Summary.
Background:
Agonist-induced inside-out signaling activates platelet integrin αIIbβ3, rendering it to bind plasma fibrinogen (Fg). Fg binding induces outside-in signaling that culminates in platelet aggregation, leading to physiological hemostasis and pathological thrombosis. How outside-in signaling through αIIbβ3 regulates hemostasis and thrombosis is not well understood. We have previously shown that CIB1 is involved in regulating αIIbβ3 function.
Objective:
To determine the in vivo role of CIB1 in the process of hemostasis and thrombosis.
Methods and Results:
Genetic ablation of Cib1 significantly increased mouse tail bleeding time. Greater than 50% of the Cib1 null mice showed a rebleeding phenotype. Time taken for complete occlusion of carotid artery upon 10% FeCl3-induced injury was significantly delayed in the absence of Cib1. This was also associated with unstable thrombus formation. The inside-out signaling appears normal as ADP-, collagen-and PAR4 peptide-induced aggregation and fibrinogen binding was unaffected. The absence of Cib1 also affected the ability of platelets to spread on immobilized Fg, but not filopodia formation. Spreading could be restored in Cib1 null platelets by the addition of exogenous ADP. Outside-in signaling-dependent tyrosine phosphorylation of the integrin β3 subunit was significantly reduced in the absence of Cib1 as determined by Western blot analysis.
Conclusion:
Using gene knockout mice, we show for the first time that lack of Cib1 results in impaired thrombosis. CIB1 regulates these processes by affecting platelet spreading, but not platelet filopodia formation. These in vivo and in vitro results clearly show that CIB1 is a key regulator of thrombosis.
Keywords: CIB1, hemostasis, integrin αIIbβ3, outside-in signaling, platelet spreading, thrombosis
Introduction
It is well established that bi-directional signaling through platelet integrin αIIbβ3 regulates physiological hemostasis and pathological thrombosis [1]. Normally, integrin αIIbβ3 is in a low affinity state on circulating platelets. Agonist-dependent signaling converts αIIbβ3 into a high affinity state capable of binding soluble ligands such as Fg. Ligand binding initiates yet another cascade of signaling events, termed outside-in signaling, which are important for platelet aggregation and clot retraction [1]. Despite the vast body of information available, the process of hemostasis is still not completely understood.
We have previously identified a novel calcium-and integrin-binding protein 1 (CIB1) that specifically interacts with the cytoplasmic domain of the αIIb subunit of integrin αIIbβ3 [2]. The interaction of CIB1 with αIIbβ3 has been confirmed by several laboratories using a variety of in vitro and in vivo techniques [3–5]. It has been reported that CIB1 may function as an endogenous inhibitor of integrin αIIbβ3 [6], an activator of agonist-induced inside-out signaling [7,8] and a regulator of outside-in signaling [3,9]. It is possible that CIB1 may regulate platelet function at multiple levels due to its ability to interact with multiple proteins in a calcium-dependent manner [3, 8]. However, the physiological consequences of the interaction of CIB1 with its binding partners in the process of hemostasis remain poorly understood. In this report, we establish a definitive role for CIB1 in the process of hemostasis, using Cib1 knockout mice.
Materials and methods
Mice
Generation and characterization of Cib1−/− mice, which were kindly provided by W. Yuan and L. Parise (University of North Carolina, Chapel Hill, NC, USA), has been described previously [10]. All animal studies performed were in accordance with an approved protocol by the University of Delaware Institutional Animal Care and Use Committee. Mice used in these studies were of the C57BL/6 genetic background (backcrossed 10 generations), and wild-type littermates were used as controls in all the experiments.
Platelet preparation
Orbital blood from 8- to 12-week-old mice was collected using a heparinized Natelson blood collecting tube and transferred into a tube containing anticoagulant. Blood was diluted using Tyrode’s buffer (1:1) without calcium and platelet-rich plasma (PRP) was obtained by centrifugation at 200 ×g for 10 min at room temperature (RT). In some experiments, PRP was pooled together from three to four mice and centrifuged at 400 ×g for 10 min at RT. Platelet pellet was resuspended, in Tyrode’s buffer containing 1 mM calcium, to a concentration of 1× 108 mL−1. Platelets were kept at RT for 30 min before experimentation and used within 3 h of isolation.
Platelet spreading
Washed platelets (1 × 106 mL−1) were allowed to adhere on Fg or BSA precoated glass coverslips or eight-chambered glass-coverslides (Nunc) for 45–60 min. Attached platelets were fixed with freshly prepared 4% paraformaldehyde (PF) then per-meabilized with 0.2% Triton X-100 for 5 min, washed and stained with Alexa-488 phalloidin (1:700) (Invitrogen, Carls-bad, CA, USA) for 1 h at RT. Stained platelets were visualized by confocal microscope (Zeiss, Ithaca, NY, USA) using a 63× objective. Differential interference contrast (DIC) images of platelets were captured using a Zeiss Axioskop II microscope (Thornwood, NY, USA) fitted with a 63× oil immersion objective. Fully-spread platelets were defined as having an extensively spread hyaloplasm with no distinct pseudopodia, using Goodman [11] criteria. The surface area of individual platelets was quantitated by determining the number of pixels within each platelet using Zeiss AIM 3.2 software (Thornwood). A minimum of 100 platelets were analyzed from five randomly chosen fields. Each experiment with duplicate samples per Fg concentration was repeated at least three times. Images were processed in Adobe Photoshop (Adobe, San Jose, CA, USA).
