Abstract
Background
Vasoactive and neuroprotective drugs such as vinpocetine are used to treat stroke in some countries.
Objectives
To assess the effect of vinpocetine in acute ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched February 2007), MEDLINE (1966 to February 2007) and Scopus (1960 to February 2007). We also searched the Internet Stroke Center Stroke Trials Registry, Google Scholar, the science‐specific search engine Scirus and Wanfang Data, the leading information provider in China. We contacted researchers in the field and four pharmaceutical companies that manufacture vinpocetine. Searches were complete to February 2007.
Selection criteria
Unconfounded randomised trials of vinpocetine compared with placebo, or any other reference treatment, in people with acute ischaemic stroke. We included trials if treatment started no later than 14 days after stroke onset.
Data collection and analysis
Two review authors independently applied the inclusion criteria. One review author extracted the data, which was then checked by the second review author. We assessed trial quality. The primary outcome measure was death or dependency.
Main results
We included two trials, involving a total of 70 participants. Data for 63 participants were reported in the two trials combined. The rate of death or dependency did not differ between the treatment and placebo groups at one and three months. The 95% confidence intervals for the outcome measures were wide and included the possibility of both significant benefit and significant harm. No adverse effects were reported.
Authors' conclusions
There is not enough evidence to evaluate the effect of vinpocetine on survival or dependency in patients with acute ischaemic stroke.
Plain language summary
Vinpocetine for acute ischaemic stroke
Stroke is a life‐threatening event in which part of the brain does not receive enough oxygen, usually because of a blood clot blocking an artery in the brain. Stroke is the third leading cause of death and is an important cause of long‐term disability. Vinpocetine is a synthetic drug that is based on a herbal vinca alkaloid; it may protect nerves by increasing blood flow in the brain. Randomised placebo‐controlled studies have reported improved cognitive function after vinpocetine administration to people with long‐term brain circulation disorders. Vinpocetine is also used in people with stroke, mostly in East European and Asian countries. This review set out to determine whether giving vinpocetine in the first two weeks after onset of stroke symptoms decreased the number of people who died or became dependent on others for care and activities of daily living. The review authors searched the medical literature but found only two controlled studies including 70 participants. There was no significant difference in the rate of death and dependency at one and three months between the treatment and placebo groups. No adverse effects were reported. This review did not provide any evidence that vinpocetine benefits patients with acute ischaemic stroke.
Background
Stroke is the third leading cause of death and is probably the most important cause of long‐term disability in most western nations (Bonita 1992). Although stroke mortality has declined in most countries of Western Europe this was not the case in Eastern European countries between 1970 and 1985; the yearly increase in stroke mortality was the highest in Hungary among 27 countries studied (Bonita 1990). After a maximum in 1994, mortality has been declining in Eastern European countries as well (Kesteloot 2006). For secondary stroke prevention recommendations for antiplatelet therapy and indications for carotid endarterectomy have been established (APT 1994; Moore 1995; Sacco 2006). In acute stroke recombinant tissue plasminogen activator (rtPA) (Wardlaw 2003) and aspirin (Sandercock 2003) are effective but there is no other medical or surgical therapy that can be uniformly recommended for routine use in each patient with acute ischaemic stroke, and all neuroprotectants tested to date failed in clinical trials (Broderick 2002; EUSI 2003; Adams 2007).
Vinpocetine, a vasoactive vinca alkaloid that is a synthetic derivative of apovincamine, has been listed among neuroprotectants even in recent reviews (Rose 2002; O'Collins 2006). The neuroprotectant activity of vinpocetine is attributed to three molecular mechanisms of action of the substance. First, the neuroprotectant as well as the anticonvulsant effect of vinpocetine may be due to the blockage of sodium channels (Lakics 1995; Molnár 1995; Tretter 1998; Bönöczk 2000; Zhou 2003; Sitges 2007). Second, vinpocetine reduces calcium‐influx into neuronal cells (Zelles 2001). Third, the antioxidant activity of vinpocetine may also contribute to its neuroprotectant effect (Pereira 2000; Santos 2000; Horvath 2002; Mendoza 2007).
In animal experiments vinpocetine was reported to protect against cell death in cell cultures of rat cerebral cortex (Erdö 1990) and in striatal slices of rat brain (Kiss 1991), and had cytoprotective activity and prevented apoptosis in hypoxia (Gabryel 2002). Vinpocetine had calcium antagonist activity in in vitro models of cerebral ischaemia (Lamar 1988), increased the neuroprotective effect of adenosine in hypoxia in cell cultures (Krieglstein 1991), decreased neuronal cell loss in a rat model of forebrain ischaemia (Sauer 1988), and prevented post ischaemic increase in glucose utilisation and decrease in local blood flow (Rischke 1990b) in the hippocampus of rats. Vinpocetine was reported to decrease the size of cerebral infarction after middle cerebral artery occlusion in rats (Rischke 1990a) and in mice (Backhauss 1992). Vinpocetine was found to be effective in restoring neuronal plasticity after toxic injury (Medina 2006).
In human observational studies vinpocetine was reported to increase cerebral blood flow in previously ischaemic cerebral regions (Tamaki 1985), to decrease platelet aggregability (Itoh 1982) in patients after transient ischaemic attack (TIA) or stroke, and to increase erythrocyte deformability in patients after ischaemic stroke (Hayakawa 1992). Based on 11C‐labelled vinpocetine positron emission tomographic studies, vinpocetine distributes mainly in the thalamus, the basal ganglia, and the visual cortex, both after oral and intravenous administration (Gulyas 2002a; Gulyas 2002b).
