Abstract
Background:
Patients with vascular stiffening may display increased arterial afterload that is out of proportion to systolic blood pressure (SBP). Since vascular and endothelial dysfunction develop in patients with coarctation of aorta (COA), we hypothesized that for any SBP, patients with mild COA (COA peak velocity <2m/s) will have a higher arterial afterload and increased left ventricular mass index (LVMI) compared to controls, and that Doppler-derived arterial load indices would be a better predictor of LVMI compared to SBP alone.
Methods:
We studied 204 COA patients (age 35±12 years) and 204 matched controls. Doppler-derived arterial afterload was assessed using effective arterial elastance index (EaI) and total arterial compliance index (TACI).
Results:
Despite similar SBP, the mild COA group displayed higher arterial afterload as evidenced by a higher EaI (3.3±0.9 vs 2.9±0.7 mmHg/mL*m2, p<0.001) and lower TACI (0.8±0.3 vs 1.2±0.5 mL/mmHg*m2, p<0.001). This was associated with higher LVMI in COA (109±35 vs 93±32, g/m2, p<0.001). Compared to SBP (β=0.24, 95%CI 0.02 to 0.45), EaI (β=20.2, 95%CI 15.8 to 44.1), and TACI (β=−32.5, 95%CI −43.8 to −123.6), were better predictors of LVMI. EaI (but not SBP) was predictive of longitudinal increases in LVMI (r=0.43, p<0.001).
Conclusion:
COA patients had higher arterial afterload compared to controls with similar SBP. In comparison to SBP, Doppler-derived arterial load indices correlate more strongly with LV hypertrophy. These data suggest that SBP may underestimate LV afterload in this population. This has important clinical implications since titration of antihypertensive therapy is currently based on SBP.
Keywords: Coarctation of aorta, Arterial load, Systolic blood pressure, Left ventricular hypertrophy
INTRODUCTION
Hypertension affects up to 50% of adults with coarctation of aorta (COA), and results in an increased arterial afterload which then leads to left ventricular (LV) remodeling and dysfunction, heart failure symptom, and cardiovascular mortality.1–6 The first step in the management of hypertension in COA patients is to identify and treat mechanical aortic obstruction with surgery or transcatheter interventions.7, 8 In the absence of significant coarctation, systemic hypertension is then treated with anti-hypertension therapy.9, 10 The goal of medical therapy is to reduce arterial afterload, and hence prevent maladaptive LV remodeling (hypertrophy) and LV dysfunction.11 Systolic blood pressure (SBP) is considered a surrogate measure of arterial afterload, and it is used to titrate anti-hypertension therapy in clinical practice.11 The prognostic role of SBP is supported by robust data demonstrating a direct correlation between SBP and cardiovascular mortality, as well as a dose-dependent reduction in cardiovascular mortality in response to a reduction in SBP during medical therapy.12, 13
A recent study compared invasively measured arterial load indices between patients with heart failure with preserved ejection fraction (HFpEF), which is a disease that is characterized by arterial stiffness and endothelial dysfunction, with that of patients with essential hypertension.14 The study showed that although both groups had similar SBP, the HFpEF group had higher arterial afterload at rest, and the between-group difference became more exaggerated during low intensity exercise.14 Arterial load indices had good correlation with LV filling pressure and cardiac output reserve in that study.14 Patients with COA also have vascular and endothelial dysfunction resulting in arterial stiffening.4, 15, 16 However, it is therefore unknown whether SBP is an accurate reflection of LV afterload and subsequent LV remodeling in this population.
The purpose of the study was to compare noninvasively measured arterial load indices between COA patients and matched controls, and to assess the correlation between arterial load indices and LV remodeling. We hypothesized that for every given SBP, patients with mild COA (without hemodynamically significant coarctation) will have a higher arterial afterload and LV hypertrophy compared to controls, and that Doppler-derived arterial afterload indices would be a better predictor of LV hypertrophy compared to SBP alone.
METHODS
Patient Selection
The data that support the findings of this study are available from the corresponding author upon reasonable request. The MACHD (Mayo Adult Congenital Heart Disease) database was queried for patients (age ≥18 years) with COA that received care at Mayo Clinic Rochester, Minnesota from January 1, 1985 through December 31, 2018. The Mayo Clinic Institutional Review Board approved this study and waived informed consent for patients that provided research authorization. Patients with COA diagnosis were eligible for the study if they met these 4 criteria based on transthoracic echocardiogram performed at rest. (1) Absence of hemodynamically significant aortic coarctation defined as a continuous wave Doppler peak velocity >2.5 m/sec at the site of coarctation. (2) Absence of significant aortic valve disease defined as prosthetic aortic valve, native aortic valve peak velocity >2 m/sec or >mild aortic regurgitation. (3) Absence of significant mitral valve disease defined as prosthetic mitral valve, native mitral valve mean gradient >3 mmHg or >mild mitral regurgitation. (4) Cuff blood pressure measurement from the right arm in the absence of aberrant origin of right subclavian artery.
