Abstract
Time perception in the second-to-minutes range is crucial for fundamental cognitive processes like decision making, rate calculation, and planning. We used a striatal beat frequency (SBF) computational model to predict the response of an interval timing network to intruders, such as gaps in conditioning stimulus (CS), or distracters presented during the uninterrupted CS. We found that, depending on the strength of the input provided to neural oscillators by the intruder, the SBF model can either ignore it or reset timing. The significant delays in timing produced by emotionally-charged distracters were numerically simulated by a strong phase resetting of all neural oscillators involved in the SBF network for the entire duration of the evoked response. The combined effect of emotional distracter and pharmacological manipulations was modeled in our SBF model by modulating the firing frequencies of neural oscillators after they are released from inhibition due to emotional distracters.
Keywords: phase resetting, computer simulations, interval timing, striatal beat frequency, gap, distracter, nomifensine
Introduction
Interval timing refers to the capability of perceiving and using the passage of time in the seconds-to-minutes range. Interval timing is essential for survival and adaptation, foraging (Moore et al. 1989), and decision making (Jozefowiez, Cerutti, and Staddon 2005), speech recognition and music (Schirmer 2004), and its impairment leads to cognitive and motor dysfunctions (Buhusi, Perera, and Meck 2005; Gallistel 1990; Meck, Penney, and Pouthas 2008). Learning and memory abilities are altered in patients with depression, schizophrenia, and phobias (Davidson and Irwin 1999; Rose and Ebmeier 2006; Etkin and Wager 2007; Gohier et al. 2009; Amir and Bomyea 2011). A recent line of pharmacological treatment for these disorders involves norepinephrine (NE) and dopamine (DA) reuptake inhibitors, which indirectly increase neurotransmission in these pathways. In turn, both DA and NE modulate the internal clock (Buhusi and Meck 2010). DA agonists speed-up, and DA antagonists slow-down timing (Buhusi, Sasaki, and Meck 2002; Buhusi and Meck 2005; M. S. Matell, King, and Meck 2004; Matthew S. Matell, Bateson, and Meck 2006; Taylor, Horvitz, and Balsam 2007; Coull, Cheng, and Meck 2011). Moreover, NE modulates interval timing in both human participants (Rammsayer 1993; Rammsayer et al. 2001) and rodents (Penney, Holder, and Meck 1996). Nevertheless, the specific roles of DA and NE in interval timing at various brain sites are less understood.
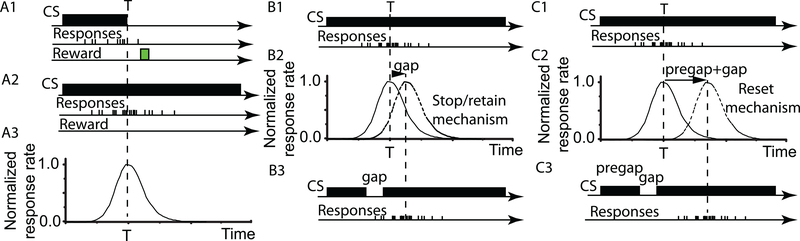
The peak interval (PI) procedure is commonly used for testing the capability of animals to perform interval timing. Temporal interval learning takes place during a fixed interval (FI) procedure (Figure 1A1). At the beginning of a FI trial, a conditioning stimulus (CS), such as light or a tone, is turned on; the first response of the subject after a certain duration (called criterion time (T)) is reinforced and turns off the to-be-timed CS (Figure 1A1). The ability to time intervals is tested in a PI procedure during which the CS is turned on for about three times longer than the learned criterion time without providing any reinforcement (Figure 1A2). Typically, the average of responses over multiple PI trials produces a normalized response rate that follows a Gaussian-shaped curve centered on T (Figure 1A3) (Church, Meck, and Gibbon 1994; Gibbon and Allan 1984).
Figure 1. Fixed interval (FI) and peak interval (PI) procedures with and without CS gaps.
(A1) During FI trials, the first response after the criterion time, T, is reinforced and turns off the CS. (A2) In PI trials, the CS is on for about three times the duration of the criterion time without providing any reinforcement. (A3) The average of responses over many PI trials produces a normalized response rate curve that peaks around T and has a Gaussian-like shape. In PI trials with gap, the CS is briefly turned off (B3 and C3). In some experiments, the response rate curve is shifted with the duration of the gap (B2) supporting the hypothesis of a stop/retain of interval timing. In other experiments, the shift equals the sum of pregap and gap duration (BC2), supporting the hypothesis of a reset.
A common variation is the PI procedure with gap during which the CS is briefly interrupted (see Figure 1B and C) and the position of the peak responses is measured. The results from PI procedures with CS gaps (S. Roberts 1981) showed that in rats the peak response is delayed with the duration of the CS gap (Figure 1B2). Such experiments support the hypothesis of a stop/retain mechanism that retains (maintains) the time of the stimulus before the gap and resumes timing when the stimulus is turned on again.
In contrast, experiments in pigeons (W. A. Roberts, Cheng, and Cohen 1989) indicated that the peak response was delayed with the sum of the pregap and gap durations (Figure 1C3). Additionally, PI procedures with gaps in starlings (Bateson 1998), black-capped chickadees (Brodbeck, Hampton, and Cheng 1998), and pigeons (Cabeza de Vaca, Brown, and Hemmes 1994; W. A. Roberts, Cheng, and Cohen 1989) support the reset mechanism hypothesis.
Recent results indicate that rats reset their timing in PI trials upon presentation of reinforcement (Thorpe, Petrovic, and Wilkie 2002), that both rats and pigeons stop or reset depending on gap’s content (Buhusi and Meck 2000), gap discriminability (Buhusi, Perera, and Meck 2005), gap/signal contrast (Buhusi, Sasaki, and Meck 2002), and subjects’ visual acuity. Recent studies showed that the outcome of PI procedure with CS gaps depends on many more factors than just the durations of gap and pregap. For example, non-temporal parameters of the to-be-timed event influence the response rule (reset or run) adopted by rats (Buhusi and Meck 2000; Buhusi, Sasaki, and Meck 2002; Buhusi and Meck 2005) and pigeons (Buhusi and Meck 2002). When timing an illuminated stimulus, a (standard) dark gap prompts rats to stop timing, and when timing a dark stimulus, a (reversed) illuminated gap prompts rats to reset timing (Buhusi and Meck 2000). Moreover, the response rule used by both rats and pigeons depends on the salience (discriminability) of the intruding event, affected by the contrast in intensity between the gap and the timed signal (Buhusi and Meck 2002; Buhusi, Perera, and Meck 2005) and by the perceptual acuity of the subjects (Buhusi and Meck 2005). Furthermore, in some PI procedures with gap no delay was found, i.e., the internal clock run through the gap and ignored it (see (Buhusi and Meck 2005) for a review).
