Abstract
Objective: The etiology and outcome of status epilepticus with continuous epileptic spasms have not been fully understood; and only rare cases have been reported in the literature. Here, we described 11 children, who manifested continuous epileptic spasms with various etiologies and different outcomes.
Methods: This is a case series study designed to systematically review the charts, video-electroencephalography (video-EEG), magnetic resonance images, and longitudinal follow-up of patients who presented continuous epileptic spasms lasting more than 30 min.
Results: Median age at onset was 2 years old, ranging from 2 months to 5.6 years. The etiology of continuous epileptic spasms for these 11 cases consisted of not only some known electro-clinical epilepsy syndromes like West Syndrome and Ohtahara Syndrome, but also secondary symptomatic continuous epileptic spasms, caused by acute encephalitis or encephalopathy, which extends the etiological spectrum of continuous epileptic spasms. The most characteristic feature of these 11 cases was prolonged epileptic spasms, lasting for a median of 13.00 days (95% CI: 7.26–128.22 days). The interictal EEG findings typically manifested as hypsarrhythmia or its variants, including burst suppression. Hospital stays were much longer in acute symptomatic cases than in primary epileptic syndromic cases (59.67 ± 50.82 vs. 15.00 ± 1.41 days). However, the long-term outcomes were extremely poor in the patients with defined electro-clinical epilepsy syndromes, including severe motor and intellectual developmental deficits (follow-up of 4.94 ± 1.56 years), despite early diagnosis and treatment. Continuous epileptic spasms were refractory to corticosteroids, immuno-modulation or immunosuppressive therapies, and ketogenic diet.
Conclusion: Continuous epileptic spasms were associated with severe brain impairments in patients with electro-clinical syndromes; and required long hospital stays in patients with acute symptomatic causes. We suggest to include continuous epileptic spasms in the international classification of status epilepticus, as a special form. Further investigations are required to better recognize this condition, better understand the etiology, as well as to explore more effective treatments to improve outcomes.
Keywords: status epilepticus, epilepsy, continuous epileptic spasms, electroencephalograms, electro-clinical epilepsy syndrome, video-EEG
Introduction
Epileptic spasms were recognized as a type of seizure by the International League against Epilepsy (ILAE) seizure classification in 2001 (1). Spasms typically last 1–2 s and can occur in clusters or in isolation (2). The ictal electroencephalogram (EEG) usually shows high amplitude slow waves with brief spindle-like fast activity. One of the characteristic features of these clinical epileptic spasms is a brief rhomboid-shape appearance on the surface electromyography (EMG) of the deltoids. Epileptic spasms occur primarily in infants but may also occur in other age groups (3–5), even in adults (6). Epileptic spasms can be the main seizure type of some primary epilepsy syndromes (7, 8), such as infantile spasms, Ohtahara Syndrome, and West Syndrome. Epileptic spasms have also been described in Lennox-Gastaut Syndrome (9), and could be seen in some acute secondary conditions (10, 11). Although burst suppression on EEG is one of the features of Ohtahara Syndrome (12–14), and hypsarrhythmia and its variants are recognized as the characteristic interictal EEG patterns of West Syndrome (15–17), patients with epileptic spasms may not have classic hypsarrhythmia EEG patterns but rather show some periodic and focal patterns (9, 18–20), such as hemispheric hypsarrhythmia (21), or even a lack of hypsarrhythmia on EEG (19). The pathogenesis and pathophysiology of epileptic spasms are mostly unknown, and their treatment remains quite challenging (22, 23). The prognosis of epileptic spasms is generally devastating and is associated with poor neurodevelopmental and behavioral outcomes, particularly in some patients with an underlying genetic etiology such as in CDKL5 encephalopathy, a rare X-linked genetic disorder, and CDKL5 genetic changes or mutations have been found in children diagnosed with infantile spasms, Lennox-Gastaut Syndrome, Rett Syndrome, West Syndrome (12).
In 1986, Dr. David L. Coulter described one case of a 3 1/2 months old boy with continuous epileptic spasms in clusters upon awakening, which were only terminated upon the administration of lorazepam and paraldehyde (24). Dr. Coulter failed to identify the etiology of the continuous epileptic spasms for this patient, despite several targeted examinations and laboratory tests, but he first suggested that continuous spasm attacks lasting over 30 min should be considered as a form of status epilepticus. Only a few similar cases have been reported afterwards (14).