For the control group, we identified patients without structural heart disease that underwent echocardiogram within the same period. The absence of structural heart disease was verified by manual review of report of echocardiogram. We performed 1:1 matching of patients with COA and control using propensity score method based on age, sex, body mass index, history of hypertension and SBP at the time of echocardiogram.
Study Endpoints and Definitions
The primary objective was to compare Doppler-derived arterial load indices between the COA patients and propensity-matched controls. Doppler-derived arterial load was assessed using 3 indices. (1) Effective arterial elastance index (EaI), which is a lumped measure of the total stiffness of the arterial system was calculated as 0.9 x brachial systolic blood pressure/stroke volume index.14, 17 (2) Total arterial compliance index (TACI), which is a linear approximation of the pressure-volume relationship of the arterial system was calculated as stroke volume index/pulse pressure.18, 19 (3) Systemic vascular resistance index (SVRI), which is a measure of non-pulsatile arterial load was calculated as (mean arterial pressure-estimated right atrial pressure/cardiac index) x 80.14 For the assessment of EaI and TACI, we calculated Doppler-derived stroke volume index using the continuity equation.20 For SVRI calculation, we used estimated mean right atrial pressure, mean arterial pressure from blood pressure cuff measurement and Doppler-derived cardiac index from continuity equation.14 An experienced sonographer (JW) performed all the offline measurements in the COA patients, and another sonographer (KT) performed the following measurements in a random sample of 50 COA patients (LV outflow tract diameter and time velocity integral, LV internal diameter, septal and posterior wall thickness). Similarly KT performed all the offline measurements in the control patients, and JW performed measurements in a random sample of 50 control patients. The 2 sonographers were blinded to the initial measurements prior to performing the repeat measurements.
The secondary objective was to determine if Doppler-derived arterial load indices (EaI, TACI, and SVRI) were better predictors of LV hypertrophy as measured by left ventricular mass index (LVMI) compared to SBP. To achieve this objective, we assessed the correlation between Doppler-derived arterial load and LVMI, and then compared the slopes and correlation coefficients of these relationship to that of SBP and LVMI. LVMI was calculated based on two-dimensional echocardiographic linear measurements of LV diastolic diameter and wall thickness.20 LV hypertrophy was defined as LVMI > 95 g/m2 or >115 g/m2 in females and males respectively.20
We performed 2 exploratory analyses in the COA group. First, an exploratory analysis was performed to determine if arterial load indices and SBP at baseline correlated with temporal change in LVMI (delta LVMI = LVMI at subsequent echocardiogram minus LVMI at baseline echocardiogram). This analysis, was performed in the subgroup of patients that met these criteria: (1) Two echocardiograms performed >10 years apart; (2) no surgical operation, transcatheter interventions, or initiation of new antihypertensive medication. A second exploratory analysis was performed to determine the incidence of cardiovascular adverse events during follow-up. We defined cardiovascular adverse events as a composite endpoint of stroke, acute coronary syndrome, heart failure hospitalization, heart transplant, and death from any cause.
Statistical Analysis
Data were presented as mean ± standard deviation or count (%). Between-group comparisons were performed with chi square test and unpaired t-test as appropriate. Propensity score matching was used to balance the differences in baseline characteristics between the COA and control groups. A propensity score, the probability of having COA was estimated using logistic regression based on age, sex, body mass index, history of hypertension, and SBP at the time of echocardiogram. One-to-one nearest neighbor caliper matching was used to match patients based on the logit of the propensity score using a caliper equal to 0.2 of the standard deviation of the logit of the propensity score.21 The balance of covariates after matching was assessed using unpaired t-test and Fisher exact test as appropriate.
Using the blinded repeat measurements obtained from the 2 observers (JW and KT), we calculated LV stroke volume index and LVMI. Intraclass correlation coefficient (ICC) with 95% confidence interval was used to assess the interobserver agreements for arterial load indices (using the LV stroke volume index) and LVMI. The difference in Doppler-derived arterial load for a given blood pressure between COA and control groups was assessed using paired t-test. Linear regression was used to assess the correlation between Doppler-derived arterial load indices and LVMI, and between SBP and LVMI in the COA group. The ability of SBP to predict LVMI vs that of Doppler-derived arterial load indices to predict LVMI was compared in using 2 methods: comparison of the β coefficients and 95% confidence interval derived from the linear regression analysis, and, comparison of correlation coefficients (r) using Meng test.22 Linear regression analysis was used to assess the correlation between SBP and arterial load indices obtained at baseline echocardiogram and temporal change in LVMI. A p<0.05 was considered statistically significant. All statistical analyses were performed with JMP software (version 14.0; SAS Institute Inc, Cary NC).