Application of vasoactive and neuroprotective drugs is one of the numerous methods used in some countries for the treatment of patients with acute ischaemic stroke. Open and blinded randomised placebo controlled studies reported improving blood flow and glucose metabolism after vinpocetine administration in perifocal regions (Szakall 1998; Szilagyi 2005) in patients after ischaemic stroke. In a double‐blind placebo‐controlled study vinpocetine was reported to increase cerebral perfusion and oxygen extraction (Bönöczk 2002). Cognitive functions (scored on the mini mental state questionnaire and clinical global impression) were reported to improve after vinpocetine administration in chronic cerebrovascular disorders (Manconi 1986; Balestreri 1987). A randomised, placebo‐controlled, double‐blind clinical trial found that vinpocetine prevented the worsening of attention in patients with multiple cerebral infarcts (Kemeny 2005).
Based on the results of pharmacological studies and data from animal experiments, vinpocetine was recommended for use (Kovács 1985) and has been administered to patients with stroke in several countries in Europe (e.g. Hungary, Poland, Germany, Russia) and in Asia (e.g. China, Japan).
Objectives
The objective of this review is to determine if vinpocetine treatment decreases the rate of early (within one month) and late (between three and six months) case fatality and dependency if administered within two weeks of ischaemic stroke onset.
Methods
Criteria for considering studies for this review
Types of studies
We attempted to identify all published and unpublished truly randomised unconfounded clinical trials that compared the effect of vinpocetine with control for acute ischaemic stroke when treatment starts no later than 14 days after stroke onset. We considered randomised comparisons between vinpocetine and other standard treatments confounded, whereas a comparison of vinpocetine plus standard treatment versus standard treatment alone was acceptable.
Types of participants
As the aim of this review is the evaluation of vinpocetine therapy in the acute phase of ischaemic stroke, we did not consider studies where participants were randomised after 14 days of stroke onset for inclusion in the review. Distinction between ischaemic and haemorrhagic stroke cannot be made unless results of computerised tomography (CT) scans are reported for all participants in the trials (examination of the cerebrospinal fluid cannot be accepted as a reliable method to exclude haemorrhage).
It is possible that vinpocetine has considerable effects on platelet and other haemostatic functions (Itoh 1982), so vinpocetine might have different effects in ischaemic and haemorrhagic strokes. Therefore, we planned two analyses for presumed and confirmed ischaemic stroke separately. In the first analysis we would include studies where CT was not routinely performed and participants with suspected cerebral haemorrhage were excluded on clinical signs or cerebrospinal fluid examination, and only in few cases by CT. We planned to do a second set of analyses on studies including participants with confirmed ischaemic stroke, therefore restricted to trials where all participants had a CT scan; that is, haemorrhage was reliably excluded before randomisation.
Types of interventions
We planned to include trials of either oral or intravenous treatment schedules in the review.
Types of outcome measures
The primary outcome of interest is death or dependency. The number of participants who are dead or dependent was estimated in the treatment groups at one month after stroke onset and at a later time point between three and six months. We planned to evaluate dependency in activities of daily living, in preference to a disability score (such as the Barthel scale), in the treatment groups. Secondary outcome measures were death at one and at three months, and adverse events. As far as safety parameters are concerned, we attempted to determine the rate of fatal and non‐fatal cerebral haemorrhages, the rate of pulmonary embolism, and the frequency of other fatal and non‐fatal adverse events in each treatment group.
Search methods for identification of studies
See: 'Specialized register' section in Cochrane Stroke Group
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Review Group Co‐ordinator in February 2007. In addition, we searched MEDLINE (1966 to February 2007) using the following search statement to include all known manufacturer code names and registered trade names for vinpocetine.
(vinpocetin$ or TCV‐3B or RGH‐4405 or CAS 42971‐09‐5 or Eusenium or Cavinton or Calan or Intelectol).tw.
We also searched Scopus (http://www.scopus.com) (1960 to February 2007), the Internet Stroke Center Stroke Trials Registry (www.strokecenter.org), Google Scholar (http://scholar.google.com), the science‐specific search engine Scirus (http://www.scirus.com) and Wanfang Data (www.wanfangdata.com), the leading information provider in China; we used the search terms 'vinpocetine and (stroke or cerebral)'. Searches were complete to February 2007.
In an effort to identify further published, unpublished and ongoing trials we contacted researchers who had participated in vinpocetine trials and four pharmaceutical companies that manufacture vinpocetine (Gedeon Richter Ltd, Budapest, Hungary; Takeda Chemical Industries Ltd, Osaka, Japan; Covex SA, Madrid, Spain; and Thiemann Arzneimittel, Waltrop, Germany). (Last contact February 2007). The main manufacturer of the drug (Gedeon Richter Ltd, Hungary) supplied English transcripts of papers on possibly relevant trials printed in Japanese. Dr L Mihálka, a neurologist, evaluated Russian and Ukrainian papers to decide if they were randomised trials or not. We used the English language abstracts of Chinese and Polish language reports to decide if the trial fulfilled the inclusion criteria and where possible sought the help of translators.
Data collection and analysis
Methods used to select trials for inclusion
Two review authors (DB and IF) independently selected the trials to be included in the review. We resolved disagreements by discussion.
Criteria and methods used to assess the methodological quality of the included trials
We extracted the following information from the included trials to assess methodological quality: (1) method of randomisation and blinding; (2) was CT performed?; (3) total number of patients randomised and the proportion lost to follow up; (4) was intention‐to‐treat analysis performed? We attempted to obtain missing data by corresponding with trialists.
Methods used to collect data from the included trials
Once we had reached agreement on which trials to include, one of the review authors (DB) extracted data from the trials and performed the data analysis. The other review author (IF) checked the accuracy of data extraction.
Methods used to synthesise the data
We used the Peto method to give odds ratios. We planned to check for heterogeneity by using the I‐squared test.
Subgroup analyses
We planned the principal analysis for all routes and doses; a second analysis would separate trials using the oral route from those using the intravenous route. We planned a third analysis to separate studies by dose: we would analyse trials separately if a daily dose of at least 40 mg was given.