The most recent ILAE classification of status epilepticus in 2015 involves four axes: 1. seizure semiology; 2. etiology; 3. EEG correlates and 4. Age (25). In the current status epilepticus ILAE classification, seizure semiology is divided between non-convulsive status epilepticus and those with prominent motor manifestation, which include convulsive, myoclonic, focal motor, tonic, and hyperkinetic seizures (25). Seizure semiology has been shown to be critical to the prognosis of status epilepticus in general (25). The nature of the etiology could be symptomatic (e.g., stroke, encephalitis), remote (e.g., posttraumatic, postencephalitic), progressive (e.g., brain tumor, Lafora's disease), unknown or cryptogenic (25). However, continuous epileptic spasms are not specifically included in this international classification, most likely due to its rare occurrence and under-recognition. Here, we describe 11 cases who manifested continuous epileptic spasms with various etiologies and report their long-term follow-up outcomes. The purpose of this study is to increase awareness of such cases and their associated causes, as well as the need to identify effective intervention and treatments.
Methods
In this case series study, we systematically reviewed the available clinical data, including medical charts, video-EEG, magnetic resonance imaging (MRI) and other auxiliary tests in 11 patients, who were admitted to the Shenzhen Children's Hospital from 2014 to 2016, with continuous epileptic spasms lasting 30 min or longer. The study was approved by the Shenzhen Children's Hospital Committee of Medical Ethics. Each patient had at least one video-EEG recording, and each video-EEG included both a waking state and a sleep state of at least one sleep cycle. When epileptic spasms were identified, the duration of the spasms, suppression on EEG, and intervals between spasms were manually measured and calculated. The spasms within an interval of <1 min were defined as being in one cluster (2). The parents and nurses were taught to recognize long-lasting continuous spasms, so when the seizure manifestation appeared or changed, video-EEG was arranged to confirm the continuous spasms. The EEG recordings (Nicolet monitor system, international 10–20 system) were reviewed by at least two experienced pediatric neurologists who were trained and certified in pediatric EEG, and confirmed by a third expert in this field. The etiological causes of the patients' continuous epileptic spasms were defined according to the ILAE task force on classification proposal (26). Statistics regarding the variables of age, duration of epileptic spasms and follow-up periods were descriptive, and variables are presented as the mean ± standard deviation (SD). To compare the clinical variables when the diagnosis of status epileptics of spasms was established, a t-test was performed for each comparison and a P-value of < 0.05 was accepted as statistically significant.
Results
Between July 2014 and August 2016, video-EEGs were performed in 3,378 in-patients at the Shenzhen Children's Hospital. The duration of the recordings varied from a minimum of 2.5 h to 15.0 h, including overnight recordings (in 725 patients, 19.60% of recordings). We identified 11 cases with status epilepticus of continuous epileptic spasms, which lasted longer than 30 min. This result represented approximately 3 cases per 1,000 admitted patients for suspected seizures during this period.
The average age at onset of continuous epileptic spasms was 2.57 ± 2.22 years (ranging from 0.14 to 5.58 years, median 2.00 years, 95% CI: 1.08–4.06 years). The duration of the continuous epileptic spasms was 67.74 ± 90.02 days, ranging from 0.02 to 240.00 days, median time 13.00 days (95% CI: 7.26–128.22 days). All the patients also presented with altered consciousness and other types of seizures. The duration of follow-up was 4.94 ± 1.56 years (range: 3.33–9.00 years), with a median follow-up of 4.25 years (95% CI: 3.89–5.59 years). The age of onset was significantly older (at 4.28 ± 1.44 years) in the acute symptomatic cases (Cases 1 to 6, see in Table 1) compared to 0.53 ± 0.4 years in the non-acute non-symptomatic cases (Cases 7–11) (P = 0.0001).
Table 1.
Patients' clinical data and follow-up.
