Supplemental Digital Content is available in the text
Keywords: cefoperazone-sulbactam, efficacy, febrile neutropenia, mortality
Abstract
Purpose:
This meta-analysis assessed the clinical efficacy and safety of cefoperazone-sulbactam for empiric therapy febrile neutropenia.
Methods:
The PubMed, Web of Science, EBSCO, Cochrane Library, Ovid Medline, EMBASE, and ClinicalTrial.gov database were searched through May 10, 2019. Only clinical trials comparing cefoperazone-sulbactam with other antibiotics for empiric treatment of febrile neutropenia were included. The primary outcome was treatment success without modification, and the secondary outcomes were all-cause mortality and adverse events (AEs).
Results:
Ten randomized controlled trials (RCTs) and 1 retrospective cohort study were included. Overall, cefoperazone-sulbactam exhibited a treatment success rate similar to those of comparator drugs for the treatment of febrile neutropenia (odds ratio [OR], 1.03; 95% confidence interval [CI], 0.85 to 1.24, I2 = 0%). A similar finding was noted in pooled analysis of 10 RCTs (OR, 1.07; 95% CI, 0.88 to 1.30, I2 = 0%). Subgroup analysis showed that cefoperazone-sulbactam had a treatment success rate similar to the rates of comparators for adults (OR, 1.10; 95% CI, 0.88 to 1.38, I2 = 0%) and children (OR, 0.96; 95% CI, 0.63 to 1.46, I2 = 0%). Cefoperazone-sulbactam did not differ significantly from comparators in the risks of all-cause mortality (OR, 0.96; 95% CI, 0.58 to 1.58, I2 = 0%) or common AEs, namely rash, nausea/vomiting, and superinfection.
Conclusion:
The clinical efficacy and tolerability of cefoperazone-sulbactam are comparable to those of comparator drugs in the treatment of febrile neutropenia.
1. Introduction
Febrile neutropenia is defined as the development of a fever during a period of significant neutropenia.[1] Despite improvements in cancer management, febrile neutropenia remains a severe complication for patients undergoing chemotherapy for cancer; approximately 1% of patients receiving chemotherapy develop febrile neutropenia.[2] Febrile neutropenia is associated with morbidity and mortality.[2] Patients with febrile neutropenia should be administered empiric antimicrobial agents intravenously; currently, broad-spectrum antibiotics such as antipseudomonal beta-lactam, carbapenems, and piperacillin-tazobactam are recommended.[3,4]
Cefoperazone-sulbactam is a broad-spectrum antibiotic and approved for the treatment of several acute bacterial infections. Even for multidrug-resistant organisms, such as extended-spectrum β-lactamase–producing Enterobacteriaceae and carbapenem-resistant Acinetobacter baumannii, cefoperazone-sulbactam exhibits potent in vitro activity that is unaffected by inoculum effects.[5–7] Therefore, cefoperazone-sulbactam can be considered a therapeutic option for febrile neutropenia. Several clinical studies[8–17] have investigated the efficacy and safety of cefoperazone-sulbactam for the treatment of febrile neutropenia. However, no meta-analysis has compared the efficacy and safety of cefoperazone-sulbactam with those of other antibiotics commonly used for treating febrile neutropenia. Therefore, we conducted a comprehensive meta-analysis to provide high-quality evidence of the efficacy and safety of cefoperazone-sulbactam for treating febrile neutropenia.
2. Methods
2.1. Data sources and search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses when searching for articles, selecting studies, evaluating article quality, and analyzing data.[18] We searched for candidate articles published before May 10, 2019, on the PubMed, Web of Science, EBSCO, Cochrane Library, Ovid Medline, EMBASE, and ClinicalTrial.gov databases. The search terms were “febrile neutropenia,” “cefoperazone,” “sulbactam,” “cefoperazone-sulbactam,” “sulperazone,” “neutropenic fever,” “and “neutropenic sepsis.” We applied no publication year or language limitations. The definitions of febrile neutropenia varied; the cutoff neutrophil counts per liter were either 500 or 1000, and the definitions of fever were either a single oral temperature of >38.3°C (101°F) or a temperature >38.0°C (100.4°F) sustained for >1 hour. We permitted simultaneous administration of granulocyte colony–stimulating factor and cefoperazone-sulbactam as well as the use of the same anti-MRSA drug or aminoglycoside in both the study and control groups. Three investigators reviewed the full texts of the candidate articles to finalize the experimental and control groups included for meta-analysis. Three investigators reviewed the study methods, site, duration, and population as well as the treatment regimen reported in the articles. Initially, 2 investigators (Lan and Chang) examined the publications independently to avoid bias, and the third author (Lu) resolved any disagreements. We recorded the year of publication; study design, duration, site, and population; antibiotic regimen of cefoperazone-sulbactam and comparators; outcomes; and adverse effects reported in the included studies.
