Abstract
Background
Aphasia describes language impairment associated with a brain lesion.
Objectives
To assess the effects of drugs on language abilities when given to people with aphasia following stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched: May 2001), and reference lists of relevant articles to December 1998. We also contacted academic institutions and other researchers to identify further published and unpublished trials. MEDLINE was searched from 1966 to 1998, and CINAHL from 1982 to 1998. We handsearched the International Journal of Disorders of Communication from 1969 to 1998.
Selection criteria
Randomised controlled trials comparing (1) any drug given to improve language versus no treatment or versus placebo, (2) any drug given to improve language versus speech and language therapy, and (3) one drug given to improve language versus another drug given with the same aim.
Data collection and analysis
One reviewer collected the data, and assessed the quality of the trials with independent data checking and methodological advice. If we could not perform a statistical combination of different studies, we sought missing data. Failing that, we provided a description.
Main results
We included 10 trials in the review. Generally, we were unable to assess methodological quality; only one trial reported sufficient detail for analysis. Drugs used were piracetam, bifemalane, piribedil, bromocriptine, idebenone, and Dextran 40. We found weak evidence that patients were more likely to have improved on any language measure at the end of the trial if they had received treatment with piracetam (odds ratio (OR) 0.46, 95% confidence interval (CI) 0.3 to 0.7). Patients who were treated with piracetam were no more likely than those who took a placebo to experience unwanted effects, including death (OR 1.29, 95% CI 0.9 to 1.7). However, the differences in death rates between the two groups give rise to some concerns that there may be an increased risk of death from taking piracetam. We could not determine if drug treatment is more effective than speech and language therapy. We could not determine whether one drug is more effective than another.
Authors' conclusions
Drug treatment with piracetam may be effective in the treatment of aphasia after stroke. Further research is needed to explore the effects of drugs for aphasia, in particular piracetam. The safety of the drug should be of primary interest. Researchers should examine the long‐term effects and whether it is more effective than speech and language therapy.
Plain language summary
Pharmacological treatment for aphasia following stroke
Drug therapy might improve recovery from loss of language function (aphasia) after stroke, but no drug has yet been proven to do more good than harm. Aphasia is a common problem after stroke. Speech and language therapy (SLT) from a speech and language therapist is the most common treatment for this disorder. A number of drugs have been used to try and improve language recovery. This review of 10 studies evaluated six different drugs. The only drug for which there was any evidence of benefit was piracetam, but the evidence of benefit was weak and there were concerns about its safety. It was not possible to conclude whether piracetam was more effective than speech and language therapy in treating aphasia after stroke. More research is needed into the effects of piracetam on aphasia, and its safety, before it can be recommended for routine use.
Background
Definition
Aphasia, a term which is used interchangeably with dysphasia, describes linguistic impairment associated with a brain lesion. Stroke is the major cause of aphasia. There is ongoing debate about the cortical location of neurological components which contribute to communication and language, and the actual mechanisms disrupted by a stroke (Goldberg 1990).
Frequency
Estimates of the incidence, prevalence and impact of aphasia following stroke vary and are uncertain (Mackenzie 1992). An estimated 11,400 people become aphasic following stroke every year in Britain (Enderby 1986). Wade et al noted that aphasia was present in one quarter of conscious patients who had suffered a stroke within the previous seven days (Wade 1986). The Scandinavian Stroke Study found that 38% of stroke patients were aphasic on admission to hospital, and 18% of stroke survivors had some degree of aphasia on discharge from hospital (Pederson 1995). The symptoms of stroke frequently persist and 12% of the survivors of stroke are still aphasic at six months (Wade 1986). The prevalence of persisting speech and language disorders 6 months following stroke has been estimated to be between 30 and 50 per 100,000 population (Enderby 1989).
Treatment
Usually it is the remit of speech and language therapists (formerly known as speech therapists in the UK, known as speech pathologists in the USA) to assess and treat people with aphasia, and this is the subject of another review (Greener 2001). In addition, however, a number of drugs have been utilised with the aim of ameliorating language functions after stroke. Nevertheless, the role of pharmacotherapy is uncertain (Bachman 1990), and the diverse theories of the neurological deficits underlying aphasia have given rise to different pharmacologic rationales for therapy (Small 1994).
Drugs and their possible mechanisms of action
The agents that have been used include meprobomate which has tranquillising and muscle relaxing effects, and L‐Dopa, a dopaminergic agent, which is used to reduce the symptoms of Parkinson's Disease (Methe 1993). Bromocriptine, also a dopamine agonist, has also been administered to aphasic patients with some evidence of success, albeit from a small group before‐and‐after study (Albert 1992). There is, therefore, some reason to suppose that dopamine has a positive effect on language.
Recently, interest has developed in the use of piracetam. This substance belongs to a unique pharmacological class known as nootropic, which affects various mental functions. A review of the uses to which piracetam has been put claimed that it improves higher cerebral integrative functions, including those involved in cognitive processes such as learning and memory (Giurgea 1976). This review, however, was not systematic, and findings should not, therefore, be considered conclusive. It has also been postulated that, in order to facilitate improvement in these cognitive processes, piracetam restores the fluidity of the neuronal membrane (Muller 1994), and that piracetam may improve the microcirculation both centrally and peripherally, for various reasons advanced by a number of researchers (Enderby 1994). A number of studies have suggested that piracetam has favourable effects on mental and motor function in patients who have suffered a stroke (Stolyarova 1978, Creytens 1980). Piracetam has been used in other circumstances, for example to improve reading ability and comprehension in dyslexic children (Wilsher 1987), and alertness and memory in elderly patients with cognitive disorders (Steginck 1972, Chouinard 1983). It is licensed in the UK for prescription for the treatment of cortical myoclonus.
It can be argued that there will be spontaneous recovery from the brain lesion after stroke, which may well account for any improvement in language function documented using observational research methods: these are, however, the most frequently used research methods in this area (Whurr 1992).
It is therefore the aim of this review to draw on the evidence of 'experimental' design studies in order to assess the effectiveness of any pharmacological substance given with the expressed aim of ameliorating language function(s), to people who are aphasic after stroke.
Objectives
To assess whether:
pharmacological treatment is more effective than no pharmacological treatment of any type in the treatment of acquired aphasia following stroke;
pharmacological treatment is more effective than speech and language therapy in the treatment of acquired aphasia following stroke;
one particular type of pharmacological substance is more effective than another in the treatment of acquired aphasia following stroke.
Methods
Criteria for considering studies for this review
Types of studies
The reports of studies formally reviewed were limited to those described as randomised controlled trials, even where the method of randomisation had not been specified. Ideally, a method of randomisation should have been used which ensured that those recruiting participants for the trial could not influence the assignment in any way. When it was unclear whether or not there was adequate allocation concealment the authors were contacted for further clarification. There was no language restriction. In addition, only those trials with a pre‐stated aim of specifically examining the effects of a drug on language function were considered eligible for formal review.
Types of participants
The participants were adults (i.e. 18 and above years of age) who had acquired aphasia due to a stroke. The definition of stroke was that given by the World Health Organization (WHO 1986). All types of aphasia were considered including expressive aphasia, receptive aphasia, global aphasia, mixed aphasia, dysphasia and non‐specific aphasia.
Types of interventions
(1) Any pharmacological substance given to a patient with aphasia, with the expressed aim of ameliorating language function lost after stroke, compared with no pharmacological treatment of any kind for aphasia, or with placebo. The comparison was required to be unconfounded, i.e. any other drugs or treatment, including speech and language therapy, must have been given to both groups in equal amounts.
(2) Any pharmacological substance given to the patient with aphasia, with the expressed aim of ameliorating language function lost as a result of stroke, compared with any type of speech and language therapy. Those receiving the drug should not be receiving speech and language therapy.
(3) One type of pharmacological substance given to aphasic patients with the expressed aim of ameliorating language function lost after stroke, compared with another pharmacological substance given with the same aim.
Types of outcome measures
Principal outcomes were measures of communication (oral expressive language, oral receptive language, functional communication, written language), and overall functional status of the patient (disability or handicap measures). Measures considered eligible were, for example: global ratings, rating scales, psychological scales, achievement tests, language tests, criterion referenced tests, psychological tests.
Other outcomes considered eligible included:
cognitive skills;
death;
further morbidity;
non‐compliance with allocated treatment;
affective state of patient;
satisfaction of patient with treatment;
carer and family outcomes, for example affective state and satisfaction with treatment;
resource use, such as the costs to the patients, carers, families, the health service and society.
Search methods for identification of studies
See: 'Specialized register' section in Cochrane Stroke Group
Relevant trials were identified in the Cochrane Stroke Group Trials Register, which was last searched by the Review Group Co‐ordinator in May 2001. We also searched MEDLINE (1966 to 1998) and CINAHL (1982 to 1998) using a search strategy which includes a combination of MeSH controlled vocabulary (/) and free text words (tw) (Appendix 1).
In addition:
(1) we handsearched the International Journal of Disorders of Communication (formerly the European Journal of Disorders of Communication and the British Journal of Disorders of Communication) from 1969 to 1998;
(2) we checked the reference lists of all relevant articles identified for other possible randomised trials;
(3) we contacted all universities and colleges where speech and language therapists are trained in Britain to enquire about any relevant past or ongoing studies;
(4) we approached colleagues and authors of randomised trials to identify other relevant studies.
References were managed using the bibliographic database Reference Manager, before transfer to the Cochrane Review Manager software, RevMan.
Data collection and analysis
One assessor assessed the quality of trials under consideration. This assessment was checked by another assessor (one of two other trained speech and language therapists) at a different location. The trials were assessed for methodological quality with attention paid to whether there was protection from the following types of bias:
selection bias, i.e. true random sequencing, and true concealment up to the time of allocation;
performance bias, i.e. differences in other types of treatment (co‐interventions), between the two groups;
exclusion bias, i.e. withdrawal after trial entry;
detection bias, i.e. 'unmasked' assessment of outcome;
over‐involvement of the drug company with the running of the trial.
The review was conducted using the Cochrane Review Manager software, RevMan. Descriptive information for each trial (in respect of methodological quality, characteristics of participants, characteristics of interventions, and characteristics of outcome) was recorded in the Characteristics of Included Studies. In the case of continuous data where a higher mean score meant a greater improvement in function, the graph labels on Metaview were reversed at the outcome level in order to allow this to be presented.
Data for all prespecified outcomes were tabulated where possible. Where trials were judged sufficiently similar in respect of their descriptive characteristics, an attempt was made to synthesise the data using standard statistics such as odds ratios or weighted mean differences. Ninety‐five per cent confidence intervals were generated throughout the review, where possible.
A narrative account of the trials entered has been given where statistical combination of different studies proved impossible, or was judged inappropriate.
Studies judged ineligible have been listed with reasons given for their exclusion (see Characteristics of Excluded Studies).
Results
Description of studies
Over 300 abstracts were screened for eligibility.
Exluded studies
Of these, 55 studies were considered and rejected from the review as ineligible on at least one criterion for the review. Twenty‐two of these were ineligible because it was clear they had used a methodology which was not a randomised controlled trial. Five of the studies judged ineligible on methodological grounds gave insufficient details of methodology to be certain what type of methods had been used. Two randomised controlled trials were rejected because the cross over design resulted in uncertainty about the effects of the intervention: learning effects from the first phase of the trials could have clouded the results from the second phase. Fifteen were rejected on methodological grounds alone, and 25 studies were rejected because they had not evaluated the effect of the intervention on aphasia separately from its effect on other disabilities. Thirteen studies were ineligible because they did not meet patient inclusion criteria: three had included patients with both head injury and stroke, three had included patients with dysarthria and/or aphasia, one included patients with apraxia, not aphasia. Of those judged ineligible on methodological grounds, eight had used a single case observational approach, studying either one patient, or a small group of patients, and five had used a before and after design with a single group of patients. Three studies were purely observational, one of these being retrospective, and one study comprised a review of past research. Two studies were judged ineligible on three or more grounds (methodological, patient type, intervention type, outcome). The range of years in which the excluded studies were published was from 1965 to 1997, with 37 of them taking place in the 1980s or 1990s.
Included studies
Ten trials were included, out of which only two were performed before 1990. Main characteristics of trials (where available in the reports):
Participants
Trials ranged from four to 927 participants, all included patients of mixed sex, apart from Gupta (Gupta 1995), and Tanaka (Tanaka 1997) who included only males. The average age within trials ranged between 53 and 78, with an absolute range of 21 to 85.
Interventions
(1) Timing of intervention
Where this was described, the timing of patient entry into the trials ranged from immediately following, to one year after the stroke, with a median of one month post stroke.
(2) Types of intervention
Piracetam versus placebo ‐ five trials: De Reuck 1995; Enderby 1994, Platt 1993, Herrschaft 1988, Poeck 1993
Bifemelane versus no active substance ‐ one trial: Tanaka 1997
Piribedil versus no active substance ‐ one trial: Bakchine 1990
Bromocriptine versus placebo ‐ one trial: Gupta 1995
Idebenone versus placebo ‐ one trial: Price 1992
Dextran 40 versus no active substance ‐ one trial: Spudis 1973
Outcome measures
See Data and analyses. A variety of tests were used to assess the abilities of the patients. Where results of tests were given, in some cases the outcome was the score achieved by the patients. This was given as a mean, often with no measure of dispersion such as a standard deviation. Other trials reported the number of people who had improved or not improved their test score after the intervention(s).
(1) Language measures used. Each of the following tests were used in two trials: Western Aphasia Battery (WAB, Kertesz 1982) was used in Gupta (Gupta 1995), and Price (Price 1992). This test comprises a group of sub tests which assess auditory and reading comprehension, and oral and written expressive language. Aaachen Aphasia Test (AAT, Huber 1983) was used in Enderby (Enderby 1994) and Poeck (Poeck 1993). This test is a psychometrically validated assessment developed using linguistic principles, allowing the detail of language breakdown to be described. Each of the following was used in only one trial: The Boston Naming Test (Kaplan 1983), used by Gupta (Gupta 1995); the Frenchay Aphasia Test (Enderby 1987), used by De Reuck (De Reuck 1995); and the Japanese Standard Language Test (SLTA 1977), used by Tanaka (Tanaka 1997). Unstandardised language measures, measures of psychological functioning and a measure of depression in patients were each reported only once, and are not considered in this section.
