Abstract
Background:
Conceptualizing cognitive aging as a step-sequential process is useful in identifying particular stages of cognitive function and impairment.
Objectives:
We applied Latent Transition Analysis (LTA) to determine i) whether the underlying structure of cognitive profiles found at every measurement occasion are uniform across three waves of assessment, ii) whether class-instability is predictive of distal outcomes, and iii) whether class-reversions from impaired to non-impaired using latent modelling is lower than when using clinical criteria of MCI.
Methods:
A mover-stayer LTA model with dementia as a distal outcome was specified to model transitions of ten neuropsychological measures over three annual waves in the Rush Memory and Aging Project (n = 1,661). The predictive validity of the mover-stayer status for incident Alzheimer’s dementia (AD) was then assessed.
Results:
We identified a five-class model across the three time-points: Mixed-Domain Impairment, Memory-Specific Impairment, Frontal Impairment, Average and Superior Cognition. None of the individuals in the Impairment classes reverted to the Average or Superior classes. Conventional MCI classification identified 26.4% and 14.1% at Times 1 and 2 as false-positive cases. “Movers” had 87% increased risk of developing dementia compared to those classified as “Stayers”.
Conclusion:
Our findings support the use of latent variable modelling that incorporates comprehensive neuropsychological assessment to identify and classify cognitive impairment.
Keywords: Alzheimer’s dementia, latent transition analysis, cognitive status, cognitive profiles, cognitive heterogeneity, individual differences, neuropsychological profiles, dementia
Introduction
Individuals differ in their cognitive abilities [1], and although individuals who typically perform well on one task also perform well on other tasks at cross-section and over time [1, 2], sometimes, they don’t follow this pattern [3, 4]. For this reason, it can be useful to consider cognitive aging as a stage-sequential process [5–7]. This conceptualization allows us to identify particular stages of cognitive impairment and distinguish amongst pathways of transitions across latent classes over time.
In previous work, we used latent class analysis (LCA) to characterize cognitive patterns of performance in two separate community-based samples [8, 9]. In both studies we identified a five-class model that characterized each sample: three cognitively impaired classes (Mixed-Domains Impairment, Memory-Specific Impairment, and Frontal Impairment) and two non-impaired classes (Average Cognition and Superior Cognition). In this study we were particularly interested to find out whether the same model holds over time. We, thus extended this cross-sectional approach to characterize transitions of cognitive classes over time, which we will refer to as statuses (see [10]), using latent transition analysis (LTA)[10, 11]. While LCA offers a taxonomic approach to classify and profile individual differences on any set of measures, LTA extends this framework to represent movement across statuses over time. Using LTA, we can build a latent variable model that represents the dynamic process of cognitive function and decline from one time point to another. This approach provides a unique opportunity to utilize the longitudinal structure of cognitive aging data to examine the relationship between cognitive function at baseline and heterogeneous patterns of cognitive performance as they unfold over time. Our rational is to evaluate our cross-sectional model, longitudinally to capture intra-individual changes on the classification of cognitive statuses. This is a novel approach that allows to test hypotheses about change, and probability of change, that is specific to particular subgroups. In other words, it allows to ask questions about the profile of the at-risk groups, and what they look like over time. It adds to the existing literature in that it
The major questions in this report are whether i) the underlying structure of cognitive profiles found at every measurement occasion are uniform across three waves of assessment, ii) whether class-instability is predictive of distal outcomes, and iii) whether class-transitions from impaired to non-impaired using latent modelling is lower than when using clinical criteria of MCI.
Materials and Methods
Participants.
Participants in the Rush Memory and Aging Project (MAP) are community-based older adults from about 40 retirement communities and senior subsidized housing facilities across northeastern Illinois. Older persons without known dementia consented for annual clinical evaluation, and signed an informed consent and an Anatomical Gift Act for organ donation at the time of death, and a repository consent that permitted data to be repurposed. The study was approved by the Institutional Review Boards of Rush University Medical Center and the Albert Einstein College of Medicine. Annual clinical evaluation includes detailed neuropsychological testing, a medical history, and neurologic examination. More detailed description of the study design can be found in previous reports [12, 13]. Cases with dementia at baseline were excluded in these analyses.
Latent Class/Status Indicators.
Ten neuropsychological measures representing a total of five cognitive domains were used:
Episodic memory: Total score of Logical Memory from the Wechsler Memory Scales – Revised [14] and Word List Recall [15];
Semantic memory: The CERAD short form [16] of the Boston Naming Test [17] and Category fluency [18];
Working memory: Digits (Forwards + Backwards) [19] and Digit Ordering [20];
Perceptual Orientation: Matrices [21] and Line Orientation[22, 23];
Perceptual Speed: Symbol Digits Modalities Test [24] and Number Composition [25].
To maintain consistency with the Rush Alzheimer’s Disease Center (RADC), we grouped cognitive measures in the domains presented in the RADC. We realize that some of these domains e.g. Perceptual Orientation are sometimes labelled as Executive function or Visuoperceptual spatial organization-planning. We also acknowledge that some measures may be grouped into different domains. However, for the sake of consistency with our previous work on Rush MAP [9, 26], and with the RADC classifications, we will maintain this terminology. Furthermore, as have other studies also illustrated, Working Memory, Perceptual Orientation (or otherwise referred to as Visuoperceptual spatial organization-planning, see: [27]), and Perceptual Speed, all tend to be referred to as Executive Functions, which essentially, includes working memory, cognitive flexibility, and attention-mental manipulation [27–29].
Mild Cognitive Impairment.