A more complex PI procedure could include another intruder, such as emotional distracters, e.g., electric shocks, paired with the uninterrupted to-be-timed CS. Delays of the peak response were obtained when the procedure includes intruders other than gaps (Buhusi and Meck 2006; Buhusi, Paskalis, and Cerutti 2006; Kaiser, Zentall, and Neiman 2002). Presentation of emotionally-charged distracters during the uninterrupted to-be-timed CS signal results in a considerable delay (over-reset) in PI procedure relative to neutral distracters (Sang Weon Aum, Brown, and Hemmes 2007; S. W. Aum, Brown, and Hemmes 2004; Brown, Richer, and Doyere 2007). For example, anxiety-inducing task-irrelevant distracters severely alter timing. When asked to keep a face in working memory (primary task), the presentation of emotional faces (secondary task) impaired recognition memory (Dolcos and McCarthy 2006). Context-dependent timing was also observed by manipulating the emotional content of stimuli (Evans 2003; Flaherty 1999; Lui, Penney, and Schirmer 2011; Matthews et al. 2012).
An abstract internal clock is the core of the influential Scalar Expectancy Theory (SET) that offers a conceptual explanation of interval timing mechanism (Gibbon 1977; Church 1984) (see also earlier work by (Fraisse 1957; Francois 1927; Hoagland 1933; Treisman 1963; Woodrow 1930). The model consists of a clock, a memory and a decision stage. The clock consists of a pacemaker that emits pulses at regular intervals that are counted and temporarily stored in an accumulator (short-term memory). At the reinforcement time, the content of the short-term memory is transferred to the long-term memory and serves as a subjective representation of T. At the decision stage, the current content of the accumulator (short-term memory) is compared against the long-term memory content and an appropriate response is produced (Church 1984).
One of the first models that closely explored the relationship between the biological structure and its interval timing functionality was the connectionist model developed by (Church and Broadbent 1990; Church and Broadbent 1991). The model assumed that a set of neural oscillators determines the peak time using multiple-period discrimination algorithms. The clock stage was represented by oscillators and the memory stage stored the oscillators’ phases at reinforcement time. At the decision stage, the content of long-term memory was compared against the current phases of all oscillators and an appropriate decision was made. This connectionist model successfully duplicated the Gaussian-like shape of response rate and the scalar property (Church and Broadbent 1991; Church, Lacourse, and Crystal 1998). However, this connectionist model is limited to timing durations that do not exceed the longest period of the set of oscillators and requires a quite large coefficient of variation (Aschoff 1989).
In this paper, we use a neurobiologically inspired Striatal Beat Frequency (SBF) model (M. S. Matell, Meck, and Nicolelis 2003; M. S. Matell and Meck 2004; Miall 1989; Sorinel A. Oprisan and Buhusi 2013; Sorinel A. Oprisan and Buhusi 2013; S. A. Oprisan and Buhusi 2011) to explain recent experimental results obtained during PI procedures with intruders, both gaps and emotional distracters.
In this paper we used a distributed neural network model that produces beats between multiple oscillators, presumably located in the prefrontal cortex, and is capable of timing intervals much longer than the durations of the intrinsic periods of individual oscillators (Miall 1989; M. S. Matell, King, and Meck 2004; M. S. Matell and Meck 2004). We implemented an SBF network with realistic, noisy Morris-Lecar (ML) model neurons (Morris and Lecar 1981; Ermentrout 1996) that mimic the activity of the frontal cortex neurons that are thought to provide the time base for the SBF (Coull et al. 2004; Olton et al. 1988). Elsewhere, we showed that the SBF-ML model produces both precise and scalar interval timing in the presence of variability of model’s parameters such as the memorized criterion time and the firing frequencies of the oscillators (Sorinel A. Oprisan and Buhusi 2013; Buhusi and Oprisan 2013; S. A. Oprisan and Buhusi 2011).
Here we showed numerically that our SBF-ML implementation is capable of producing both reset, i.e., delayed peak response equal to the sum of the pregap and gap durations, and run behavior, i.e., continue timing through the gap (ignore the gap). Crucial to the correct SBF modeling of gap effect is the ability to restart all oscillators in phase at the end of the CS gap. Such a strong phase reset could be due to postinhibitory rebound (Perkel and Mulloney 1974; Getting 1989). Rebound firing of neurons after strong inhibitions was observed in invertebrate’s central pattern generators, where the mechanism is responsible for motor response (Selverston and Moulins 1985), and in mammals’ brain where postinhibitory rebound generates sustained oscillations (Llinas 1988). In addition to the postinhibitory rebound in response to brief inhibitory pulses, the effect was also tested with long-lasting inhibitions (Goaillard et al. 2010) and was associated with a form of intrinsic short-term memory of the stimulus (Storm 1988; Egorov et al. 2002; Pulver and Griffith 2010).
We also showed that our SBF-ML model can explain the over-reset observed during the PI procedure with uninterrupted CS and emotionally-charged distracters. Furthermore, we successfully simulated the beneficial effects of the frontal cortex infusions of dopamine / norepinephrine reuptake inhibitor nomifensine in reducing the timing delay after emotional distracters (Matthews et al. 2012) by altering the frequencies of the oscillators of the SBF network.
