Abstract
A dual-wavelength excitation Raman probe with laser inputs at 866 nm or 1064 nm is customized and integrated into a compact Raman spectrometer that is based on an InGaAs detector. Under 1064 nm illumination, the spectrometer detects fingerprint Raman signals below 2000 cm−1. While under 866 nm illumination, the spectral range is extended to cover high-frequency region (2400–4000 cm−1) that includes major C–H and O–H Raman vibrations. We demonstrate that the dual excitation InGaAs Raman is beneficial in detecting high-frequency Raman signals, especially water contents in high-fluorescent biological samples such as human dental tissues, grape skin, and plum skin due to the suppressed fluorescence interference.
Keywords: Raman spectroscopy, high-frequency Raman, dual-wavelength excitation, InGaAs, hydration probing
Introduction
Raman spectroscopy is an analytical technology that measures the inelastically scattered light from matters and has been widely used in physics, chemistry, biology, medicine, and the nanosciences.1–6 Modern Raman spectrometers are often based on silicon-based CCD detectors, which normally has optimal responses between 400 nm and 900 nm; this range can be slightly extended through UV or NIR enhanced technologies.7–10 The efficiency of Raman scattering is very low (in the order of one part per million) and is inversely proportional to the fourth order of the incident wavelength, incident light with shorter wavelength is thus desired.11 On the other hand, the higher energy photons at shorter wavelength are more capable of producing fluorescence from materials (especially biological tissues) which could overwhelm the weak Raman signals of interests.12 Excitation with longer wavelength light is a major practical strategy that alleviates the strong fluorescence from biological tissues.6 In order to find the best balance between lower fluorescence interference and higher collecting efficiency, various excitation wavelengths have been employed in Raman instrumentations.13–16
A Raman spectrometer is the most critical component in a Raman spectroscopy system. High-cost research grade Raman spectrometers allow users to modify componential configurations such as replacing detectors and/or gratings, adjusting slit width, and repositioning focusing mirror, etc. The combinations of small slit width, high groove-density grating, and long focal distance allow research grade spectrometers to achieve sub-wavenumber (cm−1) spectral resolution.17 The detectors of research grade systems usually have enhanced sensitivity (through anti-reflection coating, and back illumination, etc.) and low system noise (through deep cooling). Low to middle cost Raman systems are usually compact and allow none/limited configuration modifications for the end users. By integrating high-dispersion and high-efficiency volume phase transmission gratings, these compact spectrometers may achieve comparable performance to research grade spectrometers after some level of sensitivity enhancement and noise reduction.
However, the spectral range and the resolution of compact spectrometers are fixed and are typically designed to cover the fingerprint Raman signals (i.e., ~200–2000 cm−1) with appropriate resolution (typically 6–10 cm−1). Extending the spectral range of such a compact spectrometer will reduce its resolution. Therefore, a user must select a trade-off between spectral resolution and spectral range that best fits the need for an application. An alternative method of extending the range is to use a second laser at shorter wavelength and this concept has been applied to CCD-based spectrometers in previous studies.18–20 In this note, we will demonstrate an InGaAs detector-based dual-wavelength excitation Raman system that covers both the finger print region (240–1900 cm−1) and the high-frequency region (2390–4110 cm−1). In addition, we integrated a dual-laser fiber probe using off-the-shelf components. This probe can not only be used in the current Raman system, it can but also be used for spectrometers designed for other wavelengths by replacing the Raman filter set.