Participants who did not meet accepted criteria for dementia by the clinician but were judged to have cognitive impairment by the neuropsychologist were classified with MCI. Although the neuropsychological tests were used to guide clinical judgment in order to enhance uniformity of clinical decisions across examiners and over time and to reduce bias based on age, sex, or race, both the neuropsychologist’s and clinician’s decisions were the result of clinical judgment [30].
Alzheimer’s dementia (AD):
Diagnostic classification of Alzheimer’s and other dementias in MAP were made using a three-step process that includes algorithms and clinical judgment as described previously [12, 13, 30]. A dementia diagnosis required meaningful decline in cognitive function in addition to impairment in multiple areas of cognition. Alzheimer’s dementia required presence of dementia and loss of episodic memory based on criteria by the National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) [31].
Latent transition analysis.
LTA is a Markov model that estimates latent class membership at time t+1 conditional on time t i.e. the probabilities of transitioning form one latent class at time 1 to another latent class at time t+1. The supplementary material describes the mathematical model in more detail.
We followed Collins and Lanza’s [10] steps in carrying out this procedure:
LCA at each time-point. We utilized the ten neuropsychological measures at each wave to define the latent classes. We fit 2 – 7 measurement models for Times 1, 2, and 3, as a preliminary step in model selection. Given our previous results (five classes) in the same sample [9], we were interested to find out whether the same model holds over time, thus we ran models within this framework, as previously suggested [10, 32].
Test for measurement invariance. Measurement invariance across time was assessed by comparing a model with item-response probabilities freely estimated at each time-point to a model where the item-response probabilities were constrained to be equal at each time. The hypothesis of measurement invariance assumes that any observed class differences in latent class prevalence can be interpreted simply as quantitative, hence, some latent classes are larger, and remain larger than other classes over time.
Test the hypothesis of no change between times. This hypothesis assumes that latent status membership at Time 2 is the same as latent status membership at Time 1. We tested this model by fixing all transition probabilities to 0.
Add covariates. Age at Time 1, education, and gender were incorporated as covariates on the second-order latent variable (see next step).
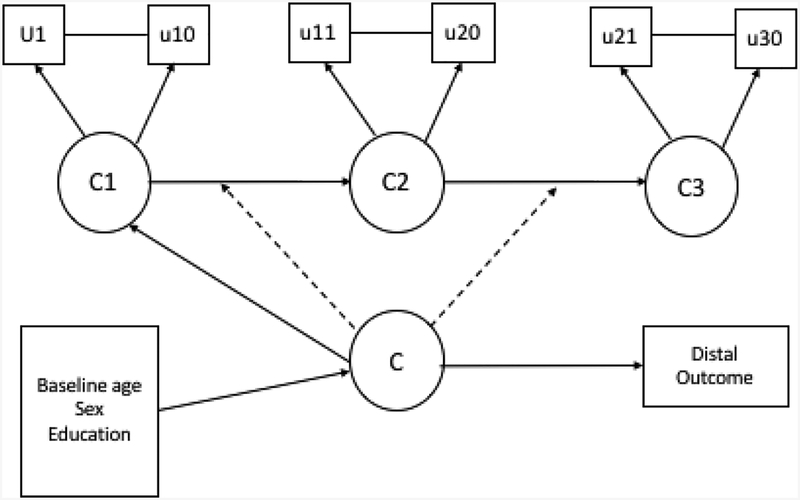
Add a distal outcome. We included all-cause dementia as a distal outcome in two different ways: dementia was related to the mover-stayer second-order latent variable to estimate proportion of stayers, and the related outcomes of dementia incidence/proportions to this second-order latent variable (Mover-Stayers). A mover-stayer LTA model with baseline age, sex, and education as covariates, and dementia as a distal outcome, and an absorbing state, was specified to model transitions across classes. A mover-stayer second order latent variable is a higher-order variable that captures unobserved heterogeneity in the transitioning probabilities. In the current application, the mover-stayer is of interest because it will identify participants who progress to impaired or more impaired classes throughout a two-year follow-up from baseline assessment. A mover is an individual who transitions at least once in or out of an impaired class, while a stayer is an individual who remains in his/her original class[33]. Figure 1 illustrates the model we fit.
Figure 1:
Mover-Stayer latent transition analysis model with baseline age, sex, and education as covariates, and dementia as a distal clinical outcome. In the model the same ten latent class indicators are measured at three time-points (u1-u10, u11-u20, u21-u30). The model assumes measurement invariance across time for the ten latent class indicators. C1, C2, and C3 indicate the latent classes at Times 1, 2, and 3. C is the higher-order mover-stayer latent variable. Time-invariant covariates and a distal outcome were related to the higher-order mover-stayer class.
In MPlus, maximum likelihood is used for model estimation, and estimates are obtained under missing at random assumptions. As a sensitivity analysis we carried out multiple imputation on five datasets under the not missing at random assumption using the same procedure as above.
We then used Cox survival analysis to model hazards of incident AD for i) the individuals who actually transitioned across classes over the three-year period, and ii) for those who were classified as ‘movers’ via the Mover-Stayer model.
Comparison to MCI.
As a final analysis, we compared reversion rates of cognitively impaired statuses to non-impaired over Times 2 and 3 of the latent variable transition model vs clinically-diagnosed MCI.
All latent class and latent transition models were fit using MPlus version 8 [34]. The rest of the analyses were done using SPSS version 25 [35].