The SBF interval timing model
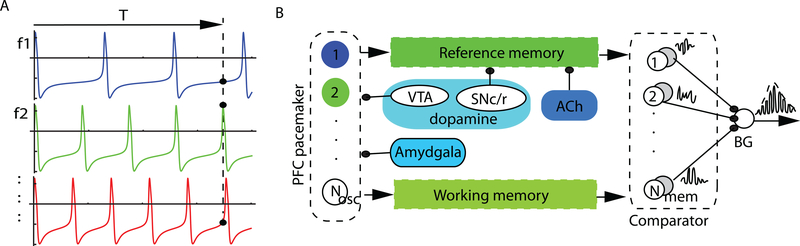
The SBF paradigm models behavioral mechanisms of interval timing that use neural structures as metaphor. It mimics the activity of cortico-striato-thalamic loops that are known to contribute to interval timing (see (S. A. Oprisan and Buhusi 2011; Buhusi and Oprisan 2013; Sorinel A. Oprisan and Buhusi 2013) for details on SBF implementation). It is assumed that a set of model oscillators provide the underlying time base for the SBF interval timing network (Figure 2A). All results presented in this paper are based on ML model oscillators (see (Sorinel A. Oprisan and Buhusi 2013) for a detailed mathematical description of the implementation of he SBF-ML model). Ubiquitous biological noise and background neural activity was implemented by allowing the intrinsic frequencies of oscillators to fluctuate according to a specified probability density function. It is assumed that at the beginning of each trial all oscillators are reset and start in phase (Figure 2A). At the criterion time, the oscillators are read out and their states, represented by solid dots in Figure 2A, are transferred from the working memory block in the reference (long-term) memory (see the block diagram in Figure 2B). Specifically, the state of the Nosc oscillators with frequencies fi in the range [8, 13] Hz (see Figure 2B) are stored at criterion time in a vector for later comparison against the vector of states at the current time (see (Sorinel A. Oprisan and Buhusi 2013) for details regarding the implementation of the model). Both writing and reading the vector of states at the criterion time to and from the memory blocks is affected by random noise, which we mimicked by allowing small variations of the vector of state drawn from a specific probability distribution. Elsewhere, we showed mathematically and checked numerically that the SBF-ML model can perform accurate, precise, and scalar interval timing regardless the type of the variability (see (Sorinel A. Oprisan and Buhusi 2013)). Additionally, the content of the long-term memory block can be altered by the cholinergic system block (see (S. A. Oprisan and Buhusi 2011) for pharmacology-related details of this SBF-ML model). The coincidence detection between the content of the reference memory and the current state of working memory (the current state of the oscillators) is ascribed to striatal spiny neurons in the basal ganglia. Matell, Meck, and Nicolelis (2003) showed that the firing patterns of striatal neurons peak around the criterion time, strongly suggesting striatal involvement in interval timing. Specifically, we implemented the coincidence detection mechanism as a dot product between the vector of states stored in the long term memory and the current vector of states (see (Sorinel A. Oprisan and Buhusi 2013) for implementation details and a discussion of other potential implementations). Additionally, pharmacological manipulations, such as administration of dopaminergic drugs both systemically (Maricq, Roberts, and Church 1981; Maricq and Church 1983; M. S. Matell and Meck 1997; M. S. Matell, King, and Meck 2004; M. S. Matell and Meck 2004; Meck 1983; Meck 1996) or directly into the anterior portion of the striatum (Neil and Herndon Jr. 1978), alter the speed of interval timing, possibly through the dopaminergic projections from ventral tegmental area to frontal cortex (Figure 2B). For example, DA agonists produce a leftward, dose-dependent, shift in the PI procedure. Experiments with dopaminergic drugs showed that the magnitude of the shift in the temporal response scales roughly linearly with the dose (Meck 1996; M. S. Matell and Meck 1997), suggesting a tight relationship between synaptic dopamine levels and clock speed. We previously shown that such a leftward shift of the peak responses in the SBF-ML implementation can be mimicked by shifting all oscillators’ frequencies fi* = (1 + α) fi, where α is a dose-dependent factor (see (S. A. Oprisan and Buhusi 2011) for a detailed implementation of the computational model). To mimic the effect of DA agonists (e.g., methamphetamine or cocaine) we considered 0 < α < 1, whereas for antagonists (e.g., haloperidol) we considered −1 < α < 0.
Figure 2. The striatal beat frequency model.
(A) All frontal cortex oscillators start in phase at the beginning of interval timing test but fire with different frequencies f1, f2, f3, … in the alpha band [8, 13] Hz. At the criterion time, T, the states (phases) of all oscillators (the solid dots along the vertical dashed line at T) are stored in the long-term (reference) memory. The comparator block (loosely associated with the basal ganglia) compares the current state of oscillators (working memory) against the content of the reference memory and generates a strong response if they coincide. (B) The oscillators are connected both with the reference and the working memory, which in turn provide input to the comparator. The SBF-ML model implemented also cholinergic and dopamine modulations. Emotional stimuli have the ability to reset the frontal cortex oscillators, presumably through amygdala link to frontal cortex (this is not shown in the figure). FC: frontal cortex; BG: basal ganglia; SNc/r: substantia nigra pars compacta/reticulata; VTA: ventral segmental area.
Reset in the peak-interval procedure with gaps
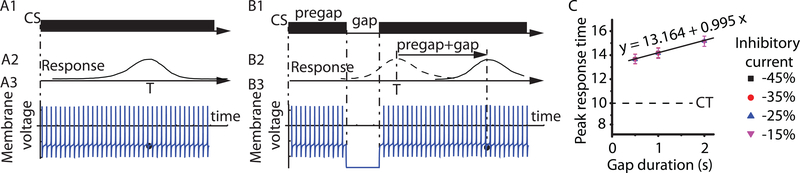
In the absence of any CS gap during the PI procedure the peak response is centered around the criterion time (see Figure 1C1). A gap in the CS (see Figure 1C2) shift the peak response with the duration of the pregap plus the gap duration (see Figure 1C3). In our SBF-ML implementation (S. A. Oprisan and Buhusi 2011; Buhusi and Oprisan 2013; Sorinel A. Oprisan and Buhusi 2013), the peak response (see Figure 3A2) is based on the coincidence detection between the memorized states of neural oscillators at the reinforcement time (such as the one shown in Figure 3A3) and the current state. The reset response could be explained by assuming that the presentation of an intruder, e.g., a CS gap (see Figure 3B2), blocks all oscillators during the entire duration of the intruder (see Figure 3B3).
Figure 3. Phase resetting of neural oscillators and PI-GAP procedure.
(A1) Schematic representation of a PI procedure with a CS stimulus (solid rectangle) presented for about three times the duration of the criterion time, T, and the corresponding Gaussian-like response (A2) centered on T. (A3) The state of oscillators, of which we only show one, at T (marked by a solid circle along the membrane voltage trace) is stored in the reference memory for later comparison with the content of the working memory. A brief CS gap (B1) shifts the peak response from the T (dashed line in panel B2) by the sum of the pregap plus the gap duration (continuous line in panel B2). This shift in behavioral response is consistent with a reset of the internal clock. (B3) In the SBF-ML model, the behavioral reset (B2) could be derived from cell-level response by the mechanism of phase resetting (B3). A strong inhibitory current that mirrors the gap could force all oscillators into a silent hyperpolarized state and reset their phases (see B3). At the end of the gap, the oscillators are released from inhibition and they rebound in phase, which mimics the behavioral reset. The memorized states from the PI procedure (panel A3) will emerge at a later time after recovering from hyperpolarization (see the solid dot in panel B3). The delay predicted by the SBF-ML model is equal to the sum of pregap and gap durations. (C) Numerical simulations using SBF-ML show that the PI peak shift form the expected T=10s is equal to the sum of the pregap = 3s and the corresponding gap duration, which was varied between 0.5s and 3s.
To mimic the behavioral reset, the computational model sets a hyperpolarizing bias current for all oscillators at the beginning of the intruder for its entire duration (see Figure 3B3). For example, when the intruder is a brief absence of the CS (a gap), the timing networks is not engaged due to a strong hyperpolarization of all oscillators of the SBF-ML model in the absence of the respective CS. When the CS is back on after the CS gap, the oscillators are released from inhibition and start oscillating with the same initial phase. As discussed in the Introduction, such a phase reset effect capable of restarting all oscillators in phase could be due to postinhibitory rebound (Perkel and Mulloney 1974; Getting 1989).