For scanning electron microscopy (SEM), adherent platelets were fixed in a mixture of freshly prepared glutaraldehyde/paraformaldehyde (2% of each) for 1 h. Coverslips were rinsed with 0.1 M sodium cacodylate buffer and further fixed with 4% PF for 10 min at RT. Fixed platelets were washed in 0.9% saline, dehydrated in graded ethanol solutions, air-dried in the presence of hexadimethyledisilazane, sprayed with gold, and examined under a Hitachi scanning microscope (Hitachi HighTechnologies, Schaumburg, IL, USA).
Tail bleeding assay
Tail bleeding was performed as described previously [12]. Four-to six-week-old offspring of Cib1+/− ×Cib1+/− were anesthetized prior to genotyping by intraperitoneal injection of a mixture of xylazine (15 mg kg−1) and ketamine (75 mg kg−1). After prewarming the tail for 5 min by immersing in 37 °C saline, the terminal 3 mm segment of the tail was amputated with a sharp sterile blade, and immediately reimmersed into saline solution. Bleeding time was measured as the time from the start of bleeding to cessation of bleeding. If the bleeding did not recur within 60 s of the cessation, it was considered stopped. The tendency to rebleed was determined by the reoccurrence of bleeding within 60 s after the cessation of the first bleeding [12]. If the bleeding continued beyond 10 min, the tail was cauterized to stop bleeding.
Platelet aggregation
Platelet aggregation was performed using platelet-rich plasma (PRP) containing 2 ×108 mL−1 platelets using a Chrono-Log lumi-aggregometer (Chrono-Log, Havertown, PA, USA). An aliquot of 250µL of PRP was taken into a siliconized aggregation tube and incubated at 37 °C for 10 min. Platelets were stimulated by various concentrations of 2-Me-S-ADP (Sigma, St. Louis, MO, USA) or PAR4 peptide (AYPGKF, AnaSpec, Fremont, CA, USA) or collagen (Chrono-log, Morrisville, NC, USA) under continuous stirring at 1000 rpm at 37 °C. Triplicate aggregation curves were recorded for each agonist and the experiments were repeated at least three times. Aggregation traces were recorded using Aggrolink software (Chrono-log).
Flow cytometry
Washed platelets (0.6 ×108 mL−1) from Cib1 +/+ and Cib1−/− mice were incubated with or without FITC-conjugated anti-αIIb (1µg mL−1) or FITC-conjugated control antibody (BD Bioscience, Franklin Lakes, NJ, USA) for 20 min at RT in the dark. For determination of the surface expression of PECAM-1, platelets were incubated for 20 min with anti-PECAM-1 (1µg mL−1) or isotype-specific IgG, followed by an additional 20-min incubation with FITC-conjugated secondary antibody. For FITC-fibrinogen binding experiments and P-selectin surface expression, platelets were stimulated with various concentrations of agonists PAR4 peptide or ADP for 10 min at RT and followed by the addition of FITC-Fg (50 µg mL−1) or FITC-conjugated anti-P-selectin (1µg mL−1, BD Biosciences) antibodies for 20 min at 37 °C. The reaction was stopped by the addition of 900 µL of ice-cold PF (1% final volume) in PBS containing 0.2% BSA and analyzed by flowcytometry (Becton Dickinson, Franklin Lakes, NJ, USA). The main platelet population was gated for the acquisition of data. Each experiment was repeated independently at least three times. Analysis of flow profile was performed using Cell Quest software (Becton Dickinson).
Thrombosis assay
A FeCl3-induced carotid artery thrombosis assay was performed as described [13] with some modifications. An adult Cib1−/− mouse of 12–14 weeks old was anesthetized and the body temperature was maintained throughout the experimental procedure, using a heating pad. Wild-type littermates were used as controls. A midline cervical incision was performed and the right common carotid artery was surgically exposed by blunt dissection. The Doppler flow probe (Taconic Systems, Ithaca, NY, USA) was placed under the artery. The surgical wound was covered with a sterile PBS solution to initiate blood flow; measurements were taken using a Transonic model T106 flow meter that interfaced with Windaq software (Dataq Instrument, Akron, OH, USA). An injury to the carotid artery was inflicted by placing a piece of Whatman 1 filter paper (1 × 1 mm) saturated with freshly prepared 10% FeCl3 (anhydrous; Sigma) on the adventitial surface of the carotid artery, proximal to the flow probe, for 2 min. The time for stable thrombotic occlusion (defined as lack of detectable blood flow) to take place after the initiation of arterial injury with 10% FeCl3, was recorded for 45 min.
Integrin β3 Y773 phosphorylation
Washed platelets isolated from Cib1+/+ and Cib1−/−(2× 108 mL−1) and suspended in Tyrode’s buffer containing 1 mM each of aspirin and apyrase (Sigma) were allowed to spread on Fg (100 µg mL−1) or 3% BSA precoated petri dishes for 45 min at 37 °C. Platelets were lysed using ice-cold lysis buffer (1% NP-40, 150 mM NaCl and 50 mM Tris–HCl pH 7.5, containing 10 µg mL−1 each of leupeptin, levisol and aprotinin, and 1 mM each of NaF, sodium orthovanadate and PMSF). An aliquot of the lysate (20–50 µg) was boiled with 5× Laemmli-sample buffer. Proteins were separated by 8% SDS-PAGE under reducing conditions, transferred to PVDF membrane, and incubated with anti-integrin β3 Y773 phospho-specific antibody (Abcam, Cambridge, MA, USA) overnight followed by the incubation of the appropriate secondary antibody at RT for 1 h. The bands were visualized by chemiluminescence using Lumiglo® substrate (New England Biolabs, Ipswich, MA, USA). The blot was stripped and reprobed with anti-integrin β3 antibody to determine the total amount of integrin β3. Quantification of band intensity was performed using gel doc software (BioRad, Hercules, CA, USA). The amount of phosphorylated β3 Y773 was normalized against the total β3 integrin.
