Abstract
Cognitive problems are common in children with neurofibromatosis type 1, representing a significant source of lifelong morbidity. Assessment of cognitive function has been challenging in the setting of clinical trials. Spatial learning deficits may be an important target for cognitive interventions. We leveraged a large, international cognitive study in affected children with NF1 treated with lovastatin to assess spatial learning using the “Arena Maze”, a portable, computerized task that allows for retesting in the same environment. As with the parent study, spatial learning assessed with this task did not improve with lovastatin treatment.
Introduction
Neurofibromatosis type 1 (NF1) is one of the most common autosomal dominant disorders,1 in which more than half of the affected children manifest cognitive impairment.2 Children with NF1 frequently demonstrate difficulty on various measures of visual spatial skills, visual‐motor integration, and visual learning.3 One of the major challenges in assessing visual spatial deficits and translating preclinical studies in mice is the lack of a suitable common assessment tool. Mice with germline mutations in the Nf1 gene demonstrate deficits in visual spatial learning, which is traditionally measured using the Morris Water Maze. Based on encouraging preclinical results in mice,4 the HMG‐CoA reductase inhibitor lovastatin was evaluated in human clinical trials, where no improvements in learning were observed.5
Since the standard tests used to measure patient spatial learning and memory in humans differ from the Morris Water Maze used in animal models, the Arena Maze was developed to assess spatial learning strategies in young adults following traumatic brain injury and in a pilot study among children with NF1 and their unaffected siblings.6 The objective of the current study was to evaluate the feasibility of the Arena Maze to assess spatial learning in children with NF1. The secondary objective was to evaluate this tool as an outcome parameter for assessing spatial learning after treatment with lovastatin (NF1 STARS, NCT00853580).
Subjects and Methods
This was an ancillary study to NF1 STARS (n = 146), a multicenter, double‐blind, placebo‐controlled, Phase II randomized trial of lovastatin, conducted by the NF Clinical Trials Consortium to determine the efficacy of lovastatin on visual spatial learning and attention abilities of children with NF1 aged 8–15 years. The same inclusion and exclusion criteria were used for the ancillary study as for the parent study.5 Participants were recruited from three sites: Boston Children’s Hospital (Boston, MA), The Children’s Hospital at Westmead (Sydney, Australia) and Children’s National Health System (Washington, DC). Informed consent was obtained from all parents/guardians, and age‐appropriate assent was obtained to participate in the ancillary study. Participants were randomized on the parent study to receive lovastatin or placebo daily.
Measures
Participants were assessed at baseline, posttreatment (week 16), and 8 weeks after cessation of treatment with all primary and secondary outcome measures for NF1 STARS. The Arena Maze was administered following completion of the assessment battery for the parent trial. The Arena Maze was administered by computer and uses a video game controller to navigate around a circular “arena” within a virtual room to locate a target on the floor. The walls of the Arena Maze contain spatial cues, such as pictures, which remain consistent through the test trials (Fig. 1). Participants are given directions, introduced to the virtual room, and allowed to familiarize themselves with the controller. For the first two practice trials, the target is visible. For the six learning trials, the target is no longer visible, but is always hidden in the same place. The child must find the invisible target, starting from a new location in the periphery each time. When the child successfully navigates to the target, it becomes visible. On the final (ninth) trial, the “probe” trial, the target is removed from the arena without the child’s knowledge and does not appear when the child navigates to the target location. Participants are told to go to the target as quickly and directly as possible for all trials.
Figure 1.