Methods and Materials
The dual-wavelength excitation Raman probe was constructed using off-the-shelf optical components ordered from Thorlabs and Semrock. A picture of the assembled Raman probe is shown in Fig. 1. A kinematic beam turning cage cube integrated with a silver prism mirror (DFM1-P01) was used to select one of the two excitation laser sources operated at 1064 nm (l0164mm0500MF, Innovative Photonics Solutions) or 866 nm (3900S, Spectra Physics). Each laser from a fiber (M43L01 105 μm) was collimated by FC-APC fiber collimator (F220FC) and then guided into the cage system through a set of adapters (AD1109F + SM05 + SM1A6, see the left inset of Fig. 1). The lock ring on the one-inch SM1 tube (SM1V05) would allow a small play of positioning the beam along the central axis of the cage. Fine alignment of the beam can also be adjusted by an x,y cage translator (CXY1, Thorlabs). The laser was then reflected by the dichroic mirror (di02-R1064–25×36, Semrock) before focused onto the sample through an objective lens. A second kinematic beam turning cage cube was used to deliver Raman signal either to the spectrometer or the image of the sample to a CCD camera for observing the sample surface and beam focusing. An SMA fiber collimator (CVH100-COL) was used to collect the Raman signal and deliver it to the fiber input of the spectrometer. This spectrometer is based on an InGaAs detector (iDus, Andor) that has optimal responses between 0.9 mm and 1.7 μm and a customized compact spectrograph (Wasatch Photonics) providing a resolution of ~8 cm−1. The incident lasers were cleaned with laser line filter (LL01–1064-12.5, Semrock) and shot-pass filter (FESH0900, Thorlabs) for 1064 and 866 nm, respectively, while the Raman signal was always filtered through a long-pass filter (FELH1100, Thorlabs). An objective turret (OT1, Thorlabs) was used to allow the selection of one of the three objectives at different magnification (10×, 40×, and 100×).
Figure 1.
Schematic layout of the dual-wavelength excitation Raman probe. The probe has two laser inputs, 1064 nm for regular Raman signals and 866 nm for high-frequency region Raman signals. The output can be routed to a spectrometer for spectral analysis or to a camera to view images of the sample with or without laser illumination.
A research grade Raman spectrograph (Kymera 328i, Andor) equipped with a NIR enhanced, back illuminated, deep cooled CCD detector (Newton 920, Andor) was used to acquire Raman spectra under 785 nm (3900S, Spectra Physics) or 633 nm excitation. The Raman signal was collected through a round to linear fiber bundle (BFL105LS02, Thorlabs) that contains seven optical fibers, each has a core diameter of 105 μm. The small cross section of the fiber works as a confocal pinhole to reduce fluorescence background.
A human tooth sample was collected from a local oral surgery clinic under the IRB approval of Jackson State University. Fruit samples were purchased from a local supermarket and were measured without further technical modification.
Results and Discussion
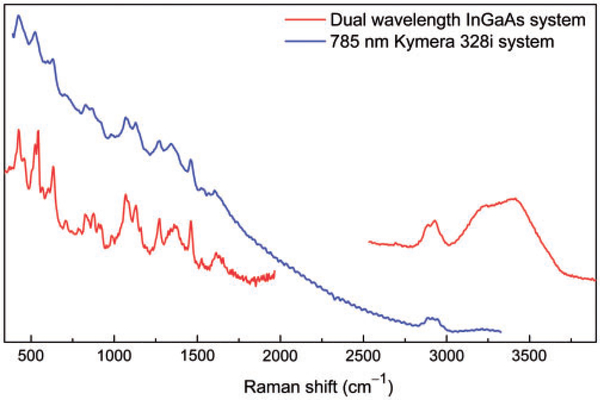
Representative spectra taken from a human tooth (enamel) using a research grade commercial Raman spectrometer (Kymera 328i) are shown in Fig. 2a. Raman spectrum taken from the same enamel piece using the customized dual-wavelength excitation system is shown in Fig. 2b for comparison. A scaled-down spectrum taken from distilled water using the same dual excitation system is also provided for reference. The red curve was taken under 15 mW, 633 nm laser excitation, while the blue curve was taken under 50 mW, 785 nm laser illumination. For the 633 nm laser illumination, the integration time was set at 1 s (100 averages) to avoid saturating the CCD detector by the fluorescence. Although the CCD detector is not saturated, the spectrum still looks distorted because the response of the fluorescence is not linear (i.e., the fluorescence baseline is not a smooth curve), multiple broad peaks appear in the range 1200–2500 cm−1 and these peaks will potentially interfere with Raman signals, if there any, in this range. For the 785 nm illumination, the laser power was set at 50 mW and integration time was set at 5 s (16 averages). In either case, high-frequency Raman signals were barely detectable due to the strong fluorescence interference (for 633 nm illumination) or significantly reduced sensitivity of the CCD detector in the region (for 785 nm excitation). For the dual-wavelength excitation Raman system, a fiber with core diameter of 600 μm was used to collect Raman signal. The large size of the fiber core improved the signal collection; however, it does not have the capability to remove out of focus fluorescence using the pinhole effect. Nevertheless, the lower frequency region taken under 100 mW 1064 nm excitation (10 s, six averages) shows nearly zero fluorescence. For the high-frequency region, dominant signals from C–H and O–H vibrations were observed under 866 nm excitation (80 mW, 30 s integration, six averages) although the fluorescence baseline was high. In addition to the reduced florescence allowing larger core fiber and longer integration time, the higher quantum efficiency of the InGaAs detector (~85%) should be the major contribution to the improvement in the high-frequency region.10
Figure 2.