Statistical analysis
Statistical analysis of the data was performed using Student’s t-test and Fisher’s exact test (mean ± SEM value, SEM, standard error of the mean). P ≤ 0.05 is regarded as statistically significant. Each experiment was repeated at least three times.
Results
Genetic ablation of Cib1 results in extended tail bleeding time and rebleeding
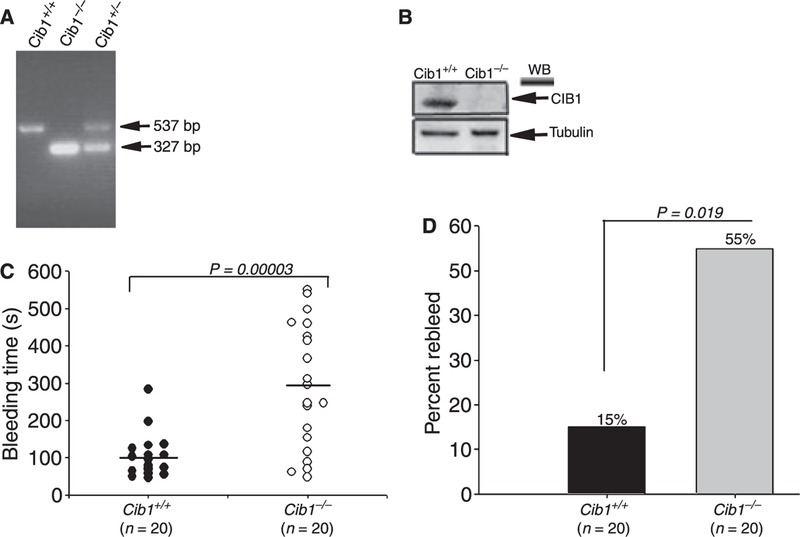
The interaction of CIB1 with several platelet proteins, including the integrin αIIb cytoplasmic tail, is well established. However, the consequences of these interactions in the physiological process of hemostasis are not known. The generation of the Cib1 knockout mouse provides the opportunity to precisely identify the role that CIB1 may play in this process. Because Cib1 null males are sterile [10], all animals used in this study were generated from heterozygous mating. Offspring were genotyped by PCR (Fig. 1A), and the expression of Cib1 protein was determined by Western blot analysis (Fig. 1B). Wild-type littermate (+/+) mice were used as controls. We first evaluated the hemostatic response of Cib1−/− mice by determining the tail bleeding time. We found that Cib1+/+ mice showed an average bleeding time of 101 s (P = 0.00003; Fig. 1C). Heterozygote (+/−) littermates had a slightly elevated average bleeding time of 121 s (P = 0.001; data not shown). Interestingly, Cib1−/−mice had a greatly extended bleeding time with an average of 289 s (Fig. 1C). Furthermore, 13 out of 20 (65%) Cib1−/− mice had a bleeding time greater than 200 s as compared with only 1 out of 20 (5%) of Cib1+/+. When evaluated for rebleeding as a measure of an unstable hemostatic response, we found that 55% of Cib1−/− mice showed rebleeding as compared with only 15% of the Cib1+/+ mice (P = 0.019; Fig. 1D). These results suggest that Cib1−/− mice may have a defective hemostatic function.
Fig. 1.
Impaired hemostasis in Cib1−/− mice. (A) Genotype of mice by PCR using Cib1 gene-specific primers. (B) Western blot using anti-Cib1. Anti-tubulin was used to ensure equal loading. (C) Tail bleeding time assay was performed to determine the hemostatic function in Cib1+/+ and Cib1−/− mice (n = 20 mice for each genotype). Horizontal lines indicate mean bleeding times. P = 0.00003. (D) Comparison of per cent rebleeding between Cib1+/+ and Cib1−/− mice.
Absence of CIB1 impairs in vivo thrombus formation
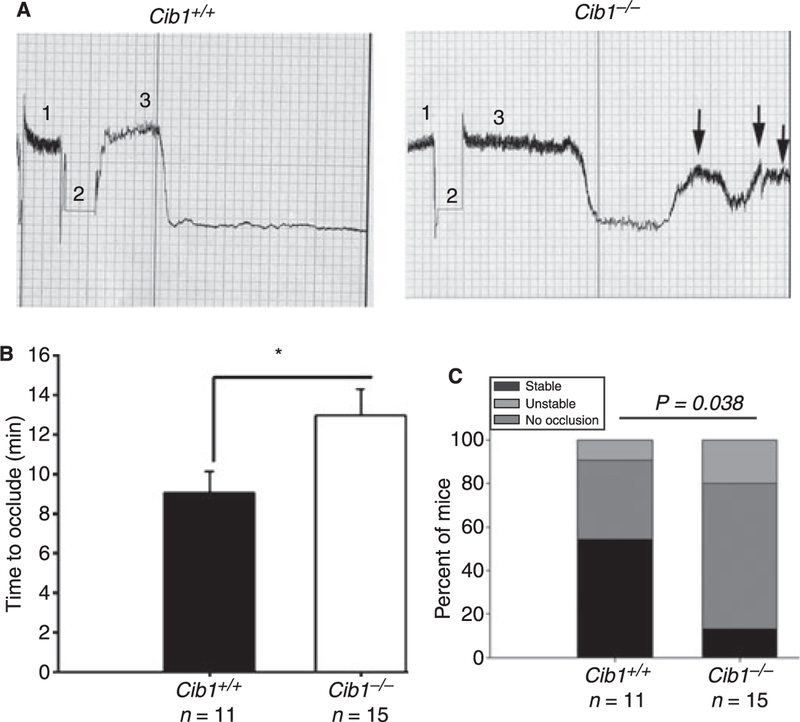
The FeCl3-induced carotid artery injury model, a well-characterized arterial thrombosis model, was used to induce an occlusive thrombus in the Cib1+/+ and Cib1−/− null mice. As expected in the Cib1+/+ mice, complete cessation of flow occurred with an average time of 9 min and remained so until 30 min, indicating the formation of a stable occlusive thrombus (Fig. 2A, left panel). In contrast, in the Cib1−/− mice the complete cessation of flow was substantially delayed, with an average time of 13 min. Further, the flow returned transiently, indicating formation of an unstable thrombus (Fig. 2A, right panel). A significant (P = 0.03) difference was observed in occlusion time between the Cib1+/+ and Cib1−/−mice (Fig. 2B). Additionally, 87% of Cib1−/− mice showed unstable/no occlusion profile as compared with 45% in the Cib1+/+ mice (P = 0.038; Fig. 2C). Similar results were observed when 7.5% FeCl3 was used in the model (data not shown). These results suggest that the absence of Cib1 affects thrombus formation or its stability.
