ABSTRACT
Background: Adverse childhood experiences (ACE) affect physical and mental health and may appear as risk factors for the development of different conditions in adult life.
Objective: To perform a literature review and meta-analysis on risk indicators for the development of chronic lung diseases in adulthood associated with ACE.
Method: We conducted a systematic literature review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the online databases PubMed, PsycINFO, and Web of Science. Quantitative studies involving male and female adults were included. Fixed- and random-effect models were used in the estimation of meta-analytical measures. The heterogeneity between studies was assessed using I2 statistics and Cochran’s Q test.
Results: A total of 19 studies were selected for the meta-analysis. The analyses showed statistically significant associations between ACE and lung diseases in general (OR = 1.41; CI 95%: 1.28–1.54), besides specific associations with asthma (OR = 1.32; CI 95%: 1.13–1.50) and COPD (OR = 1.44; CI 95%: 1.13–1.76). When the mediating effect of smoking was assessed separately we found a significant – although not quite expressive – association (OR = 1.06; CI 95%: 1.02 to 1.10), which weakens the hypothesis that a direct relationship exists between childhood trauma and the occurrence of lung diseases.
Conclusions: ACE are an important risk factor for the development of lung diseases in adulthood, whether through direct or indirect contribution to this outcome, which highlights the relevance of increasing the awareness of health staff for the early detection and intervention in situations of vulnerability or risk in childhood as an important preventative measure.
KEYWORDS: Adverse childhood experiences, chronic obstructive pulmonary disease, COPD, asthma, stress, meta-analysis
HIGHLIGHTS: • Significant associations between ACE and lung diseases exist (OR = 1.41; CI 95%: 1.28–1.54).• Controlling smoking this risk is greatly decreased, becoming weak (OR = 1.06; CI 95%: 1.02 to 1.10).• The hypothesis that a direct relationship exists between childhood trauma and the occurrence of lung diseases is weak.• The ACE risk for asthma and COPD separately did not show expressive differences.
Antecedentes: Experiencias infantiles adversas (ACE) afectan la salud física y mental y pueden aparecer como factores de riesgo para el desarrollo de diferentes afecciones en la vida adulta.
Objetivo: realizar una revisión de la literatura y un metanálisis sobre indicadores de riesgo para el desarrollo de enfermedades pulmonares crónicas en la edad adulta asociadas con ACE.
Método: Realizamos una revisión sistemática de la literatura de acuerdo con las pautas PRISMA (Elementos de Referencia para Revisiones Sistemáticas y Metaanálisis) utilizando las bases de datos en línea PubMed, PsycINFO y Web of Science. Se incluyeron estudios cuantitativos con hombres y mujeres adultos. Se utilizaron modelos de efectos fijos y aleatorios en la estimación de medidas meta-analíticas. La heterogeneidad entre los estudios se evaluó mediante estadísticas I 2 y la prueba Q de Cochran.
Resultados: Se seleccionaron un total de 19 estudios para el metanálisis. Los análisis mostraron asociaciones estadísticamente significativas entre el ACE y las enfermedades pulmonares en general (OR = 1.41; IC 95%: 1.28–1.54), además de asociaciones específicas con el asma (OR = 1.32; IC 95%: 1.13 – 1.50) y EPOC (OR = 1,44; IC 95%: 1,13–1,76). Cuando el efecto mediador del tabaquismo se evaluó por separado, encontramos una asociación significativa (aunque no del todo clara) (OR = 1.06; IC 95%: 1.02 a 1.10), lo que debilita la hipótesis de que existe una relación directa entre el trauma infantil y la ocurrencia de enfermedades pulmonares.
Conclusiónes: las experiencias ACE son un factor de riesgo importante para el desarrollo de enfermedades pulmonares en la edad adulta, ya sea a través de una contribución directa o indirecta, lo que resalta la relevancia de aumentar la conciencia del personal de salud para la detección temprana y la intervención en situaciones de vulnerabilidad o riesgo en la infancia como una medida preventiva importante.
PALABRAS CLAVE: Experiencias infantiles adversas, enfermedad pulmonar obstructiva crónica, EPOC, asma, estrés, metanálisis
背景:童年期不良经历 (ACE) 影响身体和精神健康, 可能成为成年生活中不同疾病发展的风险因素。
目标:对与ACE相关的成年期慢性肺部疾病发展的风险指标进行系统综述和元分析。
方法:我们根据PRISMA (系统综述和元分析的首选报告项目) 指南, 使用在线数据库PubMed, PsycINFO和Web of Science进行了系统的文献综述。包括涉及成年男性, 女性的定量研究。固定效应和随机效应模型用于元分析的估计。研究间的异质性使用I 2统计量和Cochran’s Q检验进行了评估。
结果:共选择了19项研究进行元分析。分析显示, 除了与哮喘 (OR = 1.32; CI 95%:1.13–1.50) 和COPD (OR = 1.44; CI 95%:1.13–1.76) 的特定关联, ACE与肺部疾病总体有显著相关。当单独评估吸烟的中介作用时, 我们发现了显著但不突出的关联 (OR = 1.06; CI 95%:1.02–1.10), 削弱了童年期创伤与肺部疾病的发生之间存在直接关系的假设。
结论:ACE是成年后肺部疾病发展的重要风险因素, 无论是通过对结果的直接还是间接影响, 都强调了提高卫生人员早期发现和干预童年期易感或风险情境的意识作为重要预防措施的相关性。
关键词: 童年期不良经历, 慢性阻塞性肺疾病, COPD, 哮喘, 应激, 元分析
1. Introduction
Adverse childhood experiences (ACE) refer to traumatic experiences in childhood including physical abuse (physical assault, beatings), sexual abuse (unconsented sexual contact), and psychological abuse (threats, insults, humiliation), in addition to neglect (lack of support to primary basic needs) and adverse events in the family setting (parental death, divorce or institutionalization; substance abuse; mental illness; financial problems, and others) (Brown et al., 2010).