|
No. of case Age at investigation Sex Hospital stay |
Diagnosis | Etiology | Spasms' duration | Other seizure types | Brain MRI findings | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| Case 1 4 years Male 30 days |
Mycoplasma-pneumoniae-related encephalopathy | Mycoplasma pneumoniae infection | Lasting 13 days, burst suppression both in sleep and awake | Focal seizures, originated from right frontal or parietal areas | At Day 15: atrophy of both cerebrum and cerebellum | IVIG, MP, 8 AEDs, KD; at investigation: TPM, LTG, CZP, LEV | At 9 years, walking, but not speaking, could not recognize parents, short stature |
| Case 2 5 years and 2 months Female 37 days |
Influenza-virus-type-one-related acute encephalopathy | H1N1 infection | Lasting 6 months, burst suppression, gradually alleviated | Focal seizures, originated bilaterally | Generalized atrophy after decompressive craniectomy | IVIG, MP, KD, prednisone, 4 AEDs | At 5 years and 1 month, walking, mental retardation, no language |
| Case 3 5 years and 7 months Male 16 days |
Anti-NMDAR encephalitis | Autoimmune encephalitis | Lasting 8 months, burst suppression EEG | Focal seizures originated right hemisphere, convulsive SE | At 10 months: MRI showed atrophy of cerebrum, and hippocampi improved | IVIG, MP, 5 AEDs; at investigation: VPA, NZP, LTG, CBZ, KD, cyclophosphamide monthly 8 times | At 4 years, nearly recovered, autoantibody remains positive in CSF |
| Case 4 5 years and 7 months Male 105 days |
Febrile-infection-related epilepsy syndrome | Unknown etiology, Mycoplasma pneumoniae infection? | Lasting 87.5 min, asymmetrical spasm | Convulsive and non-convulsive SE, multi-focal, primarily frontal in origin | Brain atrophy including hippocampi improved during the last 3 months | IVIG, PE, KD, propofol, ketamine, chloralhydrate, diazepam, 7 AEDs, with 5 AEDs when discharged. | At 4 years, walking, speaking, but memory was impaired |
| Case 5 2 years Male 29 days |
Allergenic demyelinating encephalitis | Vaccination of measles-mumps-rubella triad vaccine | Lasting 2 months, each cluster lasting more than 30 min, burst suppression and hypsarrhythmia | Focal seizures, left frontal and temporal, bilateral occipital epileptiform discharges | Atrophy of thalamus, occipital region and left hippocampus improved? | IVIG, MP, 3 AEDs, ACTH | At 4 years, nearly recovered as before, learning and language slightly impaired |
| Case 6 3 years and 4 months Female 141 days |
Acute encephalopathy | Severe allergy, post-cardiopulmonary resuscitation, iodine allergy | Lasting 30–60 min daily for 50 days. EEG hypsarrhythmia | Focal seizures generalized at early stage of disease | Caudate nucleus and thalamus abnormal signal on Diffusion-Weighted Images | TPM, ACTH, prednisone | At 4 years 2 months, left arm movement and speech not fully recovered, otherwise normal |
| Case 7 11 months Female 15 days |
Intractable epilepsy, focal seizures, developmental delay | Compound heterozygous mutation in the TBCD gene | 3 times continuous spasms, each lasing 1.5 weeks | Low voltage on EEG, non-Convulsive status epilepticus, convulsive status epilepticus | At 20 months: significant atrophy, 3 months later atrophy aggravated | IVIG, MP, KD, 3 AEDs, another 3 AEDs used | At 5.5 years, no language, rolling and walking, severe development delay |
| Case 8 5 months Male 13 days |
Intractable epilepsy, infantile spasms, Ohtahara Syndrome transition Severe asphyxia | Ohtahara Syndrome, genetic mutation? | Lasing 2 h, burst suppression and hypsarrhythmia | Focal seizures, tonic seizures frequently, Multifocal spikes | Severe brain atrophy | KD, prednisone, 4 AEDs, ACTH | At 4 years 3 months: microcephaly, no head control or language, cannot recognize parents, seizures decreased |
| Case 9 11 months Female 17 days |
Infantile spasms, psychomotor retardation, epileptic encephalopathy | Unknown | Lasing 3 months and 5 days, EEG, burst suppression | Focal seizures, refractory epilepsy | Abnormal signal at white matter, thalamus atrophy | TPM, KD, VPA, LEV, ACTH | At 6 years: developmentally delayed, could not sit, still daily seizures |
| Case 10 1 month and 20 days Male 15 days |
Ohtahara Syndrome Asphyxia | Genetic, a de novo missense mutation in the KCNQ2 gene | Lasting 108 min, spasms in cluster or singly | Tonic seizures, focal seizures | Un remarkable | ACTH, 3 AEDs | At 3 years and 4 months: spasms, no visual fixation, deceased at age of 8 months due to severe infection. |
| Case 11 4 months Male 15 days |
Pachygyria malformation, Intractable epilepsy | Genetic, 489 Kb deletion at 17p13.3 | Lasting 30 min, in cluster or single, hypsarrhythmia | Tonic seizures, focal seizures | Pachygyria | 3 AEDs, ACTH, sirolimus, KD | At 5 years: severely delayed development, no head control, language or visual following, seizures 3.5 times daily |
IVIG, intravenous immunoglobulin; PE, plasma exchange; MP, methylprednisolone; ACTH, adrenocortical tropical hormone; AEDs, antiepileptic drugs; TPM, topiramate; LTG, lamotrigine; LEV, levoparacetam; NZP, nitrozepam; CBZ, carbamazepine; KD, ketogenic diet.