Results
Descriptive analysis
Of the 1,924 participants at the time of these analyses, 79 individuals were excluded due to a baseline diagnosis of dementia, and 183 did not have a follow-up visit either because they were enrolled within the prior year, they dropped-out, or died. A total of 1,662 participants (75.4% female and 93.4% non-Hispanic White) were included in the study. All participants at baseline participated at Times 2 and 3. The mean age of the participants at baseline was 79.6 years (range = 53.3 – 100) and mean years in formal education was 14.8 (range = 0 – 28). The sample had a mean score of 28 (range = 18 – 30) on the MMSE and 7.9 (range = 0 – 10) on the NART. Mean performance on each of the 10 neuropsychological measures for Times 1, 2, and 3 is listed in Table 1. Test performance was stable over the three annual assessments.
Table 1.
Mean, standard deviation, and range of the neuropsychological measures of the Rush Memory and Aging Project at Times 1 – 3.
| Neuropsychological Performance | Time 1 (baseline) | Time 2 (follow-up 1) | Time 3 (follow-up 2) |
|---|---|---|---|
| Word List Recall | 5.3 (2.4, 0 – 10) | 5.4 (2.5, 0 – 10) | 5.4 (2.7, 0 – 10) |
| Logical Memory | 20.2 (8.5, 0 – 46) | 21.0 (8.9, 0 – 43) | 21.3 (8.9, 0 – 43) |
| Boston Naming Task | 13.9 (1.3, 0 – 15) | 13.9 (1.3, 4 – 15) | 13.9 (1.5, 4 – 15) |
| Categories | 34.1 (9.0, 6 – 69) | 33.7 (9.7, 6 – 69) | 33.1 (10.2, 2 – 73) |
| Digits Sum | 14.4 (3.5, 4 – 24) | 14.4 (3.5, 0 – 24) | 14.5 (3.5, 0 – 24) |
| Digits Ordering | 7.2 (1.6, 0 – 13) | 7.2 (1.6, 0 – 13) | 7.1 (1.8, 0 – 13) |
| Progressive Matrices | 11.7 (2.8, 0 – 16) | 11.7 (2.9, 2 – 16) | 11.6 (2.9, 1 – 16) |
| Line Orientation | 10.0 (3.1, 0 – 15) | 10.0 (3.2, 0 – 15) | 10.1 (3.3, 0 – 15) |
| Symbol Digits Modalities Test | 38.2 (10.6, 2 – 70) | 37.9 (11.2, 0 – 69) | 37.1 (11.9, 0 – 69) |
| Number Comparison | 24.4 (7.4, 0 – 46) | 24.3 (7.6, 0 – 48) | 24.0 (7.8, 0 – 46) |
Latent Class and Latent Transition Analyses
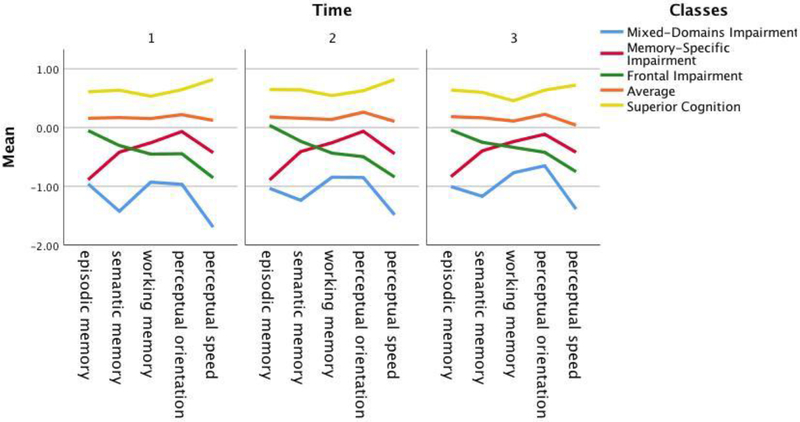
Fit statistics were compared across models (Table S1). Results showed that the structure of the five-class model and the correlations between each class and the neuropsychological measures were relatively stable over the three occasions of data in terms of class proportions (Table S2), neuropsychological performance at each time point (Table S3), and correlations between neuropsychological measures and the classes at each timepoint (Table S4). Figure S1 also shows the pattern of scores across the five cognitive domains for each class over three timepoints. We thus proceeded to test the five-class longitudinal transition model. When testing for measurement invariance, the models’ fit criteria (AIC = 244401 vs. 244456, BIC = 245441 vs. 244954, adjusted BIC = 244831 vs. 244662, entropy = 0.751 vs. 0.739) the BIC and adjusted-BIC indicated that there is no evidence that the class patterns differ across time, allowing us to impose the same measurement model over time. This implies that the nature and meaning of the latent statuses is held constant over two-years, hence there are no qualitative differences in the underlying structure of cognitive patterns of performance over time. We then tested for the hypothesis of no change between times. The model assuming change had better fit criteria than the model assuming no change (AIC = 239729, BIC = 240401, adjusted BIC = 240007, and ENT = 0.940 vs. AIC = 242141, BIC = 242639, adjusted BIC = 242347, and ENT = 0.839) thus we rejected the hypothesis of no change between times. The addition of baseline age, education, and gender on the baseline structure improved the model (AIC = 239180, BIC = 239917, adjusted BIC = 239485, ENT = 0.941). The final model, which included all-cause dementia as a distal outcome on the mover-stayer class, yielded AIC of 240968, BIC of 241693, adjusted-BIC of 241268, and entropy of 0.946. None of the classes had less the 5% of the overall population at any point in time. We proceed with describing the final model in more detail below.