Fig. 2.
Ablation of Cib1 affects in vivo thrombus formation. (A) Representative flow pattern observed in response to 10% FeCl3-induced injury. Baseline carotid blood flow [1], duration of injury [2], and recording of flow post-injury [3]. Formation of unstable thrombus in Cib1−/− mice is shown by arrows. (B) Quantitation of experiments depicts a significant difference between Cib1+/+ and Cib1−/− occlusion time (P = 0.03). (C) Quantitation of percentage of mice showing occlusive phenotype, Cib1+/+ and Cib1−/−(P = 0.038).
Inside-out signaling is normal in Cib1 null platelets
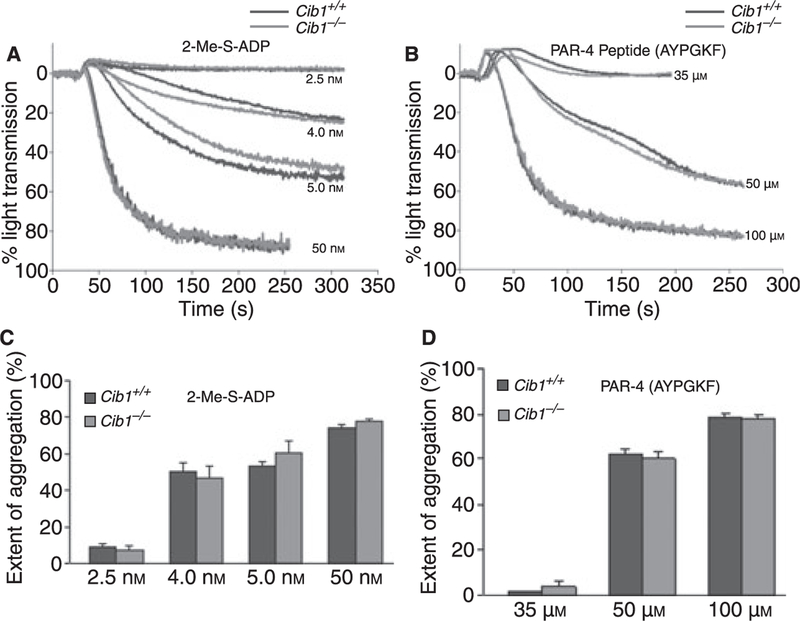
In order to determine if the inside-out signaling is affected due to the absence of Cib1, we analyzed platelet aggregation. When we stimulated the Cib1−/− and the Cib1+/+ platelets with various concentrations of agonists 2-Me-S-ADP or PAR4 peptide, we found that agonist-induced platelet aggregation was unaffected in Cib1−/− platelets, as compared with the Cib1+/+ littermates (Fig. 3A–B). Quantitation of more than three separate experiments showed no significant difference in platelet aggregation between Cib1−/− and Cib1+/+ (Fig. 3C,D). We also did not see a difference when collagen was used as an agonist (data not shown). These results indicate that the inside-out signaling is normal in the absence of Cib1, thus eliminating the possibility of defective inside-out signaling.
Fig. 3.
Platelet function is normal in the absence of Cib1. Representative aggregation in Cib1+/+ and Cib1−/− platelets induced by various concentrations of (A) 2-Me-S-ADP and (B) PAR4 peptide. Quantitation of extent of platelet aggregation induced by 2-Me-S-ADP (C) and PAR4 peptide (D). Each aggregation was performed in triplicate and repeated independently more than three times.