We performed numerical simulations using our SBF-ML implementation with a fixed pregap interval of 3s and a variable CS gap duration (Figure 3C). The criterion time was set to 10s (dashed line in Figure 3C) and the position of the peak of Gaussian response function was measured for gap durations varying between 0.5s to 3s. The inhibitory bias current injected in all oscillators of the SBF-ML model was a fraction of the lowest bias current needed in order to have all oscillators firing in the frequency range [8, 13] Hz. For strong inhibitions that mirror the CS gap, all oscillators are silent (see Figure 3B3) and they restart firing in phase after removing the inhibition. As a result, the peak of the Gaussian response is shifted from the expected T = 10s by to the sum of 3s pregap plus the corresponding gap duration (continuous trend line in Figure 3C). The strong resetting of frontal cortex oscillators followed by a postinhibitory rebound ensures that all oscillators restart in phase.
Over-reset mechanism in PI procedure with emotionally-charged distracters
In the previous section, the behavioral reset of interval timing was explained by phase resetting and postinhibitory rebound of neural oscillators at the core of the SBF-ML model due to an inhibitory stimulus that mirrors the CS whereas a run was explained by a too low inhibition of the oscillators that cannot reset them. In this subsection, we focus on the potential mechanism that could explain the over-reset observed in PI procedures when emotionally-charged distracters are presented during the to-be-timed uninterrupted CS.
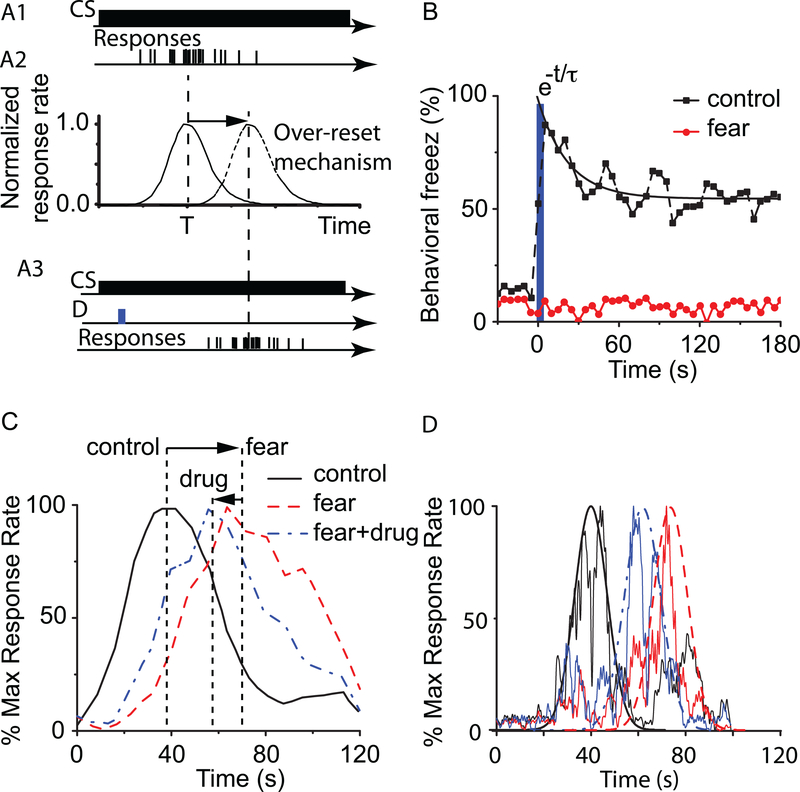
A set of recent experiments found a significantly large delay effect of anxiety-inducing task-irrelevant distracters on interval timing in a PI procedures with a 40-s visual to-be-timed signal (Matthews et al. 2012). Under normal PI procedure with a single CS without gap (Figure 4A1) the rats produced a peak response at criterion time (Figure 4A2). In the modified PI procedure rats were additionally presented with a 5-s auditory white noise stimulus (see the solid rectangle marked D (distracter) in Figure 4A3) which was paired with a 1-s foot shock in one fear group and no electric shock in a control group. We found that if the brief auditory stimulus (distracter) was not paired with the electric shock, the rats’ performance is not affected (continuous line in Figure 4A2). However, rats in the fear group show reliable freezing behavior (see Figure 4B) following the presentation of the auditory stimulus in extinction (without shock presentation) that lasted for several minutes after the noise ended (Matthews et al. 2012). The PI response of rats in the fear group is also considerably delayed (see dashed line in Figure 4A2 and Figure 4C) by the presentation of the fear-inducing distracter. However, the anxiety-induced delay of almost 31s is significantly longer than the duration of the fear stimulus (5s), which rules out a stop induced by the brief 5s presentation of the auditory stimulus. Moreover, the delay is considerably longer even than the sum of the predistracter (5s) and distracter (5s) durations, which rules out a reset based entirely on the duration of the distracter.
Figure 4. Fear-evoking stimuli and nomifensine influence on interval timing.
In a PI procedure with a single CS (A1), the peak response (continuous line in A2) occurs around the criterion time T. Concurrent presentation of a fear-evoking distracter during PI procedure (see the brief distracter D marked by a solid rectangle in panel A3) induces behavioral freeze. The average percent freezing behavior in the fear conditioning context (solid squares in panel B) show reliable, long-lasting freezing behavior after the presentation of the fear-conditioning sound (solid rectangle) at time=0s. There is no freezing behavior is rats presented with a neutral auditory distracter (that was not paired with electric shocks, solid circles in panel B). The recovery from behavioral freeze can be modeled by an exponential decay with a time constant τ ≈ 22s. The average maximum percent response (lever pressing) rate in rats trained to time a T = 40s signaled by a visual stimulus when presented with a neutral distracter was virtually identical both in the control and fear groups (continuous line in panel C - redrawn from (Matthews et al. 2012)). Emotionally charged distracter (white noise) presented to the fear group shows a considerable rightward shift of the response (dashed line in panel C) relative to neutral distracter (continuous line). Local infusions of the norepinephrine and dopamine reuptake inhibitor nomifensine (dashed-dotted line in panel C), induce a leftward shift of the peak response function in the fear group and have no effect on the control group. (D) Numerical simulations results obtained with the SBF-ML model. The jagged trace under the continuous Gaussian-shaped curve centered round 40 s represents a single trial obtained with SBF-ML model and T = 40s. The reset induces by a long-lasting behavioral freeze (dashed Gaussian and the corresponding jagged single-trial in panel D) with a duration of τ ≈ 22s shows a leftward displacement similar to experimental results from panel C. The pharmacological effect of nomifensine was simulated by speeding-up all oscillators by about 20% (dashed-dotted Gaussian and the corresponding jagged single-trial in panel D), which produced a rightward shift similar to experimental data from panel C.
