ABSTRACT
Background
Chest radiography is commonly used for diagnosing community-acquired pneumonia (CAP). Computed tomography (CT) is not routinely recommended for initial assessment of CAP patients but is more sensitive and more specific than chest radiography.
Objectives
To investigate characteristics of pneumonia with negative chest radiography in cases confirmed by CT.
Methods
We included patients diagnosed with CAP in the emergency department, and chest radiography and CT were performed and sputum cultures were collected. The CR- group was defined as patients for whom infiltration of pneumonia was detected only on CT. The CR+ group was defined as patients for whom infiltration was detected on both chest radiography and CT. Data were collected retrospectively from medical records.
Results
A total of 138 patients were included, with 58 patients in the CR- group and 80 patients in the CR+ group. Mean age was higher in the CR- group than in the CR+ group, and white blood cell counts and C-reactive protein (CRP) levels were lower in the CR- group than in the CR+ group (8.4 × 103/μL vs 12.4 × 103/μL, p = 0.01; 4.7 mg/dL vs 15.6 mg/dL, p < 0.001, respectively). Laterality of the infiltrated lungs differed between groups (right:left:bilateral = 14:30:14 vs 48:20:12, p = 0.006). Multivariate logistic analysis identified leukocytosis, elevated CRP levels (odds ratio (OR) 3.57, p = 0.003), laterality (OR 2.16, p = 0.006) as predictors of pneumonia in the CR- group.
Conclusion
In pneumonia with negative chest radiography in cases confirmed by CT, milder inflammation and infiltration in the left lung tended to be seen.
KEYWORDS: Community-acquired pneumonia, radiography, computed tomography, infiltration
1. Introduction
Pneumonia is one of the most common infections in all age groups, and the mortality of pneumonia is steadily rising, with the disease now ranked the third most common cause of death [1]. The diagnosis of pneumonia is based on clinical symptoms, physical findings, laboratory data and radiological findings consistent with pulmonary infection. Chest radiography has been the primary radiographic test used to evaluate community-acquired pneumonia (CAP) [2,3]. On the other hand, use of chest computed tomography (CT) to evaluate patients with acute respiratory symptoms has markedly increased as the clinical practice has evolved to more thoroughly image the lungs for signs of pneumonia [4]. CT is more sensitive than chest radiography for identifying radiological signs of pneumonia, resulting in some patients showing pneumonia on CT, but not on concurrent chest radiographs, even though CT has not been recommended for the initial assessment of CAP patients [5,6]. In previous studies, 7–21% of pneumonia cases with a clinical diagnosis of CAP showed negative chest radiographs on presentation [7,8]. However, little information has been available regarding the association between sensitivity of chest radiography and clinical and microbiological factors of CAP.
The aims of this study were to investigate differences in sensitivity to infiltration for plain chest radiography and chest CT of patients with CAP and to investigate clinical and microbiological characteristics of pneumonia with negative chest radiography in cases confirmed by CT.
2. Methods
2.1. Patients
We retrospectively included adult suspected cases of CAP in the emergency department of our hospital between April 2010 and April 2015 in this study. Suspected CAP was defined as acute illness associated with fever (temperature >37.5 °C) and cough, plus the presence of a new infiltrate in chest radiography or in CT. Among the CAP suspected cases, we selected clinically suspected CAP cases by the exclusion of other sites infection or non-infectious pulmonary diseases (Table 1). For radiographic and microbiologic investigation, we confirmed CAP cases in which both chest radiography and CT were performed, and pathogens were identified from sputum cultures collected on admission (Table 1). The need for informed consent was waived due to the retrospective nature of the study.
Table 1.
The criteria for confirming community-acquired pneumonia.
| A. inclusion criteria* |
| 1. fever (temperature >37.5 °C) and cough |
| 2. examinations of both plain chest radiography and chest CT on admission |
| 3. psesence of infiltration in chest CT |
| 4. collection of sputum on admission |
| 5. isolation of bacteria from sputum culture with compatible Gram stain findings |
| B. exclusion criteria# |
| 1. non-infectious pulmonary diseases (e.g. interstitial pneumonia or pulmonary embolism) |
| 2. infection of any other sites |
| 3. chronic heart failure |
| 4. aspiration pneumonia (physician-diagnosed or suspected) |
| 5. active mycobacterial infection (tuberculosis or non-tuberculous mycobacterial infection) |
| 6. pulmonary mycosis |
| 7. immunosuppressed status (e.g. current use of ≥ 10 mg/day of prednisone or other immunosuppressive agents, active treatment for cancer, or hematological malignancy) |
*A case that meets all the inclusion criteria is included. #A case that meets any of the exclusion criteria is excluded.