The consequences of ACE are not limited to the mental health of the individual, as shown by Scott et al. (2008) and Goodwin and Wamboldt (2012), but also affect physical health, appearing as risk factors for the onset of different medical conditions later in life (Afifi et al., 2016; McCrory, Dooley, Layte, & Kenny, 2015). This may happen because, as described by Sonu, Post, and Feinglass (2019), ACE trigger the experience of toxic stress, which in turn favours an increase in stress response that affects brain development, structure, and function and the physiology of other systems as the endocrine and immune systems. According to Clemens et al. (2018), our knowledge about these mechanisms is still imprecise, but evidence has implicated different pathways that may act in isolation or in combined form, including HPA axis dysregulation and resulting cortisol increases, increased levels of pro-inflammatory cytokines, and socioeconomic and individual aspects that may favour risky or dysfunctional behaviours including smoking, alcohol and drug abuse, sexual promiscuity, inadequate diets, and sedentary lifestyle (Anda et al., 2008; Felitti et al., 1998; Gilbert et al., 2014; Iniguez & Stankowski, 2016; Kamiya, Timonen, & Kenny, 2016; Scott et al., 2011). Furthermore, Sheikh (2018a) and De Hert et al. (2011) also emphasize the direct or indirect contribution of poor mental health to negative health outcomes (e.g. sedentary lifestyle, poor feeding habits, reduced self-care, and medication side effects).
Lung diseases stand out among the most common chronic diseases today due to the increase in their prevalence and their potential to cause disability in affected individuals, with important social and economic consequences (Brasil, 2010). Asthma and chronic obstructive pulmonary disease (COPD) are the most common respiratory diseases today. Prevalence data show that asthma affects around 334 million people worldwide. COPD affects over 200 million people, of which around 65 million have moderate or severe presentations of the disease, making it the third cause of death in the world. Mortality rates are estimated at around 3 million a year, with gradual annual increases (Forum of International Respiratory Societies, 2017).
Some studies have shown associations between ACE and the occurrence of different lung diseases (Cunningham et al., 2014; Iniguez & Stankowski, 2016; McCrory et al., 2015), whereas others have not confirmed this relationship (Afifi et al., 2016; Llabre et al., 2017). Exley, Norman, and Hyland (2015) performed a systematic literature review to investigate the specific association between asthma and ACE involving 10 studies with a total of 31.524 adults assessed. The authors concluded that exposure to violence early in life is related to increased susceptibility and sensitivity of the body to pro-inflammatory responses, which would in turn increase the risk of developing asthma.
Later, a non-systematic literature review investigated the relationship between child maltreatment and paediatric asthma, examining a total of seven studies. The results showed that abuse increased the risk of asthma and morbidity in adulthood and that this association appeared to depend on different variables including disease subtype, type of reported ACE, and form of ACE investigation (self-report vs. institutional records) (Schreier, Chen, & Miller, 2016).
To our knowledge, no study to date assessed the associations between ACE and lung diseases, except asthma. Also, we have found no meta-analysis on this topic. Therefore, the objective of this study was to perform a systematic review and meta-analysis to investigate risk factors for the development of chronic lung diseases in adulthood associated with ACE. This should contribute to encourage investigations on the subject, whether by expanding the variety of outcomes investigated or through the search for a combined quantitative measure based on statistically adequate methods.
2. Method
This was a systematic literature review based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Liberati et al., 2009) statement and using the online databases PubMed, PsycINFO, and Web of Science, with no time or language limits.
The search terms used were (Chronic Lung Diseases OR Asthma OR COPD OR Bronchitis OR Emphysema OR Lung Cancer OR Pulmonary Fibrosis) AND (Maltreatment OR Trauma OR Adverse Experiences OR Child Abuse OR Sexual Abuse OR Physical Abuse OR Emotional Abuse OR Negligence) AND (Childhood OR Infant OR Infancy OR Early) and the last literature search for the review was performed on 27 November 2019.
The review included clinical studies using quantitative methods, involving human samples of both sexes, and with the objective of assessing risk indicators associated with exposure to ACE and the development of lung diseases in adulthood. The exclusion criteria and the complete process of article search and selection are shown in Figure 1.
Figure 1.