Ablation of Cib1 did not alter fibrinogen binding or α-granules secretion
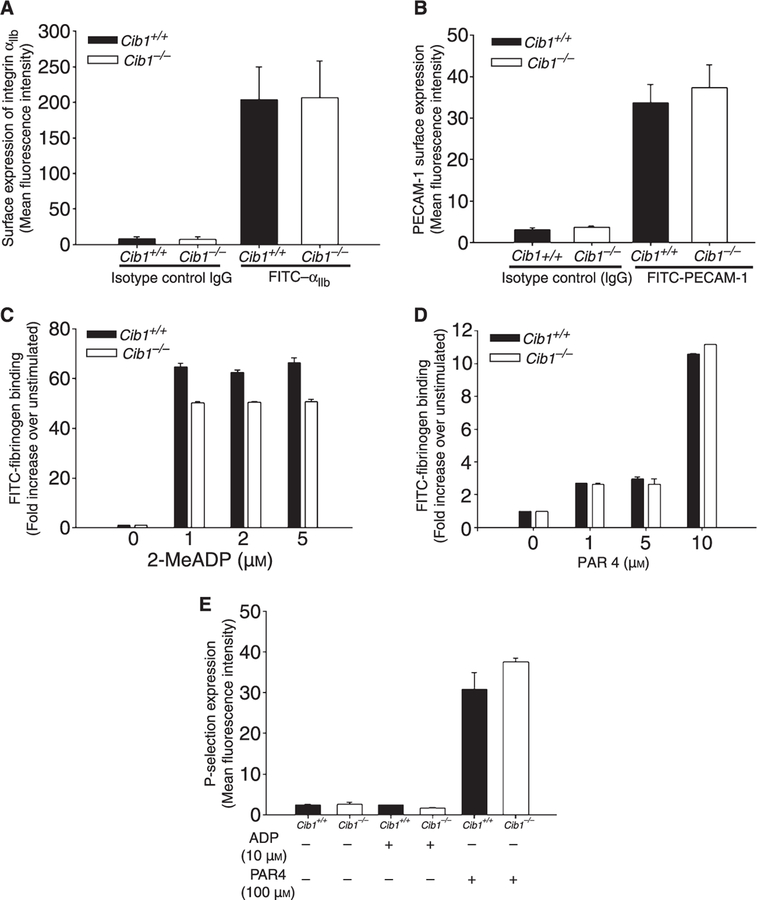
Although overall platelet aggregation is not affected by the absence of Cib1, there could be a defect in the extent of integrin αIIbβ3 activation or granular secretion. We first examined the level of expression of platelet surface proteins by flow cytometry. Expression of integrin αIIbβ3 and PECAM-1 in the Cib1+/+ and Cib1−/− platelets did not show any significant difference (Fig. 4A–B). To evaluate the role of CIB1 in αIIbβ3 integrin activation, we analyzed fibrinogen binding to resting and activated Cib1+/+ and Cib1−/− platelets by flow cytometry. We did not see any significant difference in the ability of Cib1−/− platelets to bind to Fg upon activation by various concentrations of 2-Me-S-ADP or PAR4 peptide as compared with the Cib1+/+ platelets (Fig. 4C,D). It is well known that platelet activation leads to exposure of P-selectin expression, a marker of α-granule secretion. Analysis by flow cytometry revealed that surface expression of P-selectin was minimal on resting platelets from both Cib1+/+ and Cib1−/− mice; when activated by 10 µM ADP there was no increase in P-selectin expression, as expected. Although upon stimulation with 100 µM PAR4 peptide P-selectin expression increased, there was no significant difference between Cib1+/+ and Cib1−/− platelets (Fig. 4E). These results suggest that agonist-induced integrin αIIbβ3 activation or α-granule secretion is normal in the absence of Cib1.
Fig. 4.
Normal agonist-induced inside-out signaling in Cib1−/− platelets. Flow cytometric analysis of (A) surface expression of integrin αIIb, (B) PECAM-1, (c) FITC-fibrinogen binding, upon stimulation with 2-Me-S-ADP, (D) PAR4 peptide, and (E) P-selectin exposure upon stimulation by agonists. Each flow cytometric run was performed in triplicate and each experiment was repeated at least three times.
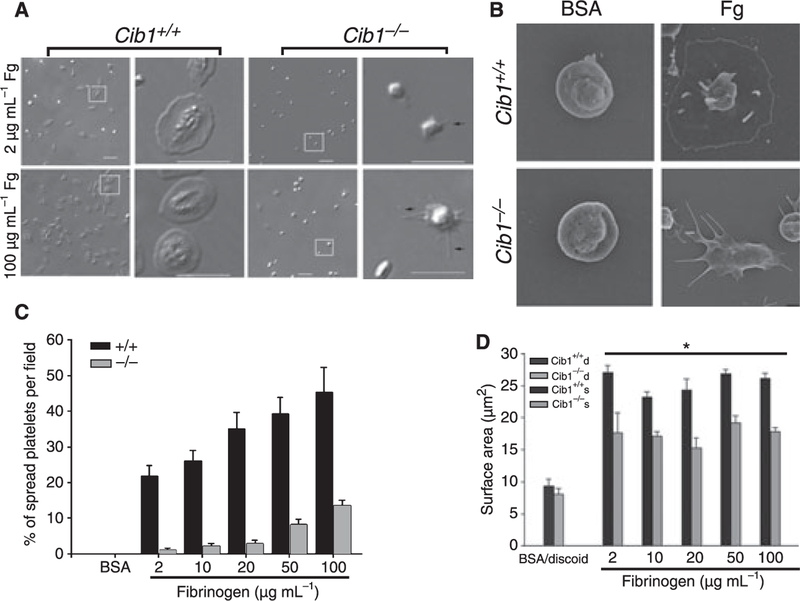
Cib1 null platelets failed to fully spread on immobilized fibrinogen
We have previously shown that association of CIB1 with integrin αIIbβ3 is required for platelet spreading on immobilized Fg [3]. Furthermore, we have also shown that CIB1 regulates FAK activity during this process [3,9]. In order to test the effect of Cib1 ablation on outside-in signaling through integrin αIIbβ3, we analyzed the ability of Cib1−/− platelets to spread on immobilized Fg. We allowed washed platelets from Cib1+/+ and Cib1−/− mice to spread on various concentrations (2–100 µg mL−1) of immobilized Fg. As can be seen in Fig. 5A, Cib1−/− platelets failed to fully spread on any given concentration of Fg. The time taken for spreading was inversely proportional to the concentration of Fg used (data not shown). Platelet filopodia formation was not affected by Cib1 deficiency as Cib1−/− platelets exhibited numerous filopodial extensions (Fig. 5A). These results were further supported by the SEM of Cib1+/+ and Cib1−/− platelets exposed to immobilized Fg or BSA. Platelets exposed to BSA remained discoid, showing that platelets were not unintentionally activated during the washing procedure (Fig. 5B). Consistent with our DIC images, Cib1+/+ platelets exposed to Fg spread completely, whereas Cib1−/− platelets formed numerous filopodia but failed to attain a fully spread morphology (Fig. 5B). Further, we found a significant decrease in the number of fully spread platelets on every concentration of Fg tested in Cib1−/− compared with Cib1+/+ platelets (Fig. 5C). The spreading defect was further confirmed by analyzing the average surface area of the spread platelets. We found that the discoid platelets from both Cib1+/+ and Cib1−/− mice had an average surface area of 8 µm2. (Fig. 5D). Cib1+/+ platelets showed a 3–4-fold increase in surface area when allowed to spread on Fg. Interestingly, Cib1−/− platelets showed only a 2-fold increase in average surface area when allowed to spread on Fg (Fig. 5D). The concentration of Fg had no effect on the average surface area of the spread platelets (Fig. 5D). These results suggest that CIB1 is required for platelets to achieve a fully spread morphology.