2.2. Methods of measurement and data collection
Interpretations of plain chest radiographs and chest CT by the attending pulmonologist and radiologist were recorded. Patients were then divided into two groups (Figure 1): those whose infiltrates were present on CT but absent or inclusive on chest radiography (CR- group; Figure 2(a, c)); and those whose infiltrates were present on both chest radiography and CT (CR+ group; Figure 2(b, d)). Infiltration of pneumonia detectable only on CT was considered to be represented by any of the following conditions: no infiltration apparent on chest radiography, but infiltration apparent on CT, or differences in infiltration sites between chest radiography and chest CT. Clinical background and medical laboratory data were collected from medical records.
Figure 1.
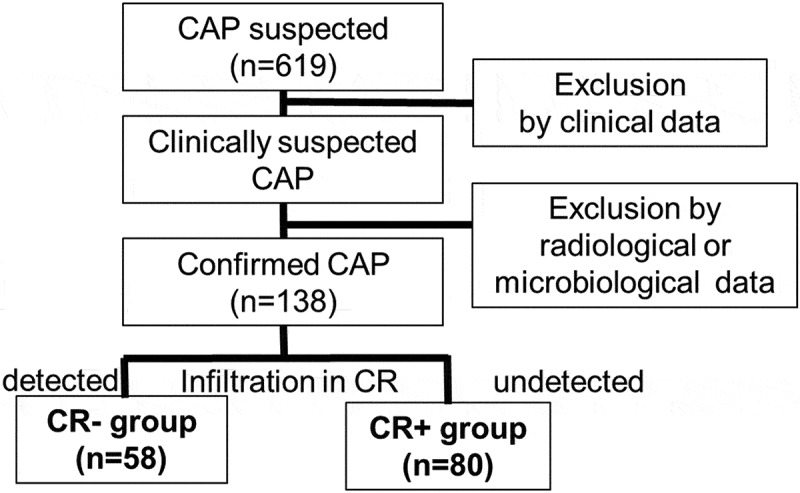
Enrollment of community-acquired pneumonia (CAP) patients and division into two groups.
Figure 2.
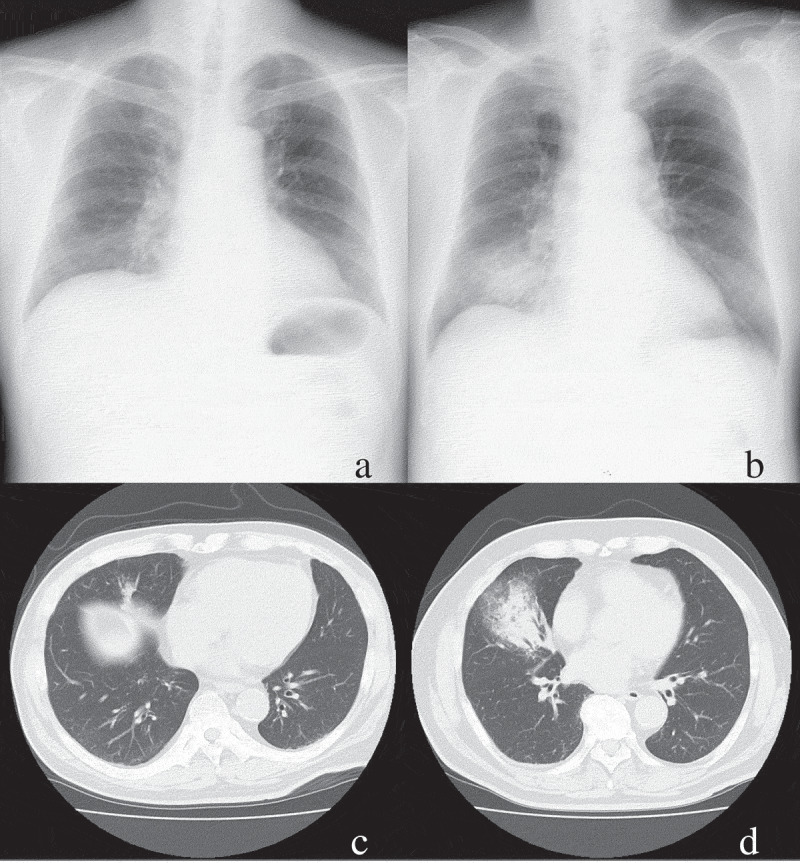
(a, c) Images from plain chest radiography (a) and CT (c) for a patient with lung infiltration detected only on CT. (b, d) Images from plain chest radiography (b) and CT (d) for a patient with lung infiltration detected only on CT.