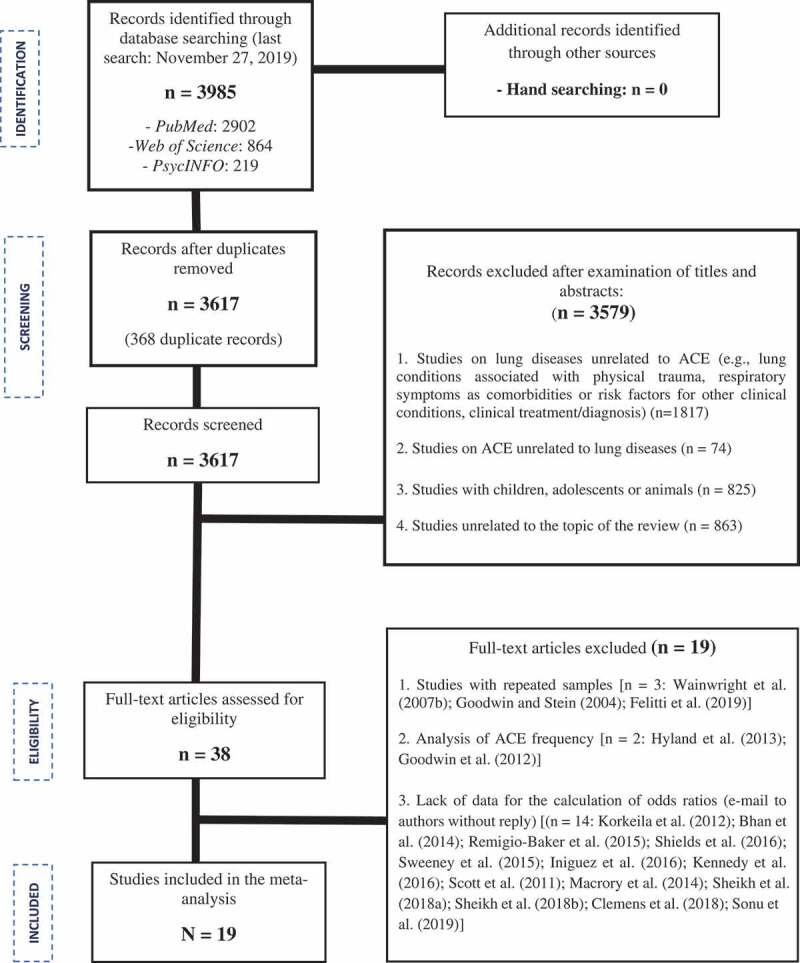
Flow diagram of the process of article search and selection for the meta-analysis based on the PRISMA protocol (Liberati et al., 2009).
The following criteria were used for the meta-analysis: (1) independent variable: any ACE (physical, sexual or emotional) assessed in isolated or combined form; (2) dependent variable: any lung disease assessed in isolation or together with other lung diseases; (3) measure of odds ratios (OR) related to the presence/absence of ACE preferably adjusted in relation to smoking (isolated or combined with other risk factors for physical and mental health) or, when smoking was absent, in relation to the subjects’ socio-demographic characteristics (note: the review included studies in which results were expressed in terms of OR or where the raw data published in the study or obtained through contact with the authors allowed the calculation of OR).
The eligibility of the studies to be included in the review was determined independently by two mental health professionals with experience in consultation-liaison psychiatry and by a statistician, with divergences resolved by consensus. The extraction of data from the articles was based on a standard form created by the authors that included the following data: (a) country of origin of the study, year of publication, and methodological design; (b) sample size; (c) subjects’ sex, age, and educational level; (d) lung disease of interest, source of diagnostic information, and instrument used for the assessment of ACE; and (e) data for the calculation of effect sizes (OR).
The methodological quality of the studies was assessed with the Newcastle-Ottawa Quality Assessment Scale (Peterson, Welch, Losos, & Tugwell, 2011) that measure methodological quality according to three parameters: sample selection (four items), comparability (two items), and exposure (three items).
Fixed- and random-effect models were used in the estimation of meta-analytical measures, and the method of DerSimonian and Lair was used in the random-effect model (Higgins, Thompson, Deeks, & Altman, 2003). The fixed-effects model assumes that differences observed across the studies are due only to sampling errors, that is, to chance. The random-effects model, in turn, considers that the studies included in a meta-analysis form a random sample from a hypothetic population of studies and that, although their effects are not considered equal, they are connected through a probability distribution generally assumed as normal. Random-effects models are desirable when the heterogeneity across studies is high, as they offer combined results with greater confidence intervals (Santos & Cunha, 2013). Data on the accuracy indicators of each study and meta-analytical measures are presented in Forest plots below.
The heterogeneity across studies was assessed with I2 statistics and Cochran’s Q test. I2 statistics is interpreted as the total percentage of variation across the studies included in a meta-analysis. Higgins et al. (2003) suggested a scale in which I2 values close to 0% indicate no heterogeneity across studies; values around 25% indicate low heterogeneity; values around 50% indicate moderate heterogeneity; and values around 75% indicate high heterogeneity across studies. All the analyses and graphs were made with the software Stata v. 13.
3. Results
A total of 19 studies were included in the meta-analysis. In respect to their methodological quality, 63% fulfilled at least two-thirds of the requirements and the main critical points found were related to the scarcity of information on the definition of cases, estimation of exposure by self-report, and absence of no-response rates. Details about these data are presented in Supplementary Material S1.
Data about the samples and study designs of the articles included in the review are presented in Table 1.
Table 1.