2.2. Definitions and outcomes
The primary outcome was treatment success without modification of the initial antibiotic regimen. Although some researchers consider success with regimen modification as treatment successes, this was not the primary outcome of our meta-analysis. The secondary outcomes were all-cause mortality and adverse events (AEs).
2.3. Quality assessment and data analysis
The investigators used the Cochrane Collaboration criteria to assess the study designs methodological quality; quality of included randomized controlled trials (RCTs), and observation studies were evaluated using the Cochrane risk-of-bias tool and standardized critical appraisal instruments from the Joanna Briggs Institute, respectively. Differences in opinion among the investigators were resolved through discussion and voting. Meta-analysis (drug efficacy and safety) was conducted using Review Manager software (RevMan, 5.3; Cochrane Informatics & Knowledge Management Department). The heterogeneity of the studies was measured using the I2 statistic and the Q test (heterogeneity X2). A Q test result of P < .1 or I2 > 50% indicates heterogeneity; in such cases, a random-effects model was used. In contrast, if heterogeneity was absent in a study, a fixed-effects model was used. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for outcome analyses.
3. Results
The search results yielded 90 records from the online databases (Appendix 1); 57 were excluded because of duplication, 19 records were deemed irrelevant after the title and abstract were screened, and 3 records were deemed irrelevant after the full text was screened. Finally, 11 studies[8–17,19] were included in the meta-analysis (Fig. 1). The risk of bias for each RCT is shown in Figure 2.
Figure 1.
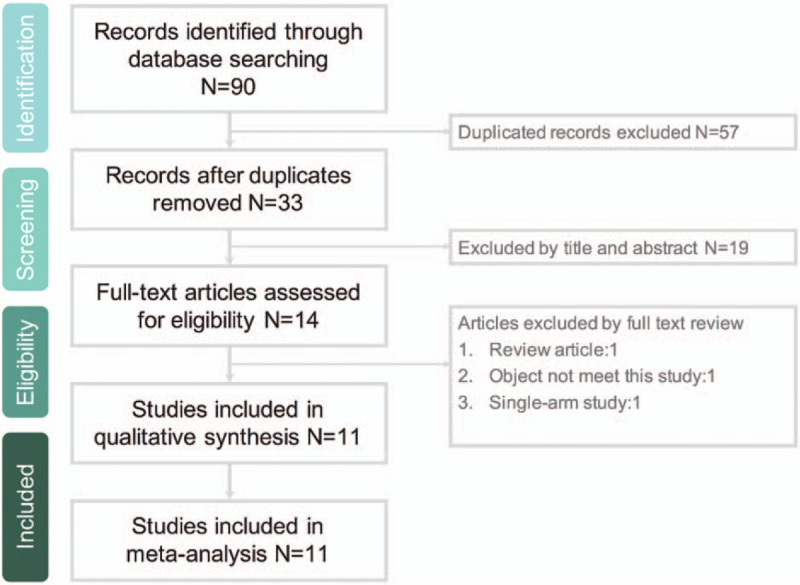
Flowchart of study selection process.
Figure 2.