Results
Description of studies
We excluded 16 studies from the review. We excluded three studies (Lipani 1984; Manconi 1986; Reneles 1986) because the participants were not only stroke patients. Lipani 1984 had six stroke patients in the total group of 44, and some, if not all, of these six were in the chronic phase of stroke. Manconi 1986 had 40 participants in 13 diagnostic groups. Of these participants, 20 had ischaemic stroke, but the term 'previously established cerebral thrombosis' most probably denoted patients long after their stroke. At the end of the report 'chronic cerebral dysfunction' was mentioned referring to the whole study population and data were not presented separately for those with previous stroke. In Reneles 1986, of the 49 participants enrolled, one died, one withdrew, and 17 were lost to follow up. The mean time from stroke onset to randomisation was 5.6 months. Of the 16 participants randomised within one month after stroke, no data were given for those randomised less than 14 days after stroke onset.
In one study (Levic 2001) the allocation of participants was unclear, the study was probably not randomised, it is not clear if treatment was started within two weeks of stroke onset, and the study evaluated the late neuropsychiatric consequences of stroke. Death and disability were not reported. One study (Wasilewski 1985) had to be excluded because it was assumed that the vinpocetine arm of the study was not truly randomly selected; we attempted to clarify this with the principal investigator, but no source data could be obtained. One study (Atarashi 1983) excluded patients less than one month after stroke onset. Three studies had to be excluded because they were confounded: vinpocetine was compared to aminophylline (Domzal 1986), to acupuncture (Zou 1990), or to another medication (venoruton) with unknown effect (Yi 2004). A Bulgarian study (Manchev 2003) had to be excluded as it turned out to be non‐randomised. A Russian study (Suslina 1999) was excluded because it was not randomised and it reported only laboratory parameters and no clinical outcomes. A Czech study reported only CT findings, and probably was non‐randomised (Kalvach 1988). Two Ukrainian studies had to be excluded as they turned out to be non‐randomised studies (Dzyak 2002; Hadjiev 2004). One study (Gliem 1988) included 23 participants with acute stroke. In this double‐blind study the method of allocation was not described and the study was excluded as no clinical outcomes were reported. In one study (Wang 2006) only patients with cerebral haemorrhage were included.
Two studies fulfilled the selection criteria for inclusion in the review; that is, they were unconfounded randomised studies in acute ischaemic stroke (Werner 1986; Feigin 2001). In Werner 1986 40 participants were randomised within 48 hours of stroke onset. The participants got either placebo or 40 mg of vinpocetine in a 200 ml dextran intravenous infusion for three weeks. Follow‐up examinations were performed weekly for three weeks. Patient inclusion was restricted to those with relatively mild clinical signs (not requiring hospitalisation, but may be dependent on help of others). Dependent participants were defined as those who could not be discharged at the end of the trial into their own care or that of the family. In Feigin 2001 30 participants were included within 72 hours of stroke onset, and all participants had a CT scan before inclusion. The participants got either low‐molecular‐weight dextran in 250 ml saline intravenously alone (in the control group) or in combination with 10 mg of vinpocetine for five to seven days followed by oral vinpocetine 10 mg three times a day. Follow up was performed at one and three months. Death, disability and dependency (the Barthel and the Rankin score), and the National Institutes of Health (NIH) scale score were the outcome measures at one and three months after stroke. Poor outcome was defined as being dead or having a Barthel index of less than 70 or a Rankin score of 3 to 5. There are no trials awaiting assessment and the review authors do not know of any ongoing trials.
Risk of bias in included studies
In the first randomised double‐blind controlled unconfounded study of vinpocetine in acute stroke (Werner 1986) the method of randomisation was not reported, CT was either not performed or the results not reported, the follow up was short and there was no real measurement of dependency. Outcome measures in seven of 40 participants were not reported as the participants were excluded prior to analysis due to protocol violation (these participants got concomitant medication that was not allowed during the study), and no intention‐to‐treat analysis was performed. The second study (Feigin 2001) was a single‐blind randomised trial (participants were unaware of the treatment assignment). The method of randomisation is described, all participants had CT before randomisation, clinically relevant outcome measures were reported and no participants were lost to follow up. The treatment groups were comparable for major prognostic factors.
Effects of interventions
Death or dependency
The primary outcome measure (death or dependency) was reported in both studies. We accepted the definitions and cut off values used in the studies when dichotomising the participants into dependent and independent. For early outcome (three to four weeks after stroke) data on 63 participants were available, and there was no significant difference between the treatment and the control groups if dependency was measured by the Rankin score (Peto odds ratio (OR) 0.44, 95% confidence interval (CI) 0.15 to 1.27) (Comparison 01.01) or disability/dependency by the Barthel score (Peto OR 0.52, 95% CI 0.11 to 2.54) (Comparison 01.02). Data on death or dependency at three months were available only in the study of Feigin 2001 for 30 participants, and there was no statistically significant difference between the treatment and control groups in the rate of death or dependency when dependency was measured by the Rankin score (Peto OR 0.34, 95% CI 0.06 to 1.79) (Comparison 01.04) or disability/dependency by the Barthel score (Peto OR 0.63, 95% CI 0.10 to 4.15) (Comparison 01.05).
Death
No participants died in the Werner 1986 study, whereas two out of 15 treated participants and one out of 15 controls died in the Feigin 2001 study by the end of follow up. Two of these deaths occurred within the first month in the vinpocetine group, and the third death occurred in the control group between one and three months after stroke. There was no statistically significant difference between the groups, and the confidence intervals for the Peto odds ratio were wide: at one month Peto OR 7.94, 95% CI 0.47 to 133.26 (Comparison 01.03) and at three months Peto OR 2.05, 95% CI 0.20 to 21.36 (Comparison 01.06).
Sensitivity analyses
As only two small studies were identified that fulfilled the inclusion criteria, we could not perform the planned sensitivity and subgroup analyses.
Safety parameters
No fatal or non‐fatal cerebral haemorrhages, no cases of pulmonary embolism, and no other fatal or non‐fatal adverse events occurred in either of the two studies.