Sample and methodological characterization of the studies analysed and included for the meta-analysis (N = 19).
| Autor/Year | Country | N Sample (♂/♀) |
Age (years) (DP) or (min-max) |
Education | Study Design/Data Source | Outcome | Clinical Diagnostc | ACE Measures |
|---|---|---|---|---|---|---|---|---|
| Romans, Belaise, Martin, Morris, and Raffi (2002) | New Zealand | 354 ♀ | 26–70 | NI | Cross-sectional Retrospective/Randon community-based sample | Asthma | SR | DS |
| Wainwright, Surtees, Wareham, and Harrison (2007a) | United Kingdom | 9081 ♂ 11,807 ♀ | 41 – 80 | NI | Cross-sectional Study/NCEPIC | Asthma | SR | DS |
| Scott et al. (2008) | America, Europe, Asia |
7394 ♂ 10,909 ♀ | 21 – 98 | NI | Cross-sectional Population Survey/WMHS | Asthma | SR | DS |
| Coogan et al. (2013) | United States | 28,456 ♀ | 21 – 69 | 49% COL | Prospective cohort/BWHS | Asthma | SR | DS |
| Gilbert et al. (2014) | United States | 21,322 ♂ 32,676 ♀ | ≥ 18 | 37,8% COL | Cross-sectional Population Survey/BRFSS | Asthma | SR | DS |
| Santaularia et al. (2014) | United States | 8160 ♀ | ≥ 18 | NI | Cross-sectional Survey/Kansas BRFSS | Asthma | SR | DS |
| Felitti et al. (1998) | United States | 3859 ♂ 4197 ♀ | = 56,1 | 43,4% COL | Cross-sectional Population Survey/KPSDHAC | COPD | SR | DS |
| Springer (2009) | United States | 3317 ♂♀ | = 53,8 | NI | Cross-sectional Population Survey/WLS | COPD | SR | CTS |
| Cunningham et al. (2014) | United States | 19,015 ♂ 26,546 ♀ | ≥ 18 | 35,8% COL | Cross-sectional Population Survey/BRFSS | COPD | SR | DS |
| Brown et al. (2010) | United States | 7970 ♂ 9367 ♀ |
≥ 18 | 39,3% COL | Prospective cohort/KPSDHAC | Lung Cancer | HR | CTS |
| Anda et al. (2008) | United States | 7117 ♂ 8355 ♀ |
= 56 (±15) | 40% COL | Prospective cohort/ACE Study Cohort | Lung Disease (Asthma + Bronchitis + Emphysema) |
SR HR |
CTS |
| Downey et al. (2017) | United States | 2541 ♂ 3820 ♀ |
≥ 18 | 30,5% COL |
Cross-sectional Population Survey/BRFSS | Lung Disease (Bronchitis + Emphysema+COPD) | SR | DS |
| Scott, Smith, and Ellis (2012) | New Zealand | 599 ♂ 814 ♀ |
= 22 | 47,7% HS | Cross-sectional Survey/NZMHS | Asthma | SR | DS |
| Afifi et al. (2016) | Canada | 23,395 ♂♀ | ≥ 18 | NI | Cross-sectional Survey/CCHS | Asthma COPD |
SR | CEVQ |
| Abajobir et al. (2017) | Australia | 1783 ♂ 1979 ♀ |
≥ 18 | NI | Prospective Cohort/MUSP | Asthma | SR+LF | SWCPR |
| Banerjee, Gelaye, Zhong, Sanchez, and Williams (2018) | Peru | 3081 ♀ | ≥ 18 | NI | Cross-sectional Survey/Outpatient from Intitution for Maternal Perinatal | Asthma | SR | CPSAQ |
| Llabre et al. (2017) | United States | 2260 ♂ 2746 ♀ |
18 – 74 | NI | Cross-sectional Study/HCHSL | Asthma COPD |
SR | DS |
| Goodwin, Wamboldt, and Pine (2003) | United States | 5877 ♂♀ | 18 – 54 | NI | Cross-sectional Population Survey/NCS | Lung Disease (Asthma + Bronchitis + Tuberculosis) |
SR | DS |
| Kamiya et al. (2016) | Ireland | 8178 ♂♀ | ≥ 50 | NI | Cross-sectional Population Survey/ILSA | Lung Disease (Asthma+COPD) |
SR | DS |
BL: Basic Level; BRFSS: Behaviour Risk Factor Surveillance Survey; BWHS: Black Women’s Health Study; CCHS: Cannadian Community Health Survey; CEVQ: Childhood Experiences of Violence Questionnaire; CPSAQ: Childhood Physical and Sexual Abuse Questionnaire; COPD: Chronic Obstrutive Pulmonar Disease; COL: College; CTQ: Childhood Trauma Questionnaire; CTS: Conflict Tatics Scale; DCFS: Department of Child and Family Services; DS: Developed for Study; HCHSL: Hispanic Community Health Study of Latinos; HR: Hospital Records; HS:High School; HSS: Health and Social Support; ILSA: Irish Longitudinal Study on Ageing; IR: Institutional Register; KPSDHAC: Kaiser Permanente’s San Diego Health Appraisal Clinic; LF: Lung Function; LT: Lung Transplantation; MIDUS: Midlife Development in the United States; MP: Medications Prescription; NCEPIC: Norfolk Cohort of the European Prospective Investigation Into Cancer; NCS: National Comorbidy Survey; NZMHS: New Zealand Mental Health SurveyMESA: Marshfiled Epidemiologic Study Area; RCMHC: Rural Community Mental Health Centre; SR: Self Reported; SWCPR: State Wide Child Protection Records; WLS: Winconsin Longitudinal Study; WMHS: World Mental Health Survey.
The studies were carried out mainly in the USA (n = 11). Together, they included a total of 276,975 subjects, with the smallest sample consisting of 354 and the largest of 53,998 subjects (=14577, SD = 14.8; median = 8160). The age of participants ranged between 18 and 98 years, with a predominance of adults aged 45–65 years and of mixed-sex samples (79%). In respect to the design, most were population studies with a cross-sectional design (n = 15) and used data from previous investigations or databases (n = 14). Information about the educational level of subjects was lacking in most of the articles.