2.3. Statistical analysis
Categorical and continuous variables were analyzed using Fisher’s exact test and Student’s t-test, respectively. All tests were two-sided, with a value of P < 0.05 considered statistically significant. Multivariate analysis was performed using binary logistic regression analysis to allow for adjustment of confounding factors. Data were statistically analyzed using PASW Statistics version 17.0.2 software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Clinical characteristics of patients with pneumonia
A total of 138 patients who were diagnosed clinically, radiographically and microbiologically were included in this study. Table 2 shows the clinical characteristics of patients with CAP. There were 58 patients in the CR- group and 80 patients in the CR+ group. Mean age was higher in the CR- group than in the CR+ group. No significant difference in body mass index (BMI) or smoking history was seen between groups. Mean white blood cell counts and C-reactive protein levels were lower in the CR- group than in the CR+ group (8.4 × 103/µL vs 12.4 × 103/µL, p = 0.01; 4.7 mg/dL vs 15.6 mg/dL, p < 0.001, respectively). As for laterality of pneumonia infiltration, patients in the CR- group showed infiltration in the left lung more frequently than those in the CR+ group (right:left:bilateral = 14:30:14 vs 48:20:12, p = 0.006).
Table 2.
Comparison of clinical factors between patients with infiltration only on CT and patients with infiltration on both CR and CT.
| Clinical factors | CR- group (n = 58) | CR+ group (n = 80) | p-value |
|---|---|---|---|
| Age (years) (mean ± SD, range) | 78.1 ± 13.9 (36–101) | 72.0 ± 16.3 (18–94) | 0.03 |
| Sex (male, %) | 70 (83) | 56 (70) | 0.68 |
| BMI (kg/m2) (mean ± SD, range) | 22.3 ± 4.5 (14.3–32.8) | 21.3 ± 3.9 (15.0–34.3) | 0.20 |
| Obesity (BMI ≥25 kg/m2) | 16 (28) | 13 (16) | 0.07 |
| Smoking history (%) | 35 (60) | 47 (59) | 0.85 |
| WBC (×103/μL) (mean ± SD, range) | 8.82 ± 3.43 (3.0–19.4) | 12.64 ± 5.85 (1.7–34.6) | <0.001 |
| CRP (mg/dL) (mean ± SD, range) | 6.51 ± 6.08 (0.10–20.62) | 11.50 ± 8.40 (0.23–34.69) | <0.001 |
| Laterality (right:left:bilateral) | 14:30:14 | 48:20:12 | <0.001 |
CT, computed tomography; CR, plain chest radiography; BMI, body mass index; WBC, white blood cells, CRP, C-reactive protein
3.2. Comparison of pneumonia pathogens in CR- and CR+ groups
We compared pathogens from sputum cultures between the CR- and CR+ groups (Table 3). Among the detected bacteria, the most frequent bacterium in the CR- group was Viridans streptococci, and the most frequent bacterium in the CR+ group was Streptococcus pneumoniae. However, no significant differences in the frequencies of pathogens were apparent between the two groups overall.
Table 3.
Comparison of cultured microorganisms between cases with infiltration only on CT and cases with infiltration on CR and CT.