Discussion
Only two small trials fulfilled the inclusion criteria for this review. The total number of randomised participants was small. In one of the studies, data for almost 20% of randomised participants were lost and the follow up was short (Werner 1986). For the primary outcome measure (death or dependency) as well as for case fatality, the confidence intervals are wide, and include both the possibility of significant benefit and significant harm. Due to the lack of sufficient data, currently no conclusion can be drawn for the use of vinpocetine in acute stroke.
Authors' conclusions
Implications for practice.
The two randomised, controlled, unconfounded studies of vinpocetine in acute ischaemic stroke had a small number of patients included. Presently there is not enough evidence to decide if the routine application of vinpocetine does or does not decrease case fatality and the proportion of dependent survivors in acute ischaemic stroke. Therefore, there is no evidence to support the routine administration of vinpocetine for all patients with acute ischaemic stroke.
Implications for research.
According to present standards of clinical research the clinical efficacy of vinpocetine in acute ischaemic stroke has not yet been properly evaluated. Based on in vitro studies and on animal experiments, as well as on human randomised studies, vinpocetine has effects that might be beneficial in acute stroke. To prove this hypothesis, placebo‐controlled unconfounded properly randomised clinical studies must be designed and performed. In these studies much larger sample sizes, the registration of early and late case fatality and of valid and reliable measures of dependency and disability would be needed to estimate the risks and benefits of this agent. Brain CT or magnetic resonance imaging (MRI) should be part of the study protocol, long‐term follow up (for example, three and six months) should be mandatory and intention‐to‐treat analysis should be performed.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 16 May 2007 | New search has been performed | The 'Background' section has been updated to include recent research results. One additional study was identified that fulfilled the inclusion criteria (Feigin 2001). This small, single‐blind randomised study included 30 patients; including it did not change the conclusions regarding the primary outcome measure (death and dependency). |
Acknowledgements
The infrastructure of the Department of Neurology, University Medical School of Debrecen supported the preparation of this review. The review authors thank the following manufacturers of vinpocetine for providing published and unpublished information for studies of vinpocetine: Gedeon Richter Ltd, Budapest, Hungary; Takeda Chemical Industries Ltd, Osaka, Japan; Covex SA, Madrid, Spain; and Thiemann Arzneimittel, Waltrop, Germany.
The authors also thank Gedeon Richter Ltd for providing English transcripts of papers originally published in Japanese, and for efforts to try to establish co‐operation between the review authors and principal investigators of the studies. The help of Dr Bálint Ernyey in collecting papers in Russian not available in Hungary and the activity of Dr László Mihálka and Dr You Hong in evaluating the methods of the Russian, Ukrainian and Chinese language studies is also appreciated.
Data and analyses
Comparison 1. Vinpocetine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency at one month: Rankin | 2 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.15, 1.27] |
| 2 Death or dependency at one month: Barthel | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.11, 2.54] |
| 3 Death at one month | 2 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.94 [0.47, 133.26] |
| 4 Death or dependency at three months: Rankin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.06, 1.79] |
| 5 Death or dependency at three months: Barthel | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.10, 4.15] |
| 6 Death at three months | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [0.20, 21.36] |
1.1. Analysis.
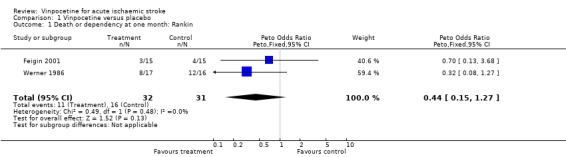
Comparison 1 Vinpocetine versus placebo, Outcome 1 Death or dependency at one month: Rankin.
1.2. Analysis.
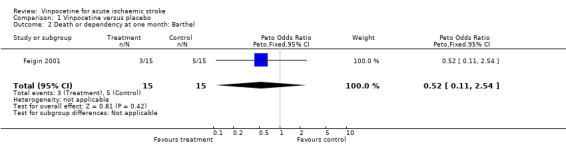
Comparison 1 Vinpocetine versus placebo, Outcome 2 Death or dependency at one month: Barthel.
1.3. Analysis.
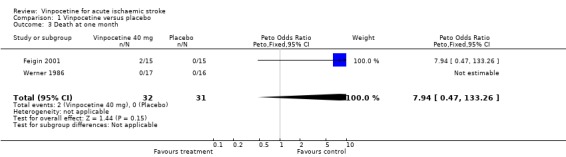
Comparison 1 Vinpocetine versus placebo, Outcome 3 Death at one month.
1.4. Analysis.
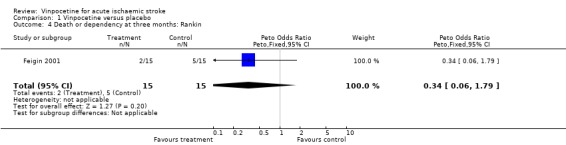
Comparison 1 Vinpocetine versus placebo, Outcome 4 Death or dependency at three months: Rankin.
1.5. Analysis.
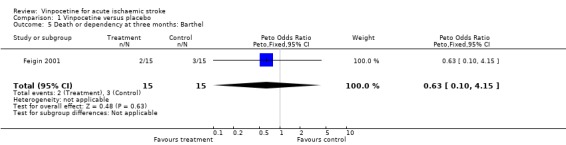
Comparison 1 Vinpocetine versus placebo, Outcome 5 Death or dependency at three months: Barthel.
