Supplemental Digital Content is available in the text
Keywords: 25-hydroxy vitamin D, 2-hour oral glucose tolerance test, fasting plasma glucose, hemoglobin A1c, meta-analysis, prediabetes, vitamin D
Abstract
Background:
Previous studies showed conflicting results for associations between vitamin D and prediabetes. The study aimed to make a systematic review and meta-analysis for the association between vitamin D and prediabetes.
Methods:
We searched for articles identifying associations between vitamin D and prediabetes published in English until July 2019 in following databases (PubMed, Web of Science, EMBASE, Medline, Google Scholar, and Cochrane databases). Finally, we conducted these analyses (heterogeneities examination, meta-regression analyses, sensitivity analysis, and publication bias examination) using STATA 12.0 software (Stata Corporation, College Station, TX, USA). Q test and I2 were applied to examine heterogeneities between studies.
Results:
Twelve studies were finally included in the present study. The study included 4 studies to explore the association between serum levels of 25-hydroxy (OH) vitamin D and risks of prediabetes (including 3094 participants). Additionally, the present study included 8 studies (including 865 individuals with prediabetes treated with vitamin D supplementation and 715 patients treated with placebo) to assess differences in therapeutic effects between individuals with prediabetes treated with vitamin D supplementation and those treated with placebo. The present study showed no significant associations between low serum levels of 25(OH) vitamin D and high risk of prediabetes. Additionally, the study showed no significant differences in changes of hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), and homeostatic model assessment of insulin resistance (HOMA-IR) between individuals with prediabetes treated with vitamin D and those patients given placebo, whereas meta-analysis showed significantly greater changes in 2-hour oral glucose tolerance test (2HPG) in individuals with prediabetes treated with vitamin D, compared with individuals with prediabetes treated with placebo.
Conclusion:
The study supported that low serum levels of 25(OH) vitamin D increased the risk of prediabetes. In addition, vitamin D supplementation improves impaired glucose tolerance in prediabetes. However, more large-scale clinical trials are essential to explore the association between vitamin D and prediabetes.
1. Introductions
Diabetes affects >400 million subjects worldwide.[1] Prediabetes was defined as participants who did not meet the criteria of diabetes but who had impaired fasting glucose or impaired glucose tolerance.[2] It is estimated that 374 million adults had prediabetes worldwide in 2017 (prevalence 7.7%) by the International Diabetes Federation (IDF).[3] Populations with prediabetes are at a 50% higher risk of developing type 2 diabetes.[4] Diabetes mellitus is associated with several vascular and nonvascular complications such as stroke, heart disease, and nerve damages.[5] Thus, it is essential to prevent the progression of prediabetes to diabetes.
Some meta-analyses indicated significant associations between vitamin D and diabetes. A recent meta-analysis indicated that hypovitaminosis D is associated with an elevated risk of future diabetes in old adults.[6] Additionally, a meta-analysis showed a reduction of hemoglobin A1c (HbA1c) after vitamin D treatment in adults with type 2 diabetes.[7] However, the studies showed conflicting results regarding the associations between vitamin D and prediabetes. Some studies indicated that individuals with prediabetes showed lower serum 25-hydroxy (OH) vitamin D concentrations compared with those with normal controls.[8,9] Additionally, individuals with prediabetes who are vitamin D deficient showed greater risk of developing diabetes.[10] Moreover, some randomized controlled trials (RCTs) explored the clinical benefit of vitamin D treatment in patients with prediabetes.[11,12] However, Sollid et al[12] reported that vitamin D supplementation does not improve glycemic indices in prediabetes patients. A systematic review and meta-analysis is essential to summary these inconsistent results. The present study aimed to make a systematic review and meta-analysis for the association between vitamin D and prediabetes.
2. Methods
We performed a meta-analysis according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guideline[13] to explore the associations between vitamin D and prediabetes. The study is a meta-analysis, an analysis with secondary processing. Thus, ethical approval was not necessary in the study.