Fig. 5.
Defective spreading of Cib1−/− platelets. (A) Representative DIC images of washed Cib1+/+ and Cib1−/− platelets allowed to spread on immobilized Fg and a zoomed image of the platelets within the representative squares are shown. Arrows indicate filopodia. (B) A representative SEM image of Cib1+/+ and Cib1−/− platelets exposed to BSA and immobilized Fg. Bar, 10 lm. (C) Quantitation of the number of spread platelets on immobilized Fg. At least 100 individual platelets per concentration were analyzed. Data shown are representative of at least three separate experiments. (D) Quantification of extent of platelet spreading on immobilized Fg. Data shown are representative of three separate experiments.
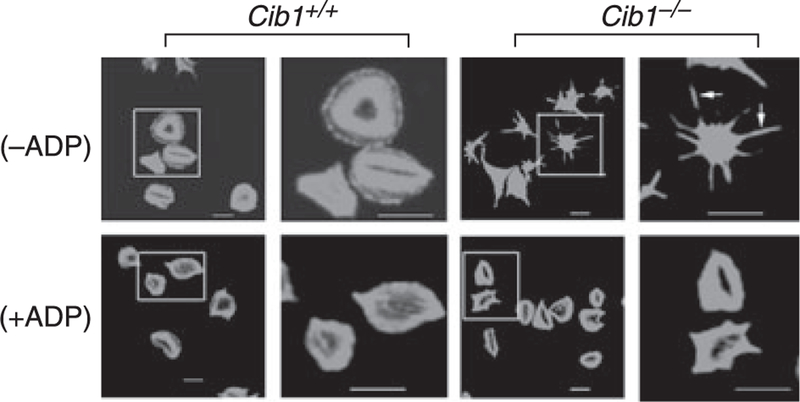
Exogenous ADP restores the ability of Cib1 null platelet to spread on immobilized Fg
CIB1 has been shown to regulate activity of signaling molecules such as FAK and PAK1, which are key regulators of cell spreading [9, 14]. It is therefore possible that in the absence of CIB1, platelets lose their ability to spread, irrespective of extracellular signals. We reasoned that if the addition of exogenous platelet agonist(s) rescues the spreading phenotype, this possibility would be excluded. Interestingly, exogenous addition of ADP completely restored the spreading phenotype in Cib1−/−platelets, suggesting that agonist-induced platelet spreading is functional in Cib1−/− platelets, but integrin outside-in signaling-induced spreading is not (Fig. 6). These results suggest that Cib1 regulates outside-in signaling dependent platelet spreading.
Fig. 6.
Rescue of Cib1−/− platelet spreading by exogenous ADP. Confocal images of FITC-phalloidin labeled Cib1+/+ and Cib1−/− platelets and zoomed images of the platelets within the representative squares are shown. Washed platelets were allowed to adhere to immobilized (100 lg mL)1) Fg and after 30 min of spreading, exogenous ADP (20 µM, +ADP) or PBS (−ADP) was added and spreading continued for additional 15 min. Bar, 10 lm. Each experiment was repeated three times.
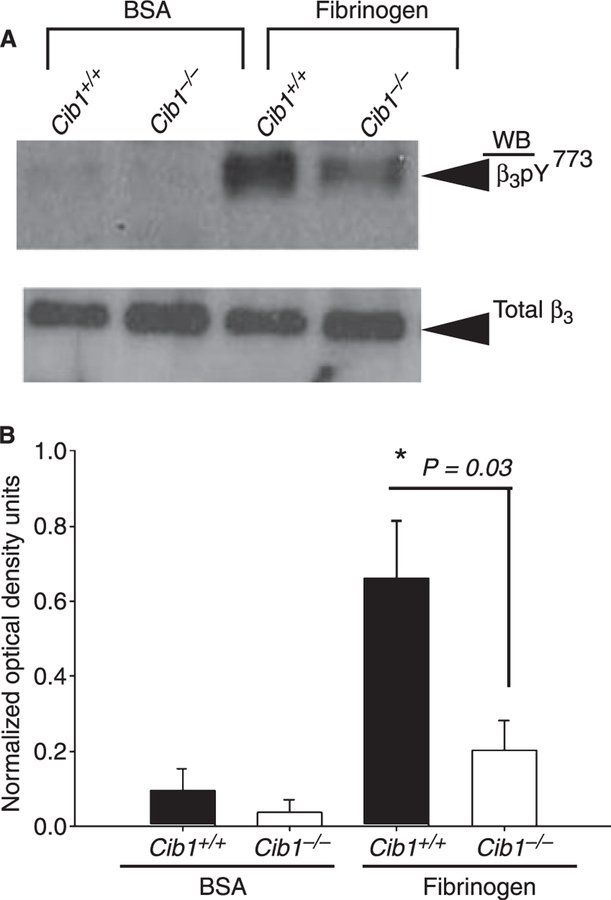
Platelets lacking Cib1 show reduced integrin β3 Y773 phosphorylation
Upon ligand binding, critical tyrosine residues in the cytoplas-mic tail of the β3 integrin become phosphorylated and therefore serve as docking sites for downstream signaling molecules [15]. We therefore assessed the role of CIB1 in β3 integrin tyrosine phosphorylation during outside-in signaling. Washed Cib1+/+ platelets, when allowed to spread on immobilized Fg, showed a robust phosphorylation on β3 Y773, as expected. Absence of Cib1 dramatically affected β3 Y773 phosphorylation in Cib1−/− platelets (Fig. 7A). Quantitation of the data revealed that Cib1−/− platelets showed a significant decrease (3-fold, P = 0.03) in β3 Y773 phosphorylation compared with the Cib1+/+ platelets (Fig. 7B). Taken together, the results presented here show for the first time an outside-in signaling defect in Cib1−/− mouse platelets that manifests into a hemostatic defect.