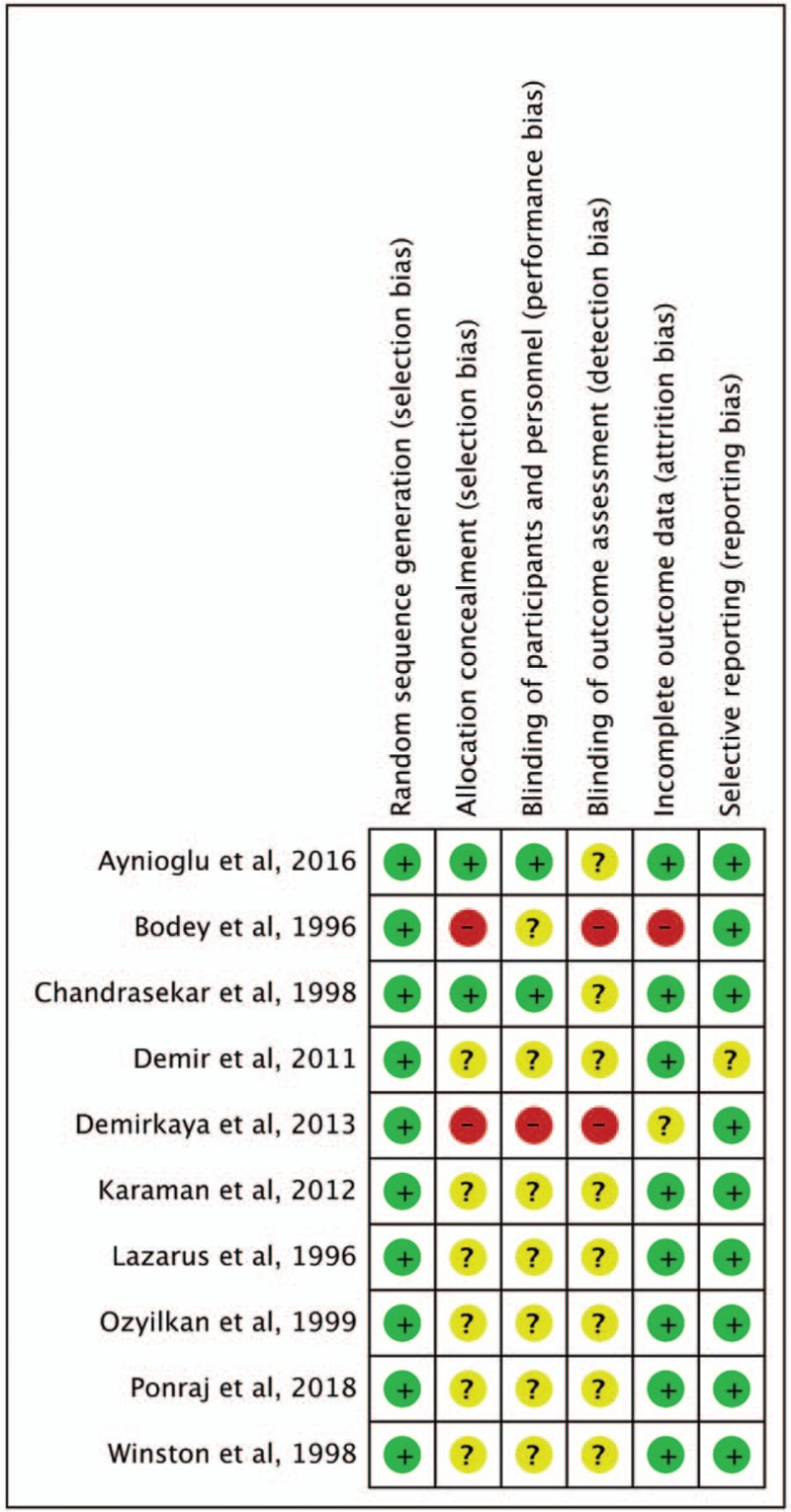
Risk of bias per study and domain of each RCT.