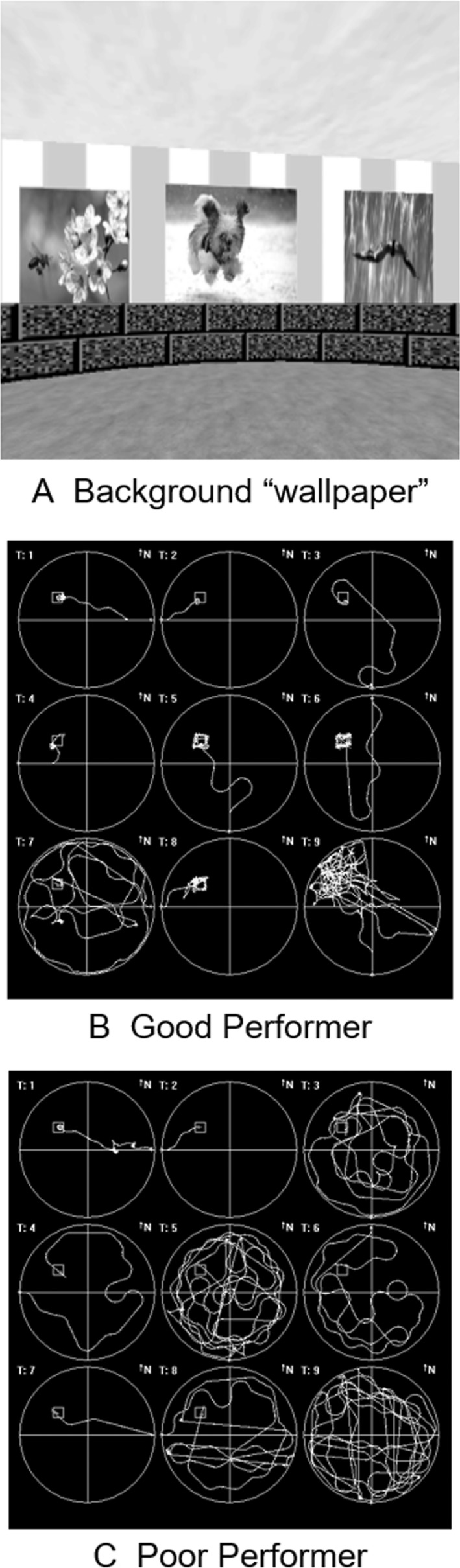
The virtual Arena Maze. (A) Sample grayscale picture of the computerized virtual water maze/computerized Arena Maze. The graphics used during the assessments were in color. (B) “Bird’s‐eye” view of the path from start point to target as navigated in a “good performer”. (C) “Bird’s‐eye” view of a “poor performer”.
Performance on the Arena Maze was assessed using several outcomes: (1) path length, the distance traveled rom start point to target; (2) latency, the time to target; and (3) dwell time, the time spent in each of the Arena Maze quadrants. During the final “probe” trial, dwell time in the quadrant, where the target was located, provides a measure of learning. The program generates a separate data file that contains a pixel‐by‐pixel recording of the participant’s path through the arena on each trial (search path). In addition, success in finding the target (target crossings) was captured.
Outcome measures/statistical analysis
The outcome measures of the Arena Maze included trial latency, path length, and dwell time in the target quadrant on the probe trial. Descriptive statistics summarized the findings (mean, standard deviation). Baseline differences between the NF1 group and normative reference data were tested using one‐sample t‐tests. Pearson correlations and spearman rank correlations were used to assess the relationship of participant characteristics and results on standardized measures of visual spatial function to Arena Maze variables at baseline. Lastly, t‐tests were used to compare the performance of the lovastatin‐treated group to the control group. Mixed effects random models were used with treatment and visit as fixed effects and participants as random effects to assess the changes within individuals and compare treatment responses over time.
Results
Forty participants were enrolled in the ancillary study; 29 completed all assessments at baseline and posttreatment. Data from these participants were analyzed. Participant characteristics and measures of spatial skills, intelligence, attention, and executive function at baseline are provided in Table 1. Apart from FSIQ and total errors from the paired associate learning (PAL) task (both, P > 0.91), baseline mean scores for study participants were significantly poorer than normative reference data (all, P < 0.02), confirming the presence of cognitive and behavioral deficits on these outcomes. There were no statistically significant differences in baseline measures between the lovastatin and placebo groups, though in the majority of areas, scores for the placebo group trended lower than for the lovastatin group.
Table 1.
Demographics and baseline neuropsychological function.