The full set of parameter estimates from the five-status model of cognitive performance is presented in Table 2. The labelling of the statuses mirrors our previous work [8, 9]: Mixed-Domains Impairment, Memory-Specific Impairment, Frontal Impairment, Average and Superior Cognition.
Table 2.
Five-Latent-Status Model of Cognitive function displaying probabilities of transitioning from one latent status to another over three time-points, N = 1,662.
| Latent Status | |||||
|---|---|---|---|---|---|
| Mixed-Domains Impairment | Memory-Specific Impairment | Frontal Impairment | Average | Superior Cognition | |
| Latent Status Prevalence | |||||
| Time 1 (%) | 68 (4.1) | 249 (15.0) | 259 (15.6) | 665 (40.0) | 420 (25.4) |
| Time 2 (%) | 89 (5.4) | 247 (14.9) | 262 (15.8) | 631 (38.0) | 432 (26.0) |
| Time 3 (%) | 111 (6.7) | 238 (14.3) | 264 (15.9) | 617 (37.1) | 431 (25.9) |
| Probability of transitioning to… Conditional on… …..Time 1 latent status | …Time 2 latent status | ||||
| Mixed-Domains Impairment | 0.950 | 0.009 | 0.041 | 0.000 | 0.000 |
| Memory-Specific Impairment | 0.055 | 0.913 | 0.032 | 0.000 | 0.000 |
| Frontal Impairment | 0.041 | 0.000 | 0.959 | 0.000 | 0.000 |
| Average | 0.000 | 0.027 | 0.022 | 0.916 | 0.036 |
| Superior Cognition | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
| Probability of transitioning to… Conditional on… …Time 2 latent status | …Time 3 latent status | ||||
| Mixed-Domains Impairment | 0.977 | 0.000 | 0.023 | 0.000 | 0.000 |
| Memory-Specific Impairment | 0.055 | 0.946 | 0.000 | 0.000 | 0.000 |
| Frontal Impairment | 0.059 | 0.000 | 0.941 | 0.000 | 0.000 |
| Average | 0.001 | 0.000 | 0.040 | 0.959 | 0.000 |
| Superior Cognition | 0.000 | 0.002 | 0.000 | 0.000 | 0.998 |
| Mover-Stayer model | |||||
| Movers | 0.057 | 0.203 | 0.207 | 0.533 | 0.000 |
| Stayers | 0.000 | 0.000 | 0.000 | 0.054 | 0.946 |
Note. BIC = 241694.1, ENT = 0.946. Bolded diagonal transitions to facilitate interpretation.
The first panel of Table 2 provides the prevalence of each cognitive profile at Times 1, 2, and 3. Prevalence rates and patterns of the five cognitive profiles were fairly stable across time (Figure 2).
Figure 2.
Patterns of the five-class model across Times 1 – 3.
The second and third panels of Table 2 shows the transition probabilities, which confirm the stability of the underlying structure of cognitive performance across classes over a two-year time frame. These parameters reflect the probability of cognitive performance at Time 2 conditional on Time 1 performance, and cognitive performance at Time 3 conditional on Time 2 performance. Diagonal elements reflect the proportion of individuals with the same cognitive profile at all three time points.
The fourth panel of Table 2 shows the probability of movers and stayers within each latent status. The stability in cognitive performance was highest among the Superior Cognition class; individuals in that status at Time 1 had a probability of 1.000 of remaining in that latent status at Time 2, and at Time 2 they had a probability of 0.998 of remaining in the same status at Time 3.
There were no back-transitions from any of the impaired to the non-impaired classes in any of the transitions.
Sensitivity analysis using multiple imputation on 5 datasets generated similar results (average BIC and average entropy of all five generated results = 263823.3 and 0.954). There were also no back-transitions from any of the impaired to the non-impaired classes in the imputed data (Table S3).
The Mover-Stayer Model
The Mover-Stayer model identified 432 participants as “Movers” although over the course of two years only 98 individuals actually moved – 62 participants transitioned across classes between Time 1 and Time 2, while 37 participants moved between Time 2 and Time 3, and 1 participant moved twice. Those who transitioned were older at study enrollment (mean = 80,7 years SD = 8.1 vs. 79.5, SD = 7.4), and had fewer years of education (14.3 years, SD = 2.9 vs. 14.8, SD = 3.2); there were no sex differences between the groups.
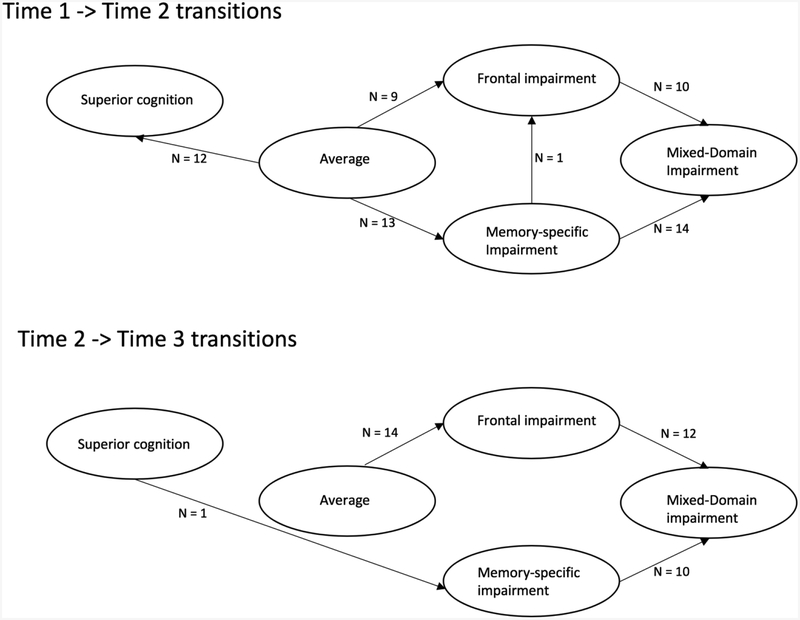
The majority of transitions (58.3% of transitions, n = 14) from enrollment to the first follow-up took place from the Memory-Specific Impairment Class to the Mixed-Domains Class. The other transitions that took place were from Frontal Impairment to Mixed-Domains Impairment (n=10) and vice-versa (n = 3), from Memory-Specific Impairment to Frontal Impairment (n =1), and from the Average Class to Memory-Specific (n =13), to Frontal Impairment (n = 9) or to Superior Class (n=12).