| Bacterial species | CR- group (n = 58) | CR+ group (n = 80) | Total (n = 138) | p-value |
|---|---|---|---|---|
| Gram-positive bacteria | 31 (53.4) | 42(52.5) | 73 (52.9) | 0.91 |
| Corynebacterium species | 0 (0.0) | 1 (1.3) | 1 (0.7) | 1.00 |
| Group A Streptococcus | 0 (0.0) | 1 (1.3) | 1 (0.7) | 1.00 |
| Group B Streptococcus | 1 (1.7) | 0 (0.0) | 1 (0.7) | 0.42 |
| Group C Streptococcus | 1 (1.7) | 1 (1.3) | 2 (1.4) | 1.00 |
| Group F Streptococcus | 1 (1.7) | 3 (3.8) | 4 (2.9) | 0.64 |
| Group G Streptococcus | 1 (1.7) | 0 (0.0) | 1 (0.7) | 0.42 |
| Staphylococcus aureus | 3 (5.2) | 5 (6.3) | 8 (5.8) | 1.00 |
| Streptococcus pneumoniae | 7 (12.1) | 19 (23.8) | 26 (18.8) | 0.12 |
| Viridans streptococci | 17 (29.3) | 12 (15.0) | 29 (21.0) | 0.06 |
| Gram-negative bacteria | 27 (46.2) | 38 (47.5) | 65 (47.1) | 0.91 |
| Acinetobacter baumannii | 1 (1.7) | 0 (0.0) | 1 (0.7) | 0.42 |
| Branhamella catarrhalis | 4 (6.9) | 8 (10.0) | 12 (8.7) | 0.76 |
| Eikenella species | 0 (0.0) | 1 (1.3) | 1 (0.7) | 1.00 |
| Enterobacter aerogenes | 0 (0.0) | 1 (1.3) | 1 (0.7) | 1.00 |
| Enterobacter cloacae | 1 (1.7) | 1 (1.3) | 2 (1.4) | 1.00 |
| Escherichia coli | 3 (5.2) | 0 (0.0) | 3 (2.2) | 0.07 |
| Haemophilus influenzae | 3 (5.2) | 13 (16.3) | 16 (11.5) | 0.06 |
| Haemophilus parainfluenzae | 2 (3.4) | 2 (2.5) | 4 (2.9) | 1.00 |
| Klebsiella pneumoniae | 8 (13.8) | 10 (12.5) | 18 (13.0) | 0.27 |
| Neisseria species | 1 (1.7) | 0 (0.0) | 1 (0.7) | 0.42 |
| Pseudomonas aeruginosa | 2 (3.4) | 1 (1.3) | 3 (2.2) | 0.57 |
| Serratia marcescens | 2 (3.4) | 1 (1.3) | 3 (2.2) | 0.57 |
CT, computed tomography; CR, plain chest radiography
3.3. Identification of independent predictive factors for pneumonia in the CR- group
Based on clinical and microbiological results, multivariate analysis was performed on items that differed significantly between CR- and CR+ groups. Age was not identified as an independent risk factor for the CR- group (Table 4). However, significant differences were evident in white blood cell counts, CRP concentration, and laterality.
Table 4.
Logistic regression analysis of risk factors for infiltration only in CT patients.
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Age (>80 years) | 1.53 | 0.68–3.45 | 0.302 |
| WBC (<10 × 103/μL) | 5.90 | 2.47–14.08 | 0.001 |
| CRP (<5 mg/dL) | 3.57 | 1.56–8.20 | 0.003 |
| Laterality (left) | 2.16 | 1.25–3.72 | 0.006 |
WBC, white blood cells; CRP, C-reactive protein
4. Discussion
Chest radiography has been the primary radiographic test used to evaluate patients for CAP, although this modality has been unreliable in achieving a definitive diagnosis of pneumonia [9]. In our study, 42% of pneumonia cases were recognizable only in CT. In a previous study of adult CAP cases, among 26 confirmed cases, high-resolution CT identified 8 cases (30.8%) [10]. In a case series of 110 cases diagnosed with pneumonia by clinical follow-up, 9 cases were confirmed by CT alone, although 17 cases of nosocomial infections were included [11]. The reason for the high rate of CR- group allocation from the diagnosis of pneumonia in our study was unclear, but we speculated that in our study, the rate of patients for whom CT was performed among patients with suspected pneumonia might have been higher than in previous studies. Indications for CT may need to be re-evaluated, given the frequency of misdiagnosis of pneumonia from chest radiography.