Concerning the clinical outcomes of interest, 58% of the studies investigated asthma alone (n = 11), 26% investigated COPD (encompassing bronchitis and emphysema; n = 5) and 21% investigated the presence of lung conditions in general without distinguishing between them (n = 4). Only one study investigated lung cancer as an outcome.
The assessment of the clinical status (presence or absence of lung disease) was based only on self-report in most studies (n = 16). Only two studies were based on medical records and one included an assessment of lung function to complement diagnostic measures.
For the assessment of ACE, around 68% of the studies (n = 13) used non-validated instruments developed specifically for the study. Six studies used validated instruments to assess ACE, the most common of which was the Conflict Tactics Scale (Straus & Gelles, 1990). One study used data from child protection institutional records to assess child abuse.
The quantitative data related to the association between ACE and risk indicators for the development of lung diseases are presented in Supplementary Material S2. Data for the meta-analysis were extracted from this dataset and the results of the meta-analysis are presented in Figure 2.
Figure 2.
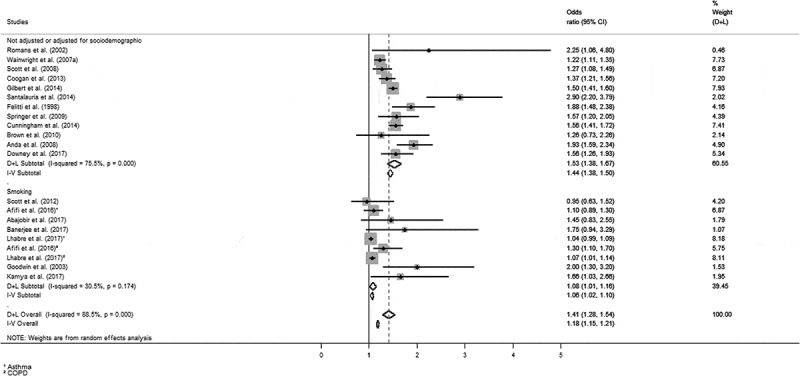
Odds ratio for the presence of lung diseases in adulthood associated with ACE [(A) Forest plot of isolated and combined measures – random (D + L) and fixed (I-V) effects.
Figure 2 shows that 71.4% of the studies found statistically significant associations between ACE and lung diseases. The combined measures also revealed that ACE were associated with the presence of lung diseases in adulthood (random effect: OR = 1.41; CI 95%: 1.28 to 1.54; fixed effect: OR = 1.18; CI 95%: 1.15 to 1.21). The analyses showed a marked heterogeneity across studies (I2 = 88.5%). For further details, please see Supplementary Material S3.
Subgroup analyses for specific lung diseases were performed separately considering the clinical outcomes asthma (n = 11) and COPD (n = 5), regardless of ACE subtype. Combined results were positive for both asthma (random effect: OR = 1.32; CI 95%: 1.13 to 1.50) and COPD (random effect: OR = 1.44; CI 95%: 1.13 to 1.76) and, differently than expected, the rate of heterogeneity across studies was higher (I2 = 90.1% for asthma and I2 = 91.5% for COPD). For more details, please see Supplementary Material S4.
Subgroup analyses were then performed to assess the mediating effect of smoking separately. The combined results were positive for both conditions (adjusted to smoking – 9 studies: random effect: OR = 1.06; CI 95%: 1.02 to 1.10; I2 = 30.5%; not adjusted to smoking – 12 studies: random effect: OR = 1.53; CI 95%: 1.38 to 1.67; I2 = 75.5%); however, this result becomes less expressive when the adjustment is considered (see Figure 2).
4. Discussion
The results of the meta-analysis show that people who have gone through ACE have around 40% more chance of presenting a lung disease in adulthood compared to unaffected individuals. However, when other risk factors for the development of lung diseases are taken into consideration, such as smoking, this risk is greatly decreased, becoming weak and challenging the hypothesis that a direct relationship exists between childhood trauma and the occurrence of lung diseases.
According to the extant literature, the physiopathological mechanisms more likely to underlie the relationship between ACE and the aetiology of different chronic diseases refer to organic reactions that occur in response to chronic psychosocial stress. Thus, chronic exposure to stress would lead to structural and functional abnormalities in the nervous, endocrine, and immune systems, which work in concert to react to stress. This negative exposure would trigger specialized adaptive responses that include the activation of different brain areas and of the sympathetic nervous system and lead to inflammatory processes, eventually triggering the neuroendocrine response to stress and the activation of the hypothalamic-pituitary-adrenal (HPA) axis. Although these integrated systems are efficient in generating short-term adaptation to acute stress, their lasting stimulation may have detrimental effects on the organism (Danese & McEwen, 2012). Acute stress is associated with an increase in cortisol levels that results in the suppression of inflammatory responses. Under the influence of stressors such as ACE, HPA axis function could be altered, leading to a decrease in cortisol levels and an increase in inflammatory markers that would favour the susceptibility to chronic diseases.