Nearly 60% of all transitions that took place from Time 2 to Time 3 were transitions from the Memory-Specific Impairment into the Mixed-Domains class (n = 10) and from the Frontal Impairment to the Mixed-Domains class (n = 12). The rest of the participants who moved were from the Average class to the Frontal Impairment (n =14), and from the Superior to the Memory-Specific Class (n = 1).
Figure 3 illustrates the movement across classes between Time 1 and Time 2, and between Time 2 and Time 3.
Figure 3:
A model illustrating movement across classes between times 1 and 2, and times 2 and 3.
Mover-Stayer model predictions.
We used Cox regression analysis to estimate the risk of developing all-cause dementia in the 90 participants who transitioned across classes. Controlling for age at baseline, sex, and education, Cox hazard’s survival model showed that individuals who transitioned had 79% risk of developing dementia compared to those who remained in the same classes: HR = 3.8, 95%CI = 2.9 – 5.1, p < 0.001). Figure 4 shows the cumulative incidence rates of dementia across individuals who transitioned vs those who did not.
Figure 4.
Cumulative incidence of developing all-cause dementia in individuals who transitioned across classes vs those who did not over three occasions of measurement.
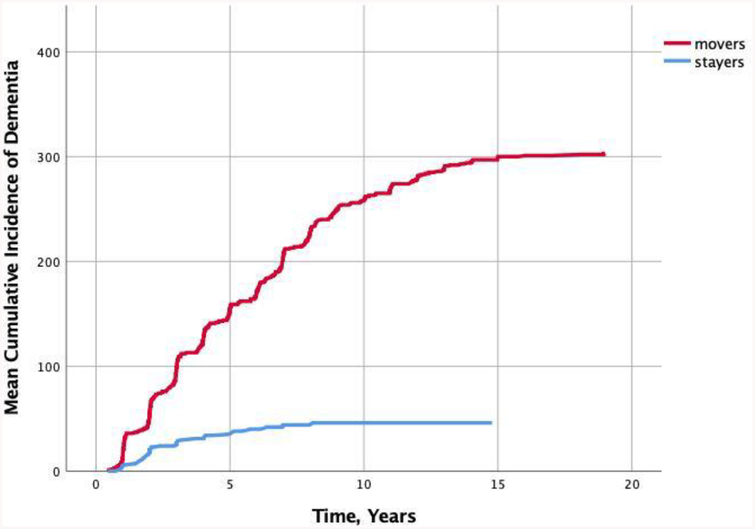
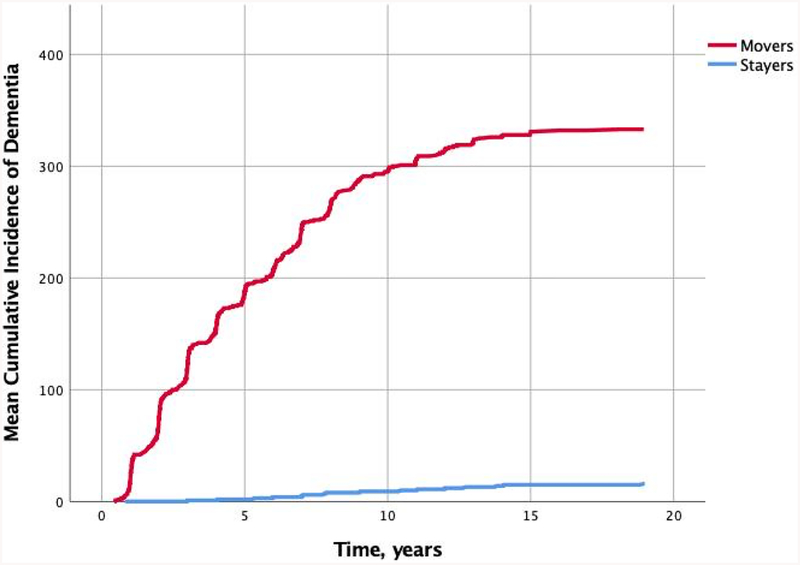
We then explored risk of ever converting to all-cause dementia among those identified as “Movers” by the Mover-Stayer model. Participants who were more likely to transition across statuses (n = 360), as identified by the Mover-Stayer model, had 86% higher risk of developing AD compared to those classified as ‘stayers’ (HR = 6.3, 95%CI = 4.0 – 10.0, p < 0.001) after controlling for age at baseline, sex, and education. Figure 5 shows the cumulative incidence rates of dementia until end of follow-up across participants classified as movers and those classified as stayers.
Figure 5.
Cumulative incidence of developing all-cause dementia in individuals who were identified as “Movers” vs those identified as “Stayers” in the Mover-Stayer model, over three occasions of measurement.