Among the clinical factors we investigated and compared between CR- and CR+ groups, differences were seen in age, white blood cell counts, CRP, and laterality of the pneumonia lesion. In a population-based cohort study of adult patients admitted with suspected pneumonia, patients with unconfirmed pneumonia were older than those with radiography-confirmed pneumonia [8]. Conversely, in a multivariate study of hospitalized adult patients with CAP, patients with CT-only pneumonia were younger (median, 53 years) than patients showing pneumonia on chest radiography (median, 58 years; p < 0.01), a result contradicting our own findings [12]. Since the results of multivariate logistic analysis were not significant in our study, we speculated that old age as a clinical factor in the CR- group might have been confounded by body size or severity of inflammation. As for body composition, a study by Upchurch et al. reported obesity as significantly more common in patients with CT-only pneumonia than in those with pneumonia also appearing on chest radiography [12]. Obese patients are well known to frequently experience atelectasis, mainly in the lung bases [13]. CT-only pneumonia appeared more common among obese patients, perhaps due to lower attenuation from adipose tissue in obese patients. In the study by Upchurch et al., the rate of obesity was higher than in our study. Mean BMI for our study population was closer to that of the general population. We considered that obesity was not associated with the diagnostic value of chest radiography. Few studies have clarified the association between laterality of infiltrations and sensitivities of chest radiography. Among 26 patients with CAP diagnosed on CT in the setting of non-diagnostic chest radiography, infiltration was detected in the right lung in 9 patients, in the left lung in 4 patients, and in both lungs in 9 patients [9], although differences in detection rate between chest radiograph and CT were not analyzed.
In our study, the CR- group was associated with lower levels of serum inflammatory biomarkers. The study by Upchurch et al. reported that white blood cell counts did not differ significantly between the CT-only pneumonia group and the pneumonia on chest radiography group [12]. They found that serum procalcitonin concentrations were lower in the CT-only pneumonia group compared with the pneumonia on chest radiography group [12]. In a previous study of pediatric cases with suspected pneumonia, CRP level showed independent diagnostic value for pneumonia, although low levels did not exclude pneumonia [14]. In a systematic review of the diagnostic value of CRP in primary care and emergency departments in terms of ruling in or ruling out CAP, CRP appeared useful for ruling out a diagnosis of CAP [15]. From our results and those findings, we considered that in cases of suspected pneumonia with low CRP level, CT might be useful for diagnosing pneumonia.
Microbiological results of our study showed no significant differences in the frequency of pathogens between CR- and CR+ groups, but proportions of S. pneumoniae and Haemophilus influenzae were higher in the CR+ group, whereas Viridans streptococci were higher in the CR- group. In the study by Upchurch et al., prevalence of major CAP pathogens, including S. pneumoniae and H. influenzae were also similar between groups, although the rate of these pathogens was higher in the CR- group than in the CR+ group [12]. Based on our findings and the results of previous reports, we considered that in cases of pneumonia with neither CRP elevation nor leukocytosis, a relatively narrow-spectrum penicillin-based drug could be selected instead of a third-generation cephalosporin.
In advanced countries and middle-income countries, the frequency of CAP has recently been increasing, as has the proportion of elderly patients [16]. Currently, nearly all hospitalized adults with CAP are treated using antibiotics [17]. Consensus guidelines by the Infectious Diseases Society of America and the American Thoracic Society on CAP management recommend the use of broad-spectrum antibiotics for CAP inpatients requiring intensive care [18]. Early diagnosis of pneumonia using CT might lead to the early start of antibiotic administration and obviate the need for broad-spectrum antibiotics.
The present study was limited by both a small sample size and the retrospective design. Furthermore, the method of the study allowed us to determine the prevalence of negative CR studies only in the presence of a positive CT result for pneumonia. The prevalence of negative CT scan in the prevalence of negative CR study were not determined. Infiltration of pneumonia has also shown poor interobserver reliability [19]. This study excluded pneumonia cases in which sputum was not collected for microbiological investigation. In the previous study of CAP, sputum culture was obtained in a small number of patients (39.6%) [20]. The exclusion of cases of unobtained sputum culture in this study could affect the results of characteristics of both groups. For clarifying the precise characteristics of CT confirmed pneumonia, additional prospective research including pneumonia patients whose sputum were uncollected will be required.