To address this finding, we simulated a PI procedure with a visual CS combined with an auditory distracter that over-reset the oscillators of the SBF-ML model, as follows: To address the over-resetting of about 30s following the presentation of a fear-evoking auditory stimulus that only lasted 5s, we hypothesized that the long-lasting freezing behavior (see Figure 4B) (Matthews et al. 2012) is mirrored in the SBF-ML network by a strong, long-lasting inhibition of the oscillators. However, the strong inhibition that explained the reset mechanism in the previous section required the inhibition of oscillators only during the duration of the distracter. To implement the over-resetting in the existing SBF-ML model we assumed that the fear-evoking auditory stimulus served only as a trigger of long-lasting frontal cortex inhibition (presumably by amygdala). The actual duration of frontal cortex oscillators inhibition had to be much longer than the duration of the fear-evoking stimulus. A reasonable duration of frontal cortex inhibition is the time-constant of the freezing behavior (τ), which can be estimated by fitting the freezing response function with an exponential decay function ke t/τ. Therefore, based on experimental data from (Matthews et al. 2012), we estimated that the time constant τ for releasing the rats from freezing behavior (see Figure 4B) is about τ = 22.2 ± 5.5s measured from the beginning of the fear stimulus (solid rectangle at time = 0s in Figure 4A3 and B).
As in the case of the reset mechanism induced in a PI procedure with gaps, once the oscillators are released from inhibition they restart in phase and begin timing. Therefore, in our SBF-ML model we simulated the effect of the emotionally-charged auditory stimulus not by its 5s behavioral distracter but rather by a distracter whose duration was the time constant τ of the behavioral freezing. As a result, in agreement with the results from the previous subsection, numerical simulations indicated that the peak response of the fear group is shifted by approximately Δtfear = tpregap + τ ≈ 5s + (22.2s ± 5.5s) = 27.2s ± 5.5s (see dashed Gaussian envelope in Figure 4D), not significantly different (p<0.05) from the 31s over-reset observed experimentally (Matthews et al. 2012), t(10)=0.69, p>0.50. The t test compared a sample of experimentally determined peak shifts from (Matthews et al. 2012) to the mean peak shift derived from our SBF-ML simulations. We only showed numerical simulations results for a single trial (see jagged traces in Figure 4D) for T = 40 (which corresponds to experimental control group from panel C). Similarly, for the FEAR case we only showed numerical results obtained with SBF-ML model for a single trial (jagged trace under the dashed Gaussian envelope).
SBF-ML model of interval timing and the combined effects of pharmacology and emotionally-charged distracters
Local infusions of nomifensine in the prelimbic cortex alter the PI response to emotional distracters only in the fear group (see dashed-dotted trace in Figure 4C) but did not change the PI response in the control group (continuous line in Figure 4C) (Matthews et al. 2012). We hypothesized that nomifensine may change the internal clock speed after the long-lasting reset effect of the fear stimulus ends. In the context of dopaminergic agonists effect on SBF-ML network (see (S. A. Oprisan and Buhusi 2011; Buhusi and Oprisan 2013; Sorinel A. Oprisan and Buhusi 2013), the leftward shift induced by nomifensine could be explained through the speeding-up of the oscillators due to elevated levels of DA and NE. Here we carried out numerical simulation with an SBF-ML model using 600 biophysically realistic ML oscillators firing in the range [8, 13] Hz, 1000 memory samples, and a drug dose effect α ≈ 0.20 (within the range of values used in (S. A. Oprisan and Buhusi 2011) that matched previous DA experiments). Numerical simulations indicated that the peak of the Gaussian response is leftward shifted (see the single-trial jagged trace under the dashed-dotted Gaussian in Figure 4D) with about 8s towards smaller durations due to an increase in the frequency of PFC oscillations that mimics the effect of nomifensine, which is in general agreement with the experimental 10-s leftward shift observed by (Matthews et al. 2012, p.7, Figure 5).
Discussion
First, under the assumption that intruders (e.g., CS gaps) produce a significant hyperpolarization of cortical oscillators that lasts for their entire duration, the SBF-ML model successfully accounted for the behavioral reset observed in PI procedures with gaps. Second, under the assumption that fear-inducing stimuli trigger long-lasting inhibition of frontal cortex oscillators (possibly through amygdalar activation), the SBF model was able to account for the considerable timing delay (over-reset) following such stimuli. In our computational implementation of the SBF-ML model the relationship between the behavioral observations (Matthews et al. 2012) and our neurobiologically inspired SBF-ML model was achieved through a strong, long-lasting hyperpolarization of the oscillators for a duration longer than the duration of the fear-inducing stimulus (i.e., a delay equal to the time constant of the behavioral freezing response). Third, we previously showed that the SBF-ML model is also able to successfully account for the effect of pharmacological agents on interval timing (S. A. Oprisan and Buhusi 2011) by speeding-up the oscillators to mimic the effect of DA agonists and slowing them down to mimic antagonists. Therefore, because nomifensine blocks DA reuptake, increases DA levels and, as a result, speed-up the oscillators, the SBF model also successfully described the reduction of the delaying effect of fear-inducing stimuli following local frontal cortex infusion of nomifensine (see dashed-dotted line in Figure 4C).
Qualitatively, there are other possible explanations of the observed behavioral resetting. For example, Staddon (1965) suggested that it is likely that poor timing performance is due to the inability to focus attention on the temporal parameters of a task (Staddon 1965). A more modern implementation of this idea is provided by the Time-Sharing model (Buhusi 2003; Buhusi 2012; Buhusi and Meck 2009a). According to the Time-Sharing model, intruders thought to stop or reset the clock, like CS gaps, rather divert attention from timing towards processing the distracter. This loss of timing resources leads to a decay of the time preceding the distracter and thus to a subjective shortening of the pre-gap time. This subjective shortening would lead to rightward peak shift and thus to a delayed response.
However, the Time-Sharing model cannot address the over-reset following emotionally-charged distracters. Presentation of emotionally-charged distracters during the uninterrupted to-be-timed signal results in a considerable delay (over-reset) in peak responses relative to neutral distracters (Sang Weon Aum, Brown, and Hemmes 2007; S. W. Aum, Brown, and Hemmes 2004; Brown, Richer, and Doyere 2007). We recently replicated this effect in our lab (Matthews et al. 2012). According to the Time-Sharing model, distracters thought to reset the clock, divert attention from timing towards processing the distracter until all accumulated time is lost, which determines subjects to restart timing (reset) after the distracter. Because accumulated time is always a positive quantity, in the Time-Sharing model the accumulated time cannot decay to negative values, and thus the model cannot address the over-reset phenomenon. Therefore, is was hypothesized that emotionally-charged distracters produce “post-cue” effects, i.e., effects that last longer than the physical stimulus (Sang Weon Aum, Brown, and Hemmes 2007; S. W. Aum, Brown, and Hemmes 2004; Brown, Richer, and Doyere 2007). In line with this alternative interpretation, here we estimated the “post-cue” effect of a fear-inducing stimulus to be the time-constant of the freezing behavior, which was determined to be much longer than the physical fear-inducing stimulus. When implementing this “post-cue” effect, the SBF model was able to account for the over-reset effect of fear-inducing stimuli (Matthews et al. 2012).