Comparison to MCI
In Time 1, there were 493 (27.1%) participants classified as MCI; by Time 2, 130 (26.4%) of those cases were classified as no cognitive impairment (NCI). In Time 2, 381 (21%) participants were classified as MCI, 55 cases (14.4%) were classified as NCI in Time 3.
Development of dementia: Out of the 493 classified as MCI at Time 1, 48 (9.7%) participants proceeded to dementia, 99% of which was AD, at Time 2, and another 35 (7.1%) proceeded to dementia at Time 3. Of the 381 participants classified as MCI in Time 2, 46 (9.3%) proceeded to dementia at Time 3, 95.7% of which was AD.
Out of the 376 participants classified as impaired (Mixed-Domains, Memory, or Frontal Impairment) in Time 1 by the five-class latent status model, 46 (12.2%), developed dementia, 99% of which was AD by Time 2. Of the 541 participants classified as impaired (Mixed-Domains, Memory, or Frontal Impairment) in Time 2, 57 (10.5%), 93.1% of which was AD, proceeded to dementia from Time 2 to Time 3.
Out of the 98 participants who transitioned across classes over the 2-year follow-up, 28 (28.5%) individuals developed dementia (8 individuals by Time 2 and another 20 by Time 3).
Discussion
In this study, latent transition analysis was used to explore whether meaningful transitions across latent statuses of cognitive performance could be identified at three measurement occasions in older adults. Results showed that the underlying latent class structure of cognition is stable over three occasions one year apart; that latent variable modelling outperformed clinical definitions of MCI in stability of impairment; and that ‘movers’ are more at risk of progressing to dementia.
The substantive contributions of these findings are threefold. First, the underlying structure of cognitive performance is relatively stable across two years: The majority of individuals have stable performance across this time scale, with most older adults showing stable performance across tests and over this time (i.e. the Superior Cognition, the Average and the Mixed-Domains subgroups showed dimensional patterns of performance), in line with theories of general cognitive aging and decline with age [2], and that subgroups with patterns of dispersion also exist and are also stable over this time scale (the Memory-Specific and the Frontal Impairment classes), in line with theories that address person-specific factors and individual differences [3, 4, 36]. The observed intra-individual heterogeneity across domains of cognitive function and over time is not consistent with the hypothesis that cognitive ability declines uniformly. Rather, results are more consistent with the accumulation of specific age-related conditions that can affect multiple cognitive systems, i.e. the disease model of aging [37]. Evidence for the existence of five subgroups over three occasions highlights the heterogeneity in cognitive aging and the stability of the underlying structure of cognitive performance across subgroups and over time. Our finding that a large segment of older adults were consistently best characterized as having stable average/superior performance indicates that most older adults maintain cognitive stability with age, consistent with previous studies [2].
Second, results showed that latent variable modelling classified stable cognitive impairment better than established definitions of MCI. Our longitudinal analyses showed that transitions across statuses mostly took place across the cognitive impairment classes, some individuals transitioned from a non-impaired to impaired status, but no transitions took place from impaired cognition to non-impaired cognition, hence there were no false-positives in our model, indicating the sensitivity of our model. Methodological arguments concerning clinical vs. actuarial predictions have been long-standing [38–40]. Research applying actuarial neuropsychological criteria has consistently fared better in making clinical predictions in general [41, 42] and in minimizing false-positive cases of cognitive impairment when compared to preestablished criteria of MCI [43–45]. This is the first study to our knowledge where the false-positive rate of the model applied was null.
Third, transitions to more impaired profiles reflected instability, and those transitions were associated with less favorable outcomes. Further, there is increasing consensus that employing larger neuropsychological test batteries that assess multiple cognitive domains is more useful in providing evidence of impaired performance in isolated cognitive domains [45, 46]. This was evident in the higher number of cases which converted from the impaired latent classes to dementia, in comparison to the MCI cases. Previously, heterogenous ability profiles characterized by intraindividual variability in cognitive performance on multiple tasks, have been dubbed vulnerable and indicative of pathological aging [47]. Over time, inconsistent performance across occasions i.e. scores on the same tests are not consistent, has been characterized as a marker of impending decline [3, 4, 36, 47–50]. Our results support and extend these findings in two ways: First, 73.5% of individuals who transitioned across statuses had a heterogeneous ability profile (Memory-Specific Impairment or Frontal-Impairment) or moved to one. Second, participants who moved were at a higher risk of progressing to dementia that those who did not transition. The implication of this finding is that within the scope of across-domain within-person variability, it is important to understand a spectrum of vulnerability traits that underlie asymmetric and unstable cognitive ability profiles that may span decades. Similarly, multivariate information across test variability has been suggested as a more sensitive indicator of cognitive status than single measures from one domain [51].
Conclusion
Our findings support the notion that the latent statuses are showing us something over and above the stage of illness. Since dispersion and inconsistency of cognitive performance seems to be stable over time, as is stability and cognitive endowment in the Superior Cognition class, we speculate that there may be underlying biological mechanisms at play, which the classes may be capturing. Better understanding on how environmental, behavioral, genetic, and disease processes are interrelated with person-specific profiles would help explain such dispersions and inconsistency in performance. This evidence can inform future prevention and intervention efforts, allowing for resources to be targeted to individuals who possess risk characteristics and vulnerability influences suggestive of poor outcomes.