The etiologies identified in the acute symptomatic spasm patients were diverse, included combined enterovirus type 71 and mycoplasma-pneumonia-related encephalopathy (Case 1), influenza-virus-type-A-related encephalopathy (Case 2), anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis (Case 3), febrile-infection-related epilepsy syndrome (FIRES, Case 4), acute post-vaccination encephalopathy from the measles-mumps-rubella vaccine (Case 5), and acute anaphylaxis from iodine disinfectant at the beginning of a tooth surgery (Case 6). The etiologies in the non-acute cases, mostly with established electro-clinical syndrome cases were also heterogenous, including compound heterozygous mutations in the TBCD gene (ENST00000355528, c.881G>A, p.Arg294Gln and c.22801C>A, p.Tyr760*, Case 7), severe perinatal asphyxia (Case 8), a de novo heterozygous missense mutation in KCNQ2 (ENST00000359125, c.1657C>T, p.Arg553Trp) in a patient with Ohtahara Syndrome (Case 10), one case of pachygyria malformation associated with a pathogenic de novo chromosomal copy number variation (489 Kb deletion at 17p13.3, chr17: 2441590-2931074 [hg19], Case 11); with only one case with unknown etiology (Case 9) (see also Table 1).
We compared different categories of cases among our series, and with the typical cases epileptic spasms in our hospital, and identified the following characteristic features in our cases. The hospital stays of the acute cases (Case 1 to Case 6) were significantly longer than the ones with primary epileptic syndromes (Case 7, Case 10 and Case 11) (59.67 ± 50.82 vs. 15.00 ± 1.41 days, P = 0.0299), while the average hospital-stay in our department during the same time period was < 7 days. The primary epilepsy syndromic cases (Case 7, 10, 11) had earlier onset of seizures, and their conditions progressively deteriorated with unresolved seizures. Neurodevelopmental and seizure outcomes after 4.94 ± 1.56 years, based on clinical interview and parental reporting, were much better in the 6 cases with acute symptomatic epileptic spasms than in the five cases with an established electro-clinical syndrome, which were mostly of genetic origin, despite the absence of any significant difference in the duration of continuous epileptic spasms between the two groups (P = 0.1885). Cases 7–11 had more severe global developmental delay, including severe intellectual deficiency, language and movement impairment; and epileptic spasms were still present at the last follow-up in all cases with epileptic syndromes. In addition, one patient (Case 8) died of severe infection at age of 8 months. Our results indicate that etiology might be an important factor in determining the outcome of the patient's epilepsy and neurodevelopment.
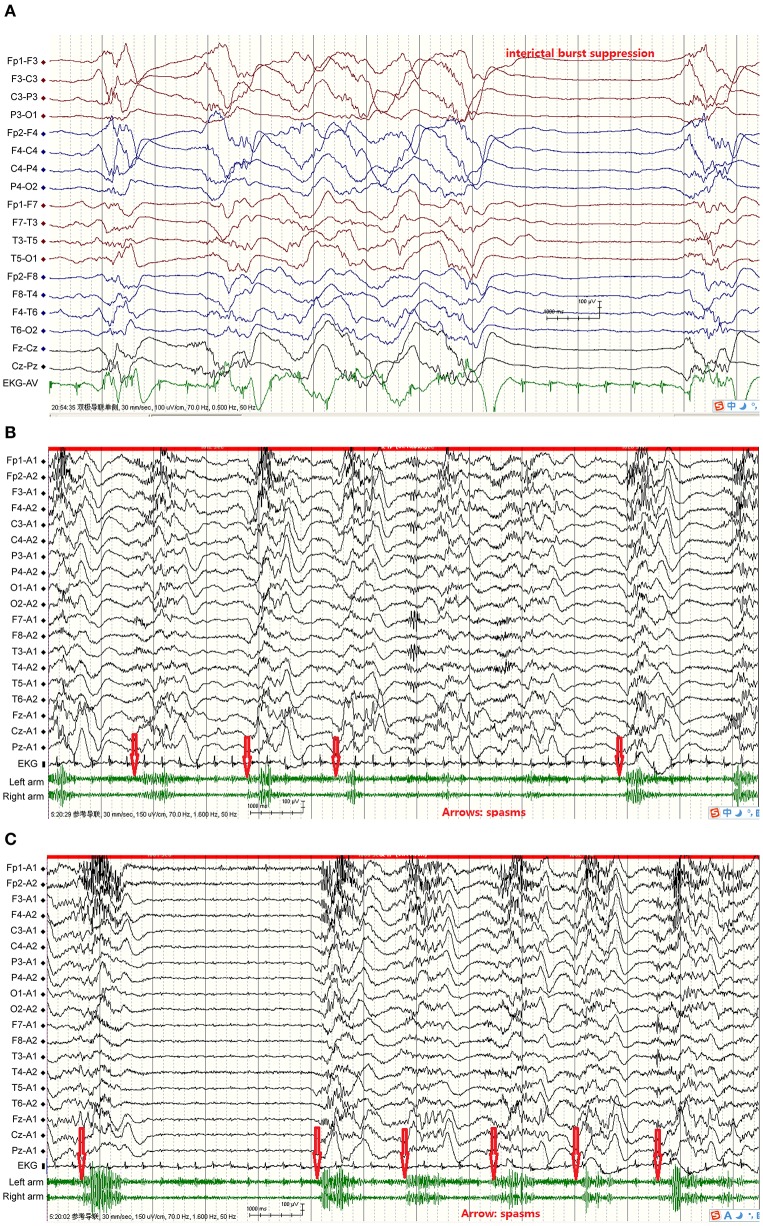
The duration of the continuous epileptic spasms ranged from 30 min to 8 months according the video-EEG recordings and the patients' seizures diaries. The attacks manifested distinctly upon arousal from sleep and decreased or disappeared during sleep. Some of the spasms were asymmetrical or weak with mild frowning and tremor of body or limbs. The inter-spasm intervals were generally longer than 1 s. The intervals between the spasm attacks varied between the patients, but also varied from one attack to another for the same individual. Ictal EEGs during the 1–2 s of spams showed mostly fast waves followed by a single high amplitude slow wave, and then voltage attenuation. In the case with Ohtahara Syndrome, the ictal EEG showed spasm attacks nearly synchronized with the burst of suppression phase (Figure 1). In the rest patients, the interictal EEGs showed hypsarrhythmia or its variants, i.e., burst suppression or periodic hypsarrhythmia pattern; and the EEG background was often generalized or multifocal slow waves.