| Entire cohort | Lovastatin | Placebo | |
|---|---|---|---|
| Demographics | N = 29 | ||
| Median age (years) | 10.77 | 10.38 | 10.77 |
| Mean age (years) | 11.22 (2.18) | 10.99 | 11.40 |
| Sex (% female) 14M 15F | 51.70% | ||
| Study randomization | 13 | 16 | |
| 8 Females | 7 Females | ||
| Screening evaluations/baseline characteristics | Mean (SD) | ||
| WASI | N = 25 | ||
| FSIQc | 95.64 (17.28) | 99.58 (19.22) | 92.00 (15.13) |
| VIQc | 100.56 (20.40) | 104.67 (18.89) | 90.08(21.74) |
| PIQc | 92.48 (15.17) | 95.08 (18.35) | 96.77 (11.77) |
| BRIEFa | |||
| Behavioral regulation indexa | 57.72 (14.35) | 54.46 (11.66) | 60.38 (16.09) |
| Metacognitive Indexa | 62.96 (13.02) | 62.75 (11.94) | 63.13 (14.15) |
| Global executive compositea | 62.07 (13.19) | 60.83 (11.29) | 63.00 (14/75) |
| TEA‐CH score!b | 4.97 (1.80) | 4.69 (1.70) | 5.19 (1.90) |
| CANTAB Paired Associate Learning (PAL)d | −0.39 (1.17) | 0.06 (0.41) | −0.82 (1.48) |
| Conners’ CPT | |||
| Omissiond | 62.54 (17.04) | 58.24 (8.81) | 66.26 (21.49) |
| Commissiond | 55.77 (8.36) | 54.52 (10.59) | 56.86 (6.01) |
| Conners’ ADHD DSM‐IV scales | |||
| DSM‐IV‐inattentiond | 67.31 (12.29) | 67.23 (12.63) | 67.38 (12.42) |
| DSM‐IV hyperactivityd | 69.17 (15.48) | 67.23 (16.41) | 70.75 (15.04) |
T‐score (mean = 50 ± 10).
Age scaled score (mean = 10 ± 3).
Composite scores (mean = 100 ± 15).
Z‐score (mean = 0 ± 1).
The computerized task required 10–12 min to complete and was easily accomplished after completion of all other neurocognitive tasks from the parent study. All participants were able to use the game controller successfully and were able to follow the necessary instructions. Only one child was unable to complete the task. The same computerized task was used across sites and scores did not differ across sites.
Outcome variables of the Arena Maze, such as Dwell Time NW Quadrant Trial 9, and Path Length Trials 3 & 8, did not correlate with other measures of spatial skills or spatial learning, nor with demographic variables. We did not find relationships between the Arena Maze outcome measures and assessments of attention and executive function at baseline.
There were also no significant differences between the lovastatin and placebo groups after treatment. For a number of tests, the changes in the lovastatin group trended in an unexpected direction. Generally, changes on the arena tasks were small after treatment. Path lengths did not significantly change (P = 0.92 for Trial 3 and P = 0.18 for Trial 9). The latencies for Trials 3 and 8 were also not significant (P = 0.17 and 0.052, respectively). The improvement in latency on Trial 8 was estimated at 11.1 sec reducing the time by slightly over 30% in both treatment groups and overall. Dwell time in the NW quadrant was also not statistically improved (P = 0.23) and the differences were in an unexpected slower direction for the lovastatin group (Table 2).
Table 2.
Spatial learning pre‐ and post‐lovastatin.
| Baseline | Week 16 | |||
|---|---|---|---|---|
| Lovastatin | Placebo | Lovastatin | Placebo | |
| Spatial tasks | ||||
| Cantab Spatial Working Memory SWM Z‐score (mean = 0 ± 1) | −0.8 (0.7) | −1.2 (0.7) | −0.4 (1.2) | −0.9 (0.8) |
| Cantab Paired Associate Learning (PAL) Z‐score (mean = 0 ± 1) | 0.06 (0.4) | −0.8 (1.5) | −0.4 (1.3) | −0.3 (1.0) |
| Judgment of Line Orientation (JLO, Z‐score (mean = 0 ± 1)) | −1.6 (1.3) | −1.4 (1.5) | −1.6 (1.6) | −1.4 (1.3) |
| Arena task | ||||
| Path length/trial accuracy Trial 3 | 380.6 (268.2) | 439.9 (254.7) | 518.9 (494.7) | 340.8 (418.2) |
| Path length/trial accuracy Trial 8 | 304.7 (426.7) | 306.1 (342.9) | 261.6 (430.3) | 230.7 (437.0) |
| Latency/time to target Trial 3 (s) | 50.3 (42.4) | 55.4 (37.0) | 49.3 (42.6) | 30.8 (31.9) |
| Latency/time to target Trial 8 (s) | 35.0 (43.1) | 34.3 (36.9) | 26.0 (34.4) | 21.5 (35.0) |
| Dwell time NW quadrant trial 9 (s) | 60.4 (27.7) | 51.0 (26.3) | 56.9 (28.2) | 64.1 (25.3) |
Discussion
Visual spatial deficits are so common in children with NF1 that some investigators have suggested that the presence of this deficit can be used to classify patients.3 Spatial learning deficits in the mouse NF1 model are best characterized by the Morris Water Maze, a task which until recently had no comparable human equivalent. This study demonstrates the feasibility of a virtual task analogous to the Morris Water Maze task for assessing spatial learning deficits in a group of children with NF1. As a measure of visual spatial learning, the computerized Arena Maze has several advantages and potential for novel applications. The task is portable, works with a desktop or laptop computer, and allows for retesting in the same environment. The task was feasible and required approximately 10 min to complete. All but one participant completed the task.