(2) Mortality was reported in De Reuck (De Reuck 1995) and Platt (Platt 1993).
(3) Drop out rates, adverse events and further morbidity were reported in Enderby (Enderby 1994), Herrschaft (Herrschaft 1988), Platt (Platt 1993), Spudis (Spudis 1973) and De Reuck (De Reuck 1995). In the last case it is not possible to separate patients who experienced a non‐fatal adverse event from those who later died after a preceding adverse event. For the purposes of this review, only deaths have been counted in this trial.
Risk of bias in included studies
The methodological quality of the trials entered for the review varied between trials.
Quality of randomisation process
One trial gave insufficient detail of the randomisation process in the published report, but contact with the author revealed that the randomisation was organised from a central location (Enderby 1994). Patient identification was taken prior to randomisation to ensure there could be no cross over ‐ or to indicate if there had been. The other nine studies gave insufficient details to be absolutely certain the trialists had ensured concealment of allocation. Four studies gave some details of the randomisation process. In the trial by De Reuck patients were stratified by centre (De Reuck 1995). Spudis (Spudis 1973) used random number tables and Tanaka (Tanaka 1997) used coin‐toss to allocate patients. In the remaining trials it is only stated that the patients were randomly allocated. Although authors have been contacted, no further details have so far been made available to the review team. Two trials, Bakchine (Bakchine 1990), and Gupta (Gupta 1995), used cross over methodology. Only the results from the first, pre‐cross‐over period of the study are considered in the review, as any improvements in people during the second period could have been due to a learning effect carried over from the first period.
Bias
The trials were assessed to determine to what degree they had eliminated the following types of bias.
Performance bias
This appears to have been eliminated from nine of the 10 trials, or five out of the six comparisons. As far as it is possible to tell, all nine were double blinded with patients unaware of their treatment status, and with both groups of patients in each trial receiving identical co‐interventions. Enderby (Enderby 1994) made efforts to minimise centre differences. However in the trial of bifemelane (Tanaka 1997) and in the trial of Dextran 40 (Spudis 1973), it appeared that the patients in the control group did not receive a placebo. Because of this, patients would therefore have been aware of their treatment status: patients would have given informed consent to be randomly assigned to receiving a treatment or not, and would then have been aware that they fell into the group not receiving treatment, since a placebo does not appear to have used.
Exclusion bias
In two trials, analysis is by intention to treat, and there appears to have been no exclusion bias (De Reuck 1995; Platt 1993). In the Herrschaft study, four (9%) of the total number randomised were lost to follow up because of adverse events (Herrschaft 1988). In the Enderby study, the number of patients available for analysis dropped between the start of the trial and the 12‐week assessment from 67 to 30 (55% lost) in the treatment group, and from 70 to 36 (49% lost) in the control group (Enderby 1994). By the 24‐week assessment these numbers had dropped to 20 and 21 respectively (70% lost from the original numbers in both cases). Further details have been sought from the author, who reports that the drop outs were mainly associated with patients attending European centres remote from their home base (a common occurrence in some countries). They were lost to follow up at various points due to being relocated to their home district which was too remote for them to remain in the trial. In the study by Poeck et al, the patients of interest have not been separated for the purposes of analysis from other patients ineligible for this review. However an overall figure of 16% drop out is given for this study, patients being mainly lost because they did not fulfil the protocol stipulations or they withdrew consent (Poeck 1993). In the study by Spudis, nine patients were lost to follow up in the treatment group ‐ one patient was excluded after the start of the study because of a serious adverse event, and eight patients died (27% lost to follow up). In the control group six patients died (21% lost) (Spudis 1973). All patients randomised were followed up in the studies by Gupta (Gupta 1995), and Tanaka (Tanaka 1997). Details of events giving rise to suspicion of exclusion bias are not given in the trial by Backchine (Bakchine 1990) or Price (Price 1992).
Detection bias
Gupta (Gupta 1995) and Enderby (Enderby 1994) gave information about the blinding of the testing therapists, who were not aware of the treatment status of the patients. The assessors in Spudis (Spudis 1973) assessors were probably blinded, and Tanaka (Tanaka 1997) blinded the assessing speech therapist until the second evaluation. No details concerning the blinding of assessors were available in the remaining studies. If the person assessing the patient is aware of the treatment status of the patient, it is possible that assessments were biased.
Potential conflicts of interest
None of the trials gives a statement about potential conflict of interest. It is possible that the manufacturers of drugs used in the trials were overly involved in the trials.
Effects of interventions
Reporting of the 10 eligible controlled trials varied in quality and a full description and analysis was only possible in one case (Enderby 1994). A summary of the results follows.
N: number randomised n: number of patients available for final assessments at the end of the trials SD: standard deviation OR: odds ratio CI: confidence interval
The trials contained six different comparisons.
(1) Piracetam compared with placebo
Five trials, N = 661, but the total number of patients available for final assessment is unknown:
De Reuck 1995: all disability N = 927, n = 927, aphasic patients N = 373, n = 373;
Enderby 1994: N = 158, n = 41;
Platt 1993: N = 56, n = 56;
Herrschaft 1988: N = 44, n = 17;
Poeck 1993: N = 30, n = unknown.
Data were available in an appropriate form for:
27 of the prespecified outcomes in the Enderby trial (Enderby 1994);
eight in the De Reuck trial (De Reuck 1995);
three in the Herrschaft trial (Herrschaft 1988);
three in the Platt trial (Platt 1993);
none in the Poeck trial (Poeck 1993).
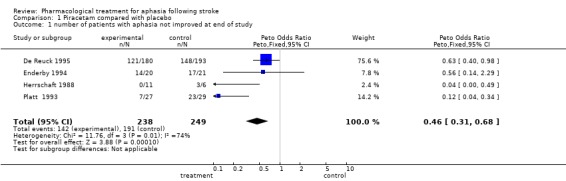
Outcome 1: Speech and language
Number of patients with aphasia not improved on any measure at the end of the trials
Data were available from four trials (De Reuck 1995; Enderby 1994; Herrschaft 1988; Platt 1993). A statistically significant difference was found in favour of treatment (i.e. more patients receiving treatment than placebo have aphasia improved at end of trial). OR = 0.46, 95% CI = 0.3 to 0.7.
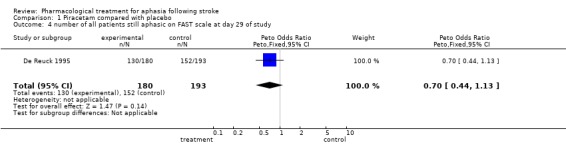
Number of patients who received early treatment still aphasic on FAST scale (Enderby 1987) at end of trial
Data were available from one trial (De Reuck 1995). A statistically significant difference was found in favour of treatment (i.e. more patients receiving early treatment than placebo have aphasia improved at end of trial). OR = 0.5, 95% CI = 0.3 to 0.9. (NB The post‐hoc analysis in this study of the relationship between the degree of impairment and improvement, if treated early, is not considered in this review.)
Patients improved on sub‐scales of Aachen Aphasia Test (Huber 1983)
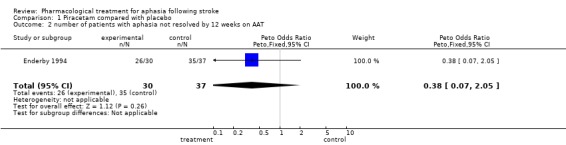
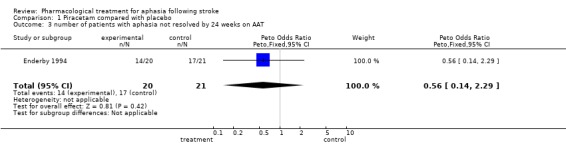
Data were available from one trial (Enderby 1994). No statistically significant differences were found between the two groups on any sub‐scales at either time interval (12 and 24 weeks) except for the score on language repetition at 24 weeks: a statistically significant difference was found in favour of treatment (patients receiving treatment had aphasia improved to greater degree on the sub‐scale than patients receiving placebo). Mean difference = 33.4, 95% CI = 3.1 to 63.7.
The very large proportion of patients lost to follow up in the Enderby trial preclude reliable intention‐to‐treat analysis. However, repeating this analysis with the Enderby trial data excluded did not materially alter the conclusions.
Outcome 2: Adverse events
Number of adverse events, defined to include death, but not drop‐out from the study, all patients by end of trial
Data were available from four trials (De Reuck 1995; Enderby 1994; Herrschaft 1988; Platt 1993). No statistically significant difference was found between the two groups. OR = 1.29, 95% CI = 0.9 to 1.7.
Number of adverse events, defined to include both deaths and drop outs from the study
These data were analysed in a variety of combinations but no statistical differences were found between the groups however the results were analysed.
(2) Bifemelane compared with no active substance
One trial: Tanaka 1997. Outcome: Speech and language ‐ Japanese Standard Language Test (SLTA 1977): Data are not in an appropriate form for consideration in this review as the meanings of the figures given are not explained in the context of the test used. The authors report that patients receiving bifemelane improved their naming ability and comprehension to a greater degree than those receiving no such support.
(3) Piribedil compared with no active substance
One trial: Bakchine 1990. Outcome: Speech and language ‐ Boston Diagnostic Aphasia Evaluation (Kaplan 1983): The authors do not report any raw data, and do not give results or conclusions in any form.
(4) Bromocriptine compared with placebo
One trial: Gupta 1995. Outcome: Speech and language ‐ Western Aphasia Battery (Kertesz 1982), Boston Naming Test (Kaplan 1983): Data are not reported in an appropriate form for re‐analysis in this review, as raw data are not reported for tests carried out when the first stage of this cross‐over trial was concluded. (This is the only stage considered eligible for consideration in this review). The authors report that no statistically significant differences were found between the two groups on any measure after this stage was over. They conclude that bromocriptine did not significantly improve the patients' speech fluency, language content, or overall degree of aphasia severity.
(5) Idebenone compared with placebo
One trial: Price 1992. Outcome: Speech and language ‐ Western Aphasia Battery (Kertesz 1982): Data are not available in an appropriate form for this review, as the numbers of patients in each group are not given. The authors report that no statistically significant differences were found between the groups.
(6) Dextran 40 compared with no active substance
One trial: Spudis 1973. Outcome 1: Speech and language ‐ number of patients worsened/not improved at end of study (unstandardised scale): No statistically significant difference was found. OR = 1.99, 95% CI = 0.6 to 7.0.
Outcome 2: Morbidity/mortality ‐ number of patients who died/experienced adverse event: No statistically significant difference was found. OR = 1.6, 95% CI = 0.5 to 5.2.
Discussion
The objectives of this review were to examine whether drug therapy was more effective for people who have aphasia following stroke than no drug support; whether drug therapy gave a better outcome than speech and language therapy alone; and whether a particular drug regimen performed better than another in this respect.
In order to identify the trials included in this review, a considerable number of study reports were appraised and rejected for a variety of reasons. It should be noted that, in the context of this review, no exhaustive or systematic attempt has been made to identify all non‐randomised studies of drug treatment for aphasia after stroke.
The quality and size of most of the eligible trials identified are such that any conclusions from this review are highly tentative. There were no eligible studies comparing speech and language therapy with a drug, and none comparing one drug with another.
For only one drug/placebo comparison was more than one trial identified. Since data were missing, not given in an appropriate form, or were from one small study only, it is possible therefore to come to a tentative conclusion about this one drug only (piracetam). However, lack of evidence of the effect of any of the interventions reviewed does not mean that the review has identified evidence of no effect.
Those agents which were single examples from various classes of drugs were idebenone, an antioxidant believed to prevent cell death, and Dextran 40 which is a glucose polymer used to expand plasma volume.
Four of the six drugs identified in the trials could be grouped together in pairs according to family or action: piracetam and bifemelane, from the nootropic class, are both believed to be metabolic activators, which may improve cognitive function, and/or act as neuroprotectors. Bromocriptine and piribedil are both dopamine agonists, which activate cerebral function. Claims by authors of a trial of the nootropic compound bifemelane about the benefits of this substance could not be verified from the report of the study.
It is possible to examine the results from the piracetam trials in more detail. Data were available for four of the five trials which studied the effects of piracetam. Only one of the trials can be considered large (De Reuck 1995). In spite of suspicions that the subgroup analysis of the aphasic patients in this study may have been performed post hoc, there is no certainty of this, and the decision was taken to include these patients. One study (Enderby 1994) lost a large proportion of patients to follow up for reasons which were later clarified through personal communications to be largely due to practical issues. The four trials were therefore judged sufficiently homogeneous to be considered together. Meta‐analysis indicated that those with aphasia who received piracetam following a stroke were more likely to have their aphasia improved at the end of the study than those who received a placebo.
Enderby also gave the results of a number of sub‐scales of a test, but in only one case (repetition test at 24 weeks), out of a total of 22 considered, was a statistically significant difference found, in favour of piracetam, and only 31 patients were available for this analysis (Enderby 1994). The very large proportion of patients lost to follow up in this trial precludes reliable intention‐to‐treat analysis.
Patients treated with piracetam were not more likely than those given placebo to experience adverse effects (no statistically significant differences, although the confidence intervals are wide). The study reports identified do not give any information about the benefits or disadvantages of piracetam in the long term. It is possible that the benefit of piracetam is lost if it is taken for months rather than weeks, although it is also feasible that the drug could have a greater benefit if given for a longer rather than shorter period.
However this review did find a non significant increase in deaths in the piracetam treated group. A Cochrane review of the effect of piracetam in acute stroke found a statistically non‐significant increase in the odds of death with piracetam (Ricci 2000). Ricci raises the possibility that in the trials they considered, there may have been an imbalance at baseline, resulting in more patients with more severe strokes being allocated to piracetam. An alternative, unproven hypothesis could be that the patients with the more severe strokes (and so more likely to have severe aphasia) allocated piracetam, were more likely to die, hence biasing the results ‐ in survivors ‐ in favour of piracetam.
Potential conflicts of interest, where the manufacturers may have been overly involved in trials, could give rise to some doubts about impartiality of the reporting of the trials identified.