Figure 1.
From Case 4 showed FIRES, acute symptomatic continuous epileptic spasms, interictal hypsarrhythmia and suppression-burst pattern, and ictal EEG, plus EKG, and EMG. (A) Interictal burst suppression, bursts 1–5 s, inhibition 1–4 s; (B) Spasms simultaneous EEG showed fast wave, then slow wave followed by voltage attenuation, spasms synchronized with initial fast waves. Red arrows indicate spasms initiation; (C) Spasms simultaneous EEG showed fast wave, then slow wave followed by voltage attenuation, spasms synchronized with initial fast waves; two slow waves consecutively appeared in spasms 1, 2, 3, and 5. Spasms more frequent and heart rate faster than interictal stage. Red arrows indicate spasms initiation.
The continuous epileptic spasms in our patients were generally resistant to multiple anticonvulsants (Table 1); and the outcomes were worse in the patients with continuous spasms mixed with other types of seizures, including focal seizures, tonic seizures etc. Immunotherapy was used in all patients, and treatments included intravenous immunoglobulin (6 cases), high doses of methylprednisolone (5–15 mg/kg/day) (3 cases), and immunosuppressors (such as cyclophosphamide in one case), but with no satisfactory results.
Discussion
We report a series of 11 cases with continuous epileptic spasms as a severe form of status epilepticus. Their clinical characteristics included: (1) each epileptic spasm episode lasted longer than 30 min though the seizure semiology was similar to the classical epileptic spasms; (2) etiologies were diverse, including acute symptomatic causes such as encephalitis, encephalopathy, and FIRES; even in the cases with known electro-clinical epilepsy syndromes showing different genetic mutations; (3) the disease course and hospital-stay in acute symptomatic cases were much longer than in those with known electro-clinical epilepsy syndromes; (4) the cases with the established electro-clinical syndromes showed the worst outcome, despite earlier diagnosis and interventions.
Although continuous epileptic spasms have only been rarely described in the literature, our experience indicate that careful review of video-EEG and prudent observation from the parents and health professionals were critical for detecting continuous epileptic spasms. Our series showed a recognizable epileptic spasms semiology pattern, which fits with the ILAE definition of status epilepticus (25). The underlying etiologies seem to be diverse, including infection, and/or inflammatory-mediated processes and genetic disorders, or still unknown causes. The etiological heterogeneity of continuous epileptic spasms is similar to those with convulsive status epilepticus, occurring in the similar age groups, including both acute encephalitis or encephalopathy and other symptomatic spasms associated with defined electro-clinical syndromes, such as the Ohtahara Syndrome and West Syndrome, which are mostly with genetic origin; and the patients often exhibited additional types of seizures, such as focal seizures, with or without generalization. Our results suggest that the long lasting spasms accompanied with disturbance of consciousness and other types of seizures could predict long hospital-stay and much worse neurodevelopmental outcomes than those with epileptic spasms lasting <30 min. The outcome was consistently poor in patients with electro-clinical epilepsy syndrome despite early recognition and treatment, which seems to be different from classical West Syndrome or other epileptic encephalopathies in which early recognition and treatment leads to improved neurologic outcomes.