In conclusion, infiltrations were detected on CT alone in 42% of CAP cases. In pneumonia with negative chest radiography in cases confirmed by CT, tendencies were seen for milder inflammation and infiltration into the left lung.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Ministry of Health LaW . Vital Statistics. 2018. Available from: https://www.mhlw.go.jp/english/database/db-hw/vs01.html
- [2].Wootton D, Feldman C.. The diagnosis of pneumonia requires a chest radiograph (x-ray)—yes, no or sometimes? Pneumonia. 2014. December 01;5(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Washington L, Palacio D.. Imaging of bacterial pulmonary infection in the immunocompetent patient. Semin Roentgenol. 2007. April;42(2):122–145. PubMed PMID: 17394925; eng. [DOI] [PubMed] [Google Scholar]
- [4].Larson DB, Johnson LW, Schnell BM, et al. National trends in CT use in the emergency department: 1995–2007. Radiology. 2011. January;258(1):164–173. PubMed PMID: 21115875; eng. [DOI] [PubMed] [Google Scholar]
- [5].Levy M, Dromer F, Brion N, et al. Community-acquired pneumonia. Importance of initial noninvasive bacteriologic and radiographic investigations. Chest. 1988. January;93(1):43–48. PubMed PMID: 3275531; eng. [DOI] [PubMed] [Google Scholar]
- [6].Beigelman-Aubry C, Godet C, Caumes E.. Lung infections: the radiologist’s perspective. Diagn Interv Imaging. 2012. June;93(6):431–440. PubMed PMID: 22658280; eng. [DOI] [PubMed] [Google Scholar]
- [7].Hagaman JT, Rouan GW, Shipley RT, et al. Admission chest radiograph lacks sensitivity in the diagnosis of community-acquired pneumonia. Am J Med Sci. 2009. April;337(4):236–240. PubMed PMID: 19365166; eng. [DOI] [PubMed] [Google Scholar]
- [8].Basi SK, Marrie TJ, Huang JQ, et al. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004. September 1;117(5):305–311. PubMed PMID: 15336579; eng. [DOI] [PubMed] [Google Scholar]
- [9].Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009. April;36(3):266–270. PubMed PMID: 18571356; eng. [DOI] [PubMed] [Google Scholar]
- [10].Syrjala H, Broas M, Suramo I, et al. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998. August;27(2):358–363. PubMed PMID: 9709887. [DOI] [PubMed] [Google Scholar]
- [11].Nagaoki K. [Usefulness of chest CT in diagnosing pneumonia]. Nihon Igaku Hoshasen Gakkai Zasshi. 1997. April;57(5):258–264. PubMed PMID: 9164115; jpn. [PubMed] [Google Scholar]
- [12].Upchurch CP, Grijalva CG, Wunderink RG, et al. Community-acquired pneumonia visualized on CT scans but not chest radiographs: pathogens, severity, and clinical outcomes. Chest. 2018. March;153(3):601–610. PubMed PMID: 28802696; PubMed Central PMCID: PMCPMC5989638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Imber DA, Pirrone M, Zhang C, et al. Respiratory management of perioperative obese patients. Respir Care. 2016. December;61(12):1681–1692. PubMed PMID: 27624632. [DOI] [PubMed] [Google Scholar]
- [14].Koster MJ, Broekhuizen BDL, Minnaard MC, et al. Diagnostic properties of C-reactive protein for detecting pneumonia in children. Respir Med. 2013. July 01;107(7):1087–1093. [DOI] [PubMed] [Google Scholar]
- [15].Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies [Review]. Fam Pract. 2009;26(1):10–21. [DOI] [PubMed] [Google Scholar]
- [16].Quan TP, Fawcett NJ, Wrightson JM, et al. Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998–2014. Thorax. 2016. June;71(6):535–542. PubMed PMID: 26888780; PubMed Central PMCID: PMCPMC4893127. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain S, Self WH, Wunderink RG. Community-acquired pneumonia requiring hospitalization. N Engl J Med. 2015. December 10;373(24):2382. 10.1056/NEJMc1511751. PubMed PMID: 26650159; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007. March 1;44(Suppl 2):S27–S72. PubMed PMID: 17278083; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boersma WG, Daniels JM, Lowenberg A, et al. Reliability of radiographic findings and the relation to etiologic agents in community-acquired pneumonia. Respir Med. 2006. May;100(5):926–932. PubMed PMID: 16337367; eng. [DOI] [PubMed] [Google Scholar]
- [20].Shariatzadeh MR, Marrie TJ. Does sputum culture affect the management and/or outcome of community-acquired pneumonia? East Mediterr Health J. 2009. Jul-Aug;15(4):792–799. PubMed PMID: 20187530; eng. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ministry of Health LaW . Vital Statistics. 2018. Available from: https://www.mhlw.go.jp/english/database/db-hw/vs01.html