(a) Representative Raman spectra of human dental enamel acquired with a research grade Raman system (Kymera 328i) under 633 nm (red curve) and 785 nm (blue curve) excitation and (b) the customized dual laser excitation InGaAs Raman system. Inset shows the magnified high-frequency region of the blue curve. No fluorescence background correction was conducted to these spectra to better present the fluorescence interference.
Comparing to the research grade Raman system, the dual-wavelength InGaAs system is not superior in detecting fingerprint Raman signals (below 2000 cm−1) if fluorescence is not a concern. However, for samples that are highly fluorescent, the 1064 nm excitation would be beneficial in preventing interference from fluorescence. The slope of the fluorescence baseline was greatly reduced on highly fluorescent red grape skin (Fig. 3). The research grade Raman is able to detect some organic components (near 3000 cm−1) from grape skin due to the high concentration of C–H bonds; however, it fails to detect water components.
Figure 3.
Raman spectra acquired from the skin a red grape acquired with research grade Raman system operated at 785 nm (blue curve), and dual-wavelength Raman system (red curve).
Sample spectra from the skins of two additional fruit, orange and red plum, are also provided (Figs. 4a and 4b) to demonstrate the capability of the dual-wavelength Raman system. These samples present stronger fluorescence than dental enamel tissue and are also challenging to study using CCD-based Raman spectrometers.
Figure 4.
Raman spectra acquired from skins of (a) orange and (b) plum.
Conclusion
In summary, we demonstrated a dual-wavelength Raman probe that allows switching between two lasers operated at 866 and 1064 nm. The dual-wavelength excitation effectively extends the scanning range of a Raman spectrometer that is designed for 1064 nm excitation and enabled the detection of high-frequency Raman signals particularly O–H and C–H bonding related Raman vibrations from biological tissues. Spectra taken from human enamel and fruit skins show that the dual-wavelength system has significantly reduced fluorescence background for acquiring fingerprint Raman signals (<2000 cm−1) due to its NIR excitation at 1064 nm. More importantly, the NIR excitation from 866 nm laser and the optimal sensitivity of the InGaAs detector at the high-frequency Raman signal regions allow the observation of water content in biological samples (especially high-fluorescent specimens) that was undetectable by CCD-based Raman spectrometers.
Acknowledgments
Funding
This work was supported by the National Institute of General Medical Sciences (NIGMS) and National Institute of Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) under award number SC2DE027240. The equipment was partially supported by the National Science Foundation (NSF) under award number 1332444.
Footnotes
Conflict of Interest
The authors report there are no conflicts of interest.