RESULTS
Baseline Characteristics
Out of 722 COA patients, 204 (28%) met the study inclusion criteria. Compared to the 204 COA patients in the study, the rest of the COA cohort were older, had lower LV ejection fraction, and had higher SBP, LV mass index, and arterial load indices (Supplementary Table 1).
A total of 204 COA patients and 204 control patients were enrolled in the study. In the COA group, 129 (63%) had bicuspid aortic valve and 181 (89%) had prior COA repair (Table 1). The initial COA repair/intervention was resection and end-to-end anastomosis (n=63, 36%), subclavian flap repair (n=25, 12%), patch aortoplasty (n=14, 7%), interposition graft repair (n=37, 18%), extra-anatomic bypass graft (n=10, 5%), balloon aortic dilation (n=10, 5%), and aortic stent implantation (n=4, 2%). The age at the time of initial COA repair/intervention was 6±4 years. In the control group, the indication for echocardiogram was screening for family history of congenital heart disease (n=95, 47%), clinical evaluation for personal/family history of genetic arrhythmia (n=54, 27%), hypertension (n=39, 19%), non-cardiac dyspnea (n=9, 4%), and others (n=7, 3%).
Table 1:
Baseline Characteristics
| COA (n=204) | Control (n=204) | p | |
|---|---|---|---|
| Age, years | 35±12 | 35±12 | 0.894 |
| Male | 117 (57%) | 117 (57%) | 0.999 |
| Body mass index, kg/m2 | 26±5 | 26±5 | 0.891 |
| Systolic blood pressure, mmHg | 126±21 | 126±16 | 0.517 |
| Hypertension | 119 (58%) | 119 (58%) | 0.999 |
| Hemoglobin, g/dl | 13.7±1.1 | 13.9±0.9 | 0.265 |
| Medications | |||
| Thiazide diuretics | 16 (8%) | 6 (3%) | 0.028 |
| Beta blockers | 50 (25%) | 17 (8%) | <0.001 |
| Calcium channel blockers | 21 (10%) | 11 (5%) | 0.066 |
| RAAS antagonist | 55 (25%) | 31 (15%) | 0.002 |
| Echocardiography | |||
| LV ejection fraction, % | 63±8 | 60±5 | 0.104 |
| LV end-diastolic dimension, mm | 51±7 | 52±3 | 0.121 |
| LV end-systolic dimension, mm | 31±6 | 33±3 | 0.095 |
| LV mass index, g/m2 | 109±35 | 93±32 | <0.001 |
| Relative wall thickness* | 0.41±0.07 | 0.37±0.06 | <0.001 |
| LV outflow tract diameter, cm | 2.2±0.3 | 2.2±0.2 | 0.374 |
| LV outflow tract TVI, cm | 23±5 | 26±5 | <0.001 |
| LV stroke volume index, mL/m2 | 39±11 | 46±8 | <0.001 |
| Heart rate, bpm | 73±10 | 68±7 | 0.014 |
| Cardiac index, L/min/m2 | 3.0±0.7 | 3.1±0.4 | 0.14 |
| EaI, mmHg/ml*m2 | 3.3±0.9 | 2.9±0.7 | <0.001 |
| TACI, mL/mmHg*m2 | 0.8±0.3 | 1.2±0.5 | <0.001 |
| SVRI, dyne-s/cm5*m2 | 2554±727 | 2470±503 | 0.243 |
COA: Coarctation of aorta; RAAS: Renin-angiotensin-aldosterone system; LV: Left ventricle; TVI: Time velocity integral; EaI: Effective arterial elastance index; TACI: Total arterial compliance index; SVRI: systemic vascular resistance index
Relative wall thickness was calculated as (2x LV posterior wall thickness)/ LV end-diastolic dimension. A relative wall thickness ≤0.42 is considered normal
Ventricular and Vascular Structure and Function
Table 1 shows a comparison of the baseline echocardiographic and vascular measures of the COA and control groups. The LVMI was higher in the COA group (109±35 vs 93±33 g/m2, p<0.001). The prevalence of LV hypertrophy was higher for any SBP in the COA group as compared to controls: SBP ≤120 mmHg (36% vs 7%, p<0.001), SBP 121-140 mmHg (49% vs 22%, p<0.001), and SBP >140 mmHg (52% vs 31%, p=0.021), Figure 1. There was good interobserver correlation for EaI (ICC 0.89; 0.84-0.93), TACI (ICC 0.89; 0.85-0.93), and LVMI (ICC 0.92; 0.90-0.94).