This could occur even in the absence of harmful lifestyle choices or conditions, such as sedentary lifestyle, smoking or unemployment, for example (Afifi et al., 2016; Anda et al., 2008; Danese & McEwen, 2012). However, our data show that the mediating effect of different risk factors in this relationship, mainly smoking, is quite expressive. As reported by Campbell, Walker, and Egede (2016) and Ford et al. (2011), victims of ACE are at increased vulnerability to becoming smokers (2.2–2.7 times). Earlier, Anda et al. (1999) described relevant relationships between the number of ACE and early onset of smoking, prevalence of smoking in adulthood (especially heavy smoking), and prevalence of self-reported COPD. These findings seem to reflect the late consequences of adverse coping methods such as smoking, food and drug abuse, and promiscuity. To Anda et al. (1999) and Brown et al. (2010), smoking may act as a resource related to self-esteem to alleviate emotional wounds or even as a surrogate treatment for psychological/affective symptoms based on the psychoactive effects of nicotine.
The subgroup analyses that considered the risk for asthma and COPD separately did not show expressive differences between the two conditions. This is an interesting finding if we consider the aetiology of the conditions since one could expect the risks for their occurrence to be different. In asthma, the most common aetiological factors described are related to physiological responses to the exposure to environmental allergens, air pollution, lower respiratory tract infections in early life, and abnormal immune responses (Forum of International Respiratory Societies, 2017). In COPD, however, harmful habits such as smoking, for example, play a major role as they favour the destruction of lung tissue and the obstruction of small airways by inflammation and mucus (Felitti et al., 1998; Forum of International Respiratory Societies, 2017; Llabre et al., 2017; Springer, 2009).
Our findings suggest, therefore, that regardless of the type of lung disease and their aetiological specificities, the impact of ACE is relevant in the assessment of risk, whether through direct or indirect action on this outcome. Despite our attempt to group studies to perform quantitative analyses of the risk indicators adjusted for confounding/mediating variables, this was limited by the heterogeneity of studies and the lack of data for the inclusion of other studies in the meta-analysis. In the investigation about the effects of mediating variables, we opted to prioritize smoking due to its direct connection with the aetiology of lung diseases and to the fact that this variable was controlled for in most studies. Although the heterogeneity across studies in this subgroup was the lowest observed, it should be noted that smoking was controlled together with a number of other health conditions and risk behaviours, and not in isolation. This is a limitation of our study and future investigations to confirm this finding and elucidate specific influences of smoking in the vulnerability to lung diseases are opportune, as well as studies about other variables including the presence of mental disorders, the lack of emotional support, or conditions of access to health, for example, which would favour the accurate design of prevention and harm reduction programmes.
An additional limitation of our study was the impossibility to assess the different types of ACE separately in order to check their impact on the occurrence of lung diseases in adulthood. This could be important since previous studies have suggested that sexual abuse and adverse events in the family setting, as well as a high incidence (>4) of traumatic experiences, are associated with increased risk of respiratory diseases in both smokers and non-smokers (Cunningham et al., 2014; Remigio-Baker, Hayes, & Reyes-Salvail, 2015; Shields, Hovdestad, Gilbert, & Tonmyr, 2016).
The studies reviewed here also had methodological limitations that deserve attention in future investigations: (a) almost all studies had a cross-sectional design, which hinder conclusions about causes and effects that could be possible with the adoption of a longitudinal design; (b) in all studies, the association between ACE and lung diseases was assessed as a secondary outcome of previous population studies and, therefore, were based on methods that were not originally designed for this specific purpose, resulting in decreased methodological quality; (c) data related to ACE were investigated through non-validated instruments and the assessment of clinical outcomes was not based on objective diagnostic tools (e.g. clinical examination), which favours heterogeneity and restrain analyses.
Additional limitations inherent to the investigation of ACE include the use of retrospective information, with the increased possibility of recall biases, especially if we consider the emotionally distressing nature of such experiences (Afifi et al., 2016; Downey, Gudmunson, Pang, & Lee, 2017; Goodwin & Wamboldt, 2012). Another issue to be considered is that almost all studies were conducted in Europe and North America, where better socio-economic and educational conditions reflect on the prevalence and impact of ACE (Abramovay, Castro, Pinheiro, Lima, & Matinelli, 2002). Thus, the results described here cannot be generalized to underdeveloped or developing countries, where specific studies should be conducted.
In conclusion, ACE are important risk factors for lung diseases in adulthood, which highlights the importance of increasing awareness among health professionals about the early detection and intervention in situations of risk or vulnerability in childhood.
Funding Statement
São Paulo Research Foundation (FAPESP, São Paulo, Brazil) (process no. 2016/01801-5).The Brazilian National Council for Scientific and Technological Development (CNPq, Brasília, Brazil) (process 301321/2016-7).
Disclosure statement
No potential conflict of interest was reported by the authors. The supporters had no role in the design, analysis, interpretation, or publication of this study.
Supplementary materials
Supplemental data for this article can be accessed here.