2.1. Search strategy and selection criteria
We searched for articles published in English until July 2019 in following databases (PubMed, Web of Science, EMBASE, Medline, Google Scholar, and Cochrane databases). We used following search terms: (“vitamin D” OR “vitamin D3” OR “cholecalciferol” OR “25(OH)D”) AND (“prediabetes” OR “insulin resistance” OR “hyperglycemia” OR “HbA1c” OR “glucose”). One thousand five hundred twelve studies were included after eliminating duplicates. Included studies explored the associations between vitamin D and prediabetes. Prediabetes was diagnosed by showing HbA1c measured in the range of 5.8% to 6.4%. For the association between serum levels of vitamin D and risks of prediabetes, we included cohort and case–control studies, reporting relative risk (RR) or odds ratio (OR) and 95% confidence intervals (CI) related to vitamin D and risks of prediabetes. Additionally, to explore the therapeutic effects of vitamin D supplementation on prediabetes, we included RCTs comparing outcomes of individuals with prediabetes treated with vitamin D supplementation and placebo. Moreover, we excluded secondary processing articles (meta-analyses and reviews) and case studies. All the abstracts and full texts were read independently by 2 researchers (LY and YZ). When the inconsistencies in the study selection appeared, the articles were discussed and decided by the 3 authors (LY, YZ, and SS).
2.2. Data extraction
Two reviewers (LY and YZ) independently used an Excel file to abstract the following data: author, publication year, study type, study location, numbers of participants, information of included participants (age and sex), mean serum levels of 25(OH) vitamin D, follow up time, endpoints, comparison, the ORs or RRs, and 95% CIs after multivariate adjustions, adjusted variables, serum levels of 25(OH) vitamin D at baseline, dose of vitamin D supplementation, duration of trial, outcomes of RCTs.
2.3. Meta-analysis
In the study, we used Q test and I2 to examine heterogeneities between included studies. Random effects models were performed as pooling methods with invariably high heterogeneity (P value for Q test ≤.05 and I2 ≥ 50%); otherwise, fixed effects models were conducted as pooling methods with invariably low heterogeneity (P value for Q test >.05 and I2 < 50%). Outcomes with an I2 of 25% to 50% were considered to have low heterogeneity, an I2 of 50% to 75% was considered as moderate heterogeneity, and an I2 of >75% demonstrated high heterogeneity.[14] To explore source of the heterogeneity, we performed meta-regression analyses. Additionally, sensitivity analysis was conducted to evaluate the stabilization of the study. Publication bias was evaluated with Begg test, Egger test, and funnel plot. We conducted these analyses using STATA 12.0 software (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Study selection and characteristics
Figure 1 illustrated the search results and selection process. After eliminating duplicates, 1512 studies were remained. After screening according to titles and abstracts, 30 articles retained for further assessment. We excluded 18 articles because of the following reasons: studies which not included control group or between-group comparisons were excluded (n = 12); Studies with insufficient information were excluded (n = 6). The characteristics of 12 finally included studies were showed in supplementary tables 1 and 2. To explore the association between serum levels of 25(OH) vitamin D and risks of prediabetes, the present study included 1 cohort study (including 490 participants),[15] 3 case–control study (including 2604 participants).[8,16,17] Additionally, the present study included 8 studies (including 865 individuals with prediabetes treated with vitamin D supplementation and 715 patients treated with placebo) to assess differences in therapeutic effects between individuals with prediabetes treated with vitamin D supplementation and those treated with placebo.
Figure 1.