Figure 1:
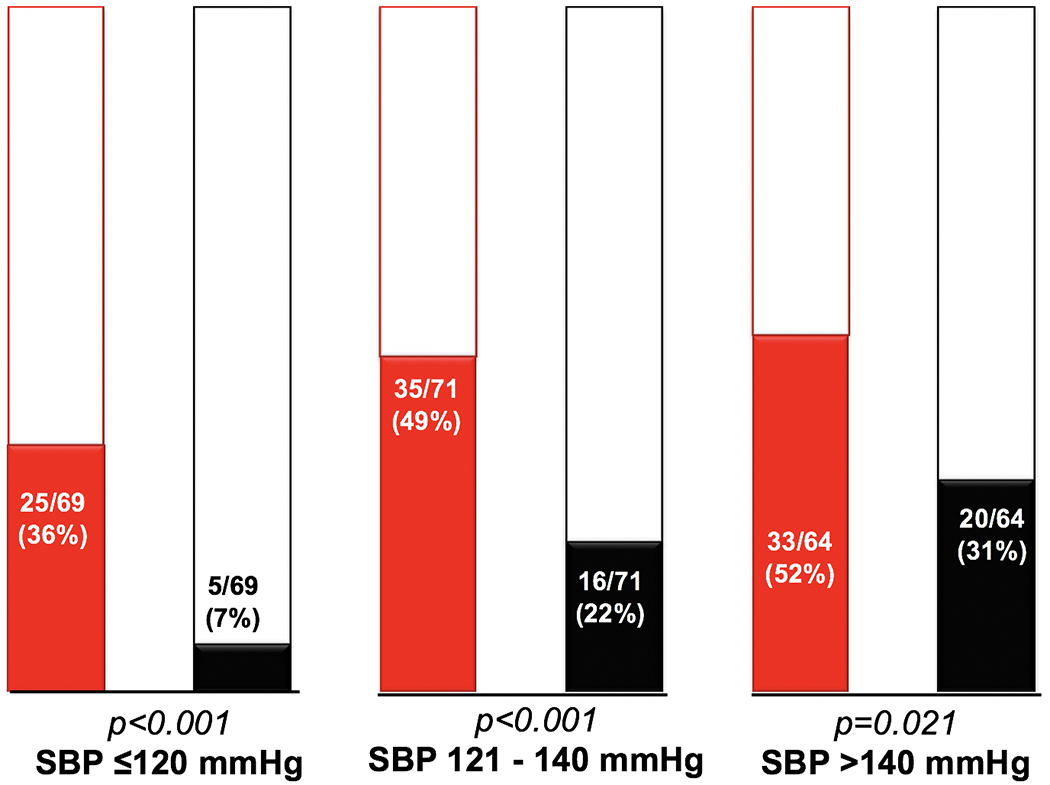
Bar graphs comparing the prevalence of LV hypertrophy between COA (red) and controls (black) for every SBP category
Although the SBP was the same in both groups (by design), the COA group had higher pulsatile LV afterload as shown by a higher EaI (3.3±0.9 vs 2.9±0.7 mmHg/mL*m2, p<0.001) and lower TACI (0.8±0.3 vs 1.2±0.5 mL/mmHg*m2, p<0.001). However, there was no difference in SVRI, a measure of non-pulsatile LV afterload, between groups (2554±727 vs 2470±503 dyne-s/cm5*m2, p=0.243). The between-group (COA minus control) mean difference of EaI was 0.40 (95% CI 0.38 - 0.41) mmHg/mL*m2 while the mean difference of TACI was −0.34 (95% CI −0.32 - −0.36) mL/mmHg*m2. There was no significant difference in the between-group mean difference of SVRI, Figure 2. Supplementary Figure 1 shows a comparison of arterial load indices between a COA patient and a control matched by age, sex, body mass index and SBP. Although both patients had similar SBP, the COA patient had higher EaI, TACI, and LVMI.
Figure 2:
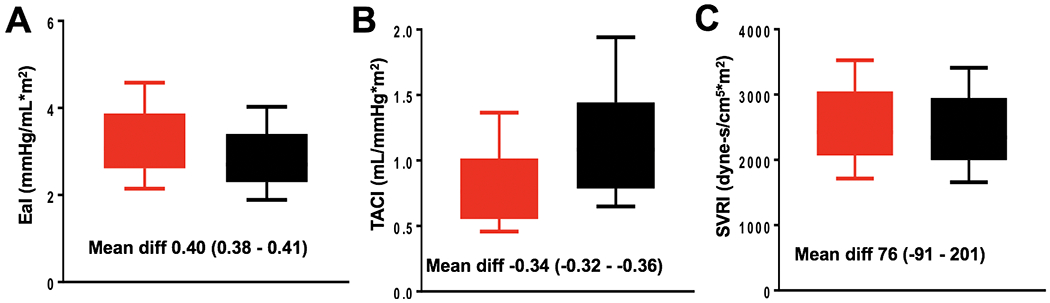
Box-and-whisker plot comparing effective arterial elastance index (EaI), (A); total arterial compliance index (TACI), (B); and systemic vascular resistance index (SVRI), (C) between patients with COA (red) and controls (black)
LV Hypertrophy in COA Group
There was strong correlation between EaI and LVMI (r=0.61, p<0.001) and a modest correlation between TACI and LVMI (r=−0.42, p<0.001), but no correlation between SVRI and LVMI (r=0.01, p=0.852), Figure 3. Compared to SBP (β=0.24, 95%CI 0.02 to 0.45), EaI (β=20.2, 95%CI 15.8 to 44.1), and TACI (β=−32.5, 95%CI −43.8 to −123.6), were better predictors of LVMI. Based on the comparison of correlation coefficients, EaI (Meng test p value <0.001) and TACI (Meng test p value <0.001) had better correlation with LVMI compared to SBP.