The effect of simultaneous presentation of CS gaps and distracters on interval timing suggests that intricate relationships exist between interval timing and associative phenomena. For example, the effect of a distracter seems to be determined by its salience (Buhusi 2012). In contrast, by and large, interval timing models, including the SBF model, do not address salience factors. Moreover, in the interval timing literature, the effect of pharmacological agents is generally understood in terms of speeding-up / slowing-down the clock, or biasing the memorized criterion (Meck 1996; S. A. Oprisan and Buhusi 2011). Instead, the effect of pharmacological agents in the PI procedure with CS gaps (Buhusi 2003; Buhusi and Meck 2002; Buhusi and Meck 2007) or distracters (Matthews et al. 2012) is credited to factors outside of the internal clock (Buhusi 2012; Buhusi and Meck 2009b). Finally, manipulations of the familiarity with the distracter (Buhusi et al., 2013, this issue), seem to be more compatible with attentional associative models (Lubow 1973) than with interval timing models.
Moreover, the present computational simulations with the SBF model suggest that such effects are relatively hard to incorporate into “pure” interval timing models, like the SBF. For example, while the present simulations show possible ways that the resetting effect of CS gaps and distracters can be implemented, they fall short of demonstrating the graded effect of distracters. Under the current set of assumptions, the SBF model seems to be able to ignore (run through) a distracter that determines no frontal cortex inhibition, or reset (restart timing) after a distracter that determines a minimal levels of frontal cortex inhibition (15%, see Figure 3C), or even over-reset by maintaining the oscillators inhibited for longer periods of time. However, under the current parameters, the model fails to mimic the graded effect of distracter when manipulating its salience (e.g., (Buhusi 2012)). As indicated in Figure 3C, varying the levels on inhibitory control failed to determine a graded delay of timing, which is contrary to findings indicating that the response used by both rats and pigeons depends on the salience (discriminability) of the distracter, affected by the contrast in intensity between the gap and the timed signal (Buhusi and Meck 2002; Buhusi, Perera, and Meck 2005) and by the perceptual acuity of the subjects (Buhusi and Meck 2005).
Instead, a graded effect of the gap (by gap duration) was demonstrated in a prototypical real-time associative model, the Spectral-Timing model (Grossberg and Schmajuk 1989; Buhusi and Schmajuk 1999). Real-time associative models assume that timing is a property of associative learning (for a similar interpretation see also Mollet and Miller 2013, this issue.) Within the framework of the Spectral Timing model, (Hopson 1999) showed that the graded effect of gaps can be addressed by assuming that (memory) traces decay during the gap. Therefore, it seems that some effects (e.g., having to do with distracter salience and duration) are more readily addressable within the framework of real-time associative models. Taken together, such results suggest the need for an integration of associative and temporal phenomena within a larger frame, possibly by the use of real-time models. While the SBF model is not yet a real-time model, here we showed that some real-time phenomena (like brief intruders) can be addressed at the computational and neurobiological level within the framework of this model, hopefully adding to the list of unexplained phenomena that the associative and temporal learning fields have yet to jointly address.
Acknowledgements
This work was supported by the National Science Foundation IOS CAREER award 1054914 to S.A.O., a Summer Undergraduate Research with Faculty-SURF award to S.D., and the National Institute of Health (National Institute of Mental Health grants MH065561 and MH073057) awards to C.V.B. Author contribution: conceived and designed the experiments: C.V.B.; implemented the SBF-ML model and performed numerical simulations: S.D., S.A.O. and C.V.B; analyzed the data: S.A.O and C.V.B; wrote the paper: S.A.O and C.V.B.
References
- Amir N, and Bomyea J. 2011. “Working Memory Capacity in Generalized Social Phobia.” Journal of Abnormal Psychology 120: 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J 1989. “Temporal Orientation: Circadian Clocks in Animals and Humans.” Animal Behaviour 37: 881–896. [Google Scholar]
- Aum SW, Brown BL, and Hemmes NS. 2004. “The Effects of Concurrent Task and Gap Events on Peak Time in the Peak Procedure.” Behavioural Processes 65: 43–56. [DOI] [PubMed] [Google Scholar]
- Aum Sang Weon, Brown Bruce L., and Hemmes Nancy S.. 2007. “The Effect of Intruded Events on Peak Time: The Role of Reinforcement History During the Intruded Event.” Behavioural Processes 74 (2) (February 22): 187–197. doi: 10.1016/j.beproc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bateson M 1998. “Species Differences in Timing Intervals with Gaps. What Do They Mean?” In Proceeding of International Conference on Comparative Cognition Melbourne, FL. [Google Scholar]
- Brodbeck DR, Hampton RR, and Cheng K. 1998. “Timing Behaviour of Blackcapped Chickadees (Parus Atricapillus).” Behavioural Processes 44: 183–195. [DOI] [PubMed] [Google Scholar]
- Brown BL, Richer P, and Doyere V. 2007. “The Effect of an Intruded Event on Peak-interval Timing in Rats: Isolation of a Postcue Effect.” Behavioural Processes 74: 300–310. [DOI] [PubMed] [Google Scholar]
- Buhusi CV 2003. “Dopaminergic Mechanisms of Interval Timing and Attention” In Functional and Neural Mechanisms of Interval Timing, edited by Buhusi CV, 317–338. Boca Raton, FL: CRC Press. [Google Scholar]
- ———. 2012. “Time-sharing in Rats: Effect of Distracter Intensity and Discriminability.” Journal of Experimental Psychology: Animal Behavior Processes 38 (1): 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, and Meck WH. 2000. “Timing for the Absence of a Stimulus: The Gap Paradigm Reversed.” Journal of Experimental Psychology: Animal Behavior Processes 26 (3): 305–322. [DOI] [PubMed] [Google Scholar]
- ———. 2002. “Differential Effects of Methamphetamine and Haloperidol on the Control of an Internal Clock.” Behavioral Neuroscience 116 (2): 291–297. [DOI] [PubMed] [Google Scholar]
- ———. 2005. “What Makes Us Tick? Functional and Neural Mechanisms of Interval Timing.” Nature Reviews Neuroscience 6 (10): 755–765. [DOI] [PubMed] [Google Scholar]
- ———. 2006. “Interval Timing With Gaps and Distracters: Evaluation of the Ambiguity, Switch, and Time-Sharing Hypotheses.” Journal of Experimental Psychology: Animal Behavior Processes 32 (3): 329–338. [DOI] [PubMed] [Google Scholar]