References
- 1.Smith E, Dent G. Modern Raman Spectroscopy: A Practical Approach. Hoboken, NJ: John Wiley and Sons, 2013. [Google Scholar]
- 2.Manoharan R, Shafer K, Perelman L, et al. “Raman Spectroscopy and Fluorescence Photon Migration for Breast Cancer Diagnosis and Imaging”. Photochem. Photobiol 1998. 67(1): 15–22. [PubMed] [Google Scholar]
- 3.Opilik L, Schmid T, Zenobi R. “Modern Raman Imaging: Vibrationa Spectroscopy on the Micrometer and Nanometer Scales”. Annu. Rev. Anal. Chem 2013. 6: 379–398. [DOI] [PubMed] [Google Scholar]
- 4.Lawson EE, Barry BW, Williams AC, et al. “Biomedical Applications of Raman Spectroscopy”. J. Raman Spectrosc 1997. 28(2–3): 111–117. [Google Scholar]
- 5.Yano Y, Ichimura T, Kuwahara S, et al. “Tip-Enhanced Nano-Raman Analytical Imaging of Locally Induced Strain Distribution in Carbon Nanotubes”. Nat. Commun 2013. 4: Art. no. 2592. [DOI] [PubMed] [Google Scholar]
- 6.Movasaghi Z, Rehman S, Rehman IU. “Raman Spectroscopy of Biological Tissues”. Appl. Spectrosc. Rev 2007. 42(5): 493–541. [Google Scholar]
- 7.Holst GC, Lomheim TS. CMOS/CCD Sensors and Camera Systems. Winter Park, FL: JCD Publishing, 2011. [Google Scholar]
- 8.Nikzad S, Hoenk ME, Greer F, et al. “Delta-Doped Electron-Multiplied CCD with Absolute Quantum Efficiency Over 50% in the Near to Far Ultraviolet Range for Single Photon Counting Applications”. Appl. Opt. 2012. 51(3): 365–369. [DOI] [PubMed] [Google Scholar]
- 9.Lesser MP. “Improving CCD Quantum Efficiency”. In: Proceedings Volume 2198, Instrumentation in Astronomy VIII. Symposium on Astronomical Telescopes and Instrumentation for the 21st Century Kailua, Kona, Hawaii: 1994. Pp. 782–791. [Google Scholar]
- 10.Adar F, Atzeni S, Gilchrist JR, et al. “Detector Choice is Vital to Spectroscopy”. Laser Focus World. April 1 2002. https://www.laserfocusworld.com/detectors-imaging/article/16552459/detector-choice-isvital-to-spectroscopy [accessed Sep 22 2019].
- 11.Penney CM, Goldman LM, Lapp M. “Raman Scattering Cross Sections”. Nature Phys. Sci 1972. 235: 110–112. [Google Scholar]
- 12.Golcuk K, Mandair GS, Callender AF, et al. “Is Photobleaching Necessary for Raman Imaging of Bone Tissue Using a Green Laser?” Biochim. Biophys. Acta, Biomembr 2006. 1758(7): 868–873. [DOI] [PubMed] [Google Scholar]
- 13.Vandenabeele P, Castro K, Hargreaves M, et al. “Comparative Study of Mobile Raman Instrumentation for Art Analysis”. Anal. Chim. Acta 2007. 588(1): 108–116. [DOI] [PubMed] [Google Scholar]
- 14.Chase B. “A New Generation of Raman Instrumentation”. Appl. Spectrosc 1994. 48(7): 14A–19A. [Google Scholar]
- 15.Schrader B, Dippel B, Erb I, et al. “NIR Raman Spectroscopy in Medicine and Biology: Results and Aspects”. J. Mol. Struct 1999. 480–481: 21–32. [Google Scholar]
- 16.Zhang J, Li M, Feng Z, et al. “UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at the Surface and in the Bulk”. J. Phys. Chem. B 2006. 110: 927–935. [DOI] [PubMed] [Google Scholar]
- 17.Strom D, Breuninger S, Fischer H, et al. “Criteria for High-Quality Raman Microscopy”. Spectroscopy. 2019. 34(6): 8–18. [Google Scholar]
- 18.Cooper JB, Marshall S, Jones R, et al. “Spatially Compressed Dual-Wavelength Excitation Raman Spectrometer”. Appl. Opt 2014. 53(15): 3333–3340. [DOI] [PubMed] [Google Scholar]
- 19.Masson LE, O’Brien CM, Pence IJ, et al. “Dual Excitation Wavelength System for Combined Fingerprint and High Wavenumber Raman Spectroscopy”. Analyst. 2018. 143(24): 6049–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiefer J. “Dual-Wavelength Raman Fusion Spectroscopy”. Anal. Chem 2019. 91(3): 1764–1767. [DOI] [PubMed] [Google Scholar]