1.6. Analysis.
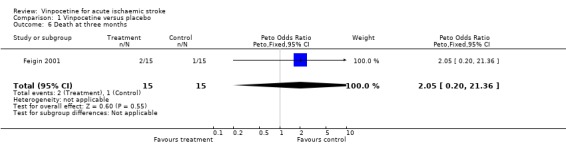
Comparison 1 Vinpocetine versus placebo, Outcome 6 Death at three months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Feigin 2001.
| Methods | Computer generated randomisation codes, sequentially numbered opaque sealed envelopes No participants lost to follow up | |
| Participants | Patients within 72 hours of CT‐verified acute ischaemic stroke | |
| Interventions | Low‐molecular‐weight dextran alone (3 g in 250 ml of isotonic saline) in the control group, or in combination with 10 mg iv vinpocetine for 5 to 7 days, followed by oral vinpocetine 3 x 10 mg in the treatment group | |
| Outcomes | Glasgow Coma Scale, NIH Stroke Scale, Barthel and Rankin scores at 1 and 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Werner 1986.
| Methods | Double‐blind, placebo‐controlled Method of randomisation not stated CT either not performed or not reported 7 out of 40 patients dropped from analysis after randomisation (protocol violators) Intention‐to‐treat analysis not performed | |
| Participants | Patients of either sex, no more than 48 hours after stroke onset, relatively mild clinical signs (no need for hospitalisation) The study was performed in Germany, the mean age of participants was 67.7 years (range: 48 to 82 years) | |
| Interventions | Vinpocetine 40 mg or placebo in 200 ml dextran iv infusion over one hour daily for 3 weeks | |
| Outcomes | Dependency on other than self or family, cognitive functions on mini mental state examination, self assessment by visual analogue scale, clinical global impression | |
| Notes | Independence defined as 'dischargable into their own care or that of the family' or 'able to live relatively independently in their home environments' No deaths within 3 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
CT: computerised tomography iv: intravenous NIH: National Institutes of Health
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atarashi 1983 | Patients with stroke within one month were excluded from the study |
| Domzal 1986 | Confounded study: vinpocetine (27 participants) was compared with aminophylline (30 participants) |
| Dzyak 2002 | Not a randomised trial |
| Gliem 1988 | The method of allocation was not described No clinical outcomes were reported |
| Hadjiev 2004 | Not a randomised trial |
| Kalvach 1988 | No clinical outcomes reported, and probably a non‐randomised trial |
| Levic 2001 | The study was probably not randomised, it is not clear if treatment was started within 2 weeks of stroke onset, and the study evaluated the late neuropsychiatric consequences of stroke Death and disability are not reported |
| Lipani 1984 | In addition to stroke patients, patients with other diagnostic categories (TIA, dementia, Huntington's chorea) also entered the study The number of participants with stroke was small (6 participants), and some, if not all, of these were in the chronic phase of stroke |
| Manchev 2003 | 253 patients treated with 10 to 20 mg vinpocetine for 5 to 30 days iv or 3 to 30 days per os and 40 controls Not a randomised study |
| Manconi 1986 | Patients with diagnostic categories other than stroke also entered the study The term 'previously established cerebral thrombosis' most probably denoted patients a long time after stroke and not patients with acute stroke At the end of the report ‘chronic cerebral dysfunction’ is mentioned referring to the whole study population and data are not presented separately for those with previous stroke |
| Reneles 1986 | Mixed patient group (TIA, acute and chronic stroke) The mean time from stroke onset to randomisation was 5.6 months Of the 16 participants randomised within one month after stroke no data were given for those randomised less than 14 days after stroke onset |
| Suslina 1999 | Non‐randomised study Lipid peroxidation was measured No clinical outcome |
| Wang 2006 | Study performed in acute hemorrhagic stroke Patients with ischaemic stroke were excluded |
| Wasilewski 1985 | There were 50 participants in both the placebo and cinnarizine groups whereas in the vinpocetine group there were only 32 This imbalance in sample size plus the term 'the patient groups were selected at random' suggests that randomisation, if performed at all, was improper We attempted to clarify this with the principal investigator, but no source data could be obtained |
| Yi 2004 | 83 patients were screened, 70 were randomised: 35 to vinpocetine and 35 to venoruton Therefore the study is confounded |
| Zou 1990 | Based on the MEDLINE abstract the study was confounded because vinpocetine was compared with acupuncture |
iv: intravenous per os: by mouth TIA: transient ischaemic attack
Contributions of authors
Dr Istvan Fekete: participated in data collection for the review, screened retrieved papers against inclusion criteria, appraised the quality of papers, screened data for published and unpublished studies, interpreted the data, and provided general advice on the review.
Dr Daniel Bereczki: participated in all phases of the protocol and review preparation.
Sources of support
Internal sources
University Medical School of Debrecen, Hungary.
External sources
Grant No. ETT 178/2006, Ministry of Health, Hungary.
Declarations of interest
One of the review authors (DB) was supported by an unrestricted travel grant for a Cochrane training course, and both review authors participated in clinical studies sponsored by one of the manufacturers of vinpocetine (Gedeon Richter Ltd, Budapest, Hungary). The company did not have any influence on selection of subject, or on the design, conduct, analysis and reporting of this systematic review.
Edited (no change to conclusions)
References
References to studies included in this review
Feigin 2001 {published data only}
- Feigin VL. Safety and efficacy of Kavinton in the treatment of acute ischemic insult. A randomised clinical trial [Bezopasnost i effektivnost kavintona v lechenii ostrova ishemicheskovo insulta. Randomizirovannoe klinicheskoe issledovanie]. Russki Medicinski Zhurnal 2000;8(15‐16):638‐42. [Google Scholar]
- Feigin VL, Doronin BM, Popova TF, Gribatcheva EV, Tchervov DV. Vinpocetine treatment in acute ischaemic stroke: a pilot single‐blind randomized clinical trial. European Journal of Neurology 2001;8:81‐5. [DOI] [PubMed] [Google Scholar]
Werner 1986 {published and unpublished data}
- Werner J. Final medical report on the testing of the efficacy and tolerance of vinpocetine after the intravenous application to outpatients suffering from apoplectic attack. RGD document numbers 36350/E; 34885/G 1986.