Figure 3:
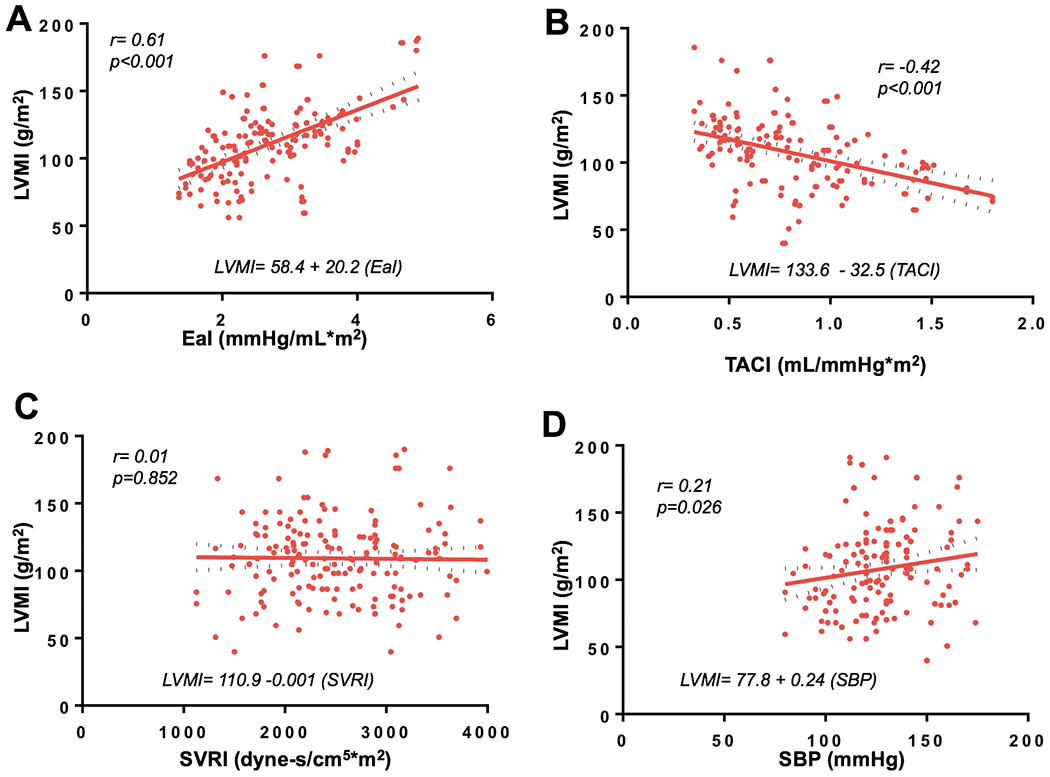
Linear regression of left ventricular mass index (LVMI) and EaI (A); LVMI and TACI (B); LVMI and SVRI (C); LVMI and systolic blood pressure (SBP), (D)
Afterload Indices and Longitudinal Changes in LV remodeling
Of the 204 COA patients, 92 (45%) underwent a repeat echocardiogram within the specified window to allow for assessment of temporal changes in LV structure. The mean interval between baseline and subsequent echocardiogram was 11.6±0.8 years, and the mean temporal change in LVMI was 6.1 (95%CI 3.2 to 9.6) g/m2. There was a positive correlation between EaI from the baseline echocardiogram and temporal change in LVMI (r=0.43, p<0.001) and weak correlation between TACI and temporal change in LVMI (r=−0.22, p=0.057). In contrast, there was no correlation between SBP and temporal change in LVMI (r=−0.10, p=0.146).