We have chosen to concentrate on evidence from randomised controlled trials to minimise the likelihood of being misled by bias; the problem in interpretation is that all estimates of effect are very imprecise. Reviews based on a wider literature will be less prone to imprecision, but the problem with their interpretation is that the estimates are much more likely to be biased.
Authors' conclusions
Implications for practice.
It is not possible, based on the results of this review, to give prescribing advice to doctors who manage patients who are aphasic following stroke. Speech and language therapists cannot prescribe, but this review found no basis for advising that any drug is either superior or inferior to speech and language therapy. The findings concerning piracetam may be of interest to doctors, therapists, patients and carers, but it should be noted that piracetam is not licensed for prescription in the UK for people with aphasia (it is licensed for prescription to people with cortical myoclonus). Piracetam can be obtained without prescription in parts of Europe and the US, and is also available through the World Wide Web.
Implications for research.
All the drugs identified in this review have been under‐studied. The only drug which appears to give some promise based on this review is piracetam. While no conclusive evidence about the benefit of piracetam could be identified, it would appear that there is sufficiently rigorously established information to encourage further research of this particular substance. If researchers wish to respond to this, consideration should be given to a large multi‐centre trial, with sufficient attention paid to patients' language performance in the short and long term. Outcome measures used should be robust.
The contrasting effects of piracetam compared to speech and language therapy could also be usefully studied, and would avoid the need to randomise patients to a 'no treatment' group. Alternatively., speech and language therapy could be given to both groups of patients, with only one group receiving piracetam. Ethical objections to the withholding of speech and language therapy treatment could thus be avoided.
Outcomes focusing on the views and perceptions of the patients and those who care for them should be used in any further studies of treatment for aphasia.
There are a number of methodological traps that any researcher undertaking such a trial would need to take care to avoid, wherever possible. For example trialists must be clear about whether they are evaluating the effects of a drug on stroke recovery generally, or just on language recovery. Assessment of outcome should be blinded, and any side effects should be reported. In view of some (unproven) concerns about the selective culling of severely aphasic patients, numbers of patients 'dead or aphasic at end of trial' should be reported.
Care should be taken to ensure full randomisation, with blinding of outcome.
Feedback
Incorrect citation, 23 March 2010
Summary
Regarding the reference: Huber W, Willmes K, Poeck K, Van Bleyman B, Deberdt W. Piracetam as an adjuvent to language therapy for aphasia: a randomised double‐blind placebo‐controlled pilot study. Archives of Physical Medicine and Rehabilitation 1997;78(3):345‐50.
This reference is cited incorrectly. It should be pages 245‐50. This made its discovery difficult.
Reply
The reference has been corrected.
Contributors
Submitter: William Jones Responder: Hazel Fraser
What's new
| Date | Event | Description |
|---|---|---|
| 23 March 2010 | Feedback has been incorporated | The following reference for the included study Poeck 1993 has been corrected: Huber W, Willmes K, Poeck K, Van Bleyman B, Deberdt W. Piracetam as an adjuvent to language therapy for aphasia: a randomised double‐blind placebo‐controlled pilot study. Archives of Physical Medicine and Rehabilitation 1997;78(3):245‐50 |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 24 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The reviewers would like to thank Professor Adrian Grant, Health Services Research Unit, University of Aberdeen.
Appendices
Appendix 1. MEDLINE search strategy
1. exp. aphasia/ 2. Language disorders/ 3. Speech disorders/ 4. Speech‐language pathology/ 5.Language therapy 6. Speech therapy/ 7 (aphasi$ or dysphasi$)tw. 8. ((speech or language) adj 10 (disorder$ or therap$ or treat$ or rehabilitat$ or remediat$)or intervention or patholog$)) tw. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. drug evaluation/ 11. drug therapy/ 12. (drug or pharma$).tw. 13. (therp$ or treat$ or evaluat$ or interven$).tw. 14. 12 and 13 15. Bromocriptine/ or Ergolines/or Ergot alkaloids/ 16. Piracetam/ or Piribedil/ 17. Dopa/ or Levodopa 18. Meprobamate/ 19. pharmacotherapy.tw 20. (piracetam or nootropil or piribedil or neurotrop$ or bromocriptine or dopamin$ or l‐dopa or idebenone or bifemalene).tw. 21. 10 or 11 or 145 or 15 or 16 or 17 or 18 or 19 or 20 22. 9 and 21 23. exp aphasia/dt (subheading drug therapy) 24. speech disorders 25. 22 or 23 or 24
Data and analyses
Comparison 1. Piracetam compared with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 number of patients with aphasia not improved at end of study | 4 | 487 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.31, 0.68] |
| 2 number of patients with aphasia not resolved by 12 weeks on AAT | 1 | 67 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.07, 2.05] |
| 3 number of patients with aphasia not resolved by 24 weeks on AAT | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.14, 2.29] |
| 4 number of all patients still aphasic on FAST scale at day 29 of study | 1 | 373 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.44, 1.13] |
| 5 number of all patients still aphasic on FAST scale at day 84 of study | 1 | 373 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 6 number of patients with early treatment still aphasic on FAST scale at day 29 of study | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.34, 1.22] |
| 7 number of patients with early treatment still aphasic on FAST scale at day 84 of study | 1 | 197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.25, 0.86] |
| 8 difference in aphasia scale scores before and after treatment | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.07, 1.27] |
| 9 spontaneous language rating (1) at week 12 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.33, 0.93] |
| 10 spontaneous language rating (1) at week 24 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.76, 0.96] |
| 11 spontaneous language rating (2) at week 12 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.41, 1.01] |
| 12 spontaneous language rating (2) at week 24 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.86, 0.86] |
| 13 spontaneous language rating (3) at week 12 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.42, 1.42] |
| 14 spontaneous language rating (3) at week 24 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.30, 1.10] |
| 15 spontaneous language rating (4) at week 12 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.13, 1.73] |
| 16 spontaneous language rating (4) at week 24 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.20, 1.00] |
| 17 spontaneous language rating (5) at week 12 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.39, 1.39] |
| 18 spontaneous language rating (5) at week 24 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.93, 1.33] |
| 19 spontaneous language rating (6) at week 12 | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.20, 1.40] |
| 20 spontaneous language rating (6) at week 24 | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.77, 1.37] |
| 21 score on token test at 12 weeks | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐3.66, 11.06] |
| 22 score on token test at 24 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐9.90, 9.50] |
| 23 score on repetition test at 12 weeks | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 8.30 [‐16.10, 32.70] |
| 24 score on repetition test at 24 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 33.40 [3.12, 63.68] |
| 25 score on written language test at 12 weeks | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 9.70 [‐4.81, 24.21] |
| 26 score on written language test at 24 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐17.20, 22.20] |
| 27 score on confrontation naming test at 12 weeks | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐9.43, 27.43] |
| 28 score on confrontation naming test at 24 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐14.90, 21.30] |
| 29 score on comprehension test at 12 weeks | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 10.20 [‐0.23, 20.63] |
| 30 score on comprehension test at 24 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 8.30 [‐4.52, 21.12] |
| 31 Aachen Aphasia Test score at end of 42 days of treatment | Other data | No numeric data | ||
| 32 Frenchay Aphasia Test score after 4 weeks of treatment | Other data | No numeric data | ||
| 33 Frenchay Aphasia Test score after 12 weeks of treatment | Other data | No numeric data | ||
| 34 Orgogozo Scale at end of 4 weeks | 1 | 927 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐3.97, 4.17] |
| 35 number of deaths, any type of patient, at end of trial | 4 | 1160 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.95, 1.77] |
| 36 number of dropouts, any cause, by end of trial (ie people not included in test score analyses). | 3 | 258 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.31, 1.66] |
| 37 number of patients experiencing adverse events, including death, at end of trial | 4 | 1185 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.97, 1.74] |
| 38 number of deaths of aphasic patients at end of study | 3 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.05, 5.41] |
1.1. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 1 number of patients with aphasia not improved at end of study.
1.2. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 2 number of patients with aphasia not resolved by 12 weeks on AAT.
1.3. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 3 number of patients with aphasia not resolved by 24 weeks on AAT.
1.4. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 4 number of all patients still aphasic on FAST scale at day 29 of study.
1.5. Analysis.
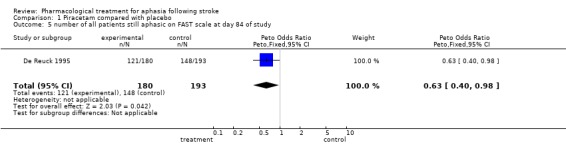
Comparison 1 Piracetam compared with placebo, Outcome 5 number of all patients still aphasic on FAST scale at day 84 of study.
1.6. Analysis.
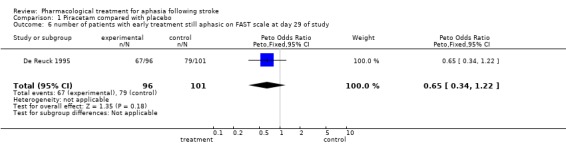
Comparison 1 Piracetam compared with placebo, Outcome 6 number of patients with early treatment still aphasic on FAST scale at day 29 of study.
1.7. Analysis.
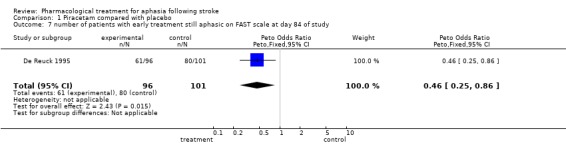
Comparison 1 Piracetam compared with placebo, Outcome 7 number of patients with early treatment still aphasic on FAST scale at day 84 of study.
1.8. Analysis.
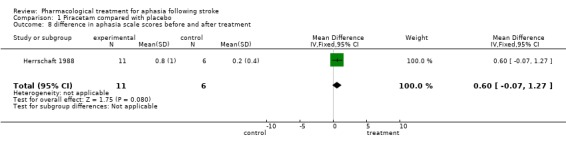
Comparison 1 Piracetam compared with placebo, Outcome 8 difference in aphasia scale scores before and after treatment.
1.9. Analysis.
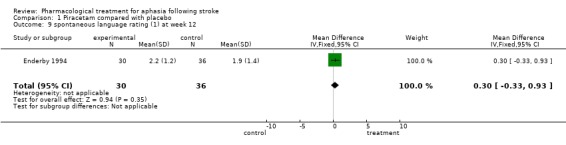
Comparison 1 Piracetam compared with placebo, Outcome 9 spontaneous language rating (1) at week 12.
1.10. Analysis.
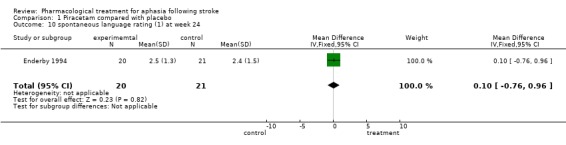
Comparison 1 Piracetam compared with placebo, Outcome 10 spontaneous language rating (1) at week 24.
1.11. Analysis.
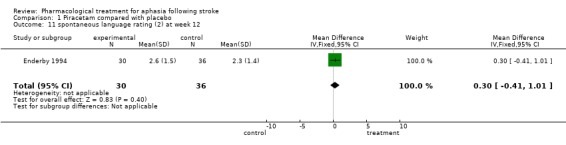
Comparison 1 Piracetam compared with placebo, Outcome 11 spontaneous language rating (2) at week 12.
1.12. Analysis.
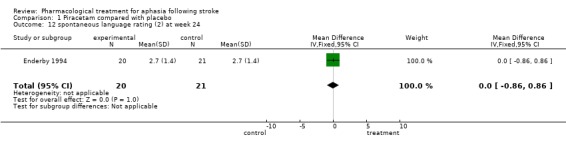
Comparison 1 Piracetam compared with placebo, Outcome 12 spontaneous language rating (2) at week 24.
1.13. Analysis.
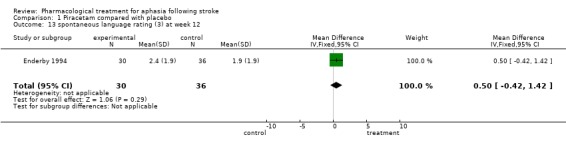
Comparison 1 Piracetam compared with placebo, Outcome 13 spontaneous language rating (3) at week 12.
1.14. Analysis.
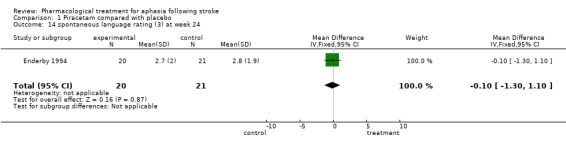
Comparison 1 Piracetam compared with placebo, Outcome 14 spontaneous language rating (3) at week 24.
1.15. Analysis.
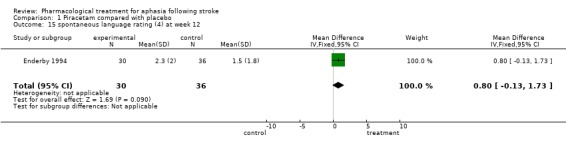
Comparison 1 Piracetam compared with placebo, Outcome 15 spontaneous language rating (4) at week 12.
1.16. Analysis.
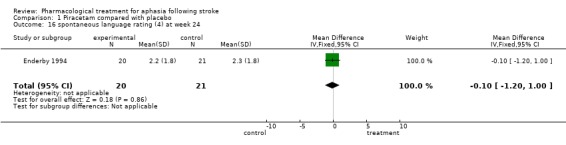
Comparison 1 Piracetam compared with placebo, Outcome 16 spontaneous language rating (4) at week 24.
1.17. Analysis.
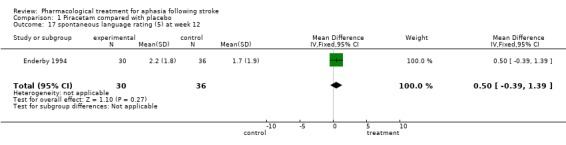
Comparison 1 Piracetam compared with placebo, Outcome 17 spontaneous language rating (5) at week 12.
1.18. Analysis.
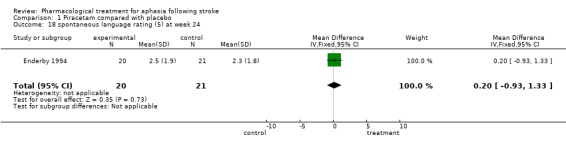
Comparison 1 Piracetam compared with placebo, Outcome 18 spontaneous language rating (5) at week 24.