The recognition and diagnosis of continuous epileptic spasms is challenging, particularly in acutely and critically ill children when first admitted to the pediatric intensive care unit (PICU). The epileptic spasms are not as easily noticeable as focal motor seizures and generalized tonic-clonic seizures. Lack of clinical experience, inadequate observation and short video-EEG monitoring may result in misdiagnosis or underdiagnosis. In our study, the patients were sometimes in a supine position and were so weak that their movements manifesting epileptic spasms were quite subtle, therefore easily overlooked by the observers. At times, the subtle spasm movements could also be misinterpreted as extra-pyramidal motor dyskinesia. In addition, the patients often had co-occurring focal motor seizures or tonic-clonic seizures so the faint epileptic spasms were unnoticed.
Hypsarrhythmia is a characteristic interictal EEG feature of epileptic spasms (15–17). This condition emerges gradually and can be used to predict the onset of infantile spasms or the recurrence of spasms after discontinuing adrenocorticotropic hormone (ACTH) administration (27, 28). However, the classic EEG pattern of hypsarrythmia is recorded in fewer than half of cases of epileptic spasms; instead, variants similar to paroxysmal or periodic hypsarrhythmia have been recorded and such variants of hypsarrhythmia may be as high as 69% (16), which could easily be overlooked or misinterpreted.
Continuous video EEG monitoring has been recommended for at least 24 h in critically ill or complex cases, who are resistant to initial treatment, to rule out the presence of status epilepticus of epileptic spasms or non-convulsive status epilepticus (26, 29, 30). Physicians, nurses, or EEG technicians trained in cerebral function monitoring must be on-site to observe and detect possible epileptic spasms or other seizures types since long-lasting seizures or epileptiform discharges may cause brain damage and result in poor prognoses (26). Early diagnosis is extremely important so that early and aggressive intervention could be undertaken to improve outcomes and prognosis, especially in children with acute symptomatic spasms status epilepticus.
Among all the currently available therapies, ACTH and vigabatrin have the best response rate in infantile spasms (31–35). Anecdotal reports and animal experiments indicate that lorazepam, clobazam, paraldehyde, ACTH, and other corticosteroids may be effective in the treatment of continuous epileptic spasms (27). Our preliminary experience indicated that acute symptomatic spasms could also be treated similarly to autoimmune encephalitis (36, 37), which includes ACTH, intravenous immunoglobulin, high doses of methylprednisolone, immunosuppressors such as cyclophosphamide and rapamycin (38), and a ketogenic diet. Acute symptomatic cases seemed to have responded better to immuno-therapy.
Obviously, our study was limited by the small sample size and the nature of a retrospective study. Future prospective studies with larger sample size, and/or randomized, controlled, multi-center clinical trials could help to further characterize this particular type of status epilepticus, for example, to quantify precisely the duration of epileptic spasms, the number of spasms in each cluster attack, the statistical cutoff for continuous epileptic spasms as status epilepticus, as well as age and video-EEG and their correlation with the prognosis.
In summary, continuous epileptic spasms is most likely a clinically distinct form of status epilepticus. Etiologies include severe acute encephalopathy, encephalitis or FIRES in acute symptomatic conditions, as well as various genetic causes in defined electro-clinical syndromes. The acute symptomatic cases often need longer hospital-stay, and usually have changes in level of consciousness and other types of seizures. However, the cases with known electro-clinical epileptic syndromes showed the worst long-term outcome. All the cases were generally resistant to conventional AEDs and ACTH treatments; therefore, more effective treatment is urgently needed. We suggest that continuous epileptic spasms be considered to be included into the classification of ILAE status epilepticus in the future.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shenzhen Children's Hospital. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JL, TH, YC, and YX were responsible for EEG reviewing. JL, MS, and LX were responsible for the design and completion of the manuscript and figures. All authors were responsible for the acquisition and analysis of data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Sándor Beniczky, Francois Dubeau, and Hui Ming Khoo for their advice and for critically reviewing the manuscript. JL thanks the Montreal Neurological Institute and Montreal Children's Hospital, McGill University for hosting his 6 months clinical observation, during which this manuscript was completed.
Footnotes
Funding. This study was supported by Shenzhen Medical Key Specialty Development Grants 2016026 and SZSM20181205.