Of the 204 COA patients, 112 (55%) did not meet the inclusion criteria for exploratory analysis. There were no significant differences between the clinical characteristics of the 92 patients included in the exploratory analysis vs the other 112 patients (Supplementary Table 2). Of the 112 patients, 19 (17%) did not have any echocardiographic follow-up, 4 (4%) underwent intervention within 10 years from the baseline echocardiogram, and 89 (80%) patients had echocardiographic follow-up less than 10 years from the baseline echocardiogram. Among these 89 patients, the mean interval between baseline and subsequent echocardiogram was 5.9±2.2 years, and the mean temporal change in LVMI was 3.6 (95%CI 1.4 to 5.5) g/m2. There was a positive correlation between EaI from the baseline echocardiogram and temporal change in LVMI (r=0.41, p<0.001). However, TACI (r=−0.20, p=0.102) and SBP (r=0.04, p=0.512) did not have significant correlations with temporal changes in LVMI. Compared to the correlation between EaI and temporal change in LVMI observed in the 92 patients with adequate follow-up, there was no significant difference in the strength of correlation between EaI and temporal change in LVMI observed in the 89 patients with inadequate follow-up (r=0.43 vs r=0.41, Meng test p=0.436).
Clinical Outcomes in COA and Control groups
The mean follow-up in the COA group was 7.6±2.8 years, and during this period, 2 (1%) patients had stroke, 1 (0.5%) patient had acute coronary syndrome, 1 (0.5%) patient had heart failure hospitalization, and 4 (2%) patients died. The cause of death was end-stage heart failure in 2 patients, stroke-related death 1 patient, and unknown cause of death in 1 patient. Altogether, 6 (3%) patients met the composite cardiovascular event endpoint in the COA group, and the freedom from cardiovascular adverse event was 94% in 5 years. In contrast, there were no cardiovascular events during a mean follow-up of 4.6±2.1 years in the control group. We did not perform correlation analysis between arterial load indices and cardiovascular events because of low event rates in both groups.
DISCUSSION
Based on the comparison of Doppler-derived arterial afterload indices between COA patients and propensity matched controls, we showed that COA patients had higher arterial afterload (higher EaI and lower TACI) for the same SBP. Compared to SBP, Doppler-derived arterial load indices had a better correlation with LV hypertrophy at baseline, and also had a better correlation with progression of LV hypertrophy. The prevalence of LV hypertrophy was significantly higher in COA patients for every SBP category. These data suggest that SBP may underestimate the severity of LV pressure overload, and that Doppler-derived EaI and TACI were better surrogate measures of LV afterload in the setting of vascular dysfunction such as COA.
Several studies have shown that patients with COA have abnormal arterial stiffness due to endothelial dysfunction.4, 15, 16, 23 Reduced arterial vascular reactivity resulting from abnormal arterial stiffness is associated with hypertension, LV hypertrophy, and LV diastolic dysfunction in this population.4, 16 Concordant with previous data about arterial stiffness in COA patients, we demonstrated a higher arterial afterload and lower LV stroke volume for every given SBP in COA patients compared to controls. It is important to emphasize that these data were derived from a selected cohort of mild COA patients (without hemodynamically significant COA), suggesting that an even worse hemodynamic profile might be observed from the general COA population.
In a case-control study of 72 COA patients and 53 healthy controls, daytime ambulatory SBP but not nighttime ambulatory BP (SBP at rest) correlated with LVMI.4 The difference between daytime ambulatory SBP and BP at rest was significantly higher in the COA patient, and correlated with the severity of endothelial dysfunction.4 In another study in which 20 COA patients and 20 controls were matched by SBP at rest, the COA patients had higher ambulatory SBP and LVMI, and worse LV diastolic function indices.3 Similarly we also observed higher LVMI in the COA group even though both groups had similar SBP at rest. Additionally, the current study demonstrated that compared to SBP, Doppler-derived arterial load indices had a stronger correlation with LVMI. We postulate that the pathophysiologic mechanism underpinning these differences in ambulatory SBP, LV adaptation (LVMI) and LV function (diastolic function indices) between COA patients and controls, despite have similar SBP at rest, is due to vascular and endothelial dysfunction which blunts the obligatory sympathetically-mediated vasodilation during activity.24, 25 Very low intensity exercise such as activities of daily living requires an increase in LV stroke volume.24, 25 This physiologic increase in LV stroke volume during exercise results in higher LV afterload in the setting of endothelial and vascular dysfunction in COA patients compared to controls despite having similar SBP at rest. Concordant with our postulation, we also showed, for the first time, that arterial load indices (but not SBP) were predictive of temporal change in LVMI, further emphasizing the effect of Doppler-derived arterial load indices on LV remodeling. These data highlight the limitations of relying upon SBP alone as an indicator of LV afterload in COA patients.
Clinical Implications and Future Directions
LVMI is an independent risk factor for cardiovascular mortality and morbidity, and reflects LV adaptation to chronic pressure and/or volume overload.26 In COA patients where LV pressure overload results from a combination of aortic coarctation (mechanical obstruction) and arterial stiffness, LVMI provides a composite metric of the effect of pressure overload on the LV.4, 27 Since LV hypertrophy is a secondary event, identifying and modifying the inciting risk factors such as hypertension should result in regression of LV hypertrophy and potentially improvement in clinical outcomes. In the absence of hemodynamically significant coarctation, the treatment of hypertension in COA patients currently follows the general recommendations for management of systemic arterial hypertension where resting SBP is used for titration of medical therapy.9–11 However, we observed that the prevalence of LV hypertrophy was significantly higher in COA patients compared to controls for SBP category, suggesting that SBP may underestimate the severity of LV pressure overload in this population.