1.19. Analysis.
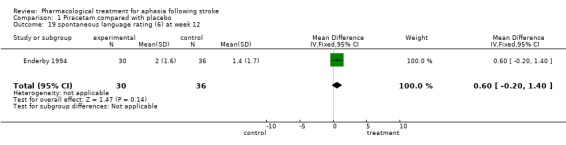
Comparison 1 Piracetam compared with placebo, Outcome 19 spontaneous language rating (6) at week 12.
1.20. Analysis.
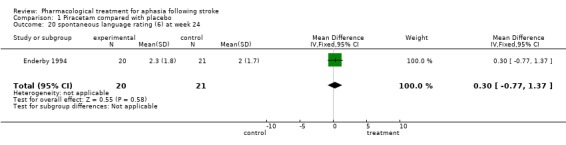
Comparison 1 Piracetam compared with placebo, Outcome 20 spontaneous language rating (6) at week 24.
1.21. Analysis.
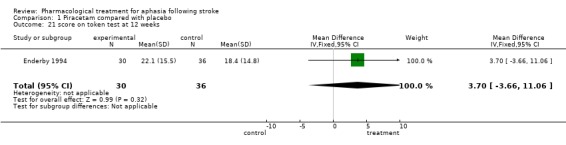
Comparison 1 Piracetam compared with placebo, Outcome 21 score on token test at 12 weeks.
1.22. Analysis.
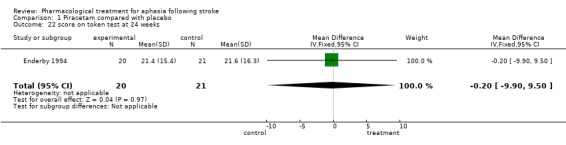
Comparison 1 Piracetam compared with placebo, Outcome 22 score on token test at 24 weeks.
1.23. Analysis.
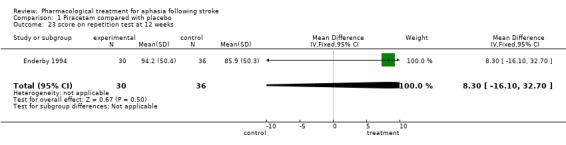
Comparison 1 Piracetam compared with placebo, Outcome 23 score on repetition test at 12 weeks.
1.24. Analysis.
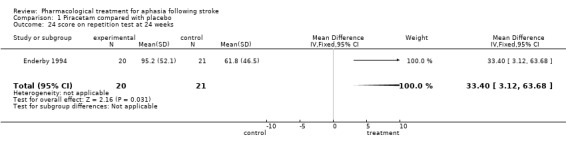
Comparison 1 Piracetam compared with placebo, Outcome 24 score on repetition test at 24 weeks.
1.25. Analysis.
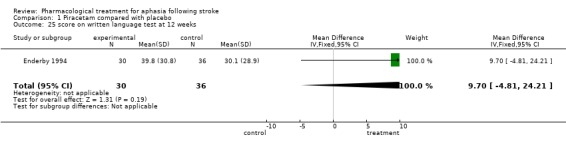
Comparison 1 Piracetam compared with placebo, Outcome 25 score on written language test at 12 weeks.
1.26. Analysis.
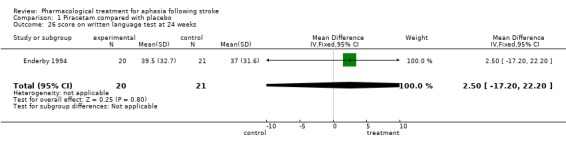
Comparison 1 Piracetam compared with placebo, Outcome 26 score on written language test at 24 weeks.
1.27. Analysis.
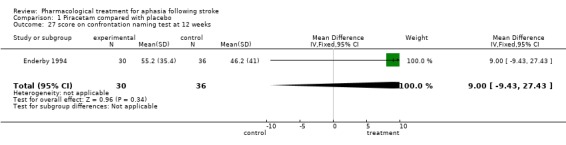
Comparison 1 Piracetam compared with placebo, Outcome 27 score on confrontation naming test at 12 weeks.
1.28. Analysis.
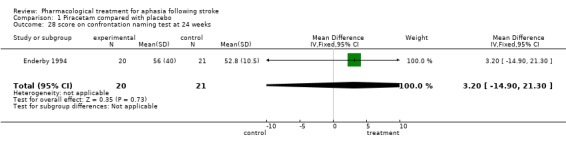
Comparison 1 Piracetam compared with placebo, Outcome 28 score on confrontation naming test at 24 weeks.
1.29. Analysis.
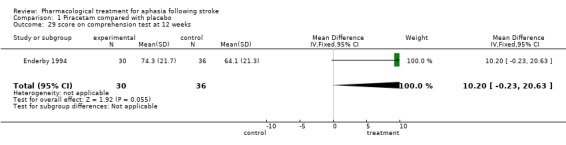
Comparison 1 Piracetam compared with placebo, Outcome 29 score on comprehension test at 12 weeks.
1.30. Analysis.
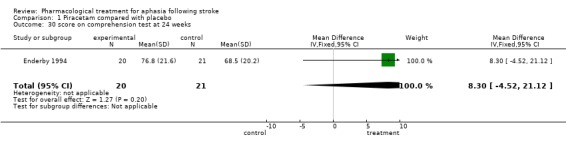
Comparison 1 Piracetam compared with placebo, Outcome 30 score on comprehension test at 24 weeks.
1.31. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 31 Aachen Aphasia Test score at end of 42 days of treatment.
| Aachen Aphasia Test score at end of 42 days of treatment | |
|---|---|
| Study | |
| Poeck 1993 | No raw data of any type are available in the reports of the pilot study, which is the only part of the study as a whole that is eligible for the review. |
1.32. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 32 Frenchay Aphasia Test score after 4 weeks of treatment.
| Frenchay Aphasia Test score after 4 weeks of treatment | |
|---|---|
| Study | |
| De Reuck 1995 | Neither mean test scores nor standard devations are available in the report. |
1.33. Analysis.
Comparison 1 Piracetam compared with placebo, Outcome 33 Frenchay Aphasia Test score after 12 weeks of treatment.
| Frenchay Aphasia Test score after 12 weeks of treatment | |
|---|---|
| Study | |
| De Reuck 1995 | Mean scores are given in the report, but without standard deviations. They are, therefore, not in an appropriate form for statistical analysis in this review. |
1.34. Analysis.
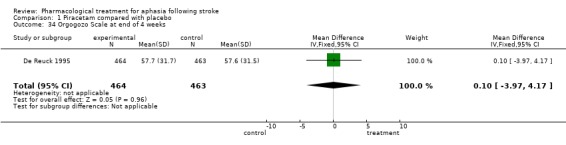
Comparison 1 Piracetam compared with placebo, Outcome 34 Orgogozo Scale at end of 4 weeks.
1.35. Analysis.
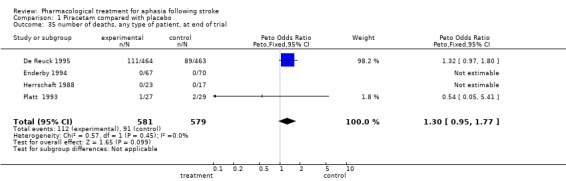
Comparison 1 Piracetam compared with placebo, Outcome 35 number of deaths, any type of patient, at end of trial.
1.36. Analysis.
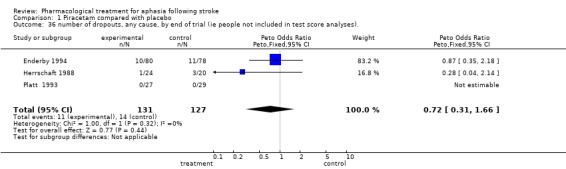
Comparison 1 Piracetam compared with placebo, Outcome 36 number of dropouts, any cause, by end of trial (ie people not included in test score analyses)..
1.37. Analysis.
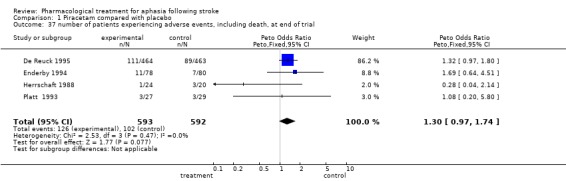
Comparison 1 Piracetam compared with placebo, Outcome 37 number of patients experiencing adverse events, including death, at end of trial.
1.38. Analysis.
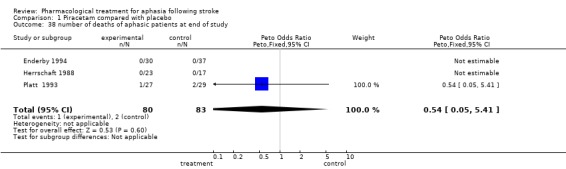
Comparison 1 Piracetam compared with placebo, Outcome 38 number of deaths of aphasic patients at end of study.
Comparison 2. Bifemalane compared with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Score on SLTA test one month after treatment | Other data | No numeric data |
2.1. Analysis.
Comparison 2 Bifemalane compared with placebo, Outcome 1 Score on SLTA test one month after treatment.
| Score on SLTA test one month after treatment | |
|---|---|
| Study | |
| Tanaka 1997 | Results are not given in a form appropriate for this review, as the meaning of the figures given are not explained in the context of the test used. |
Comparison 3. Piribedil compared with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Boston Diagnostic Aphasia Evaluation at end of trial | Other data | No numeric data | ||
| 2 Score Aphasiologique de la Saltpetriere at end of trial | Other data | No numeric data |
3.1. Analysis.
Comparison 3 Piribedil compared with placebo, Outcome 1 Boston Diagnostic Aphasia Evaluation at end of trial.
| Boston Diagnostic Aphasia Evaluation at end of trial | |
|---|---|
| Study | |
| Bakchine 1990 | Abstract only, no raw data or any results given. |
3.2. Analysis.
Comparison 3 Piribedil compared with placebo, Outcome 2 Score Aphasiologique de la Saltpetriere at end of trial.
| Score Aphasiologique de la Saltpetriere at end of trial | |
|---|---|
| Study | |
| Bakchine 1990 | Abstract only, no raw data or any results given. |
Comparison 4. Bromocriptine compared with placebo.
4.1. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 1 Western Aphasia Quotient at end of first period of study.
| Western Aphasia Quotient at end of first period of study | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.2. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 2 Western Aphasia Battery Auditory Comprehension score at end of first period of test.
| Western Aphasia Battery Auditory Comprehension score at end of first period of test | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. . |
4.3. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 3 Western Aphasia Battery Repetition score at end of first period of trial.
| Western Aphasia Battery Repetition score at end of first period of trial | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.4. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 4 Western Aphasia Battery Reading Comprehension score at end of first period of trial.
| Western Aphasia Battery Reading Comprehension score at end of first period of trial | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.5. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 5 Western Aphasia Battery Writing score at end of first period of trial.
| Western Aphasia Battery Writing score at end of first period of trial | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.6. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 6 Boston Naming Test score at end of first period of trial.
| Boston Naming Test score at end of first period of trial | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.7. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 7 Rey‐Osterrieth Figure Test score at end of first period of trial.
| Rey‐Osterrieth Figure Test score at end of first period of trial | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.8. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 8 Raven's Progressive Matrices Test score at end of first period of trial.
| Raven's Progressive Matrices Test score at end of first period of trial | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.9. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 9 Wechsler Memory Scale ‐ Revised figure memory score at end of test.
| Wechsler Memory Scale ‐ Revised figure memory score at end of test | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.10. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 10 Wechsler Memory Scale ‐ Revised visual paired associates score at end of test.
| Wechsler Memory Scale ‐ Revised visual paired associates score at end of test | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.11. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 11 Wechsler Memory Scale ‐ Revised visual memory span score at end of test.
| Wechsler Memory Scale ‐ Revised visual memory span score at end of test | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.12. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 12 Wechsler Memory Scale ‐ Revised Visual reproduction score at end of test.
| Wechsler Memory Scale ‐ Revised Visual reproduction score at end of test | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.13. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 13 mean phrase length at end of first period of study.
| mean phrase length at end of first period of study | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
4.14. Analysis.
Comparison 4 Bromocriptine compared with placebo, Outcome 14 information index score at end of first period of study.
| information index score at end of first period of study | |
|---|---|
| Study | |
| Gupta 1995 | Data are in an inappropriate form for reanalysis in the review as raw data are not given for the end of the first stage of the study, which is the period relevant for this review. |
Comparison 5. Idebenone compared with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Western Aphasia Battery cortical quotient test score at end of trial | Other data | No numeric data | ||
| 2 Western Aphasia Battery aphasia quotient test score at end of trial | Other data | No numeric data | ||
| 3 Mini Mental State Test score at end of trial. | Other data | No numeric data | ||
| 4 Hamilton Depression Scale score at end of trial | Other data | No numeric data | ||
| 5 Barthel Index score at end of trial | Other data | No numeric data | ||
| 6 Fugl‐Meyer Motor Score test at end of trial | Other data | No numeric data |
5.1. Analysis.
Comparison 5 Idebenone compared with placebo, Outcome 1 Western Aphasia Battery cortical quotient test score at end of trial.
| Western Aphasia Battery cortical quotient test score at end of trial | |
|---|---|
| Study | |
| Price 1992 | Data are not reported in an appropriate form for reanalysis in this review, as neither the numbers of patients in the two groups nor standard deviations are given. |
5.2. Analysis.
Comparison 5 Idebenone compared with placebo, Outcome 2 Western Aphasia Battery aphasia quotient test score at end of trial.
| Western Aphasia Battery aphasia quotient test score at end of trial | |
|---|---|
| Study | |
| Price 1992 | Data are not reported in an appropriate form for reanalysis in this review, as neither the numbers of patients in the two groups nor standard deviations are given. |
5.3. Analysis.
Comparison 5 Idebenone compared with placebo, Outcome 3 Mini Mental State Test score at end of trial..
| Mini Mental State Test score at end of trial. | |
|---|---|
| Study | |
| Price 1992 | Data are not reported in an appropriate form for reanalysis in this review, as neither the numbers of patients in the two groups nor standard deviations are given. |
5.4. Analysis.
Comparison 5 Idebenone compared with placebo, Outcome 4 Hamilton Depression Scale score at end of trial.
| Hamilton Depression Scale score at end of trial | |
|---|---|
| Study | |
| Price 1992 | Data are not reported in an appropriate form for reanalysis in this review, as neither the numbers of patients in the two groups nor standard deviations are given. |
5.5. Analysis.
Comparison 5 Idebenone compared with placebo, Outcome 5 Barthel Index score at end of trial.
| Barthel Index score at end of trial | |
|---|---|
| Study | |
| Price 1992 | Data are not reported in an appropriate form for reanalysis in this review, as neither the numbers of patients in the two groups nor standard deviations are given. |
5.6. Analysis.
Comparison 5 Idebenone compared with placebo, Outcome 6 Fugl‐Meyer Motor Score test at end of trial.
| Fugl‐Meyer Motor Score test at end of trial | |
|---|---|
| Study | |
| Price 1992 | Data not reported in an appropriate form for reanalysis in this review, as neither the numbers of patients in the two groups nor standard deviations are given. |
Comparison 6. Dextran 40 compared with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 number of patients worsened or not improved on four item language scale at end of trial | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [0.57, 7.00] |
| 2 number of patients died/experienced an adverse effect | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [0.51, 5.18] |
6.1. Analysis.
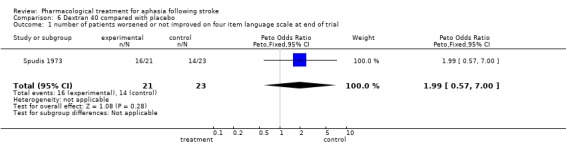
Comparison 6 Dextran 40 compared with placebo, Outcome 1 number of patients worsened or not improved on four item language scale at end of trial.