References
- 1.Blume WT, Lüders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. (2001) 42:1212–8. 10.1046/j.1528-1157.2001.22001.x [DOI] [PubMed] [Google Scholar]
- 2.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. (2004) 45:1416–28. 10.1111/j.0013-9580.2004.02404.x [DOI] [PubMed] [Google Scholar]
- 3.Auvin S, Lamblin MD, Pandit F, Vallée L, Bouvet-Mourcia A. Infantile epileptic encephalopathy with late-onset spasms: report of 19 patients. Epilepsia. (2010) 51:1290–6. 10.1111/j.1528-1167.2010.02534.x [DOI] [PubMed] [Google Scholar]
- 4.Ronzano N, Valvo G, Ferrari AR, Guerrini R, Sicca F. Late-onset epileptic spasms: clinical evidence and outcome in 34 patients. J Child Neurol. (2015) 30:153–9. 10.1177/0883073814532547 [DOI] [PubMed] [Google Scholar]
- 5.Metsähonkala L, Gaily E, Valanne L, Blomstedt G. Etiology and long-term outcomes of late-onset infantile spasms. Neuropediatrics. (2015) 46:269–76. 10.1055/s-0035-1554099 [DOI] [PubMed] [Google Scholar]
- 6.Shirai K, Saito Y, Yokoyama A, Nishimura Y, Tamasaki A, Maegaki Y. Stimulus-induced reflex epileptic spasms in 5p- syndrome. Brain Dev. (2016) 38:261–5. 10.1016/j.braindev.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuka Y, Kobayashi K, Ogino T, Oka E. Spasms in clusters in epilepsies other than typical West syndrome. Brain Dev. (2001) 23:473–81. 10.1016/S0387-7604(01)00289-3 [DOI] [PubMed] [Google Scholar]
- 8.Yamatogi Y, Ohtahara S. Early-infantile epileptic encephalopathy with suppression-bursts, Ohtahara syndrome; its overview referring to our 16 cases. Brain Dev. (2002) 24:13–23. 10.1016/S0387-7604(01)00392-8 [DOI] [PubMed] [Google Scholar]
- 9.Caraballo RH, Reyes G, Falsaperla R, Ramos B, Ruiz AC, Fernandez CA, et al. Epileptic spasms in clusters with focal EEG paroxysms: a study of 12 patients. Seizure. (2016) 35:88–92. 10.1016/j.seizure.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Saito Y, Sugai K, Nakagawa E, Komaki H, Okazaki T, et al. Late-onset epilepsy in children with acute febrile encephalopathy with prolonged convulsions: a clinical and encephalographic study. Brain Dev. (2013) 35:531–9. 10.1016/j.braindev.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Natsume J, Kidokoro H, Ishihara N, Azuma Y, Tsuji T, et al. Seizure characteristics of epilepsy in childhood after acute encephalopathy with biphasic seizures and late reduced diffusion. Epilepsia. (2015) 56:1286–93. 10.1111/epi.13068 [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz S, Tekgul H, Serdaroglu G, Akcay A, Gokben S, et al. Evaluation of ten prognostic factors affecting the outcome of West syndrome. Acta Neurol Belg. (2016) 116:519–27. 10.1007/s13760-016-0611-8 [DOI] [PubMed] [Google Scholar]
- 13.Ohtahara S, Yamatogi Y. Epileptic encephalopathies in early infancy with suppression-burst. J Clin Neurophysiol. (2003) 20:398–407. 10.1097/00004691-200311000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Fusco L, Pachatz C, Di Capua M, Vigevano F. Video/EEG aspects of early-infantile epileptic encephalopathy with suppression-bursts (Ohtahara syndrome). Brain Dev. (2001) 23:708–14. 10.1016/S0387-7604(01)00280-7 [DOI] [PubMed] [Google Scholar]
- 15.Hrachovy RA, Frost JD, Jr, Kellaway P. Hypsarrhythmia: variations on the theme. Epilepsia. (1984) 25:317–25. 10.1111/j.1528-1157.1984.tb04195.x [DOI] [PubMed] [Google Scholar]
- 16.Kramer U, Sue WC, Mikati MA. Hypsarrhythmia: frequency of variant patterns and correlation with etiology and outcome. Neurology. (1997) 48:197–203. 10.1212/WNL.48.1.197 [DOI] [PubMed] [Google Scholar]
- 17.Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, et al. Infantile spasms: a U.S. consensus report. Epilepsia. (2010) 51:2175–89. 10.1111/j.1528-1167.2010.02657.x [DOI] [PubMed] [Google Scholar]
- 18.Gobbi G, Bruno L, Pini A, Giovanardi Rossi P, Tassinari CA. Periodic spasms: an unclassified type of epileptic seizure in childhood. Dev Med Child Neurol. (1987) 29:766–75. 10.1111/j.1469-8749.1987.tb08822.x [DOI] [PubMed] [Google Scholar]