This is consistent with previous studies that reported LV hypertrophy in COA patients whose SBP are within the upper reference limit of 120 to 140 mmHg.27 Data from the Systolic Blood Pressure of Intervention (SPRINT) trial showed a significant reduction in cardiovascular mortality among patients that received intensive BP treatment with target SBP of <120 vs standard therapy with target SBP of 120-140 mmHg.28 Considering the added burden of vasculopathy, endothelial dysfunction, and risk of intracranial aneurysm in COA patients, perhaps these patients may benefit from intensive BP treatment with lower target SBP target.4, 15, 16, 23, 29 Furthermore, Doppler-derived arterial elastance and compliance can easily be calculated using cuff BP and Doppler-derived LV stroke volume, both of which are routinely obtained as part of comprehensive echocardiography. Perhaps these indices may be considered in clinical decision making regarding anti-hypertension therapy. However, further studies are required to determine if these proactive measures will result in improved LV reverse remodeling and clinical outcomes.
Limitations
All measurements of arterial afterload were taken at a single time point (at the time of index echocardiographic evaluation), and therefore, they do not necessarily reflect the overall time-dependent burden of LV pressure load. However, all measures of arterial afterload in the study were derived from this single-time point assessment, such that the limitations of using a single BP measurement will affect all indices similarly, and hence should not significantly influence the observed result. Ambulatory BP data were not available and requires further study. Although we controlled for differences in baseline characteristics using propensity analysis, we did not account for the effect of other factors such smoking, diabetes etc that can also affect arterial load indices. We measured LVMI using 2D echocardiography instead of cardiac magnetic resonance imaging which has better spatial resolution. Although we postulated that endothelial dysfunction may be one of the potential causes of higher arterial load indices in COA group, we did not measure this variable in this study. Moreover the data about endothelial dysfunction and its clinical importance in COA is still somewhat controversial.
Conclusions
Patients with mild COA display higher arterial afterload compared to control group of patients without COA but with similar SBP. This may explain the greater prevalence of LV hypertrophy in COA patients for any SBP category. In comparison to SBP, Doppler-derived arterial load indices are more robustly related to cardiac remodeling, both at baseline and importantly during long term follow-up. These data suggest that SBP may underestimate LV afterload in the setting of vascular dysfunction such as COA, and that more sophisticated indices of arterial afterload may improve treatment for patients with COA.
Supplementary Material
Clinical Perspective.
Patients with mild coarctation of aorta (COA) display higher arterial afterload compared to control group of patients without COA, but with similar systolic blood pressure (SBP). This is likely responsible for the higher prevalence of left ventricular (LV) hypertrophy in COA patients for any SBP category. In comparison to SBP, Doppler-derived arterial load indices are more robustly related to cardiac remodeling, both at baseline and importantly during long term follow-up. These data suggest that SBP may underestimate LV afterload in the setting of vascular dysfunction such as COA. Systemic hypertension is one of the strongest predictors of cardiovascular mortality in the COA population, and the standard of care is to titrate antihypertensive therapy based on SBP obtained at rest. Considering the results of the current study suggesting that SBP may underestimate LV afterload in this population, the use of resting SBP to guide antihypertensive therapy may result in ‘under-treatment’ of hypertension in this population. It is therefore important to explore other metrics for assessing adequacy of antihypertensive therapy in this population.
Acknowledgement:
James Welper and Katrina Tollefsrud for performing offline measurements of the echocardiographic indices used in this study.