Strengths and weaknesses
Several strengths of this study are notable. First, modelling multiple cognitive changes in a single model is of particular interest during developmental transitions such as from normative to non-normative cognitive decline. LTA provides a powerful tool to answer these questions and help pinpoint important targets for designing effective intervention programs. Second, multiple tests of cognitive function reflecting multiple domains of cognition were assessed, making it possible to investigate heterogeneous cognitive asymmetric profiles across domains. Third, three evenly-spaced observations made it possible to characterize patterns of change in individuals.
These findings also have important limitations. First, 93.4% of this sample was white; more diverse cohorts may provide different insights into the structure and stability of the classes as well as dispersion and inconsistency of cognitive performance, and risk and vulnerability factors. Second, more proximate cognitive evaluations may identify individuals displaying dispersion and inconsistency more efficiently than a typical annual routine, this would offer the opportunity for earlier characterization and possible intervention. Third, although we used dementia as a distal outcome, a number of participants developed dementia between Times 1 and 2, and Times 2 and 3. Fourth, the neuropsychological measures used in this cohort are different from measures applied in other cohorts, and although we have shown that the classes replicate well across two different cohorts [8, 9], this may not always be the case due to large differences in demographics and the nature and number of neuropsychological measures assessed. However, the facilitation of replication, co-ordinated analyses, and harmonization efforts help in identifying homogeneous profiles across multiple studies that may have more widespread applications [52]. We encourage future studies to engage in these efforts for validation. Fifth, although in this study we utilized three time-points, individual paths of cognition may continue to change as they approximate death as may the underlying cognitive structure; this was not accounted for here. Participants in the Memory and Aging Project have agreed to brain donation at the time of death, and we will have the opportunity to examine if /how class structure changes as participants approximate death, and explore the relation of disease pathology to person-specific performance. Future studies should also address the question on whether diverse cognitive profiles are related to rate of decline.
Supplementary Material
Acknowledgments:
The authors thank the many Illinois residents who have participated in the Rush Memory and Aging Project; Traci Colvin, MPH, for coordination of the clinical data collection; Karen Skish, MS, for coordination of the pathologic data collection; and John Gibbons, MS, and Greg Klein, MS, for data management.
Funding sources: This work was supported by the Memory and Aging Project (R01AG17917, R01AG343749, and R01AG42210) from the National Institute on Aging, the Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program, by the National Institute On Aging of the National Institutes of Health under Award Number K01AG054700, and by the Sylvia and Leonard Foundation. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts: All authors declare that there are no financial, personal, or other potential conflicts of interest.
References
- [1].Deary I (2000) Looking Down on Human Intelligence: From Psychometrics to the Brain, Oxford University Press UK. [Google Scholar]
- [2].Salthouse TA (1996) The processing-speed theory of adult age differences in cognition. Psychol Rev 103, 403–428. [DOI] [PubMed] [Google Scholar]
- [3].Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA (2002) Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 17, 179–193. [PubMed] [Google Scholar]
- [4].Hultsch DF, Dixon RA, MacDonald SWS (2002) Variability in Reaction Time Performance of Younger and Older Adults. The Journals of Gerontology: Series B 57, P101–P115. [DOI] [PubMed] [Google Scholar]
- [5].Gunstad J, Paul RH, Brickman AM, Cohen RA, Arns M, Roe D, Lawrence JJ, Gordon E (2006) Patterns of cognitive performance in middle-aged and older adults: A cluster analytic examination. J Geriatr Psychiatry Neurol 19, 59–64. [DOI] [PubMed] [Google Scholar]
- [6].Eppig JS, Edmonds EC, Campbell L, Sanderson-Cimino M, Delano-Wood L, Bondi MW (2017) Statistically Derived Subtypes and Associations with Cerebrospinal Fluid and Genetic Biomarkers in Mild Cognitive Impairment: A Latent Profile Analysis. J Int Neuropsychol Soc 23, 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ritchie K, Leibovici D, Ledesert B, Touchon J (1996) A typology of sub-clinical senescent cognitive disorder. Br J Psychiatry 168, 470–476. [DOI] [PubMed] [Google Scholar]
- [8].Zammit AR, Hall CB, Katz MJ, Muniz-Terrera G, Ezzati A, Bennett DA, Lipton RB (2018) Class-Specific Incidence of All-Cause Dementia and Alzheimer’s Disease: A Latent Class Approach. J Alzheimers Dis 66, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zammit AR, Muniz-Terrera G, Katz MJ, Hall CB, Ezzati A, Bennett DA, Lipton RB (2019) Subtypes Based on Neuropsychological Performance Predict Incident Dementia: Findings from the Rush Memory and Aging Project. J Alzheimers Dis 67, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Collins LM, Lanza ST (2010) Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences, John Wiley & Sons, Hoboken, New Jersey. [Google Scholar]
- [11].Hyatt SL, Collins LM (2000) Using latent transition analysis to examine the relationship between perceived parental permissiveness and the onset of substance use In Multivariate applications in substance use research: New methods for new questions. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, US, pp. 259–288. [Google Scholar]
- [12].Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS (2012) Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res 9, 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 64, S161–s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wechsler D (1987) Wechsler Memory Scale - Revised, The Psychological Corporation, San Antonio. [Google Scholar]
- [15].Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A (1994) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44, 609–614. [DOI] [PubMed] [Google Scholar]
- [16].Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39, 1159–1165. [DOI] [PubMed] [Google Scholar]
- [17].Kaplan EF, Goodglass H, Weintraub S (1983) The Boston Naming Test, Lea & Febiger, Philadelphia. [Google Scholar]
- [18].Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ (1992) Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol 49, 1253–1258. [DOI] [PubMed] [Google Scholar]
- [19].Wechsler D (1997) Adult Intelligence Scale-III, Psychological Corporation, San Antonio, TX. [Google Scholar]
- [20].Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV (1991) Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain 114 (Pt 5), 2095–2122. [DOI] [PubMed] [Google Scholar]
- [21].Raven J. Standard Progressive Matrices.