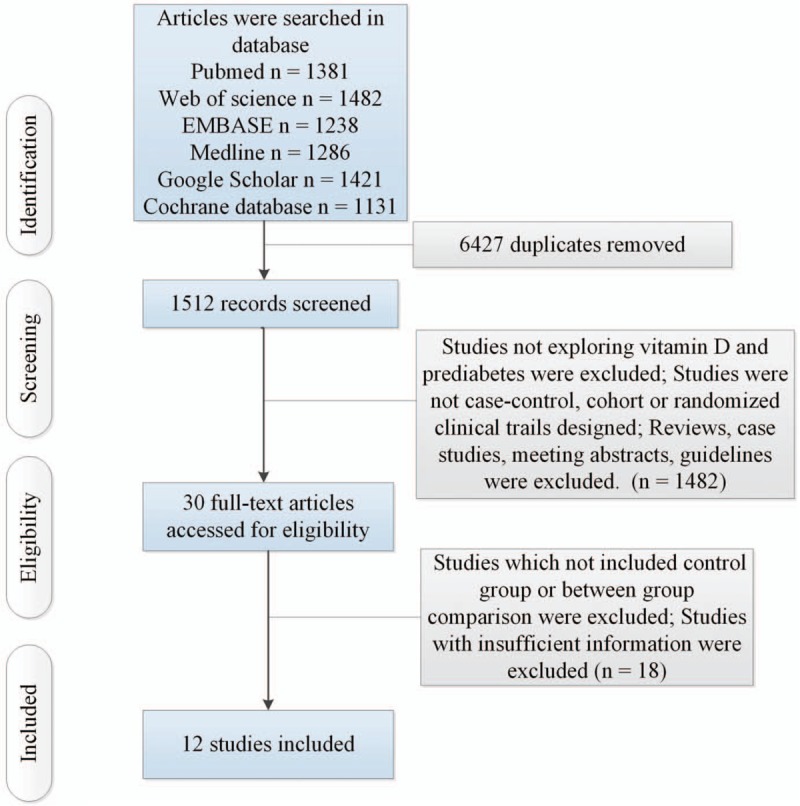
Flow of information through the different phases of a systematic review.
3.2. Results of meta-analysis
The present study showed that the low serum levels of 25(OH) vitamin D is significantly associated with high risk of prediabetes (OR/RR = 1.54, 95% CI 1.26–1.89, I2 = 48.9%, P = .118, Fig. 2). In addition, the study showed no significant differences in changes of HbA1c, fasting plasma glucose (FPG) and homeostatic model assessment of insulin resistance (HOMA-IR) between individuals with prediabetes treated with vitamin D and those patients given placebo (HbA1c: standardized mean difference [SMD] = 0.01, 95% CI –1.08 to 1.10, I2 = 98.8%, P < .001, Fig. 3; FPG: SMD = 1.23, 95% CI –1.05 to 3.51, I2 = 99.2%, P < .001, Fig. 4; HOMA-IR: SMD = 0.81, 95% CI –2.92 to 4.55, I2 = 99.6%, P < .001, Fig. 5), whereas meta-analysis showed significantly greater changes in plasma glucose after 2-hour oral glucose tolerance test (2HPG) in individuals with prediabetes treated with vitamin D, compared with individuals with prediabetes treated with placebo (SMD = 1.80, 95% CI 0.29–3.31, I2 = 98.6%, P < .001, Fig. 6). Meta-regression analysis showed that ages, serum levels of 25(OH) vitamin D at baseline, doses of vitamin D and follow-up durations were not responsible for heterogeneity across studies, serum levels of 25(OH) vitamin D at baseline, doses of vitamin D, and follow-up durations (all P > .05, supplementary table 3). Sensitivity analysis showed no changes in the direction of effect when any one study was excluded in the meta-analyses (Supplementary figure 1). In addition, Begg test, Egger tests, and funnel plots indicated no significant risks of publication bias for these meta-analyses (supplementary table 4 and supplementary figure 2).
Figure 2.