- ———. 2007. “Effect of Clozapine on Interval Timing and Working Memory for Time in the Peak-interval Procedure with Gaps.” Behavioural Processes 74: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2009a. “Relativity Theory and Time Perception: Single or Multiple Clocks?” PLoS One 4 (7): 6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2009b. “Relative Time Sharing: New Findings and an Extension of the Resource Allocation Model of Temporal Processing.” Philosophical Transactions of the Royal Society London B Biological Sciences 364 (1525): 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2010. “Timing Behavior” In Encyclopedia of Psychopharmacology, edited by Stolerman IP, 2:1319–1323. Berlin: Springer. [Google Scholar]
- Buhusi CV, and Oprisan SA. 2013. “Time-scale Invariance as an Emergent Property in a Perceptron with Realistic, Noisy Neurons.” Behavioural Processes 95 (May): 60–70. doi: 10.1016/j.beproc.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Paskalis J-PG, and Cerutti DT. 2006. “Time-sharing in Pigeons: Independent Effects of Gap Duration, Position and Discriminability from the Timed Signal.” Behavioural Processes 71: 116–125. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Perera D, and Meck WH. 2005. “Memory for Timing Visual and Auditory Signals in Albino and Pigmented Rats.” Journal of Experimental Psychology: Animal Behavior Processes 31 (1): 18–30. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Sasaki A, and Meck VH. 2002. “Temporal Integration as a Function of Signal and Gap Intensity in Rats (Rattus Norvegicus) and Pigeons (Columba Livia).” Journal of Comparative Psychology 116 (4): 381–390. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, and Schmajuk NA. 1999. “Timing in Simple Conditioning and Occasion Setting: a Neural Network Approach.” Behavioural Processes 45 (1–3): 33–57. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Brown BL, and Hemmes NS. 1994. “Internal Clock and Memory Processes in Animal Timing.” Journal of Experimental Psychology: Animal Behavior Processes 20: 184–198. [DOI] [PubMed] [Google Scholar]
- Church RM 1984. “Properties of the Internal Clock” In Timing and Time Perception, edited by Gibbon J and Allan L, 566–582. New York: New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Church RM, and Broadbent HA. 1990. “Alternative Representations of Time, Number, and Rate.” Cognition 37 (1–2): 55–81. [DOI] [PubMed] [Google Scholar]
- ———. 1991. “A Connectionist Model of Timing” In Neural Network Models of Conditioning and Action. Quantitative Analyses of Behavior Series, edited by Michael SGJERS and Commons L, 225–240. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Church RM, Lacourse DM, and Crystal JD. 1998. “Temporal Search as a Function of the Variability of Interfood Intervals.” Journal of Experimental Psychology. Animal Behavior Processes 24: 291–315. [DOI] [PubMed] [Google Scholar]
- Church RM, Meck WH, and Gibbon J. 1994. “Application of Scalar Timing Theory to Individual Trials.” Journal of Experimental Psychology: Animal Behavior Processes 20: 135–155. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, and Meck WH. 2011. “Neuroanatomical and Neurochemical Substrates of Timing.” Neuropsychopharmacology 36 (1): 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, and Macar F. 2004. “Functional Anatomy of the Attentional Modulation of Time Estimation.” Science 303: 1506–1508. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, and Irwin W. 1999. “TThe Functional Neuroanatomy of Emotion and Affective Style.” Trends in Cognitive Science 3: 11–21. [DOI] [PubMed] [Google Scholar]
- Dolcos F, and McCarthy G. 2006. “Brain Systems Mediating Cognitive Interference by Emotional Distraction.” Journal of Neuroscience 26: 2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, and Alonso AA. 2002. “Graded Persistent Activity in Entorhinal Cortex Neurons.” Nature 420: 173–178. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB 1996. “Type I Membranes, Phase Resetting Curves, and Synchrony.” Neural Computation 8 (5): 979–1001. [DOI] [PubMed] [Google Scholar]
- Etkin A, and Wager TD. 2007. “Functional Neuroimaging of Anxiety: a Meta-analysis of Emotional Processing in PTSD.” American Journal of Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans V 2003. The Structure of Time. Amsterdam: John Benjamins Publishing Company. [Google Scholar]
- Flaherty MG 1999. “A Watched Pot: How We Experience Time.” [Google Scholar]
- Fraisse P 1957. Psychologie Du Temps. Paris, France:: P.U.F. [Google Scholar]
- Francois M 1927. “Contributions a L’etude Du Sens Du Temps: La Temperature Interne Comme Facteur de Varuation de L’appreciation Subjective Des Durees.” Annee Psychologique 27: 186–204. [Google Scholar]
- Gallistel CR 1990. The Organization of Behavior. Cambridge, MA: MIT Press. [Google Scholar]
- Getting PA 1989. “Emerging Principles Governing the Operation of Neural Networks.” Annual Review of Neuroscience 12: 185–204. [DOI] [PubMed] [Google Scholar]
- Gibbon J 1977. “Scalar Expectancy Theory and Weber’s Law in Animal Timing.” Psychological Review 84 (3): 279–325. [Google Scholar]
- Gibbon J, and Allan L. 1984. “Time Perception - Introduction.” Annals of the New York Academy of Sciences 423 (May): 1.6588776 [Google Scholar]
- Goaillard Jean-Marc, Taylor Adam L., Pulver Stefan R., and Marder Eve. 2010. “Slow and Persistent Postinhibitory Rebound Acts as an Intrinsic Short-Term Memory Mechanism.” Journal of Neuroscience 30 (13): 4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohier B, Ferracci L, Surguladze SA, Lawrence E, El Hage W, Kefi MZ, Allain P, Garre JB, and Le Gall D. 2009. “Cognitive Inhibition and Working Memory in Unipolar Depression.” Journal of Affective Disorders 116: 100–105. [DOI] [PubMed] [Google Scholar]
- Grossberg S, and Schmajuk N. 1989. “Neural Dynamics of Adaptive Timing and Temporal Discrimination During Associative Learning.” Neural Networks 2: 79–102. [Google Scholar]
- Hoagland H 1933. “The Psychological Control of Judgements of Duration: Evidence for a Chemical Clock.” Journal of General Psychology 9: 267–287. [Google Scholar]
- Hopson JW 1999. “Gap Timing and the Spectral Timing Model.” Behavioural Processes 45: 23–31. [DOI] [PubMed] [Google Scholar]
- Jozefowiez J, Cerutti DT, and Staddon JE. 2005. “Timing in Choice Experiments.” Journal of Experimental Psychology: Animal Behavior Processes 31: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser DH, Zentall TR, and Neiman E. 2002. “Timing in Pigeons: Effects of the Similarity Between Intertrial Interval and Gap in a Timing Signal.” Journal of Experimental Psychology: Animal Behavior Processes 28: 416–422. [PubMed] [Google Scholar]
- Llinas RR 1988. “The Intrinsic Electrophysiological Properties of Mamma- Lian Neurons: Insights into Central Nervous System Function.” Science 242: 1654–1664. [DOI] [PubMed] [Google Scholar]
- Lubow JW 1973. “Latent Inhibition.” Psychological Bulletin 79 (6): 398–407. [DOI] [PubMed] [Google Scholar]