Fig. 7.
Absence of Cib1 affected β3 Y773 phosphorylation. (A) Western blot showing β3 Y773 phosphorylation in Cib1+/+ and Cib1−/− platelets attached to immobilized Fg (upper panel). The blot was reprobed with anti-integrin β3 to ensure equal loading. A representative example of three independent experiments is shown. (B) Quantitation of normalized optical density of β3 Y773 phosphorylation in Cib1−/− platelets as compared with Cib1+/+ platelets.
Discussion
We have previously identified CIB1, a calcium binding protein, abundantly expressed in platelets as an αIIb subunit cytoplasmic domain-interacting protein of platelet fibrinogen receptors, integrin αIIbβ3 [2]. Further studies from several laboratories, including ours, have established that CIB1 interacts with many proteins and regulates several key steps in platelet integrin signaling [4–8]. It has been reported that CIB1 is an endogenous inhibitor of αIIbβ3 activation [6], an activator of αIIbβ3 [7,8], and an effecter of outside-in signaling [3,9]. We found a significant defect in hemostasis in Cib1−/− mice, which was further attributed to the defect in outside-in signaling. While this manuscript was in preparation, DeNofrio et al. reported their failure to detect any significant hemostatic defect in these mice [16]. The observed discrepancy in these two studies could be due to the differences in the experimental procedures used.
Tail bleeding time is an excellent test for in vivo hemostatic function [17–19]. We found a statistically significant difference in tail bleeding time (P = 0.00003) between Cib1+/+ and Cib1−/− mice. The observed wide range in the bleeding times among Cib1−/− mice was not surprising because such spread has been observed in many knockout mice [17,19]. Although not significant, DeNofrio et al. also found a slightly extended bleeding time in Cib1−/− mice [16]. In their experiments, several of their wild-type (Cib1+/+) mice had bleeding times greater than 10 min, which appears to be uncommon. Inclusion of such data points could have affected the statistical significance between groups. In contrast, none of the Cib1+/+ or Cib1+/− mice in our study bled for longer than 5 min. DeNofrio et al. also did not determine the tendency of these mice to rebleed. We found about 55% of Cib1−/− mice rebled, which is highly significant (P = 0.019). The augmented tendency of Cib1−/− mice to rebleed further supports the notion of impaired hemostasis. Another widely accepted in vivo model used to test defective hemostasis/thrombosis is the FeCl3-induced carotid artery injury model [13, 17]. Consistent with tail bleeding times, both 7.5% and 10% FeCl3-induced thrombosis was also found to be defective in Cib1−/− mice. This is in contrast to the report of DeNofrio et al., where no difference in the time of occlusion among Cib1+/+ and Cib1−/− mice was reported. It should be noted that they used 20% FeCl3 in their experiments; this high concentration of FeCl3 may have masked any difference between the two mouse genotypes.
The impairment in hemostasis observed in Cib1−/− mice could be due to a consequence of altered inside-out or outside-in signaling. Our finding of normal agonist-induced platelet aggregation and α-granule secretion as well as normal expression of platelet surface proteins such as integrin αIIbβ3 and PECAM-1 in Cib1−/− platelets suggests that the problem is not in inside-out signaling. These findings are consistent with the results reported by DeNofrio et al. [16]. The significant difference in hemostatic response observed suggests that the likely source of the problem could be in outside-in signaling. Altered hemostasis due to impaired outside-in signaling, but normal inside-out signaling, has been documented. Megakaryocyte-restricted MYH9 gene inactivation dramatically affects hemo-stasis while preserving platelet aggregation and secretion [18].
When resting platelets are exposed to immobilized Fg, they attach and become spiky with numerous filopodia and ultimately spread through the formation of lamellipodia [3]. The primary signal for platelet spreading is initiated by outside-in signaling through αIIbβ3 [20]. Our observation of impaired Cib1−/− platelet spreading on Fg and its rescue by exogenous ADP is consistent with our previously reported findings where introduction of an anti-CIB1 antibody or αIIb peptide inside the human platelets blocked platelet spreading but not filopodia formation, and was rescued by exogenous ADP [3].
Upon ligand binding, the β3 cytoplasmic domain of integrin αIIbβ3 is phosphorylated on tyrosine [15, 21]. Rebleeding phenotype has been observed in mice in which tyrosine residues are mutated to phenylalanine, indicating that outside-in signaling dependent tyrosine phosphorylation plays a key role during thrombosis and hemostasis [21]. Our observation of significant attenuation of β3 Y773 phosphorylation in Cib1−/− platelets strongly suggests a role for Cib1 in regulating platelet outside-in signaling.