References
- Abajobir, A. A., Kisely, S., Williams, G., Strathearn, L., Suresh, S., & Najman, J. M. (2017). The association between substantiated childhood maltreatment, asthma and lung function: A prospective investigation. Journal of Psychosomatic Research, 101, 58–9. [DOI] [PubMed] [Google Scholar]
- Abramovay, M., Castro, M. J., Pinheiro, L. C., Lima, F. S. E., & Matinelli, C. C. (2002). Juventude, violência e vulnerabilidade social na América Latina: Desafios para políticas públicas. Brasília: UNESCO, BID. [Google Scholar]
- Afifi, T. O., MacMillan, H. L., Boyle, M., Cheung, K., Taillieu, T., Turner, S., & Sareen, J. (2016). Child abuse and physical health in adulthood. Health Reports, 27(3), 10–18. [PubMed] [Google Scholar]
- Anda, R. F., Brown, D. W., Dube, S. R., Bremner, J. D., Felitti, V. J., & Giles, W. H. (2008). Adverse childhood experiences and chronic obstructive pulmonary disease in adults. American Journal of Preventive Medicine, 34(5), 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda, R. F., Croft, J. B., Felitti, V. J., Nordenberg, D., Giles, W. H., Williamson, D. F., & Giovino, G. A. (1999). Adverse childhood experiences and smoking during adolescence and adulthood. JAMA, 282(17), 1652–1658. [DOI] [PubMed] [Google Scholar]
- Banerjee, D., Gelaye, B., Zhong, Q. Y., Sanchez, S. E., & Williams, M. A. (2018). Childhood abuse and adult-onset asthma among Peruvian women. Journal of Asthma, 55(4), 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan, N., Glymour, M. M., Kawachi, I., & Subramanian, S. V. (2014). Childhood adversity and asthma prevalence: Evidence from 10 US states (2009–2011). BMJ Open Respiratory Research, 1(1), e000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil . (2010). Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Doenças respiratórias crônicas/Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica. Brasília: Ministério da Saúde. 160 p.: il. – (Série A. Normas e Manuais Técnicos) (Cadernos de Atenção Básica, n. 25). [Google Scholar]
- Brown, D. W., Anda, R. F., Felitti, V. J., Edwards, V. J., Malarcher, A. M., Croft, J. B., & Giles, W. H. (2010). Adverse childhood experiences are associated with the risk of lung cancer: A prospective cohort study. BMC Public Health, 10(1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. A., Walker, R. J., & Egede, L. E. (2016). Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. American Journal of Preventive Medicine, 50(3), 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, V., Huber-Lang, M., Plener, P. L., Brähler, E., Brown, R. C., & Fegert, J. M. (2018). Association of child maltreatment subtypes and long-term physical health in a German representative sample. European Journal of Psychotraumatology, 9(1), 1510278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan, P. F., Wise, L. A., O’Connor, G. T., Brown, T. A., Palmer, J. R., & Rosenberg, L. (2013). Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. Journal of Allergy and Clinical Immunology, 131(4), 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, T. J., Ford, E. S., Croft, J. B., Merrick, M. T., Rolle, I. V., & Giles, W. H. (2014). Sex-specific relationships between adverse childhood experiences and chronic obstructive pulmonary disease in five states. International Journal of Chronic Obstructive Pulmonary Disease, 9, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A., & McEwen, B. S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106, 29–39. [DOI] [PubMed] [Google Scholar]
- De Hert, M., Correll, C. U., Bobes, J., Cetkovich‐Bakmas, M., Cohen, D. A. N., Asai, I., … Newcomer, J. W. (2011). Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 10(1), 52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey, J. C., Gudmunson, C. G., Pang, Y. C., & Lee, K. (2017). Adverse childhood experiences affect health risk behaviors and chronic health of Iowans. Journal of Family Violence, 1–8, 557–564. [Google Scholar]
- Exley, D., Norman, A., & Hyland, M. (2015). Adverse childhood experience and asthma onset. A Systematic Review European Respiratory Review, 24(136), 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., … Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., … Marks, J. S. (2019). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine, 56(6), 774–786. [DOI] [PubMed] [Google Scholar]
- Ford, E. S., Anda, R. F., Edwards, V. J., Perry, G. S., Zhao, G., Li, C., & Croft, J. B. (2011). Adverse childhood experiences and smoking status in five states. Preventive Medicine, 53(3), 188–193. [DOI] [PubMed] [Google Scholar]
- Forum of International Respiratory Societies . (2017). The global impact of respiratory disease (2nd ed.). Sheffield: European Respiratory Society. [Google Scholar]
- Gilbert, L. K., Breiding, M. J., Merrick, M. T., Thompson, W. W., Ford, D. C., Dhingra, S. S., & Parks, S. E. (2014). Childhood adversity and adult chronic disease: An update from ten states and the District of Columbia, 2010. American Journal of Preventive Medicine, 48(3), 345–349. [DOI] [PubMed] [Google Scholar]
- Goodwin, R. D., & Stein, M. B. (2004). Association between childhood trauma and physical disorders among adults in the United States. Psychological Medicine, 34(3), 509–520. [DOI] [PubMed] [Google Scholar]
- Goodwin, R. D., & Wamboldt, F. S. (2012). Childhood physical abuse and respiratory disease in the community: The role of mental health and cigarette smoking. Nicotine & Tobacco Research, 14(1), 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, R. D., Wamboldt, M. Z., & Pine, D. S. (2003). Lung disease and internalizing disorders: Is childhood abuse a shared etiologic factor? Journal of Psychosomatic Research, 55(3), 215–219. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland, M. E., Alkhalaf, A. M., & Whalley, B. (2013). Beating and insulting children as a risk for adult cancer, cardiac disease and asthma. Journal of Behavioral Medicine, 36(6), 632–640. [DOI] [PubMed] [Google Scholar]