- Werner J, Apececha M, Schaltenbrand R, Fenzl E. Clinical study to evaluate the efficacy and tolerance of vinpocetine i.v. added to standard therapy in patients suffering from an acute apoplectic insult. In: Bés A editor(s). Senile Dementias: Early Detection. John Libbey Eurotext, 1986:636‐41. [Google Scholar]
- Werner JP, Lohaus R. Statistical evaluation of the tests of the efficacy and tolerance of the intravenous administration of vinpocetine as an additional therapy in the case of outpatients with an apoplectic shock in the context of a controlled double‐blind study. Thiemann Arzneimittel, Test No. 045.290 1984.
References to studies excluded from this review
Atarashi 1983 {published and unpublished data}
- Atarashi J, Araki G, Ito E, Otomo E, Omae T, Kuzuya F, et al. Usefulness of TCV‐3B tablets in the treatment of patients with cerebrovascular disorders ‐ a multicenter double‐blind clinical evaluation using ifenprodil tartarate tablets as control. Igakumo Ayumi 1983;124:66‐90. [Google Scholar]
Domzal 1986 {published data only}
- Domzal T, Kozlowski P, Zaleska B. Cavinton in the treatment of ischaemic brain stroke. Clinical and computer‐tomographic evaluation. Neurologia i Neurochirurgia Polska 1986;20(36):234‐40. [PubMed] [Google Scholar]
Dzyak 2002 {published data only}
- Dzyak LA, Golik VA, Rozhkova IV, Mizyakina EV. Efficacy of Cavinton in the treatment of cerebral ischemias, caused by the pathology of main arteries of the head. Ukrainskij Medichnij Chasopis 2002;6(32):39‐45. [Google Scholar]
Gliem 1988 {published data only}
- Gliem S, Mielke U. Rheoencephalographic examination of patients with acute stroke receiving Cavinton [Rheoenzephalographische untersuchungen bei patienten mit akutem zerebraleninsult unter Cavinton]. Zeitshrift für Klinische Medizin 1988;43(8):653‐5. [Google Scholar]
Hadjiev 2004 {published data only}
- Hadjiev D. Latent insufficiency of cerebral blood circulation and neuroprotective therapy as a primary prevention of ischemic stroke. Ukrainski Medichni Chasopis 2004;5(43):47‐53. [Google Scholar]
Kalvach 1988 {published data only}
- Kalvach P, Bauer J, Vymazal J. Computed tomography study of cerebral infarctions treated with vinpocetine. Drug Development Research 1988;14(3‐4):227‐30. [Google Scholar]
Levic 2001 {published data only}
- Levic Z, Ziklic M, Ocic GG, Diklic‐Jeremic VV, Dujmovic I. Efficacy and safety of Cavinton in the treatment of cerebrovascular diseases [Efikasnost i bezbednost Cavintona u tretiranju cerebrovaskularnih bolesti]. Aktuelnosti iz Neurologije, Psihijatrije i Granicnih Podrucja 2001;9(1‐2):1‐17. [Google Scholar]
Lipani 1984 {unpublished data only}
- Lipani G. A double‐blind parallel group placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular or degenerative senile cerebral dysfunction. Garibaldi Provincial General Hospital, Catania, Italy: Protocol 84‐175, 1984.
- Lipani G. Clinical/experimental evaluations of the therapeutic efficacy and tolerability of the medical preparation "Cavinton" of Ayerst Italiana S.p.A. Ayerst Report AX‐27, 255‐35, 1984.
Manchev 2003 {published data only}
- Manchev I, Tsolova M, Mancheva V. Cavinton (vinpocetine) treatment in acute ischaemic cerebrovascular disorders [Lechenie s kavinton (vinpocetine) pri ostri ischemichni narushenia na mozhchnot krovoobrashenie]. Bulgarian Medicine 2003;9(4):17‐21. [Google Scholar]
Manconi 1986 {published data only}
- Manconi E, Binaghi F, Pitzus F. A double‐blind clinical trial of vinpocetine in the treatment of cerebral insufficiency of vascular and degenerative origin. Current Therapeutic Research 1986;40:702‐9. [Google Scholar]
Reneles 1986 {unpublished data only}
- Reneles LD, Villadolid L, Florete OG. Cavinton in the treatment of thrombotic strokes. Ayerst International Central file #AX/27, 255/128, 1986.
Suslina 1999 {published data only}
- Suslina ZA, Fedorova TN, Kistenev BA, Khrapova EV, Maksimova MY. Dynamics of lipid peroxidation in patients with acute ischemic stroke. Zhurnal Nevrologii i Psichiatrii 1999;7:33‐6. [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang L, Li J, Zhang Y. A clinical observation of efficacy of vinpocetine injection on acute cerebral hemorrhage. Progress in Pharmaceutical Sciences 2006;30(12):563‐5. [Google Scholar]
Wasilewski 1985 {unpublished data only}
- Wasilewski R. Effect of Cavinton treatment on the dynamics of the clinical syndromes of acute and chronic cerebral vascular disturbances. Richter Gedeon document number: 32140, 1985.