The results presented in this report provide evidence that CIB1 plays an important regulatory role in the process of hemostasis by modulating post-occupancy signaling events through integrin αIIbβ3.
Acknowledgements
The authors would like to thank S. Seta for her help in drawing the blood from mice, as well as D. Powell for SEM. This work was supported by Grants from the National Institutes of Health (HL57630) to U. P. Naik.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood 2004; 104: 1606–15. [DOI] [PubMed] [Google Scholar]
- 2.Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J Biol Chem 1997; 272: 4651–4. [DOI] [PubMed] [Google Scholar]
- 3.Naik UP, Naik MU. Association of CIB with GPIIb/IIIa during outside-in signaling is required for platelet spreading on fibrinogen. Blood 2003; 102: 1355–62. [DOI] [PubMed] [Google Scholar]
- 4.Shock DD, Naik UP, Brittain JE, Alahari SK, Sondek J, Parise LV. Calcium-dependent properties of CIB binding to the integrin alphaIIb cytoplasmic domain and translocation to the platelet cytoskeleton. Biochem J 1999; 342 Pt 3: 729–35. [PMC free article] [PubMed] [Google Scholar]
- 5.Vallar L, Melchior C, Plancon S, Drobecq H, Lippens G, Regnault V, Kieffer N. Divalent cations differentially regulate integrin alphaIIb cytoplasmic tail binding to beta3 and to calcium-and integrin-binding protein. J Biol Chem 1999; 274: 17257–66. [DOI] [PubMed] [Google Scholar]
- 6.Yuan W, Leisner TM, McFadden AW, Wang Z, Larson MK, Clark S, Boudignon-Proudhon C, Lam SC, Parise LV. CIB1 is an endogenous inhibitor of agonist-induced integrin alphaIIbbeta3 activation. J Cell Biol 2006; 172: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuboi S Calcium integrin-binding protein activates platelet integrin alpha IIbbeta 3. J Biol Chem 2002; 277: 1919–23. [DOI] [PubMed] [Google Scholar]
- 8.Tsuboi S, Nonoyama S, Ochs HD. Wiskott-Aldrich syndrome protein is involved in alphaIIb beta3-mediated cell adhesion. EMBO Rep 2006; 7: 506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik MU, Naik UP. Calcium-and integrin-binding protein regulates focal adhesion kinase activity during platelet spreading on immobilized fibrinogen. Blood 2003; 102: 3629–36. [DOI] [PubMed] [Google Scholar]
- 10.Yuan W, Leisner TM, McFadden AW, Clark S, Hiller S, Maeda N, O’Brien DA, Parise LV. CIB1 is essential for mouse spermatogenesis. Mol Cell Biol 2006; 26: 8507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman SL. Sheep, pig, and human platelet-material interactions with model cardiovascular biomaterials. J Biomed Mater Res 1999; 45: 240–50. [DOI] [PubMed] [Google Scholar]
- 12.Severin S, Gratacap MP, Lenain N, Alvarez L, Hollande E, Penninger JM, Gachet C, Plantavid M, Payrastre B. Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J Clin Invest 2007; 117: 944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuchay SM, Kim N, Grunz EA, Fay WP, Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol Cell Biol 2007; 27: 6038–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leisner TM, Liu M, Jaffer ZM, Chernoff J, Parise LV. Essential role of CIB1 in regulating PAK1 activation and cell migration. J Cell Biol 2005; 170: 465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips DR, Prasad KS, Manganello J, Bao M, Nannizzi-Alaimo L. Integrin tyrosine phosphorylation in platelet signaling. Curr Opin Cell Biol 2001; 13: 546–54. [DOI] [PubMed] [Google Scholar]
- 16.Denofrio JC, Yuan W, Temple BR, Gentry HR, Parise LV. Characterization of calcium- and integrin-binding protein 1 (CIB1) knockout platelets: potential compensation by CIB family members. Thromb Haemost 2008; 100: 847–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Goschnick MW, Lau LM, Wee JL, Liu YS, Hogarth PM, Robb LM, Hickey MJ, Wright MD, Jackson DE. Impaired ‘‘outside-in’’ integrin alphaIIbbeta3 signaling and thrombus stability in TSSC6-deficient mice. Blood 2006; 108: 1911–8. [DOI] [PubMed] [Google Scholar]
- 18.Leon C, Eckly A, Hechler B, Aleil B, Freund M, Ravanat C, Jourdain M, Nonne C, Weber J, Tiedt R, Gratacap MP, Severin S, Cazenave JP, Lanza F, Skoda R, Gachet C. Megakaryocyte-restricted MYH9 inactivation dramatically affects hemostasis while preserving platelet aggregation and secretion. Blood 2007; 110: 3183–91. [DOI] [PubMed] [Google Scholar]
- 19.Schwer HD, Lecine P, Tiwari S, Italiano JE Jr, Hartwig JH, Shivda-sani RA. A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol 2001; 11: 579–86. [DOI] [PubMed] [Google Scholar]
- 20.Shattil SJ, Haimovich B, Cunningham M, Lipfert L, Parsons JT, Ginsberg MH, Brugge JS. Tyrosine phosphorylation of pp125FAK in platelets requires coordinated signaling through integrin and agonist receptors. J Biol Chem 1994; 269: 14738–45. [PubMed] [Google Scholar]
- 21.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for out-side-in alphaIIbbeta3 signalling and platelet function. Nature 1999; 401: 808–11. [DOI] [PubMed] [Google Scholar]