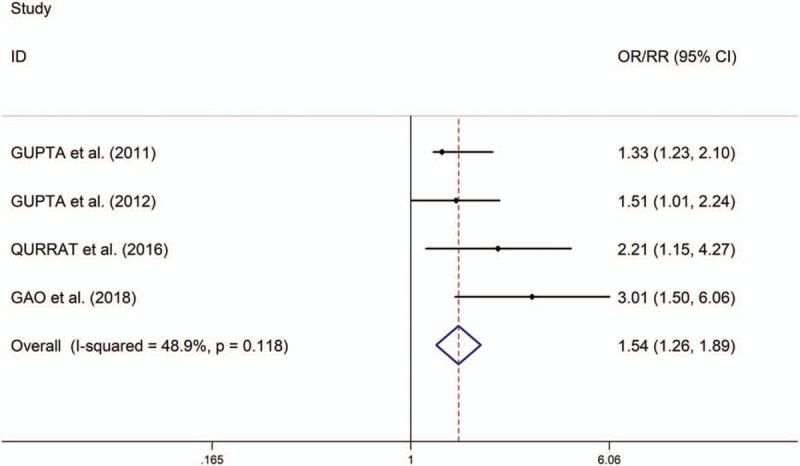
Forest plots of the association between serum levels of 25-hydroxy (OH) vitamin D and risks of prediabetes.
Figure 3.
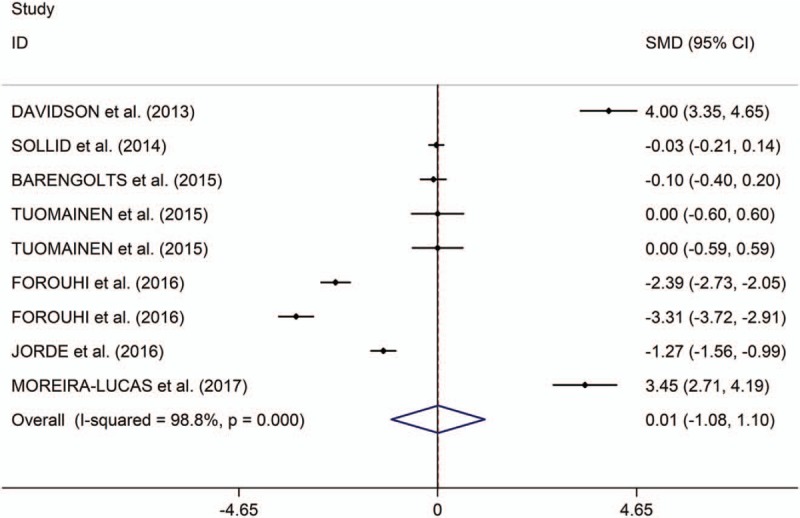
Forest plots of changes of HbA1c comparing groups (individuals with prediabetes given vitamin D and those given placebo). HbA1c indicates hemoglobin A1c.
Figure 4.
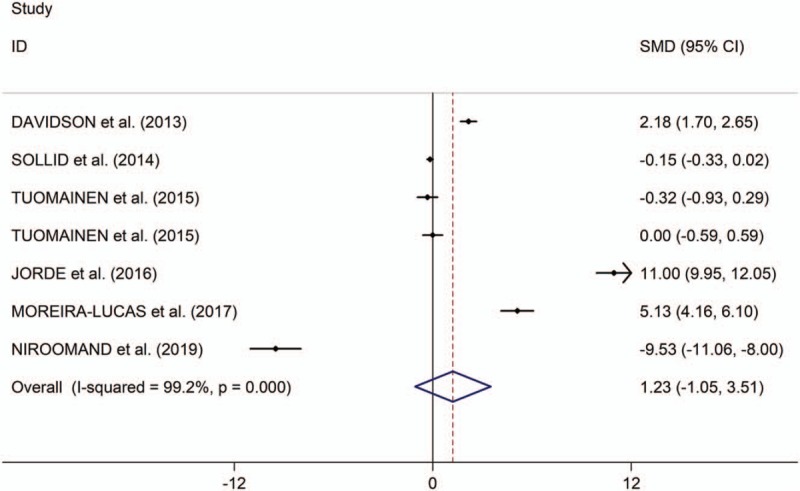
Forest plots of changes of FPG comparing groups (individuals with prediabetes given vitamin D and those given placebo). FPG indicates fasting plasma glucose.
Figure 5.