3.1. Study characteristics and study quality
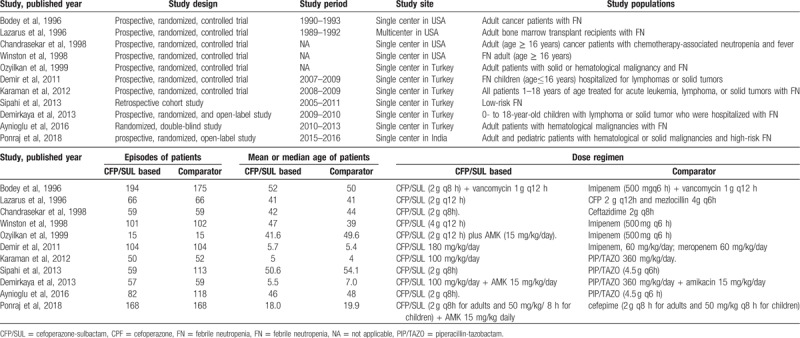
Ten prospective RCTs[8–15,17,19] and 1 retrospective cohort study[16] published between 1996 and 2018 met the inclusion criteria (Table 1). Except for 1 multicenter study,[13] all were conducted in a single center.[8,12,14–17,19] Six studies[8,10–12,14,16] were conducted in Turkey, 4 in the United States,[9,13,17,19] and 1 in India[15]; 3 focused on children,[10–12] and the other 8 involved mainly adults.[8,9,13–17,19] One study[13] focused on bone marrow transplant recipients; the other 10 involved patients with either solid or hematologic cancer.[8,12,14–17,19] Four studies[8,11,12,16] used piperacillin-tazobactam as the comparator, and 4 used carbapenems. One study each used cefepime,[15] cefoperazone plus mezlocillin,[13] and ceftazidime[19] as the comparator.
Table 1.
Characteristics of included studies.
3.2. Treatment success without modification
Treatment success without modification was reported in all 11 studies,[8–17,19] which together comprise 2054 patients. Among 983 patients receiving cefoperazone-sulbactam, 565 (57.9%) achieved treatment success. Among 1071 patients receiving comparators, the treatment success rate was 56.9% (n = 609). Cefoperazone-sulbactam had a treatment success rate similar to the comparators in empiric treatment of febrile neutropenia (OR, 1.03; 95% CI, 0.85 to 1.24, I2 = 0%, Fig. 3). In the pooled analysis of the 10 RCTs, no significant difference was found between cefoperazone-sulbactam and comparators (OR, 1.07; 95% CI, 0.88 to 1.30, I2 = 0%). The similarity between cefoperazone-sulbactam and comparators remained unchanged in the sensitivity test after individual studies were randomly excluded. No significant publication bias was found, according to a funnel plot (Fig. 4).
Figure 3.
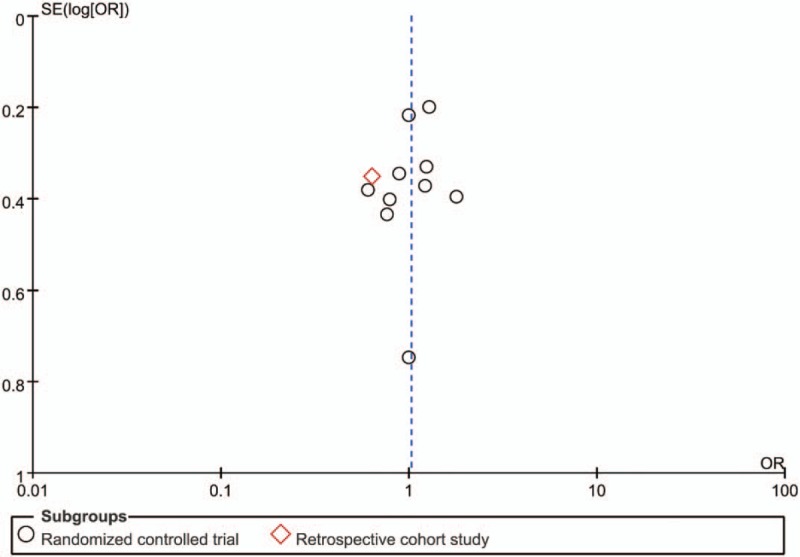
Funnel plot of overall clinical cure rates of cefoperazone-sulbactam and comparators in empiric treatment of febrile neutropenia.
Figure 4.
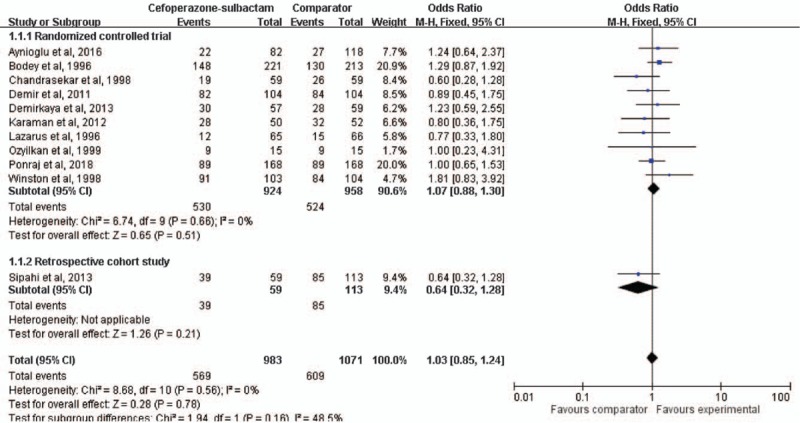
Forest plot for clinical cure rates of cefoperazone-sulbactam and comparators in empiric treatment of febrile neutropenia.