6.2. Analysis.
Comparison 6 Dextran 40 compared with placebo, Outcome 2 number of patients died/experienced an adverse effect.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakchine 1990.
| Methods | Randomised double blind cross‐over placebo controlled trial, no details are available of this process in the abstract. | |
| Participants | N and setting ‐ not possible to determine these from the abstract., but the setting was most likely to have been France. Participants were people with aphasic symptoms with pure subcortical extrathalamic lesions. People were only included if the improvement of their aphasic symptoms was less than 40% during a one month period of preinclusion. | |
| Interventions | Piribedil 100mg per day in two daily doses versus placebo, given for one four week treatment period and then for one four week control period without wash out at cross‐over. 'Usual speech therapy' was provided to all participants during preinclusion and essay periods. This therapy is not described in the report. Piribebil is a dopamine D2 agonist. | |
| Outcomes | Participants were evaluated at the end of each treatment period. They were tested on 5 sub tests of the Boston Aphasia Examination (BDAE, Goodlass 1972), and on the Score Aphasiologique de la Saltpetriere (no reference to this test is available). | |
| Notes | An abstract is the only report available. The author has written for further details, but no reply has been received at the time of the completion of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
De Reuck 1995.
| Methods | Double blind randomised placebo controlled trial. Participants were randomised to treatment or placebo groups using a computer generated randomisation schedule stratified by study centre. Results were analysed by intention to treat, missing data were estimated by the last available score. No details are available about whether assessors were blinded to the treatment status of the participants. Participants or relatives provided written consent. | |
| Participants | 927 people, from 55 hospitals in 10 European countries, of which 373 were aphasic and were analysed separately from the others (placebo group n=193, treatment group n= 180). The age range of participants was 40 to 80 years (the mean age of aphasic people was 72.9 years). All had been admitted to hospital with a clinical diagnosis of an acute ischaemic supratentorial stroke. People entered the study if arousable, and if their symptoms within the preceding 12 hours were judged disabling on the Orgogozo scale (Orgogozo 1986). The diagnosis was confirmed by one or two CT scans. Exclusion criteria: A scan showing first evidence of cerebral haemorrhage and significant midline shift Significant stupor or coma A previous stroke with clinical sequelae Confounding neurologic or systemic illness Dipyridamole and ticlopidine were prohibited during the first 4 weeks of the study. Concomitant aspirin was not recommended for at least 24 hours after the stroke. Thrombolytic agents, haemodilution and drugs acting on cerebral vasculature were forbidden throughout. Specific entry criteria for the aphasic patients: People were only admitted whose mother tongue was the same as the language of the presented FAST (Enderby 1987) test version, and who could read before the stroke. People were deemed to be aphasic if they scored 13/20 or less on the expression/comprehension subscales of the FAST scale (Enderby 1987). | |
| Interventions | Piracetam 12 gram in bolus intravenously within 12 hours post stroke onset, followed by piracetam 12 gram per day intravenously until 4th day. Thereafter 12 gram piracetam per day orally until 4 weeks, then 4.8 gram per day for 8 weeks. Piracetam belongs to a unique pharmacologic class and has been suggested to act as both a neuroprotective and neurotropic agent. | |
| Outcomes | Aphasic participants were assessed with the Frenchay Aphasia Test (FAST, Enderby 1987). Tests of neurological functioning (Orgogozo 1983), and behaviour (Barthel Index, Mahoney 1965) were also given, the analysis being performed on an intention‐to‐treat basis, with missing data estimated by the last available score. The authors report that a statistically significant difference was found in favour of the treated aphasic group on the FAST test at day 84 (the level of significance is variously reported in the papers written concerning this study). The authors also report that no statistically significant differences were found between the two groups on other parameters tested in the study population as a whole.
However in a predefined sub‐group analysis of a group who were aphasic, and treated within 7 hours of the stroke, the authors reported statistically significant differences in language function on aphasia testing, and in other parameters of functioning at the end of the study period, in favour of the treated group.
A post hoc analysis of the early treated group reportedly showed that those treated within 7 hours of the stroke performed better on treatment than on placebo if they were originally diagnosed as having a moderate to marked impairment. This difference in favour of piracetam did not apply to those with mild impairment. No difference in mortality rates was found for the population as a whole, and it is not possible to separate those who had an adverse event and died later, from those experiencing only an adverse event. Death rates for the aphasic participants are not reported separately. An economic analysis showed that total cost per participant was lower in the treated group than in the placebo group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Enderby 1994.
| Methods | Randomised multi‐centre, double blind parallel group study. Computerisation was used to allocate participants. Meetings were held to minimise differences between centres and data collectors. |
|
| Participants | The study was performed at six centres, three in Belgium, three in The Netherlands (contact with author confirmed that these centres were either hospitals, or rehabilitation hospitals). A total of 158 people were enrolled, 80 to placebo, 78 to piracetam. 66 of the total were aphasic at entry; 30 of these were in the piracetam group, 37 received placebo. Eligible people were aged between 21 and 85 years and recovering from intracerebral haemorrhage or thromboembolic infarction sustained more than five and less than ten weeks previously. Inclusion criteria: A clinical deficit affecting motor, peripheral or language functions Suitable for rehabilitation therapy Exclusion criteria: A premorbidly low IQ Unable to care for themselves premorbidly A defect in sight or hearing sufficient to limit testing A history of previous cerebral infarction A history of other organic cerebral disease A psychotic or other psychiatric disorder requiring neuroleptic or anti‐depressant therapy The two groups were generally comparable, but there was a significantly higher incidence of hypercholesteraemia in the placebo group. Also, the treatment group had a higher score on the Rivermead Perceptual Assessment Battery, i.e. disability was less severe. 46% of those in the treatment group and 37% of those in the placebo group had a left sided hemiparesis. People were enrolled after a washout period of one week. The results of a total of 137 people were available for analysis at week 12; 88 were available at week 24. Exclusions from the analysis were because deviations from the protocol had occurred. | |
| Interventions | Piracetam versus placebo for twelve weeks, piracetam given was 4.8 gram per day, in a dosage of 12 ml twice daily as a 20% solution. Smell, appearance and taste of placebo and piracetam solutions were identical.
Piracetam belongs to a unique pharmacologic class and has been suggested to act as both a neuroprotective and neurotropic agent. All aphasic participants also received speech and language therapy for the duration of the study. |
|
| Outcomes | Assessments were carried out at weeks five, 12 and 24, final assessment being 12 weeks after cessation of treatment. Participants were tested on the Barthel Index (Mahoney 1965), the Kurianski Daily Living test (Kurianski 1976), and the Rivermead Perceptual Assessment Battery (Whiting 1985). The principal test of language performance was the Aachen Aphasia Test (AAT, Huber 1983), which was broken down into sub‐scales, with scores given for each. Authors report that differences between the two groups approached significance at 12 weeks in certain sub tests of the AAT (written language and comprehension). No significant differences were reportedly found between the two groups at 24 weeks on any tests. Adverse events occurred in 11 who had taken piracetam and 7 who had received the placebo. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gupta 1995.
| Methods | Randomised controlled cross‐over trial. The method of randomisation is not described in the report. The trial consisted of two phases, at the start of the second phase participants crossed over to receive the treatment they had not received in the first phase. The testing therapist was not aware of the treatment status of participant. Participants were examined by a physician at each visit for monitoring of side effects and compliance. All participants signed to give informed consent indicating their understanding of the risks and benefits of the study. | |
| Participants | As far as can be determined from the report, the study took place in a single centre in the USA. Participants were 20 adult men, from 43 screened, all with cerebral infarction incurred during the previous year, which had resulted in aphasia. All had to be able to understand and sign to give the informed consent. The average age of the participants was 62 years, with a median 61. Two were left handed. Seventeen had a right hemi‐paresis. The mean phrase length of the participants' utterances was one to five words, with a score greater than 5 on the Auditory Comprehension subscore of the Western Aphasia Battery (WAB, Kertesz 1982). Exclusion criteria: Significant dysarthria Language other than English as first language Education level less than eighth‐grade or unable to read and write before the stroke Already receiving language therapy Uncontrolled hypertension Sensitivity to ergot alkaloids Significant renal or hepatic disease Receiving concurrent therapy with phenothiazines or butyrophenones. | |
| Interventions | During first phase of study (weeks 1‐8) people received either bromocriptine or placebo. The drug or placebo was given as one capsule (5 mg) daily, increasing to 3 capsules (15 mg) by the third week.
During the second phase the participants crossed over to the alternative arm of the trial. A washover period of six weeks elapsed between the two stages, with the dose gradually reduced over 2 weeks, followed by 4 drug free weeks. Bromocriptine is a semi‐synthetic ergot alkaloid, which acts as a dopamine D2 agonist. |
|
| Outcomes | Each participant's speech and language and nonverbal cognitive abilities were evaluated at the beginning and end of each phase of the study, and also 6 weeks after the completion of the second phase (cross over period). Five evaluation sessions were performed in all. Language tests used were the Western Aphaaia Battery (WAB, Kertesz 1982), and the Boston Naming Test (Kaplan 1983). Non verbal skills were tested with selected subtests of the Wechsler Memory Scale‐ Revised, including Figure Memory, Visual Paired Associates, Visual Reproduction 1, and Visual Memory subtests (Wechsler 1987), Raven's Progressive Matrices (Raven 1962), and the Rey‐Osterrieth Figure (Rey‐Osterrieth 1944). All measures were taken by a speech and language pathologist who was blinded to the treatment status of the person being assessed. Participants were also blinded to their own treatment status. | |
| Notes | It is not made explicit in the report whether the placebo and the bromocriptine tablets tasted or smelled the same. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Herrschaft 1988.
| Methods | Randomised prospective double blind controlled trial. No details of the randomisation process are available in the report. | |
| Participants | The setting was a single hospital in Germany. 44 people entered the study, who had been admitted to one clinic in this hospital. 40 were assessable at the end of the study. Participants were aged between 29 and 80 years, with an average age of 56.5, with acute cerebral ischaemic cerebral infarct that had existed from zero to maximum of 5 days before the beginning of the treatment. 23 patients (17 male) were assessable in the treatment group, 17 patients (10 male) were assessable in the placebo group. Exclusion criteria: Severe accompanying diseases, in particular cardiac, pulmonary, liver or renal insufficiency, insulin dependant diabetes mellitus, fixed hypertonia, neoplasia, haematological and systemic diseases Earlier neurological diseases of a different nature A history of alcohol or drug abuse Participants were not significantly different at baseline regarding sex, age, duration of illness before start of therapy, risk factors, or test scores. | |
| Interventions | Piracetam 3x4 g/20 ml in bolus, or placebo from first to fourteenth day, followed by either 4.8 g piracetam orally, or placebo in identical formulation for further 14 days. No other medication allowed during the 28 day treatment period. Participants were given remedial exercises five times weekly and, if necessary, speech therapy 3 times a week. No note is available in the report of who was thought to be in need of this therapy, in either of the two groups. All were treated with 1000 ml Dextran 40 as continuous infusion plus 2x150 ml Sorbit 40% daily during first three days, then 500 ml Dextran 40 (duration of infusion 4‐6 hours) from fourth to fourteenth day. |
|
| Outcomes | Aphasia was graded from 0 to 4 in severity. This was measured on the day of reception into the study, then on days 7, 14, and 28 thereafter. One person receiving piracetam and 3 receiving the placebo developed complications which led to them being withdrawn from the study. They were lost to follow up and have been treated as having suffered an adverse effect for the purposes of this review. The authors did not believe that the complications experienced were connected to the treatment. | |
| Notes | It is not made explicit in the report whether the tablets containing piracetam tasted the same as those which were a placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Platt 1993.
| Methods | Clinically controlled double blind study. No details are available of the allocation procedure. | |
| Participants | As far as can be determined from the text, the setting was a single hospital in Germany. 56 people were admitted to the study, with an acute disturbance of the cerebral blood flow, aged 73‐84 years. 52% of the treatment group and 66% of the placebo group were male. Inclusion criteria: The event had occurred within preceding 3 days Stenosis of internal carotid or intracranial arteries Aged more than 56 years Acute supratentorial first cerebral ischaemia confirmed by a CT scan Exclusion criteria: Contra indication of hypervolemic hemodilution. Acute myocardial infarction Severe cardiac insufficiency Severe renal insufficiency Malignant hypertension Polyglobulism Hemorrhagic diathesis Hemorrhagic infarct, cerebral hemorrhage Cerebral oedema confirmed by CT Disturbance of blood brain barrier Reinfarction Repeated TIAs or PRIND attacks Progressive cerebral degeneration Insulin dependant diabetes Malignancy Systemic disease | |
| Interventions | Once daily fast infusion (20 mins) of 12 g piracetam versus saline as placebo. 27 were treated with piracetam, 29 with placebo, for 14 days.