In a pilot study, performance on the Arena Maze was compared in children with NF1 to their unaffected siblings; children with NF1 were able to learn the task and navigate the virtual environment but performed more poorly on standard measures of spatial learning. In the pilot study, we were able to demonstrate a correlation between measures of working memory/executive function and the Arena Maze variables. In the current study, however, we did not find a relationship between Arena Maze performance and other measures of visual spatial skills, visual learning, attention, or executive function.6 There was some improvement seen in latency on one trial over the course of the study for the whole group, but there was no evidence of treatment response.
Given that the parent study did not demonstrate efficacy of lovastatin, it is perhaps not surprising that we did not find a difference in spatial learning using the Arena Maze. Potential explanations for the lack of correlation with traditional measures and the lack of difference after lovastatin may include that our paradigm was not sufficiently complex or challenging for our cohort of participants. Children have much more experience and facility with video games and a simple spatial task may not be sensitive to their visual spatial learning deficits. In addition, participants in the parent study completed “standard” measures of spatial learning and were required to demonstrate below average scores on either a measure of spatial learning or a measure of auditory attention.5 The majority of participants in the ancillary study, however, qualified for the clinical trial on the basis of low performance on the measure of auditory attention (Score!), not visual memory/spatial learning (PAL). Lastly, the placebo group trended lower on baseline measures and IQ compared to the lovastatin group.
Spatial learning deficits may still be an important target for cognitive interventions in children with NF1. Consequently, a paradigm to assess visual spatial learning is needed. This study using the Arena Maze represents an effort to bridge the gap between the mouse model and human clinical trials by testing a treatment endpoint that can be used in humans and employing a paradigm that evaluates functions analogous to those known to be impaired in Nf1 mouse models. Performance in virtual environments, such as the one used for this study, is thought to transfer readily to real‐world contexts.
Conflict of Interest
The authors report no conflict of interest associated with this study.
Author Contributions
Conception and design of the work: N.J.U. and C.R.C. Material preparation, data collection, and analysis: N.J.U., K.W., J.P., C.R.C., G.C. First draft: N.J.U. Revising work critically for important intellectual content: All authors. Final approval of manuscript: All authors.
Acknowledgments
This pilot study was funded through a Clinical Research Award (N.J.U) through the Children’s Tumor Foundation. The primary clinical trial was supported by the United States Army Medical Research and Materiel Command, Office of the Congressionally Directed Medical Research Programs, Department of Defense Neurofibromatosis Research Program, Grant Number W81XWH‐05‐1‐0615. We thank the children and parents who participated in this study.
Funding Information
This pilot study was funded through a Clinical Research Award (N.J.U) through the Children’s Tumor Foundation. The primary clinical trial was supported by the United States Army Medical Research and Materiel Command, Office of the Congressionally Directed Medical Research Programs, Department of Defense Neurofibromatosis Research Program, Grant Number W81XWH‐05‐1‐0615.
Funding Statement
This work was funded by Children’s Tumor Foundation grant ; United States Army Medical Research and Materiel Command grant ; Office of the Congressionally Directed Medical Research Programs grant ; Department of Defense Neurofibromatosis Research Program grant W81XWH‐05‐1‐0615.
References
- 1. Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor‐prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 2010;152A:327–332. [DOI] [PubMed] [Google Scholar]
- 2. Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 2005;65:1037–1044. [DOI] [PubMed] [Google Scholar]
- 3. Payne JM, Barton B, Shores EA, North KN. Paired associate learning in children with neurofibromatosis type 1: implications for clinical trials. J Neurol 2013;260:214–220. [DOI] [PubMed] [Google Scholar]
- 4. Li W, Cui Y, Kushner SA, et al. The HMG‐CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol 2005;15:1961–1967. [DOI] [PubMed] [Google Scholar]
- 5. Payne JM, Barton B, Ullrich NJ, et al. Randomized placebo‐controlled study of lovastatin in children with neurofibromatosis type 1. Neurology 2016;87:2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ullrich NJ, Ayr L, Leaffer E, et al. Pilot study of a novel computerized task to assess spatial learning in children and adolescents with neurofibromatosis type 1. J Child Neurol 2010;25:1195–1202. [DOI] [PubMed] [Google Scholar]