In the subgroup analysis by comparator, cefoperazone-sulbactam had a treatment success rate similar to those of piperacillin-tazobactam (OR, 0.95; 95% CI, 0.67 to 1.36, I2 = 0%) and carbapenems (OR, 1.25; 95% CI, 0.92 to 1.69, I2 = 0%). The pooled analysis of 7 studies[8,9,13,14,16,17,19] involving only adult patients revealed that cefoperazone-sulbactam had a treatment success rate therein similar to that of comparators (OR, 1.10; 95% CI, 0.88 to 1.38, I2 = 0%). The pooled analysis of 3 studies[10–12] involving only children also revealed a treatment success rate similar to that of comparators (OR, 0.96; 95% CI, 0.63 to 1.46, I2 = 0%). Moreover, this trend persisted despite changes in cefoperazone dosage (≥6 g/day, OR, 1.05; 95% CI, 0.79 to 1.39, I2 = 55.4%; 4 g/day, OR, 0.82; 95% CI, 0.39 to 1.72, I2 = 0%).
3.3. All-cause mortality
All-cause mortality was reported in 6 studies[8,10–12,15,16]; the mortality rate was 6.0% (31/520) and 6.5% (40/614) in patients receiving cefoperazone-sulbactam and those receiving comparators, respectively. No significant difference between cefoperazone-sulbactam and comparators in mortality was found through pooled analysis (OR, 0.96; 95% CI, 0.58 to 1.58, I2 = 0%, Fig. 5).
Figure 5.
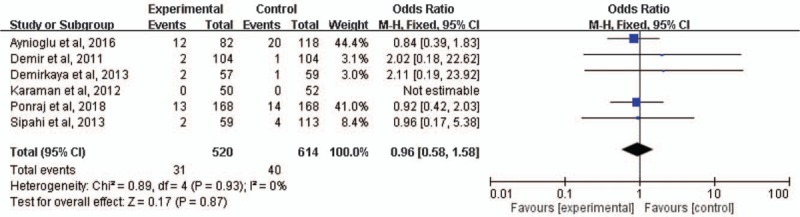
Forest plot of all-cause mortality rates of cefoperazone-sulbactam and comparators in treatment of febrile neutropenia.
3.4. Adverse events
Among patients using cefoperazone-sulbactam, rash (10.1%, 71/703) was the most common AE, followed by nausea/vomiting (4.4%, 18/410). The risks of these 2 AEs were similar in the cefoperazone-sulbactam and comparator groups (rash, OR, 1.05; 95% CI, 0.71 to 1.53, I2 = 0%, nausea/vomiting, OR, 0.32; 95% CI, 0.03 to 3.74, I2 = 80%). In addition, pooled analysis revealed no significant difference in superinfection between the cefoperazone-sulbactam and comparator groups (OR, 0.73; 95% CI, 0.46 to 1.16, I2 = 0%). Prolongation of prothrombin time occurred in 10% (10/101) of patients receiving cefoperazone-sulbactam in one study[17]; however, no hemorrhage related to the study drug was observed.
4. Discussion
This meta-analysis of 11 clinical studies[8–17,19] determined that cefoperazone-sulbactam has a clinical efficacy similar to those of comparators in empiric treatment of febrile neutropenia. First, the success rate of cefoperazone in treating febrile neutropenia was similar to those of comparators in the pooled population of all 11 studies.[8–17,19] The similar clinical efficacy persisted in the analysis of only the 10 RCTs[8–15,17,19] and subsequent sensitivity test. Second, comparing cefoperazone-sulbactam with 2 antimicrobial agents, piperacillin-tazobactam and carbapenems, commonly recommended for the treatment of febrile neutropenia in subgroup analysis revealed no significant differences in the clinical efficacy. Third, the treatment success rate of cefoperazone-sulbactam was similar to those of comparators in the pooled analyses of both pediatric and adult populations. Finally, the pooled all-cause mortality was only 6.0% among patients receiving cefoperazone-sulbactam, similar to that among patients receiving comparators. Overall, the findings suggest that cefoperazone-sulbactam can be as effective for the treatment of patients with febrile neutropenia as other available antibiotics.