From day 15 people in the treatment group were given 2x800mg piracetam 3 times a day, those in the control group were given placebo tablets. A background therapy of 500mls HAES/200 10% was administered in 4 hours plus low dose heparin with 15000 U/day over the entire period of the study. |
|
| Outcomes | Participants were assessed on a 4 point scale of language function. The authors report that a statistically significant difference was found in favour of the treatment group regarding aphasia and all other parameters tested, except sensitivity to the piracetam. 3 people died, 1 from the treatment group, 2 from those receiving the placebo. 2 further people were withdrawn from the treatment group and one from the placebo group because of illness. All these people were considered therapeutic failures for the statistical analysis. Authors reported that they did not believe there to have been any unwanted effects found during the trial. All the remaining 50 participants completed the 4 week period of the study. | |
| Notes | No explicit mention was made in the report about whether the placebo tablets looked and tasted like piracetam tablets. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Poeck 1993.
| Methods | Double blind randomised parallel‐group study. No details of the randomisation process are available in the report. | |
| Participants | Setting was the RWTH‐Aachen Hospital in Germany. Participants were 30 people with chronic aphasia of cerebrovascular origin and of at least 6 weeks duration.
Inclusion criteria:
In‐patients
Right handed
Pre‐stroke speakers of German as a native language
Exclusion criteria:
Rcent myocardial infarction
Severe cardiac insufficiency
Severe hypertension
Carcinoma
Severe renal insufficiency
Serious diabetes mellitus
Certain concomitant medications At baseline both groups were comparable for age and severity of the stroke, and distribution of their Aachen Aphasia Test scores (AAT, Huber 1984). This was a pilot study for a later study. |
|
| Interventions | Piracetam 4.8 gram per day plus intensive speech therapy compared to placebo plus intensive speech therapy. The study ran for 6 weeks. |
|
| Outcomes | Performance on Aachen Aphasia Test (AAT, Huber 1984) before and after 6 weeks of treatment. There are no results available for the pilot study. Authors report that those receiving piracetam plus speech therapy scored higher on 'profile height' (i.e. weighted sum score of different sub‐tests of AAT), than those receiving speech therapy alone, when results from those in both the pilot and the later study were analysed together. However, some of those in the later study had aphasia of an origin other than stroke, and these people are not eligible for consideration by this review. | |
| Notes | No details of the characteristics of the placebo are available in the report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Price 1992.
| Methods | Randomised, placebo controlled, double blind study. No details of the randomisation process are available in the report. | |
| Participants | It is not possible to be certain of the setting, but it was probably a single hospital in the USA, as far as can be determined from the abstract. Participants were all people who had been admitted for inpatient rehabilitation following ischaemic stroke. 57 people were enrolled at a mean of 3 days after a stroke for a duration of 40 plus or minus 4 days. | |
| Interventions | Idebenone versus placebo. Idebenone is a coenzyme Q analogue believed to have antioxidant properties. These are the only details concerning the intervention available in the abstract. | |
| Outcomes | Assessed at baseline and at 9 weeks, or upon discharge from the study. Language was assessed on the Western Aphasia Battery (Kertesz 1982) ‐ the authors report that no statistically significant differences were found between the two groups. Participants were also assessed on the Hamilton Depression Scale (Hamilton 1960) ‐ the authors report that no differences were found between the two groups. | |
| Notes | An abstract is the only report available. The author has written for further details, but no reply has been received at the time of the completion of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Spudis 1973.
| Methods | Randomised controlled trial. Participants were divided according to random number tables. | |
| Participants | It is not possible to be certain of the setting, but it was probably a single hospital in the USA, as far as can be determined from the report. Participants were 59 people of any age and both sexes with onset of moderate to severe paralysis of less than 24 hours duration. 30 people with an average age of 70 years received the experimental intervention. 29 people with an average age of 71 years who received a placebo.
Exclusion criteria:
Hypertension
Suspected intracranial haemorrhage
Insulin‐dependant diabetes
Potential emboli
Pulmonary or renal disease. Usual medications were continued, but all who had been given any new medication between the time of onset and initial examination were rejected. |
|
| Interventions | Low molecular weight Dextran was given to people in the experimental group (500 cc in 10% glucose in water over one hour as a loading dose, followed by 1000 cc each 24 hours for three days. No details are available about whether the control group received a placebo transfusion. Dextran 40 is a glucose polymer used as a plasma volume expander and anticoagulant. All other medications, nursing and rehabilitative treatments were carried out as normal. | |
| Outcomes | Language function was divided into 4 categories for assessment‐ normal, mild nonfluency, dysphasia, aphasia. No definitions of these terms was given in the report. The authors report that there were fewer cases of restoration of language in the treatment group than in the control group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tanaka 1997.
| Methods | Randomised controlled trial, using coin‐toss to allocate participants. It would appear, although it is not made explicit in the report, that people in the control group did not receive a placebo. | |
| Participants | It is not possible to be certain of the setting, but this was probably a single hospital in Japan, as far as can be determined from the report. Four people were randomised. All were male, and right handed with fluent aphasia and anomia after unilateral left cerebral infarction. 6‐8 weeks had passed since the stroke. The age range of participants was 54‐65 years. Brain lesions were all confirmed by CT scan to be in the left temporal area. Computerised tomography was performed at the same time as the initial language assessment, and no differences were found in lesion site and size between the two groups. The treatment group and the control group comprised two people each. | |
| Interventions | Those in the treatment group received bifemelane 33mg daily. People in the control group appeared to have received no placebo, although this is not certain. People in both groups received conventional aphasia therapy thrice weekly from a blinded speech therapist until after the second evaluation. This therapy was started after administration of the drug in the treated group, and co‐incidentally in the non‐treated group. Bifemelane appears to have a cerebro‐protective action, and may be an antioxidant. |
|
| Outcomes | All participants were assessed with the Japanese Standard Aphasia Test (SLTA 1977). Additionally all underwent an examination of cerebrospinal fluid before and after the administration of bifemalane in the treated group, and twice at one monthly intervals in the untreated group. The authors state that those taking bifemalane were improved in naming ability and comprehension, compared to those not receiving the drug. | |
| Notes | Bifemelane is a recently developed drug, the action of which is not fully understood. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Admani 1978 | Study did not evaluate the effect of the treatments on aphasia. |
| Albert 1988 | Study was not a randomised controlled trial, but a one participant, single case study. |
| Albert 1992 | Study was not a randomised controlled trial, but a small group before and after study. |
| Anonymous 1990 | Study did not evaluate the effect of a drug on aphasia. |
| Bachman 1988 | Study was not a randomised controlled trial, but a single case study of three people. |
| Bergmann 1951 | Study was not a randomised controlled trial, but a before and after single case study on a set of people. |
| Beyreder 1983 | Study did not evaluate the effect of a drug on aphasia. |
| Bragoni 1997 | Although the study was a randomised controlled trial, the participants were randomised in the first instance to either speech therapy or placebo, then in the next stage of the study they received either drug or placebo, so conclusions cannot be drawn about differences between the two groups in the second (drug) stage, as any differences could be due to a carry over from the first (speech therapy) stage. |
| Capon 1990 | Study was not a randomised controlled trial, but a placebo controlled double blind study, with no mention of randomisation. Author has been contacted, but no reply has been received. |
| Clark 1979 | Study did not evaluate the effect of a drug on aphasia. |
| Darley 1977 | Not all participants in the study were aphasic as result of stroke, some were so because of trauma. |
| Dekoninck 1987 | Study included people with dysarthria as well as those with aphasia. |
| Ducarne 1986 | Study did not evaluate the effect of a drug on aphasia. |
| Feeney 1990 | Study was not on humans. |
| Franke 1996 | Study did not evaluate the effect of a drug on aphasia. |
| Frei 1987 | Study did not evaluate the effect of the treatment on aphasia. |
| Gelmers 1988 | Study did not evaluate the effect of a drug on aphasia. |
| Gelmers 1990 | Study did not evaluate the effect of a drug on aphasia. |
| Gupta 1992 | Study was not a randomised controlled trial, but a single case study of two people. |
| Hartmann 1993 | Study did not evaluate the effect of a drug on aphasia, and was not a randomised controlled trial, but a group observational study with neither control nor experimental group |
| Hrbek 1978 | Study did not evaluate the effect of a drug on aphasia. Also it was not a randomised controlled trial but a small group single case study, and participants were not aphasic, and had not had a stroke. |
| Hulser 1988 | Study did not evaluate the effect of treatment on aphasia. In addition it was probably not a randomised controlled trial. |
| IASSG 1988 | Study did not evaluate the effect of treatment on aphasia. |
| Jacobs 1996 | Study employed a number of methodologies, one element of which was a randomised controlled trial with each participant acting as their own control. However one person was included who was aphasic due to tumour not stroke. |
| Kabasawa 1994 | Study was not a randomised controlled trial, but a small group before and after study. |
| Kartin 1979 | Study gave no detail of whether people were randomly allocated into the two groups, and did not evaluate the effect of a drug on aphasia separately from other disorders. |
| Kaste 1976 | Not clear if the study was a randomised controlled trial, but also it did not evaluate the effect of treatment on aphasia. |
| Koller 1990 | Study did not evaluate the effect of treatment on aphasia. |
| Markov 1973 | Study was not a randomised controlled trial, but a small group before and after study, with participants having aphasia or dysarthria. |
| McNeil 1997 | Study was a single participant, double blind placebo‐controlled, multiple baseline design, but for the first nine weeks of the study comparisons appear to have been other than drug compared to a type of speech and language therapy. Any improvements during this phase could, therefore, have been due to a learning effect. No statement was made about any randomisation process. |
| Motomura 1993 | Study was not a randomised controlled trial. |
| Muller 1994 | Study evaluated the effects of treatment on patents with apraxia, not aphasia. |
| Ozeren 1995 | Study was not a randomised controlled trial but a case study of four patients. |
| Patten 1972 | Study did not evaluate the effects of a drug on aphasia separately from other disorders. |
| Popa 1989 | Study did not evaluate the effect of treatment on aphasia. |
| Porch 1981 | The study was retrospective. There was no control group. |
| Porch 1985 | Study was not a randomised controlled trial but a retrospective study. |
| Roquefeuil 1975 | Study did not evaluate the effect of a drug on aphasia. Also, it was not a randomised controlled trial, but a small group single case study which included people with aphasia of causes other that stroke. |
| Sabe 1992 | Study was not a randomised controlled trial, but a small group before and after study of the drug given to seven consecutive people. |
| Sabe 1995 | Although this was a randomised controlled trial, all the seven people studied were randomised to receive the drug in the first treatment arm, and placebo later in the second arm. There was therefore no control group receiving placebo at the same time as the treatment group, to allow comparisons to be made. Any better performance found in the placebo period may have been due to practice effects. |
| Sarno 1972 | Study was not a randomised controlled trial, but a small group before and after study. |
| Schneider 1986 | Study was not a randomised controlled trial, and does not evaluate effect of drug on aphasia separately from other disorders. |
| Sciclounoff 1934 | As far as can be determined from the report, which is in French, the study was not a randomised controlled trial, but a before and after observational study of single cases. Also, the study does not appear to have evaluated the effect of the drug on aphasia. |
| SSSG 1997 | Study did not evaluate the effect of treatment on aphasia. |
| Steiner 1986 | Study did not evaluate effect of drug on aphasia |
| Steiner 1994 | It is possible that some people in the study were dysarthric, not aphasic. Tests of function at end of trial did not evaluate aphasia separately from dysarthria. |
| Strand 1984 | People evaluated in the study were a mixed group of some with aphasia and some with dysarthria. |
| Voinescu 1978 | Study included people aphasic after trauma as well as those aphasic after stroke. |
| Walker‐Batson 1991 | Study was not a randomised controlled trial but a one participant single case study. |
| Walker‐Batson 1992 | Study was not a randomised controlled trial, but a small group single case study. |
| Walker‐Batson 1995 | Study did not evaluate the effect of a drug on aphasia separately from other disorders. |
| West 1965 | There is no mention of whether or not the study was a randomised controlled trial, however there was a control group and an experimental group. Study used a double blind method to ensure that neither participants nor the research team knew which group participants were in, but the pharmacist may have chosen people rather than randomly allocated them. Efforts to elicit further details have not been made due the fact that this study is 35 years old. |
| Willmes 1984 | Study was not a randomised controlled trial, but a descriptive study of spontaneous recovery, with neither experimental nor control group. |
| Witzmann 1977 | Paper comprises a study which did not look at effects of a drug on aphasia, and a retrospective overview of 33 other studies. |
| Zeigler 1993 | Study evaluated the effect of a drug on apraxia, not aphasia. |
Contributions of authors
Pam Enderby (PE) and Renata Whurr (RW) advised concerning the background, objectives, criteria for eligible studies, and the key words for use in the search strategy. Jenny Greener (JG) carried out the searching and screened potentially eligible abstracts. Those which seemed to hold further promise were obtained by JG. JG assessed the quality of trials under consideration. These were checked by PE or RW at a different location. Results and conclusions were considered by all members of the team. All contributed to the writing of the review, and all drafts were approved by the entire team before submission.
Sources of support
Internal sources
Health Services Research Unit, Aberdeen University, Scotland, UK.
External sources
No sources of support supplied
Declarations of interest
The principal reviewer no longer practices speech and language therapy. The other two reviewers are both speech and language therapists with many years experience in the treatment of aphasia after stroke. Professor Enderby was an investigator in the Enderby 1994 trial.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bakchine 1990 {published data only}
- Bakchine S Fiorelli M. Double blind placebo controlled study of Pirebedil in aphasic patients with pure subcortical extrathalamic vascular lesions: Clinical assessment and metabolic imaging with PET scan. Journal of Neurology 1990;Vol 237:S107. [Google Scholar]
De Reuck 1995 {published and unpublished data}
- Deyn P P, Reuck J. Piracetam in acute stroke study. Unpublished 1995.