- Lui Ming Ann, Penney Trevor B., and Schirmer Annett. 2011. “Emotion Effects on Timing: Attention Versus Pacemaker Accounts.” PLoS ONE 6 (7): e21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, and Church RM. 1983. “The Differential Effects of Haloperidol and Methamphetamine on Time Estimation in the Rat.” Psychopharmacology 79: 10–15. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Roberts S, and Church RM. 1981. “Methamphetamine and Time Estimation.” Journal of Experimental Psychology. Animal Behavior Processes 7: 18–30. [DOI] [PubMed] [Google Scholar]
- Matell MS, King GR, and Meck WH. 2004. “Differential Modulation of Clock Speed by the Administration of Intermittent Versus Continuous Cocaine.” Behavioral Neuroscience 118: 150–156. [DOI] [PubMed] [Google Scholar]
- Matell MS, and Meck WH. 1997. “A Comparison of the Tri-peak and Peak- Interval Procedure in Rats: Equivalency of the Clock Speed Enhancing Effect of Methamphetamine on Interval Timing.” Abstracts-Society for Neuroscience 23. [Google Scholar]
- ———. 2004. “Cortico-striatal Circuits and Interval Timing: Coincidence Detection of Oscillatory Processes.” Cognitive Brain Research 21 (2): 139–70. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, and Nicolelis MA. 2003. “Interval Timing and the Encoding of Signal Duration by Ensembles of Cortical and Striatal Neurons.” Behavioral Neuroscience 117: 760–773. [DOI] [PubMed] [Google Scholar]
- Matell Matthew S., Bateson Melissa, and Meck Warren H.. 2006. “Single-trials Analyses Demonstrate That Increases in Clock Speed Contribute to the Methamphetamine-induced Horizontal Shifts in Peak-interval Timing Functions.” Psychopharmacology. [DOI] [PubMed] [Google Scholar]
- Matthews Alexander R., He Olivia H., Buhusi Mona, and Buhusi Catalin V.. 2012. “Dissociation of the Role of the Prelimbic Cortex in Interval Timing and Resource Allocation: Beneficial Effect of Norepinephrine and Dopamine Reuptake Inhibitor Nomifensine on Anxiety-inducing Distraction.” Frontiers in Integrative Neuroscience 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH 1983. “Selective Adjustment of the Speed of Internal Clock and Memory Processes.” Journal of Experimental Psychology. Animal Behavior Processes (9): 171–201. [PubMed] [Google Scholar]
- ———. 1996. “Neuropharmacology of Timing and Time Perception.” Cognitive Brain Research 3 (3–4): 227–242. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, and Pouthas V. 2008. “Cortico-striatal Representation of Time in Animals and Humans.” Current Opinion in Neurobiology 18 (2): 145–152. [DOI] [PubMed] [Google Scholar]
- Miall RC 1989. “The Storage of Time Intervals Using Oscillating Neurons.” Neural Computation 1: 359–371. [Google Scholar]
- Moore D, Siegfried D, Wilson R, and Rankin MA. 1989. “The Influence of Time of Day on the Foraging Behavior of the Honeybee, Apis Mellifera.” Journal of Biological Rhythms 4: 305–325. [DOI] [PubMed] [Google Scholar]
- Morris C, and Lecar H. 1981. “Voltage Oscillations in the Barnacle Giant Muscle Fiber.” Biophysical Journal 35: 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil DB, and Herndon JG Jr. 1978. “Anatomical Specificity Within Rat Striatum for the Dopaminergic Modulation of DRL Responding and Activity.” Brain Research 153: 529–538. [DOI] [PubMed] [Google Scholar]
- Olton DS, Wenk GL, Church RM, and Meck WH. 1988. “Attention and the Frontal Cortex as Examined by Simultaneous Temporal Processing.” Neuropsychologia 26: 307–318. [DOI] [PubMed] [Google Scholar]
- Oprisan SA, and Buhusi CV. 2011. “Modelling Pharmacological Clock and Memory Patterns of Interval Timing in a Striatal Beat-frequency Model with Realistic, Noisy Neurons.” Frontiers in Integrative Neuroscience 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprisan Sorinel A., and Buhusi Catalin V.. 2013. “How Noise Contributes to Time-scale Invariance of Interval Timing.” Physical Review E 87 (5) (May 29): 052717. doi: 10.1103/PhysRevE.87.052717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney TB, Holder MD, and Meck WH. 1996. “Clonidine-induced Antagonism of Norepinephrine Modulates the Attentional Processes Involved in Peak-interval Timing.” Experimental and Clinical Psychopharmacology 4: 10. [Google Scholar]
- Perkel DH, and Mulloney B. 1974. “Motor Pattern Production in Reciprocally Inhibitory Neurons Exhibiting Postinhibitory Rebound.” Science 185: 181–183. [DOI] [PubMed] [Google Scholar]
- Pulver SR, and Griffith LC. 2010. “Spike Integration and Cellular Memory in a Rhythmic Network from Na/K Pump Current Dynamics.” Nature Neuroscience 13: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammsayer TH 1993. “On Dopaminergic Modulation of Temporal Information Processing.” Biological Psychology 36: 209–222. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH, Hennig J, Haag A, and Lange N. 2001. “Effects of Noradrenergic Activity on Temporal Information Processing in Humans.” Quarterly Journal of Experimental Psychology B 54: 247–258. [DOI] [PubMed] [Google Scholar]
- Roberts S 1981. “Isolation of an Internal Clock.” Journal of Experimental Psychology: Animal Behavior Processes 7: 242–268. [PubMed] [Google Scholar]
- Roberts WA, Cheng K, and Cohen JS. 1989. “Timing Light and Tone Signals in Pigeons.” Journal of Experimental Psychology: Animal Behavior Processes 15: 23–35. [PubMed] [Google Scholar]
- Rose EJ, and Ebmeier KP. 2006. “Pattern of Impaired Working Memory During Major Depression.” Journal of Affective Disorders 90: 149–161. [DOI] [PubMed] [Google Scholar]
- Schirmer A 2004. “Timing Speech: A Review of Lesion and Neuroimaging Findings.” Brain Research: Cognitive Brain Research 21: 269–287. [DOI] [PubMed] [Google Scholar]
- Selverston AI, and Moulins M. 1985. “Oscillatory Neural Networks.” Annual Review of Physiology 47: 29–48. [DOI] [PubMed] [Google Scholar]
- Staddon JER 1965. “Some Properties of Spaced Responding in Pigeons.” Journal of Experimental Analysis of Behavior 8: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF 1988. “Temporal Integration by a Slowly Inactivating K Current in Hippocampal Neurons.” Nature 336: 379–381. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Horvitz JC, and Balsam PD. 2007. “Amphetamine Affects the Start of Responding in the Peak Interval Timing Task.” Behavioral Processes 74 (1–3): 168–175. [DOI] [PubMed] [Google Scholar]
- Thorpe CM, Petrovic V, and Wilkie DM. 2002. “How Rats Process Spatiotemporal Information in the Face of Distraction.” Behavioral Processes 58: 79–90. [DOI] [PubMed] [Google Scholar]
- Treisman M 1963. “Temporal Discrimination and the Indifference Interval. Implications for a Model of the ‘Internal Clock’.” Psychological Monograph 77 (13): 1–31. [DOI] [PubMed] [Google Scholar]
- Woodrow H 1930. “The Reproduction of Temporal Intervals.” Journal of Experimental Psychology 13: 473–499. [Google Scholar]