- [22].Benton AL, Varney NR, Hamsher KS (1978) Visuospatial judgment: A clinical test. Archives of Neurology 35, 364–367. [DOI] [PubMed] [Google Scholar]
- [23].Benton A, Hamshar KD, Varney N, Spreen O (1983) Contribution to Neuropsychological Assessment, Oxford University Press, New York. [Google Scholar]
- [24].Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, Wong MM, Zucker RA (2006) Normative Symbol Digit Modalities Test performance in a community-based sample. Archives of Clinical Neuropsychology 21, 23–28. [DOI] [PubMed] [Google Scholar]
- [25].Ekstom RB, French JW, Harman HH, Dermen D (1976) Kit of Factor-Referenced Cognitive Tests, Educational Testing Service, Prinston, NJ. [Google Scholar]
- [26].Zammit AR, Hall CB, Bennett DA, Ezzati A, Katz MJ, Muniz-Terrera G, Lipton RB (2019) Neuropsychological latent classes at enrollment and postmortem neuropathology. Alzheimers Dement 15, 1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lamar M, Zonderman AB, Resnick S (2002) Contribution of specific cognitive processes to executive functioning in an aging population. Neuropsychology 16, 156–162. [DOI] [PubMed] [Google Scholar]
- [28].Cristofori I, Cohen-Zimerman S, Grafman J (2019) Executive functions. Handb Clin Neurol 163, 197–219. [DOI] [PubMed] [Google Scholar]
- [29].Fuster J (1997) The Prefrontal Cortex Anatomy, Physiology and Neuropsychology of the Frontal Lobe.
- [30].Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J (2002) Natural history of mild cognitive impairment in older persons. Neurology 59, 198–205. [DOI] [PubMed] [Google Scholar]
- [31].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nylund KL (2007) Citeseer.
- [33].Nylund K (2007) University of California, Los Angeles.
- [34].Muthén LK, Muthén BO (1998–2016) Muthén & Muthén, Los Angeles, CA.
- [35].SPSS Inc. (Released 2016) IBM Corp., Armonk, NY.
- [36].Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA (1999) Change in Cognitive Function in Older Persons From a Community Population: Relation to Age and Alzheimer Disease. Archives of Neurology 56, 1274–1279. [DOI] [PubMed] [Google Scholar]
- [37].Weintraub S, Wicklund AH, Salmon DP (2012) The Neuropsychological Profile of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Meehl PE (1954) Clinical versus statistical prediction: A theoretical analysis and a review of the evidence.
- [39].Grove WM, Meehl PE (1996) Comparative efficiency of informal (subjective, impressionistic) and formal (mechanical, algorithmic) prediction procedures: The clinical–statistical controversy. Psychology, Public Policy, and Law 2, 293–323. [Google Scholar]
- [40].Westen D, Weinberger J (2004) When clinical description becomes statistical prediction. American Psychologist 59, 595–613. [DOI] [PubMed] [Google Scholar]
- [41].Grove WM, Zald DH, Lebow BS, Snitz BE, Nelson C (2000) Clinical versus mechanical prediction: A meta-analysis. Psychological Assessment 12, 19–30. [PubMed] [Google Scholar]
- [42].Ægisdóttir S, White MJ, Spengler PM, Maugherman AS, Anderson LA, Cook RS, Nichols CN, Lampropoulos GK, Walker BS, Cohen G, Rush JD (2006) The Meta-Analysis of Clinical Judgment Project: Fifty-Six Years of Accumulated Research on Clinical Versus Statistical Prediction. The Counseling Psychologist 34, 341–382. [Google Scholar]
- [43].Edmonds EC, McDonald CR, Marshall A, Thomas KR, Eppig J, Weigand AJ, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW (2019) Early versus late MCI: Improved MCI staging using a neuropsychological approach. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thomas KR, Eppig JS, Weigand AJ, Edmonds EC, Wong CG, Jak AJ, Delano-Wood L, Galasko DR, Salmon DP, Edland SD, Bondi MW (2019) Artificially low mild cognitive impairment to normal reversion rate in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wong CG, Thomas KR, Edmonds EC, Weigand AJ, Bangen KJ, Eppig JS, Jak AJ, Devine SA, Delano-Wood L, Libon DJ, Edland SD, Au R, Bondi MW (2018) Neuropsychological Criteria for Mild Cognitive Impairment in the Framingham Heart Study’s Old-Old. Dement Geriatr Cogn Disord 46, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP (2008) Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychology review 18, 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Salthouse TA, Soubelet A (2014) Heterogeneous ability profiles may be a unique indicator of impending cognitive decline. Neuropsychology 28, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Salmon DP, Bondi MW (2009) Neuropsychological assessment of dementia. Annu Rev Psychol 60, 257–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, Rodgers B (1999) An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a function of age. Psychol Aging 14, 365–379. [DOI] [PubMed] [Google Scholar]
- [50].Dixon R, Hertzog C, Friesen I, Hultsch DJNdini, adults na (1993) Assessment of intraindividual change in text recall of elderly adults. 77–101. [Google Scholar]
- [51].Kliegel M, Sliwinski M (2004) MMSE cross-domain variability predicts cognitive decline in centenarians. Gerontology 50, 39–43. [DOI] [PubMed] [Google Scholar]
- [52].Hofer SM, Piccinin AM (2009) Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychological methods 14, 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.