic hemoptysis in our study to be explained by differences in study designs and low prevalences of other etiologies such as tuberculosis.
The two most frequent non-cryptogenic etiologies for hemoptysis in this study, respiratory tract infection (12.6%) and bronchiectasis (2.2%), are consistently reported as major etiologies in previous studies. However, for both of these, considerable variations in reported frequencies exist due to differences in definitions and reporting methods. Most other studies have reported in terms of pneumonia rather than respiratory tract infection, the definition of which varying markedly between reports. Nielsen et al. reported pneumonia as the etiology in 34% of non-cryptogenic cases [4] and the corresponding proportion was approx. 46% in the French population [13], whereas Thirumaran et al. reported only 10%, in part because acute bronchitis constituted 67% of diagnoses [7]. Similar variations can also be found and correspondingly explained for bronchiectasis [4,5,7,9,10,19]. In our study, radiological findings of bronchiectasis were considerably more prevalent than the final diagnosis of bronchiectasis. Also, 20% of patients with a radiological finding of bronchiectasis had respiratory tract infection recorded as their etiology while bronchiectasis was the recorded etiology in just 11% of patients with bronchiectasis on CT. Thus, a considerable proportion of the respiratory tract infections identified may be associated with underlying bronchiectasis, suggesting an underestimation of the proportion of bronchiectasis as the cause of hemoptysis. Nonetheless, it is widely agreed that respiratory tract infection and bronchiectasis, diagnoses not consistently requiring bronchoscopy, are leading causes of hemoptysis.
The only etiologies identified in our study strictly requiring bronchoscopy and bronchoscopic sampling are the two hemangiomas, the one leiomyoma, and the seven cases of interstitial lung diseases, which are seen in only 0.8% of patients. Mycobacterial and fungal infections may be suspected from sputum prior to performing bronchoscopy, although most cases do require bronchoscopy to confirm the diagnosis before initiation of treatment.
With a considerably higher number of study subjects than most previous studies, our data suggest that only very few patients with no malignancy suspected on CT will benefit more from bronchoscopy than from non-invasive investigations. This is consistent with previous reports of slightly different perspectives [4,5,11]. Thus, we argue that bronchoscopy is reserved for patients with specific conditions suspected or with persistent symptomatology. We recommend that bronchoscopy should be performed only after careful consideration of the necessity of invasive investigation rather than as a routine investigation in hemoptysis patients with no malignancy suspected on CT.
Since the predominant etiology is respiratory tract infection, sputum culture may be a relevant second-line investigation in patients with no etiology evident from CT. Since microbiological evaluation was only performed in 60% of patients, and since one-fourth of these samples turned out positive, sputum culture may even be considered before CT in order to optimize the detection of respiratory tract infections. Ideally at the first contact with the medical system, which in our case would be to primary care.
Risk factors for non-cryptogenic hemoptysis
The only identified risk factors for non-cryptogenic hemoptysis in our study were age >60, previous or current lung disease and a positive CT scan. Risk factors for respiratory tract infection were previous or current lung disease and a positive CT scan. The latter was also a risk factor for bronchiectasis. Smoking history was not found to be an independent risk factor for any etiology. The literature only considers age and smoking history in relation to hemoptysis caused by cancer and is therefore not comparable to this study.
Few studies have assessed risk factors for etiologies other than lung cancer in hemoptysis patients. Bronchiectasis has been reported to be associated with female gender [9] and moderate to severe hemoptysis [19], while pulmonary embolism has been found associated with mild to moderate hemoptysis [12]. Others, however, found no associations between the amount of hemoptysis and etiology [6] or between gender or race and etiology [20]. No such correlations were found in the present study.
Strengths and limitations
The retrospective design of the study has multiple built-in limitations especially regarding missing data from the clinical records. Also, some results are based on interpretations of clinical reports and procedure descriptions that did not explicitly state the outcome we were looking for. However, a strict methodology and well-defined variables along with the large sample provide a solid foundation for analyses.
In a prospective design with all patients investigated by all diagnostic modalities, it is possible that more etiologies would be identified. However, with the very low proportion of non-cryptogenic hemoptysis in our study and the high proportion of investigations performed, we do not believe that this would markedly alter the overall interpretation of the study.
With the present design, we cannot know whether or not some conditions were overlooked by the diagnostic workup. This may be addressed by a study on the same patient population to identify new diagnoses within a follow-up period, as was done by Tsoumakidou et al. who found no new cases of lung cancer in a 2.7-year follow-up [9].
With regard to the inclusion of patients, 59 of the identified patients were excluded because they declined evaluation or did not show up while one patient died before evaluation. This may have been due to poor health, which might have influenced the rate of serious diagnoses in our population. Conversely, 82 patients were excluded because they did not have CT performed, which may be due to very little suspicion of serious disease. All things considered, we believe selection bias to be minimal in our study.