In addition to the clinical response, AEs during antibiotic treatment are a concern in the management of patients with febrile neutropenia. The most common AEs among patients receiving cefoperazone-sulbactam in this meta-analysis were rash and nausea/vomiting. The pooled risks of rash, nausea/vomiting, and superinfection were similar for cefoperazone-sulbactam and comparators. Another side effect of the study drug is the inhibition of vitamin K metabolism; such inhibition can induce abnormal coagulation and hemorrhage.[20,21] In this meta-analysis, only Winston et al[17] reported data relevant to this AE, reporting that the incidence of prolonged prothrombin time was 10%. However, no significant hemorrhage related to cefoperazone-sulbactam was noted in this report.[17] These findings suggest that cefoperazone is as safe as its comparators in the treatment of febrile neutropenia.
However, this meta-analysis has several limitations. First, we did not evaluate the efficacy of cefoperazone-sulbactam by sex, age, or underlying conditions, such as the type of cancer (eg., solid or hematologic) or risk of febrile neutropenia. Second, we did not assess the specific association between the in vitro activity and in vivo response of different microorganisms, particularly antibiotic-resistant ones, among patients with febrile neutropenia and documented microbial infection. Third, the numbers of studies and patients were low in this meta-analysis; therefore, a large-scale study is warranted to confirm our findings.
The findings of 11 clinical trials indicate that the efficacy and tolerability of cefoperazone-sulbactam are as high as those of its comparators for empiric treatment of patients with febrile neutropenia.
Author contributions
Conceptualization: Shao-Huan Lan, Chih-Cheng Lai, Hung-Jen Tang.
Data curation: Shao-Huan Lan, Shen-Peng Chang, Li-Chin Lu.
Formal analysis: Shao-Huan Lan, Shen-Peng Chang, Li-Chin Lu, Hung-Jen Tang.
Investigation: Hung-Jen Tang.
Writing – original draft: Chih-Cheng Lai.
Writing – review & editing: Hung-Jen Tang.
Supplementary Material
Footnotes
Abbreviations: AE = adverse event, CI = confidence interval, MRSA = methicillin-resistant Staphylococcus aureus, OR = odds ratio, RCT = randomized controlled trial.
How to cite this article: Lan SH, Chang SP, Lai CC, Lu LC, Tang HJ. Efficacy and safety of cefoperazone-sulbactam in empiric therapy for febrile neutropenia: A systemic review and meta-analysis. Medicine. 2020;99:8(e19321).
The authors report no conflicts of nterest.
Supplemental Digital Content is available for this article.