- Iniguez, K. C., & Stankowski, R. V. (2016). Adverse childhood experiences and health in adulthood in a rural population-based sample. Clinical Medicine & Research, 14(3–4), 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, Y., Timonen, V., & Kenny, R. A. (2016). The impact of childhood sexual abuse on the mental and physical health, and healthcare utilization of older adults. International Psychogeriatrics, 28(3), 415–422. [DOI] [PubMed] [Google Scholar]
- Kennedy, C. C., Zubair, A., Clark, M. M., & Jowsey-Gregoire, S. (2016). Childhood abuse is associated with worse survival following lung transplantation. Progress in Transplantation, 26(2), 178–182. [DOI] [PubMed] [Google Scholar]
- Korkeila, J., Lietzen, R., Sillanmäki, L. H., Rautava, P., Korkeila, K., Kivimäki, M., … Vahtera, J. (2012). Childhood adversities and adult-onset asthma: A cohort study. BMJ Open, 2(5), e001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P. A., … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700. pmid:1962255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabre, M. M., Schneiderman, N., Gallo, L. C., Arguelles, W., Daviglus, M. L., Franklyn Gonzalez, I. I., … Penedo, F. J. (2017). Childhood trauma and adult risk factors and disease in Hispanics/Latinos in the US: Results From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) sociocultural ancillary study. Psychosomatic Medicine, 79(2), 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory, C., Dooley, C., Layte, R., & Kenny, R. A. (2015). The lasting legacy of childhood adversity for disease risk in later life. Health Psychology, 34(7), 687. [DOI] [PubMed] [Google Scholar]
- Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2011). The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. [Google Scholar]
- Remigio-Baker, R. A., Hayes, D. K., & Reyes-Salvail, F. (2015). Adverse childhood events are related to the prevalence of asthma and chronic obstructive pulmonary disorder among adult women in Hawaii. Lung, 193(6), 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans, S., Belaise, C., Martin, J., Morris, E., & Raffi, A. (2002). Childhood abuse and later medical disorders in women. Psychotherapy and Psychosomatics, 71(3), 141–150. [DOI] [PubMed] [Google Scholar]
- Santaularia, J., Johnson, M., Hart, L., Haskett, L., Welsh, E., & Faseru, B. (2014). Relationships between sexual violence and chronic disease: A cross-sectional study. BMC Public Health, 14(1), 1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, D., & Cunha, M. (2013). Critical interpretation of statistical results of a meta-analysis: Methodological strategies. Millenium, 44, 85–98. [Google Scholar]
- Schreier, H. M., Chen, E., & Miller, G. E. (2016). Child maltreatment and pediatric asthma: A review of the literature. Asthma Research and Practice, 2(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, K. M., Smith, D. A., & Ellis, P. M. (2012). A population study of childhood maltreatment and asthma diagnosis: Differential associations between child protection database versus retrospective self-reported data. Psychosomatic Medicine, 74(8), 817–823. [DOI] [PubMed] [Google Scholar]
- Scott, K. M., Von Korff, M., Alonso, J., Angermeyer, M. C., Benjet, C., Bruffaerts, R., & Ono, Y. (2008). Childhood adversity, early-onset depressive/anxiety disorders, and adult-onset asthma. Psychosomatic Medicine, 70(9), 1035–1043. [DOI] [PubMed] [Google Scholar]
- Scott, K. M., Von Korff, M., Angermeyer, M. C., Benjet, C., Bruffaerts, R., De Girolamo, G., & Tachimori, H. (2011). Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Archives of General Psychiatry, 68(8), 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, M. A. (2018a). Childhood adversities and chronic conditions: Examination of mediators, recall bias and age at diagnosis. International Journal of Public Health, 63(2), 181–192. [DOI] [PubMed] [Google Scholar]
- Sheikh, M. A. (2018b). Child maltreatment, psychopathological symptoms, and onset of diabetes mellitus, hypothyroidism and COPD in adulthood. Journal of Affective Disorders, 241, 80–85. [DOI] [PubMed] [Google Scholar]
- Shields, M. E., Hovdestad, W. E., Gilbert, C. P., & Tonmyr, L. E. (2016). Childhood maltreatment as a risk factor for COPD: Findings from a population-based survey of Canadian adults. International Journal of Chronic Obstructive Pulmonary Disease, 11, 2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonu, S., Post, S., & Feinglass, J. (2019). Adverse childhood experiences and the onset of chronic disease in young adulthood. Preventive Medicine, 123, 163–170. [DOI] [PubMed] [Google Scholar]
- Springer, K. W. (2009). Childhood physical abuse and midlife physical health: Testing a multi-pathway life course model. Social Science & Medicine, 69(1), 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus, M., & Gelles, R. J. (1990). Physical violence in American families: Risk factors and adaptations to violence in 8,145 families. New Brunswick, NJ: Transaction Press. [Google Scholar]
- Sweeney, S., Air, T., Zannettino, L., Shah, S. S., & Galletly, C. (2015). Gender differences in the physical and psychological manifestation of childhood trauma and/or adversity in people with psychosis. Frontiers in Psychology, 6, article1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright, N. W., Surtees, P. G., Wareham, N. J., & Harrison, B. D. (2007a). Psychosocial factors and asthma in a community sample of older adults. Journal of Psychosomatic Research, 62(3), 357–361. [DOI] [PubMed] [Google Scholar]
- Wainwright, N. W. J., Surtees, P. G., Wareham, N. J., & Harrison, B. D. W. (2007b). Psychosocial factors and incident asthma hospital admissions in the EPIC‐Norfolk cohort study. Allergy, 62(5), 554–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