Yi 2004 {published data only}
- Yi YX, Yang Y, Qu XB, Liu YL. Effect of vinpocetine on hemodynamics and neurologic impairment in senile patients with acute cerebral infarction. Zhongguo Linchuang Kangfu 2004;8(28):6122‐3. [Google Scholar]
Zou 1990 {published data only}
- Zou X, Wang D. Comparative study of cerebral infarction treated with acupuncture at 6 acupoints of yang meridian and calan. Chung Hsi I Chieh Ho Tsa Chih 1990;10:199‐202. [PubMed] [Google Scholar]
Additional references
Adams 2007
- Adams HP, Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for early management of adults with ischemic stroke. A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke 2007;38:1655‐711. [DOI] [PubMed] [Google Scholar]
APT 1994
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomized trials of antiplatelet therapy ‐ I. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81‐106. [PMC free article] [PubMed] [Google Scholar]
Backhauss 1992
- Backhauss C, Karkoutly C, Welsch M, Krieglstein J. A mouse model of focal cerebral ischemia for screening neuroprotective drug effects. Journal of Pharmacological Methods 1992;27:27‐32. [DOI] [PubMed] [Google Scholar]
Balestreri 1987
- Balestreri R, Fontana L, Astengo F. A double‐blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. Journal of the American Geriatric Society 1987;35:425‐30. [DOI] [PubMed] [Google Scholar]
Bonita 1990
- Bonita R, Stewart A, Beaglehole R. International trends in stroke mortality: 1970‐1985. Stroke 1990;21:989‐92. [DOI] [PubMed] [Google Scholar]
Bonita 1992
- Bonita R. Epidemiology of stroke. Lancet 1992;339:342‐4. [DOI] [PubMed] [Google Scholar]
Broderick 2002
- Broderick JP, Hacke W. Treatment of acute ischemic stroke. Part II: Neuroprotection and medical management. Circulation 2002;106:1736‐40. [DOI] [PubMed] [Google Scholar]
Bönöczk 2000
- Bönöczk P, Gulyas B, Adam‐Vizi V, Nemes A, Karpati E, Kiss B, et al. Role of sodium channel inhibition in neuroprotection: effect of vinpocetine. Brain Research Bulletin 2000;53(3):245‐54. [DOI] [PubMed] [Google Scholar]
Bönöczk 2002
- Bönöczk P, Panczel G, Nagy Z. Vinpocetine increases cerebral blood flow and oxygenation in stroke patients: a near infrared spectroscopy and transcranial Doppler study. European Journal of Ultrasound 2002;15:85‐91. [DOI] [PubMed] [Google Scholar]
Erdö 1990
- Erdö SL, Ning‐Sheng C, Wolff JR, Kiss B. Vinpocetine protects against excitotoxic cell death in primary cultures of rat cerebral cortex. European Journal of Pharmacology 1990;187:551‐3. [DOI] [PubMed] [Google Scholar]
EUSI 2003
- Hacke W, Kaste M, Bogusslavsky J, Brainin M, Chamorro A, Lees K, et al. European Stroke Initiative recommendations for stroke management ‐ update 2003. Cerebrovascular Diseases 2003;16(4):311‐37. [DOI] [PubMed] [Google Scholar]
Gabryel 2002
- Gabryel B, Adamek M, Pudelko A, Malecki A, Trzeciak HI. Piracetam and vinpocetin exert cytoprotective activity and prevent apoptosis of astrocytes in vitro in hypoxia and reoxygenation. Neurotoxicology 2002;23:19‐31. [DOI] [PubMed] [Google Scholar]
Gulyas 2002a
- Gulyas B, Halldin C, Sandell J, Karlsson P, Sovago J, Karpati E, et al. PET studies on the brain uptake and regional distribution of 11C‐vinpocetine in human subjects. Acta Neurologica Scandinavica 2002;106:325‐32. [DOI] [PubMed] [Google Scholar]
Gulyas 2002b
- Gulyas B, Halldin C, Sovago J, Sandell J, Cselenyi Z, Vas A, et al. Drug distribution in man: a positron emission tomography study after oral administration of the labelled neuroptrotective drug vinpocetine. European Journal of Nuclear Medicine and Molecular Imaging 2002;29:1031‐8. [DOI] [PubMed] [Google Scholar]
Hayakawa 1992
- Hayakawa M. Effect of vinpocetine on red blood cell deformability in stroke patients. Arzneimittel‐Forschung 1992;42:425‐7. [PubMed] [Google Scholar]
Horvath 2002
- Horvath B, Marton Z, Halmosi R, Alexy T, Szapary L, Vekasi J, et al. In vitro antioxidant properties of pentoxifylline, piracetam and vinpocetine. Clinical Neuropharmacology 2002;25:37‐42. [DOI] [PubMed] [Google Scholar]
Itoh 1982
- Itoh T. Effect of vinpocetine on platelet aggregation in patients with cerebrovascular diseases. Japanese Pharmacology and Therapeutics 1982;10:1481‐5. [Google Scholar]
Kemeny 2005
- Kemeny V, Molnar S, Andrejkovics M, Makai A, Csiba L. Acute and chronic effects of vinpocetine on cerebral hemodynamics and neuropsychological performance in multi‐infarct patients. Journal of Clinical Pharmacology 2005;45:1048‐54. [DOI] [PubMed] [Google Scholar]
Kesteloot 2006
- Kesteloot H, Sans S, Kromhout D. Dynamics of cardiovascular and all‐cause mortality in Western and Eastern Europe between 1970 and 2000. European Heart Journal 2006;27:107‐13. [DOI] [PubMed] [Google Scholar]
Kiss 1991
- Kiss B, Ning‐Sheng C, Erdo SL. Vinpocetine preferentially antagonizes quisqualate/AMPA receptor responses: evidence from release and ligand binding studies. European Journal of Pharmacology 1991;209:109‐12. [DOI] [PubMed] [Google Scholar]
Kovács 1985
- Kovács L. Cavinton in the treatment of acute stroke. Therapia Hungarica 1985;33:50‐7. [PubMed] [Google Scholar]
Krieglstein 1991
- Krieglstein J, Rischke R. Vinpocetine increases the neuroprotective effect of adenosine in vitro. European Journal of Pharmacology 1991;205:7‐10. [DOI] [PubMed] [Google Scholar]
Lakics 1995