- Deyn P, Reuck J, Milonas I, Vlietinck B, Deberdt W. Piracetam acute stroke study. European Journal of Neurology 1996;3(Supp 5):37. [Google Scholar]
- Deyn P, Orgogozo JM, Reuck J. Acute treatment of stroke. PASS group. Piracetam acute stroke study (letter). Lancet 1998;352(9124):326. [DOI] [PubMed] [Google Scholar]
- Deyn PP. The piracetam in acute stroke study. European Journal of Neurology 1995;2(Supp2):7. [Google Scholar]
- Deyn PP, Reuck J, Orgogozo JM, Deberdt W, for the Piracetam Study Group. The piracetam in acute stroke study. Stroke 1996;27(1):33 (Abstr 165). [Google Scholar]
- Deyn PP, Reuck J, Orgogozo JM, Vlietinck R, Deberdt W. Piracetam in acute stroke study. Cerebrovascular Disorders 1996;6(suppl 2):32‐178. [Google Scholar]
- Deyn PP, Minderhoud JM, Deberdt W, Bleyman B. Piracetam in acute stroke study (PASS). Journal of Neurology 1996;243(6):S22. [Google Scholar]
- Deyn PP, Reuck JD, Deberdt W, Vlietinck R, Orgogozo JM. Members of the piracetam in acute stroke study (PASS) group. Treatment of acute ischemic stroke with piracetam. Stroke 1997;28(2):2347‐52. [DOI] [PubMed] [Google Scholar]
- Reuck J, Bleyman B. The clinical safety of high‐dose dose piracetam ‐ its use in the treatment of acute stroke. Pharmacopsychiatry 1999;32(suppl):33‐7. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Deberdt WG, UCB Braine l'Alleud, Belgium. The piracetam study in acute stroke. Journal of Neurology 1995;Suppl 2:242. [Google Scholar]
- Huber W. The role of piracetam in the treatment of acute and chronic aphasia. Pharmacopsychiatry 1999;32(supp 1):38‐43. [DOI] [PubMed] [Google Scholar]
- Marien P, Saerens J, Bleyman B, Deyn PP. Piracetam in the treatment of aphasia in acute stroke. European Journal of Neurology 1997;4(Supp 1):S105. [Google Scholar]
- Murphy N, Kazek MP, Bleyman B, et al. Economic evaluation of Nootropil in the treatment of acute stroke. Pharmacol Res 1997;36(5):373‐80. [DOI] [PubMed] [Google Scholar]
- Murphy N, Nazek MP, Bleyman B, Melac M, Souetre E. Economic evaluation of Nootropil in the treatment of acute stroke in France. Pharmacological Research 1997;36(5):373‐80. [DOI] [PubMed] [Google Scholar]
- Orgogozo J‐M. Piracetam in the treatment of acute stroke. CNS Drugs 1998;9(suppl 1):41‐9. [Google Scholar]
- Orgogozo JM. Piracetam in the treatment of acute stroke. Pharmacopsychiatry 1999;32(Suppl 1):25‐32. [DOI] [PubMed] [Google Scholar]
- Saerens J, Marien P, Bleyman B, et al. Piracetam in the treatment of aphasia in acute stroke. Journal of Neurology 1997;244(Suppl 3):S71. [Google Scholar]
- UCB Pharma. Piracetam versus placebo in the treatment of acute ishemic supratentorial cerebrovascular accidents (protocol). A double blind randomised controlled multicentre study. Unpublished 1993.
Enderby 1994 {published and unpublished data}
- Broeckx J. Piracetam in post stroke rehabilitation: a three month, double‐blind, parallel group, multicentre study to compare the efficacy and safety of piracetam versus placebo in facilitating the rehabilitation process in patients recovering from ischemic infarction or intracerebral haemorrhage in the carotid areas. External report MRCE93B1101, UCB Pharma, Belgium 1994.
- Deberdt W. Interaction between psychological and pharmacological treatment in cognitive impairment. Life Sciences 1994;55(25/26):2057‐2066. [DOI] [PubMed] [Google Scholar]
- Enderby P. The efficacy and safety of piracetam in the rehabilitation of stroke patients.. Racagni G, Brunello N, Langer SZ (eds) Recent advances in the treatment of neurodegenerative disorders and cognitive dysfunction. International Academic Biomedical Drug Research 1994;7:249‐255. [Google Scholar]
- Enderby P, Broeckx J, Hospers W, et al. Effect of piracetam on recovery and rehabilitation after stroke: A double blind, placebo controlled study. Clinical Neuropharmacology 1994;17(4):320‐331. [DOI] [PubMed] [Google Scholar]
- Schildermans F, Broeckx J, Hospers W, Deberdt W. Efficacy of piracetam in the rehabilitation of stroke patients. Proceedings of 2nd International Conference on Stroke, Geneva, Switzerland. World Federation of Neurology, 1993.
Gupta 1995 {published data only}
- Gupta SR, Mlcoch AG, Scolaro C, Moritz T. Bromocriptine treatment of non‐fluent aphasia. Neurology 1995;45:2170‐2173. [DOI] [PubMed] [Google Scholar]
Herrschaft 1988 {published data only}
- Herrschaft H. Die Wirkamseit von Piracetam bie der acuten zerebralen Ischamie des Menschen. Medizinische Klinik 1983;20:667‐677. [PubMed] [Google Scholar]
Platt 1993 {published data only}
- Platt D, Horn J, Summa J‐D, et al. On the efficacy of piracetam in gerietric patients with acute cerebral ischemia: A clinically controlled double blind study. Archives of Gerontology and Geriatrics 1993;16:149‐164. [DOI] [PubMed] [Google Scholar]
- Platt D, Horn J, Summa J‐D, Schmitt‐Ruth R, Reinlein B, et al. Zur Wirkamseit von Piracetam bei geriatrischen Patienten mit akuter zerebraler Ischamie. Die Medizinische Welt 1992;43:181‐190. [Google Scholar]
Poeck 1993 {published and unpublished data}
- Deberdt W. Interaction between psychological and pharmacological treatment in cognitive impairment. Life Sciences 1994;55(25/26):2057‐2066. [DOI] [PubMed] [Google Scholar]
- Deberdt W, Poeck K, Huber W. Piracetam as an add‐on treatment to intensive speech therapy for aphasia. Meeting of the European Federation of Neurological Societies. 1993:S 89.
- Huber W, Willmes K, Poeck K. Piracetam as an add‐on treatment to intensive speech therapy. Journal of Neurology 1994;241(suppl 1):S152. [Google Scholar]
- Huber W, Willmes K, Poeck K, Bleyman B, Deberdt W. Piracetam as an adjuvent to language therapy for aphasia: a randomised double‐blind placebo‐controlled pilot study. Archives of Physical Medicine and Rehabilitation 1997;78(3):245‐50. [DOI] [PubMed] [Google Scholar]
- Poeck K, Deberdt W. Uberprufung der wirking von Piracetam bei der logopadischen intensivtherapie von chronisch aphaschen patienten. Unpublished report, UCB Pharma,. UCB Pharma, 1993:1‐22.
- Poeck K, Huber W, WiIlmes K. Piracetam as an add‐on treatment to intensive speech therapy. 2nd International Conference on Stroke. Geneva, 1993.
Price 1992 {published data only}
- Price J, Kheyferts S, Reding MJ. The effect of Idebenone on recovery from stroke. Neurology 1992;42 (Suppl 3):328. Neurology 1992;42 Suppl 3:328. [Google Scholar]
Spudis 1973 {published data only}
- Spudis E, Torre E, Pikula L. Management of completed strokes with Dextran 40. A community hospital failure. Stroke 1974;4:895‐897. [DOI] [PubMed] [Google Scholar]
Tanaka 1997 {published data only}
- Tanaka Y, Miyazaki M, Albert ML. Effects of cholinergic activity on naming in aphasia. Lancet 1997;350:116‐117. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Admani 1978 {published data only}
- Admani AK. New approach to treatment of recent stroke. BMJ Dec 16 1978;2(6153):1678‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 1988 {published data only}
- Albert ML, Bachmann DL, Morgan A, Helm‐Estabrooks N. Pharmacotherapy for aphasia. Neurology 1988;38(6):877‐879. [DOI] [PubMed] [Google Scholar]
Albert 1992 {published data only}
- Albert M, McNamara P, Rosen J, Siu C. Pharmacotherapy for nonfluent aphasia. Neurology 1992;42(suppl 30):181 (abstract). [Google Scholar]
Anonymous 1990 {published data only}
- Anonymous. Randomised, double‐blind, placebo controlled trial of nimipodine in acute stroke. Lancet 1990;336(8725):1205‐9. [PubMed] [Google Scholar]
Bachman 1988 {published data only}
- Bachman DL, Morgan A. The role of pharmacotherapy in the treatment of aphasia: preliminary results. Aphasiology 1988;2(3/4):225‐228. [Google Scholar]
Bergmann 1951 {published data only}
- Bermann PS, Green M. Aphasia: effect of intravenous sodium amytal. Neurology 1951;1:471‐5. [DOI] [PubMed] [Google Scholar]
Beyreder 1983 {published data only}
- Beyreder J. Use of pentoxifyllene in the treatment of acute cerebral insufficiency. European Neurology 1983;22(suppl 1):116‐123. [DOI] [PubMed] [Google Scholar]
Bragoni 1997 {published data only}
- Bragoni M, Peiro V, Cacioppo M, Padrovani A, et al. Non fluent stable chronic aphasia: effects of bromocriptine and speech therapy. Neurology 1997;48:A214. [Google Scholar]
- Bragoni M, Piero V, Capocci MM, et al. Bromocriptine and speech therapy: a possible treatment of stroke patients with non‐fluent chronic aphasia. Journal of Neural Transmission 1996;103:23. [Google Scholar]
Capon 1990 {published data only}
- Capon A, Lehert P, Opsomer L. Naftidrofuryl in the treatment of sub‐acute stroke. Journal of Cardiovascular Pharmacology 1990;16(suppl 3):S63‐66. [PubMed] [Google Scholar]
Clark 1979 {published data only}
- Clark A, Manikar GD. d‐Amphetamine in elderly patients refractory to rehabilitation procedures. Journal of the American Geriatrics Society 1979;27(4):174‐177. [DOI] [PubMed] [Google Scholar]
Darley 1977 {published data only}
- Darley FL, Keith RL, Sasanuma S. The effect of alerting and tranquillizing drugs upon the performance of aphasic patients. Clinical Aphasiology 1977;7:91‐96. [Google Scholar]
Dekoninck 1987 {published data only}
- Dekoninck WJ, Jocquet P, Jacquy J, Henriet M. Comparative study of the clinical effects of vincamine + glycerol vs glycerol + placebo in acute phase of stroke. Arzeimittel Forschung 1978;28(11):1654‐1657. [PubMed] [Google Scholar]
Ducarne 1986 {published data only}
- Ducarne H. Evaluation of a vasoactive ubstance, naftidrofuryl, during the rehabilitation phase after an ischaemic insult. Current Medical Research and Opinion 1986;10(1):58‐71. [DOI] [PubMed] [Google Scholar]
Feeney 1990 {published data only}
- Feeney DM, Westerberg VS. Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery. Canadian Journal of Psychology 1990;44(2):233‐52. [DOI] [PubMed] [Google Scholar]
Franke 1996 {published data only}
- Franke CL, Palm R, Dalby M, Schoonderwaldt HC, Hantson L, et al. Fluarizine in stroke treatment (FIST); a double blind, placebo controlled trial in Scandinavia and the Netherlands. Acta Neurologica Scandinavia 1996;93(1):56‐60. [DOI] [PubMed] [Google Scholar]
Frei 1987 {published data only}
- Frei A, Cottier C, Wunderlich P, Ludin E. Glycerol and dextran combined in the therapy of acute stroke. A placebo‐controlled, double‐blind trial with a planned interim analysis. Stroke 1987;18(2):373‐9. [DOI] [PubMed] [Google Scholar]
Gelmers 1988 {published data only}
- Gelmers HJ, Gorter K, Weerdt CJ, Weizer HJ. A controlled trial of nimodipine in acute ischemic stroke. New England Journal of Medicine 1988;318(4):203‐7. [DOI] [PubMed] [Google Scholar]
Gelmers 1990 {published data only}
- Gelmers HJ, Hennerici M. Effect of nimipodine on acute ischaemic stroke. Pooled results from five randomized trials. Stroke 1990;21(12 suppl):81‐4. [PubMed] [Google Scholar]
Gupta 1992 {published data only}
- Gupta S, Mlcoch A. Bromocriptine treatment of nonfluent aphasia. Archives of Physical and Medical Rehabilitation 1992;73:373‐76. [DOI] [PubMed] [Google Scholar]
Hartmann 1993 {published data only}
- Hartmann A, Dettmers C, Lagreze H, Tsuda Y. Blood flow and clinical course in patients with ischaemic stroke without cerebrospecific therapy. Acta Neurochirugica Supplementum 1993;57:130‐135. [DOI] [PubMed] [Google Scholar]
Hrbek 1978 {published data only}
- Hrbek J, Macakova J, Komenda S, Dostalova K, Medek A, Navratil J. Effects of pyrithioxine, centrophenoxine, and piracetam on verbal learning. Activitas Nervosa Superior (Praha) 1978;20(4):260‐1. [PubMed] [Google Scholar]
Hulser 1988 {published data only}
- Hulser PJ, Bernhart H, Marbach C, Kornhuber HH. Treatment with an i.v. calcium overload blocker (flunarizine) in acute stroke. A pilot study. European Archives of Psychiatry and Neurological Sciences 1988;237(50):253‐7. [DOI] [PubMed] [Google Scholar]
IASSG 1988 {published data only}
- Anonymous. Haemodilition in acute stroke: results of the Italian haemodilution trial. Italian Acute Stroke Study Group. Lancet 1988;1(8581):318‐21. [PubMed] [Google Scholar]
Jacobs 1996 {published data only}
- Jacobs DH, Shuren J, Gold M, et al. Physostigmine pharmacotherapy for anomia. Neurocase 1996;2:83‐91. [Google Scholar]
Kabasawa 1994 {published data only}
- Kabasawa H, Matsubara M, Kamimoto K, Hibino H, Banno T, Nagai H. Effects of bifemalane on cerebral circulation and metabolism in patients with aphasia. Clinical Therapeutics 1994;16:471‐82. [PubMed] [Google Scholar]
Kartin 1979 {published data only}
- Kartin P, Povse M, Skondia V. Clinical study of piracetam in patients with subacute cerebrovascular accidents. Acta Therapeutica 1979;5:235‐243. [Google Scholar]
Kaste 1976 {published data only}
- Kaste M, Fogelholm R, Waltimo O. Combined dexamethasone and low‐molecular‐weight dextran in acute brain infarction: double blind study. BMJ 1976;2(6049):1409‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koller 1990 {published data only}
- Koller M, Heanny P, Hess K, Weniger D, Zangger P. Adjusted hypervolemic hemodilution in acute ischemic stroke. Stroke 1990;21(10):1429‐34. [DOI] [PubMed] [Google Scholar]
Markov 1973 {published data only}
- Markov G. The treatment of aphasia and dysarthria using psychoforin (Tofranil) and Myocalm. Zhural Nevpatologi i Psikhatri s‐s korsova 1973;73(4):512‐3. [PubMed] [Google Scholar]
McNeil 1997 {published data only}
- McNeil MR, Doyle PJ, Spencer KA, Goda AJ, Flores D, Small SL. A double‐blind, placebo controlled study of pharmacological and behavioural treatment of lexical‐semantic deficits in aphasia. Aphasiology 1997;11(4/5):385‐400. [Google Scholar]
Motomura 1993 {published data only}
- Motomura S. Aphasia in stroke‐ pathogenesis, recovery and treatment. Nippon Rinsho 1993;Suppl:278‐290. [PubMed] [Google Scholar]
Muller 1994 {published data only}
- Muller U, Zeigler W, Cramon DY. Amphetamine in apraxia of speech.. Neuropsychopharmacology 1994;10(35, Part 2):226S (Abst. P174‐56). [Google Scholar]
Ozeren 1995 {published data only}
- Ozeren A, Sarica Y, Mavi H, Demikiran M. Bromocriptine is ineffective in the treatmnt of chronic nonfluent aphasia. Acta Neurologica Belga 1995;95(4):235‐8. [PubMed] [Google Scholar]
Patten 1972 {published data only}
- Patten BM, Mendel J, Bruun B, Curtin W, Carter S. Double‐blind study of the effects of dexamethasone on acute stroke. Neurology 1972;22:377‐383. [DOI] [PubMed] [Google Scholar]
Popa 1989 {published data only}
- Popa G, Popa C, Stanescu A, Ionescu G, Lugoji G, Radula D, Popescu A. Haemodilution therapy in acute iscaemic stroke. Neurologie et Psychiatrie 1989;2792:79‐90. [PubMed] [Google Scholar]
Porch 1981 {published data only}
- Porch B, Feeney D. Effects of antihypertensive drugs on recovery from aphasia. Clinical Aphasiology Proceedings. 1981:187‐200.