References
- [1].Patel K, West HJ. Febrile Neutropenia. JAMA Oncol 2017;3(12):1751. [DOI] [PubMed] [Google Scholar]
- [2].Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 2016;27: suppl 5: v111–8. [DOI] [PubMed] [Google Scholar]
- [3].Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 2011;52(4):e56–93. [DOI] [PubMed] [Google Scholar]
- [4].Masaoka T. Evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clin Infect Dis 2004;39: Suppl 1: S49–52. [DOI] [PubMed] [Google Scholar]
- [5].Chang PC, Chen CC, Lu YC, Lai CC, Huang HL, Chuang YC, et al. The impact of inoculum size on the activity of cefoperazone-sulbactam against multidrug resistant organisms. J Microbiol Immunol Infect 2018;51(2):207–13. [DOI] [PubMed] [Google Scholar]
- [6].Lai CC, Chen CC, Lu YC, Chuang YC, Tang HJ. In vitro activity of cefoperazone and cefoperazone-sulbactam against carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Infect Drug Resist 2019;12:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lai CC, Chen CC, Lu YC, Lin TP, Chuang YC, Tang HJ. Appropriate composites of cefoperazone-sulbactam against multidrug-resistant organisms. Infect Drug Resist 2018;11:1441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aynioglu A, Mutlu B, Hacihanefioglu A. A comparison of the efficacy of piperacillin-tazobactam and cefoperazone-sulbactam therapies in the empirical treatment of patients with febrile neutropenia. Rev Esp Quimioter 2016;29(2):69–75. [PubMed] [Google Scholar]
- [9].Bodey G, Abi-Said D, Rolston K, Raad I, Whimbey E. Imipenem or cefoperazone-sulbactam combined with vancomycin for therapy of presumed or proven infection in neutropenic cancer patients. Eur J Clin Microbiol Infect Dis 1996;15(8):625–34. [DOI] [PubMed] [Google Scholar]
- [10].Demir HA, Kutluk T, Ceyhan M, Yagci-Kupeli B, Akyuz C, Cengiz B, et al. Comparison of sulbactam-cefoperazone with carbapenems as empirical monotherapy for febrile neutropenic children with lymphoma and solid tumors. Pediatr Hematol Oncol 2011;28(4):299–310. [DOI] [PubMed] [Google Scholar]
- [11].Demirkaya M, Celebi S, Sevinir B, Hacimustafaoglu M. Randomized comparison of piperacillin-tazobactam plus amikacin versus cefoperazone-sulbactam plus amikacin for management of febrile neutropenia in children with lymphoma and solid tumors. Pediatr Hematol Oncol 2013;30(2):141–8. [DOI] [PubMed] [Google Scholar]
- [12].Karaman S, Vural S, Yildirmak Y, Emecen M, Erdem E, Kebudi R. Comparison of piperacillin tazobactam and cefoperazone sulbactam monotherapy in treatment of febrile neutropenia. Pediatr Blood Cancer 2012;58(4):579–83. [DOI] [PubMed] [Google Scholar]
- [13].Lazarus HM, Creger RJ, Gucalp R, Fox RM, Ciobanu N, Carlisle PS, et al. Cefoperazone/sulbactam versus cefoperazone plus mezlocillin: empiric therapy for febrile, neutropenic bone marrow transplant patients. Int J Antimicrob Agents 1996;7(2):85–91. [DOI] [PubMed] [Google Scholar]
- [14].Ozyilkan O, Yalcintas U, Baskan S. Imipenem-cilastatin versus sulbactam-cefoperazone plus amikacin in the initial treatment of febrile neutropenic cancer patients. Korean J Intern Med 1999;14(2):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ponraj M, Dubashi B, Harish BH, Kayal S, Cyriac SL, Pattnaik J, et al. Cefepime vs. cefoperazone/sulbactam in combination with amikacin as empirical antibiotic therapy in febrile neutropenia. Support Care Cancer 2018;26(11):3899–908. [DOI] [PubMed] [Google Scholar]
- [16].Sipahi OR, Arda B, Nazli-Zeka A, Pullukcu H, Tasbakan M, Yamazhan T, et al. Piperacillin/tazobactam vs. cefoperazone/sulbactam in adult low-risk febrile neutropenia cases. Int J Clin Pract 2014;68(2):230–5. [DOI] [PubMed] [Google Scholar]
- [17].Winston DJ, Bartoni K, Bruckner DA, Schiller GJ, Territo MC. Randomized comparison of sulbactam/cefoperazone with imipenem as empirical monotherapy for febrile granulocytopenic patients. Clin Infect Dis 1998;26(3):576–83. [DOI] [PubMed] [Google Scholar]
- [18].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- [19].Chandrasekar PH. Safety and efficacy of cefoperazone plus sulbactam versus ceftazidime in the empiric teatment of febrile neutropenia. J Pharm Technol 1998;14(2):63–9. [Google Scholar]
- [20].Alagozlu H, Cindoruk M, Unal S. Severe INR elevation in a patient with choledocholithiasis receiving cefoperazone. Clin Drug Investig 2006;26(8):481–4. [DOI] [PubMed] [Google Scholar]
- [21].Cai Z, Yang W, He Y, Chen Q, Wang S, Luo X, et al. Cefoperazone/sulbactam-induced abdominal wall hematoma and upper gastrointestinal bleeding: a case report and review of the literature. Drug Saf Case Rep 2016;3(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