Funding: Dr. Egbe is supported by NHLBI grant K23 HL141448-01. Dr. Borlaug is supported by RO1 HL128526 and U10 HL110262. Dr. Obokata is supported by a research fellowship from the Uehara Memorial Foundation, Japan
Abbreviations
- COA
Coarctation of aorta
- SBP
Systolic blood pressures
- LV
Left ventricular mass index
- HFpEF
Heart failure with preserved ejection fraction
- EaI
Effective arterial elastance
- TACI
Total arterial compliance index
- SVRI
Systemic vascular resistance index
- ICC
Intraclass correlation
Footnotes
Disclosures: none
REFERENCES
- 1.Rinnstrom D, Dellborg M, Thilen U, Sorensson P, Nielsen NE, Christersson C and Johansson B. Hypertension in adults with repaired coarctation of the aorta. Am Heart J. 2016;181:10–15. [DOI] [PubMed] [Google Scholar]
- 2.Vriend JW and Mulder BJ. Late complications in patients after repair of aortic coarctation: implications for management. Int J Cardiol. 2005;101:399–406. [DOI] [PubMed] [Google Scholar]
- 3.Leandro J, Smallhorn JF, Benson L, Musewe N, Balfe JW, Dyck JD, West L and Freedom R. Ambulatory blood pressure monitoring and left ventricular mass and function after successful surgical repair of coarctation of the aorta. J Am Coll Cardiol. 1992;20:197–204. [DOI] [PubMed] [Google Scholar]
- 4.de Divitiis M, Pilla C, Kattenhorn M, Donald A, Zadinello M, Wallace S, Redington A and Deanfield J. Ambulatory blood pressure, left ventricular mass, and conduit artery function late after successful repair of coarctation of the aorta. J Am Coll Cardiol.. 2003;41:2259–2265. [DOI] [PubMed] [Google Scholar]
- 5.Canniffe C, Ou P, Walsh K, Bonnet D and Celermajer D. Hypertension after repair of aortic coarctation--a systematic review. Int J Cardiol. 2013;167:2456–61. [DOI] [PubMed] [Google Scholar]
- 6.Wu MH, Chen HC, Kao FY and Huang SK. Risk of Systemic Hypertension and Cerebrovascular Accident in Patients With Aortic Coarctation Aged <60 Years (from a National Database Study). Am J. Cardiol 2015;116:779–84. [DOI] [PubMed] [Google Scholar]
- 7.Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, Oliver WC, Warnes CA and Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–5. [DOI] [PubMed] [Google Scholar]
- 8.Chen CK, Cifra B, Morgan GJ, Sarkola T, Slorach C, Wei H, Bradley TJ, Manlhiot C, McCrindle BW, Redington AN, et al. Left Ventricular Myocardial and Hemodynamic Response to Exercise in Young Patients after Endovascular Stenting for Aortic Coarctation. J Am Soc Echocardiogr 2016;29:237–46. [DOI] [PubMed] [Google Scholar]
- 9.Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, et al. ; Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of C, Association for European Paediatric C and Guidelines ESCCfP. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–57. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; Group ESCSD. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 12.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A and Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 13.Thomopoulos C, Parati G and Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hyperten. 2014;32:2285–95. [DOI] [PubMed] [Google Scholar]
- 14.Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP and Borlaug BA. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MGY, Hemmes RA, Mynard J, Lambert E, Head GA, Cheung MMH, Konstantinov IE, Brizard CP, Lambert G and d’Udekem Y. Elevated sympathetic activity, endothelial dysfunction, and late hypertension after repair of coarctation of the aorta. Int J Cardiol. 2017;243:185–190. [DOI] [PubMed] [Google Scholar]
- 16.Lombardi KC, Northrup V, McNamara RL, Sugeng L and Weismann CG. Aortic stiffness and left ventricular diastolic function in children following early repair of aortic coarctation. Am Heart J. 2013;112:1828–33. [DOI] [PubMed] [Google Scholar]
- 17.Taghavi S, Esmaeilzadeh M, Amin A, Naderi N, Abkenar HB, Maleki M and Chitsazan M. Measurement of pulmonary arterial elastance in patients with systolic heart failure using Doppler echocardiography. Anatol J Cardiol. 2016;16:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus RH, Korcarz C, McCray G, Neumann A, Murphy M, Borow K, Weinert L, Bednarz J, Gretler DD, Spencer KT. Noninvasive method for determination of arterial compliance using Doppler echocardiography and subclavian pulse tracings. Validation and clinical application of a physiological model of the circulation. Circulation. 1994;89:2688–99. [DOI] [PubMed] [Google Scholar]
- 19.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P and Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–5. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng test: https://www.psychometrica.de/correlation.html. Updated June 4, 2010. Accessed January 2, 2018
- 23.Ong CM, Canter CE, Gutierrez FR, Sekarski DR and Goldring DR. Increased stiffness and persistent narrowing of the aorta after successful repair of coarctation of the aorta: relationship to left ventricular mass and blood pressure at rest and with exercise. Am Heart J. 1992;123:1594–600. [DOI] [PubMed] [Google Scholar]
- 24.Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O’Donnell CJ, Mitchell GF, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim PO, MacFadyen RJ, Clarkson PB and MacDonald TM. Impaired exercise tolerance in hypertensive patients. Ann Intl Med. 1996;124:41–55. [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Garrison RJ, Savage DD, Kannel WB and Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 27.Rinnstrom D, Dellborg M, Thilen U, Sorensson P, Nielsen NE, Christersson C and Johansson B. Left ventricular hypertrophy in adults with previous repair of coarctation of the aorta; association with systolic blood pressure in the high normal range. Int J Cardiol. 2016;218:59–64. [DOI] [PubMed] [Google Scholar]
- 28.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly HM, Huston J 3rd, Brown RD Jr., Warnes CA, Ammash NM and Tajik AJ. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clinic Pro. 2003;78:1491–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