Conclusion
The majority of hemoptysis patients with no malignancy suspected on CT had cryptogenic hemoptysis and all etiologies identified were benign, generally not requiring bronchoscopy. Bronchoscopy may be reserved for patients with specific conditions suspected or persistent symptomatology. Sputum culture may be an alternative second-line investigation, while bronchoscopy should be performed only after careful consideration of the necessity of invasive investigation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Ketai LH, Mohammed TH, Kirsch J, et al. ACR appropriateness criteria® hemoptysis. J Thorac Imaging. 2014;29(3):W19–7. [DOI] [PubMed] [Google Scholar]
- [2].Earwood JS, Thompson TD.. Hemoptysis: evaluation and management. Am Fam Physician. 2015;91(4):243–249. [PubMed] [Google Scholar]
- [3].Lenner R, Schilero GJ, Lesser M.. Hemoptysis: diagnosis and management. Compr Ther. 2002;28(1):7–14. [DOI] [PubMed] [Google Scholar]
- [4].Nielsen K, Gottlieb M, Colella S, et al. Bronchoscopy as a supplement to computed tomography in patients with haemoptysis may be unnecessary. Eur Clin Respir J. 2016;3:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bønløkke S, Guldbrandt LM, Rasmussen TR. Bronchoscopy in patients with haemoptysis and normal computed tomography of the chest is unlikely to result in significant findings. Dan Med J. 2015;62(8):A5123. [PubMed] [Google Scholar]
- [6].Estarriol MH, M V S, Lopez JJ, et al. Etiology of hemoptysis: prospective analysis of 752 cases. Rev Clin Esp. 2001;201(12):696–700. [DOI] [PubMed] [Google Scholar]
- [7].Thirumaran M, Sundar R, Sutcliffe IM, et al. Is investigation of patients with haemoptysis and normal chest radiograph justified? Thorax. 2009;64(10):854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pires FS, Teixeira N, Coelho F, et al. Hemoptises-etiologia, avaliação e tratamento num hospital universitário. Rev Port Pneumol (English Ed). 2011;17(1): 7–14. [DOI] [PubMed] [Google Scholar]
- [9].Tsoumakidou M, Chrysofakis G, Tsiligianni I, et al. A prospective analysis of 184 hemoptysis cases: diagnostic impact of chest X-ray, computed tomography, bronchoscopy. Respiration. 2006;73(6):808–814. [DOI] [PubMed] [Google Scholar]
- [10].Lee BR, Yu JY, Ban HJ, et al. Analysis of patients with hemoptysis in a tertiary referral hospital. Tuberc Respir Dis (Seoul). 2012;73(2):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arooj P, Bredin E, Henry MT, et al. Bronchoscopy in the investigation of outpatients with hemoptysis at a lung cancer clinic. Respir Med. 2018;139:1–5. [DOI] [PubMed] [Google Scholar]
- [12].Uzun O, Atasoy Y, Findik S, et al. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin Respir J. 2010;4(3):131–138. [DOI] [PubMed] [Google Scholar]
- [13].Abdulmalak C, Cottenet J, Beltramo G, et al. Haemoptysis in adults: a 5-year study using the French nationwide hospital administrative database. Eur Respir J. 2015. August;46(2):503–511. [DOI] [PubMed] [Google Scholar]
- [14].Davoodi M, Kordi M, Gharibvand MM, et al. Hemoptysis: comparison of diagnostic accuracy of multi detector CT scan and bronchoscopy. Glob J Health Sci. 2015. April;7(3):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jeschke KN, Knudsen T, Larsen KR, et al. Danish society of respiratory medicine: hæmoptyse. [Internet]; 2017. [cited 2018 October23]. Available from: https://www.lungemedicin.dk/fagligt/53-haemoptyse/file.html
- [16].Petersen CL, Weinreich UM. Five-year follow-up of hemoptysis with no malignancy suspected on chest computed tomography: recurrence, lung cancer and mortality. Eur Clin Respir J. 2019. January 1;6(1):1616519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Prignot J. Quantification and chemical markers of tobacco-exposure. Eur J Respir Dis. 1987. January;70(1):1–7. [PubMed] [Google Scholar]
- [18].Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hirshberg B, Biran I, Glazer M, et al. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112(2):440–444. [DOI] [PubMed] [Google Scholar]
- [20].Wong CMM, Lim KH, Liam CK. The causes of haemoptysis in Malaysian patients aged over 60 and the diagnostic yield of different investigations. Respirology. 2003;8(1):65–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jeschke KN, Knudsen T, Larsen KR, et al. Danish society of respiratory medicine: hæmoptyse. [Internet]; 2017. [cited 2018 October23]. Available from: https://www.lungemedicin.dk/fagligt/53-haemoptyse/file.html