Porch 1985 {published data only}
- Porch B, Wyckes J, Feeney DM. Haloperidol, thiazides and some hypertensives slow recovery from aphasia. Proceedings from the annual meeting of the Society for Neuroscience. 1985:52 (abst).
Roquefeuil 1975 {published data only}
- Roquefeuil E, Escuret E. Etude metabolique du Piracetam en reanimation neuro‐chirugicale. Agressologie 1975;16:43‐62. [PubMed] [Google Scholar]
Sabe 1992 {published data only}
- Sabe L, Leiguarda R, Starkstein SE. An open‐label trial of bromocriptine in nonfluent aphasia. Neurology 1992;73:373‐376. [DOI] [PubMed] [Google Scholar]
Sabe 1995 {published data only}
- Sabe L, Salvarezza F, Cuerva A, Leiguarda R, Starkstein S. A randomised double blind placebo controlled study of bromocriptine in nonfluent aphasia. Neurology 1995;45(12):2272‐2274. [DOI] [PubMed] [Google Scholar]
Sarno 1972 {published data only}
- Sarno JE, Rusk HA, Diller L, Sarno MT. The effect of hyperbaric oxygen on the mental and verbal ability of stroke patients. Stroke 1972;3:10. [DOI] [PubMed] [Google Scholar]
- Sarno M, Sarno J, Diller L. The effect of hyperbaric oxygen on communication function in adults with aphasia secondary to stroke. Journal of Speech and Hearing Research 1972;14:42‐48. [DOI] [PubMed] [Google Scholar]
Schneider 1986 {published data only}
- Schneider R, Brockmann M, Keisewetter H, et al. Behandlung lakunarer Infarkte mit Pentoxyfyllin. Medwelt 1986;37:1340‐1343. [Google Scholar]
Sciclounoff 1934 {published data only}
- Sciclounoff F. L'acetylcholine dans le traitement de l'ictus hemiplegique. La Presse Medicale 14 July 1934;56:1140‐1142. [Google Scholar]
SSSG 1997 {published data only}
- Anonymous. Multicenter trial of hemodilution in acute ischemic stroke. I. Results in the total patient population. Scandinavian Stroke Study Group. Stroke 1987;18(4):691‐9. [DOI] [PubMed] [Google Scholar]
Steiner 1986 {published data only}
- Steiner TJ, Clifford Rose F. Towards a model stroke trial. Neuroepidemiology 1986;5:121‐147. [DOI] [PubMed] [Google Scholar]
Steiner 1994 {published data only}
- Steiner 1994. Praxiline in stroke treatment in Northern Europe (PRISTINE). (Abstract) Stroke 1994;25(6):1318. [Google Scholar]
- Steiner T. Personal correspondence 1995.
- Steiner T. Personal correspondence 1996.
Strand 1984 {published data only}
- Strand T, Aspund MD, Eriksson S, Hagg E, et al. A randomised controlled trial of hemodilution therapy in acute ischemic stroke. Stroke 1984;15(6):980‐989. [DOI] [PubMed] [Google Scholar]
Voinescu 1978 {published data only}
- Voinescu I, Georghita N. Adjuvent drug therapy with psychologopaedic rehabilitation of aphasic patients. R Roum Med, Neurol Psychiat 1987;16:155‐161. [PubMed] [Google Scholar]
Walker‐Batson 1991 {published data only}
- Walker‐Batson D, Devous MD, Curtis S, Unwin H, Greenlee RG. Response to amphetamine to facilitate recovery from aphasia subsequent to stroke. Clinical Aphasiology 1991;21:137‐143. [Google Scholar]
Walker‐Batson 1992 {published data only}
- Walker‐Batson D, Unwin H, Curtis S, Allen E, Wood M, et al. Use of amphetamine in the treatment of aphasia. Restorative Neurology and Neuroscience 1992;4:47‐50. [DOI] [PubMed] [Google Scholar]
Walker‐Batson 1995 {published data only}
- Walker‐Batson D, Smith P, Curtis MA, Unwin H, Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Stroke 1995;26(12):2254‐2259. [DOI] [PubMed] [Google Scholar]
West 1965 {published data only}
- West R, Stockel S. The effect of meprobamate on recovery from aphasia. Journal of Speech and Hearing Research 1965;8:57‐62. [DOI] [PubMed] [Google Scholar]
Willmes 1984 {published data only}
- Willmes K, Poeck K. Ergebnisse einer multizentrischen Untersuchung uber die Spontanprognose von Aphasien vaskularer Atiologie. Nervenarzt 1984;55:62‐71. [PubMed] [Google Scholar]
Witzmann 1977 {published data only}
- Witzmann von HK, Blechacz W. On the role of vincamine in the therapy of cerebrovascular diseases and impairment of cerebral function. Arzneimittel Forschung 1977;27(6A):1238‐1247. [PubMed] [Google Scholar]
Zeigler 1993 {published data only}
- Zeigler W, Muller U. Amphetamine in apraxia of speech. Proceedings of the International Congress on stroke rehabiltation. 1993:71.
References to studies awaiting assessment
Wade 1994 {published data only}
- Wade D. Personal communication 1999.
- Wade D. Piracetam in post stroke aphasia. Unpublished protocol 1994.
Walker‐Batson 2001 {published and unpublished data}
- Walker‐Batson D. Unpublished paper 2001.
- Walker‐Batson D. Use of amphetamine in the treatment of aphasia. Unpublished book chapter.
Additional references
Bachman 1990
- Bachman DL, Albert ML. The pharmacotherapy of aphasia: historical perspectives. Aphasiology 1990;4:407‐413. [Google Scholar]
Chouinard 1983
- Chouinard G, Annable L, Ross‐Chouinard A, Olivier M, Fontaine F. Piracetam in elderly psychiatric patients with mild diffuse cerebral impairment. Psychopharmacology (Berlin) 1983;81:100‐6. [DOI] [PubMed] [Google Scholar]
Creytens 1980
- Creytens G. Nouveautes dans la traitement de la pathologie cerebrale. Acta Ther 1980;6:33‐53. [Google Scholar]
Enderby 1986
- Enderby P, Philipp R. Speech and language handicap: Towards knowing the size of the problem. British Journal of Disorders of Communication 1986;21(2):151‐165. [DOI] [PubMed] [Google Scholar]
Enderby 1987
- Enderby P, Wood DT, Hewer RL. The Frenchay Aphasia Test: a short simple test for aphasia appropriate for non‐specialists. International Rehabilitation Medicine 1987;8(4):166‐170. [DOI] [PubMed] [Google Scholar]
Enderby 1989
- Enderby P, Davies P. Communication disorders: Planning a service to meet the needs. British Journal of Disorders of Communication 1989;24(3):301‐331. [DOI] [PubMed] [Google Scholar]
Giurgea 1976
- Giurgea C. Piracetam: nootropic pharmacology of neurointegrative activity. Current Developments in Psychopharmacology. Vol. 3, New York: Spectrum Publications, 1976:222‐273. [Google Scholar]
Goldberg 1990
- Goldberg E. Contemporary Neuropsychology and the Legacy of Luria. Hove and London: Lawrence Earlbaum Associates, 1990. [Google Scholar]
Goodlass 1972
- Goodlass HK, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Febiger, 1972. [Google Scholar]
Greener 2001
- Greener J, Enderby P, Whurr R. Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews 1999, Issue 4. [Art. No.: CD000425. DOI: 10.1002/14651858.CD000425] [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huber 1983
- Huber W, Poeck K, Willmes K. Der Aachen Aphasie Test. Gottingen: Hogrefe, 1983. [Google Scholar]
Kaplan 1983
- Kaplan E, Goodlass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger, 1983. [Google Scholar]
Kertesz 1982
- Kertesz A. Western Aphasia Battery. New York: Grune and Stratton, 1982. [Google Scholar]
Kurianski 1976
- Kurianski J, Gurland B. The performance test of activities of daily living. International Journal of Aging and Human Development 1976;7:343‐352. [DOI] [PubMed] [Google Scholar]
Mackenzie 1992
- Mackenzie C. The diversity of speech and language therapy services for aphasic adults in the United Kingdom. Disability and Rehabilitation 1992;14(3):146‐151. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. A simple index of independence useful in scoring improvement in the rehabilitation of chronically ill. Maryland State Medical Journal 1965;14:62‐65. [PubMed] [Google Scholar]
Methe 1993
- Methe, S, Huber, W, Paradis M. in Paradis M, editor. Foundation of Aphasia Rehabilitation. Oxford: Pergamon Press, 1993. [Google Scholar]
Orgogozo 1986
- Orgogozo J‐M, Capildeo R, Anagnostou, Juge O, Pere JJ, et al. Development of a neurological score for the evaluation of sylvian infarctions [French]. Presse Medical 1983;12(48):3039‐44. [PubMed] [Google Scholar]
Pederson 1995
- Pederson PM, Jorgenson HS, Nakayama H, Raasschou HO, Olsen TS. Aphasia in Acute Stroke:incidence, determinants, and recovery. Ann Neurol 1995;38:659‐666. [DOI] [PubMed] [Google Scholar]
Rey‐Osterrieth 1944
- Osterrieth P, Rey A. Le test de copie d'une figure complex. Archives de Psychologie 1944;30:205‐221. [Google Scholar]
Ricci 2000
- Ricci S, Celani MG, Cantisani AT, Righetti E. Piracetam for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2000, Issue 4. [Art. No.: CD000419. DOI: 10.1002/14651858.CD000419] [DOI] [PubMed] [Google Scholar]
SLTA 1977
- SLTA Committee. Standard Language Test of Aphasia: manual of directions. 2. Tokyo: Homeido, 1977. [Google Scholar]
Small 1994
- Small S. Pharmacotherapy of aphasia. Stroke 1994;25(6):281‐289. [DOI] [PubMed] [Google Scholar]
Steginck 1972
- Stegink, KJ. The clinical use of piracetam, a new nootropic drug. Arzniemittelforsch 1972;22:975‐7. [PubMed] [Google Scholar]
Stolyarova 1978
- Stolyarova L, Kadykov A, Kisrenev B, Varykin YU, Yurchenko Z, Davylov V. The role of piracetam in complex rehabilitation therapy of patients with residual manifestations of a cerebral stroke. Proceedings of a symposium: Nootropil in Neurological and Psychiatric Practice. Moscow, 1978:74‐7.
Wade 1986
- Wade DT, Langton‐Hewer R, David RM, Enderby PM. Aphasia after stroke: Natural history and associated deficits. Journal of Neurology Neurosurgery and Psychiatry 1986;49(1):11‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wechsler 1987
- Wechsler D. Memory Scale ‐ Revised. New York: Psychological Corporation, 1987. [Google Scholar]
Whiting 1985
- Whiting SE, Lincoln NB, Bhavani G, Cockburn J. The Rivermead Perceptual Assessment Battery. Windsor: NFER Nelson, 1985. [DOI] [PubMed] [Google Scholar]
WHO 1986
- World Health Organisation. International classification of diseases: 1975 Revision. Geneva: WHO, 1986. [Google Scholar]
Whurr 1992
- Whurr R, Lorch M, Nye CA. Meta‐analysis of studies carried out between 1946 and 1988 concerned with the efficacy of speech and language therapy treatment for aphasic patients. European Journal of Disorders of Communication 1992;27(1):1‐18. [DOI] [PubMed] [Google Scholar]
Wilsher 1987
- Wilsher C. Piracetam and dyslexia ‐ effects on reading tests. Journal of Clinical Psychopharmacology 1987;7:230‐7. [PubMed] [Google Scholar]