- 19.Marchi LR, Seraphim EA, Corso JT, Naves PV, Carvalho KC, Ramirez MD, et al. Epileptic spasms without hypsarrhythmia in infancy and childhood: tonic spasms as a seizure type. Epileptic Disord. (2015) 17:188–93. 10.1684/epd.2015.0738 [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa N, Kobayashi Y, Fujii Y, Tajima G, Kobayashi M. Ictal electroencephalography and electromyography features in symptomatic infantile epileptic encephalopathy with late-onset spasms. Neuropediatrics. (2014) 45:36–41. 10.1055/s-0033-1357479 [DOI] [PubMed] [Google Scholar]
- 21.Tjiam AT, Stefanko S, Schenk VW, de Vlieger M. Infantile spasms associated with hemihypsarrhythmia and hemimegalencephaly. Dev Med Child Neurol. (1978) 20:779–98. 10.1111/j.1469-8749.1978.tb15310.x [DOI] [PubMed] [Google Scholar]
- 22.Galanopoulou AS, Moshé SL. Pathogenesis and new candidate treatments for infantile spasms and early life epileptic encephalopathies: a view from preclinical studies. Neurobiol Dis. (2015) 79:135–49. 10.1016/j.nbd.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek H, Yi MH, Pandit S, Park JB, Kwon HH, Zhang E, et al. Altered expression of KCC2 in GABAergic interneuron contributes prenatal stress-induced epileptic spasms in infant rat. Neurochem Int. (2016) 97:57–64. 10.1016/j.neuint.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 24.Coulter DL. Continuous infantile spasms as a form of status epilepticus. J Child Neurol. (1986) 1:215–7. 10.1177/088307388600100307 [DOI] [PubMed] [Google Scholar]
- 25.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–report of the ILAE task force on classification of status epilepticus. Epilepsia. (2015) 56:1515–23. 10.1111/epi.13121 [DOI] [PubMed] [Google Scholar]
- 26.Abend NS. Electrographic status epilepticus in children with critical illness: epidemiology and outcome. Epilepsy Behav. (2015) 49:223–7. 10.1016/j.yebeh.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K, Toribe Y, Kimizu T, Kimura S, Ikeda T, Mogami Y, et al. Predictive value of EEG findings at control of epileptic spasms for seizure relapse in patients with West syndrome. Seizure. (2014) 23:703–7. 10.1016/j.seizure.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 28.Altunel A, Sever A, Altunel EO. Hypsarrhythmia paroxysm index: a tool for early prediction of infantile spasms. Epilepsy Res. (2015) 111:54–60. 10.1016/j.eplepsyres.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. (2012) 17:3–23. 10.1007/s12028-012-9695-z [DOI] [PubMed] [Google Scholar]
- 30.Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. (2015) 32:96–108. 10.1097/WNP.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen-Sadan S, Kramer U, Ben-Zeev B, Lahat E, Sahar E, Nevo Y, et al. Multicenter long-term follow-up of children with idiopathic West syndrome: ACTH versus vigabatrin. Eur J Neurol. (2009) 16:482–7. 10.1111/j.1468-1331.2008.02498.x [DOI] [PubMed] [Google Scholar]
- 32.Schwarz MD, Li M, Tsao J, Zhou R, Wu YW, Sankar R, et al. A lack of clinically apparent vision loss among patients treated with vigabatrin with infantile spasms: the UCLA experience. Epilepsy Behav. (2016) 57:29–33. 10.1016/j.yebeh.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 33.Westall CA, Wright T, Cortese F, Kumarappah A, Snead OC, Buncic JR. Vigabatrin retinal toxicity in children with infantile spasms: an observational cohort study. Neurology. (2014) 83:2262–8. 10.1212/WNL.0000000000001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riikonen R. Long-term outcome in children with infantile spasms treated with vigabatrin: a cohort of 180 patients. Epilepsia. (2015) 56:807–9. 10.1111/epi.12953 [DOI] [PubMed] [Google Scholar]
- 35.Knupp KG, Coryell J, Nickels KC, Ryan N, Leister E, Loddenkemper T, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. (2016) 79:475–84. 10.1002/ana.24594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadoya M, Onoue H, Kadoya A, Ikewaki K, Kaida K. Refractory status epilepticus caused by anti-NMDA receptor encephalitis that markedly improved following combination therapy with rituximab and cyclophosphamide. Intern Med. (2015) 54:209–13. 10.2169/internalmedicine.54.2047 [DOI] [PubMed] [Google Scholar]
- 37.Scheer S, John RM. Anti-N-methyl-D-aspartate receptor encephalitis in children and adolescents. J Pediatr Health Care. (2016) 30:347–58. 10.1016/j.pedhc.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 38.Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. (2011) 43:322–9. 10.1016/j.nbd.2011.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.