- Lakics V, Sebestyen MG, Erdo SL. Vinpocetine is a highly potent neuroprotectant against veratradine induced cell death in primary cultures of rat cerebral cortex. Neuroscience Letters 1995;185:127‐30. [DOI] [PubMed] [Google Scholar]
Lamar 1988
- Lamar J‐C, Poignet H, Beaughard M, Dureng G. Calcium antagonist activity of vinpocetine and vincamine in several models of cerebral ischaemia. Drug Development Research 1988;13:297‐304. [Google Scholar]
Medina 2006
- Medina AE, Krahe TE, Ramoa AS. Restoration of neuronal plasticity by a phosphodiesterase type 2 inhibitor in a model of fetal alcohol exposure. Journal of Neuroscience 2006;26(3):1057‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendoza 2007
- Mendoza G, Alvarez AI, Pulido MM, Molna AJ, Merino G, Real R, et al. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydrate Research 2007;342:96‐102. [DOI] [PubMed] [Google Scholar]
Molnár 1995
- Molnár P, Erdo SL. Vinpocetine is as potent as phenytoin to block voltage‐gated Na channels in rat cortical neurons. European Journal of Pharmacology 1995;273:303‐6. [DOI] [PubMed] [Google Scholar]
Moore 1995
- Moore WS, Barnett HJM, Beebe HG, Bernstein EF, Brener BJ, Brott T, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke 1995;26:188‐201. [DOI] [PubMed] [Google Scholar]
O'Collins 2006
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, Worp BH, Howells DW. 1026 experimental treatments in acute stroke. Annals of Neurology 2006;59:467‐77. [DOI] [PubMed] [Google Scholar]
Pereira 2000
- Pereira C, Agostinho P, Oliveira CR. Vinpocetine attenuates the metabolic dysfunction induced by amyloid beta‐peptides in PC12 cells. Free Radical Research 2000;33:497‐506. [DOI] [PubMed] [Google Scholar]
Rischke 1990a
- Rischke R, Krieglstein J. Protective effects of vinpocetine against brain damage caused by focal or global cerebral ischemia. In: Krieglstein J, Oberpichler H editor(s). Pharmacology of Cerebral Ischemia. Stuttgart: Wissenschaftliche Verlagsgesellschaft, 1990:527‐32. [Google Scholar]
Rischke 1990b
- Rischke R, Krieglstein J. Effects of vinpocetine on local cerebral blood flow and glucose utilization seven days after forebrain ischaemia in the rat. Pharmacology 1990;41:153‐60. [DOI] [PubMed] [Google Scholar]
Rose 2002
- Rose SPR. 'Smart drugs': Do they work? Are they ethical? Will they be legal?. Nature Reviews Neuroscience 2002;3(12):975‐9. [DOI] [PubMed] [Google Scholar]
Sacco 2006
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention. Circulation 2006;113:e409‐49. [PubMed] [Google Scholar]
Sandercock 2003
- Sandercock P, Gubitz G, Foley P, Counsell C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2003, Issue 2. [Art. No.: CD000029. DOI: 10.1002/14651858. CD000029] [DOI] [PubMed] [Google Scholar]
Santos 2000
- Santos MS, Duarte AI, Moreira PI, Oliveira CR. Synaptosomal response to oxidative stress: effect of vinpocetine. Free Radical Research 2000;32(1):57‐66. [DOI] [PubMed] [Google Scholar]
Sauer 1988
- Sauer D, Rischke R, Beck T, Rossberg C, Mennel H‐D, Bielenberg GW, et al. Vinpocetine prevents ischemic cell damage in rat hippocampus. Life Sciences 1988;43:1733‐9. [DOI] [PubMed] [Google Scholar]
Sitges 2007
- Sitges M, Chiu LM, Guarneros, Nekrassov V. Effects of carbamazepine, phenytoin, lamotrigine, oxcarbazepine, topiramate and vinpocetine on Na(+) channel‐mediated release of 3H‐glutamate in hippocampal nerve endings. Neuropharmacology 2007;52:598‐605. [DOI] [PubMed] [Google Scholar]
Szakall 1998
- Szakall S, Boros I, Balkay L, Emri M, Fekete I, Kerenyi L, et al. Cerebral effects of a single dose of intravenous vinpocetine in chronic stroke patients: a PET study. Journal of Neuroimaging 1998;8(4):197‐204. [DOI] [PubMed] [Google Scholar]
Szilagyi 2005
- Szilagyi G, Nagy Z, Balkay L, Boros I, Emri M, Lehel S, et al. Effects of vinpocetine on redistribution of cerebral blood flow and glucose metabolism in chronic ischemic stroke patients: a PET study. Journal of the Neurological Sciences 2005;229‐30:219‐23. [DOI] [PubMed] [Google Scholar]
Tamaki 1985
- Tamaki N, Kusunoki T, Matsumoto S. The effect of vinpocetine on cerebral blood flow in patients with cerebrovascular disorders. Therapia Hungarica 1985;33:13‐21. [PubMed] [Google Scholar]
Tretter 1998
- Tretter L, Adam‐Vizi V. The neuroprotective drug vinpocetine prevents veratridine‐induced Na+i and Ca2+i rise in synaptosomes. Neuroreport 1998;9(8):1849‐53. [DOI] [PubMed] [Google Scholar]
Wardlaw 2003
- Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD000213. DOI: 10.1002/14651858. CD000213] [DOI] [PubMed] [Google Scholar]
Zelles 2001
- Zelles T, Franklin L, Koncz I, Lendvai B, Zsilla G. The nootropic drug vinpocetine inhibits veratradine‐induced Ca2+i increase in rat hippocampal CA1 pyramidal cells. Neurochemical Research 2001;26:1095‐100. [DOI] [PubMed] [Google Scholar]
Zhou 2003
- Zhou G, Dong XW, Crona J, Maguire M, Priestley T. Vinpocetine is a potent blocker of rat NaV 1.8 tetrodotoxin resistant sodium channels. Journal of Pharmacology and Experimental Therapeutics 2003;306:498‐504. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bereczki 1997
- Bereczki D, Fekete I. Vinpocetine for acute ischaemic stroke. Cochrane Database of Systematic Reviews 1997, Issue 4. [Art. No.: CD000480. DOI: 10.1002/14651858. CD000480] [DOI] [PubMed] [Google Scholar]
Bereczki 1999
- Bereczki D, Fekete I. A systematic review of vinpocetine therapy in acute ischaemic stroke. European Journal of Clinical Pharmacology 1999;55(5):349‐52. [DOI] [PubMed] [Google Scholar]