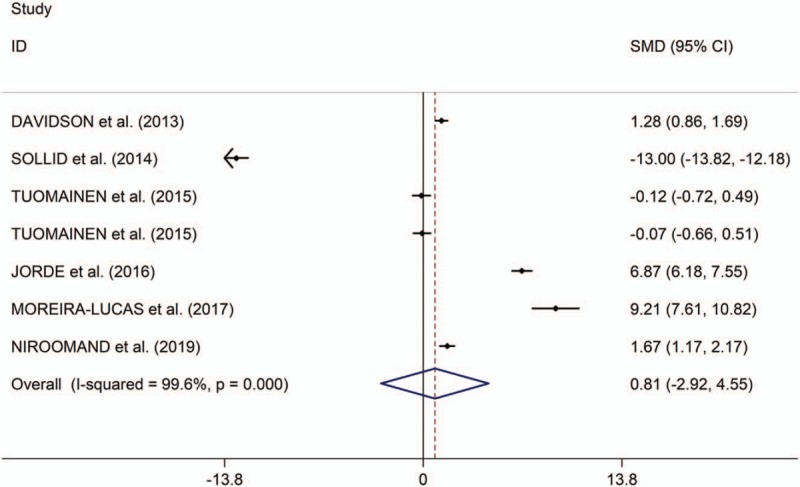
Forest plots of changes of HOMA-IR comparing groups (individuals with prediabetes given vitamin D and those given placebo). HOMA-IR indicates homeostatic model assessment of insulin resistance.
Figure 6.
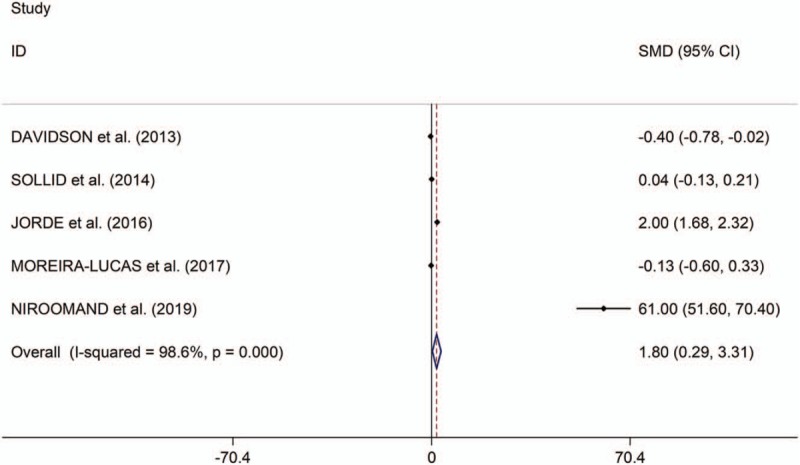
Forest plots of changes of 2HPG comparing groups (individuals with prediabetes given vitamin D and those given placebo). 2HPG indicates 2-hour oral glucose tolerance test.
4. Discussion
The present study showed significant associations between low serum levels of 25(OH) vitamin D and high risk of prediabetes. Additionally, the study showed no significant differences in changes of HbA1c, FPG, and HOMA-IR between individuals with prediabetes treated with vitamin D and those patients given placebo, whereas meta-analysis showed significantly greater changes in 2HPG in individuals with prediabetes treated with vitamin D, compared with individuals with prediabetes treated with placebo.
Some studies showed that the prevalence of metabolic syndrome, central obesity, hyperglycemia, and hypertension were higher in the vitamin D deficient group.[18] However, the effect of vitamin D supplementation against the progression of diabetes has been reported in several trials with mixed results.[19,20] Our study showed significant associations between low serum levels of 25(OH) vitamin D and high risk of prediabetes.
Additionally, the study showed no significant differences in changes of HbA1c, FPG, and HOMA-IR between individuals with prediabetes treated with vitamin D and those patients given placebo, whereas meta-analysis showed significantly greater changes in 2HPG in individuals with prediabetes treated with vitamin D, compared with individuals with prediabetes treated with placebo. Vitamin D supplementation improved glucose metabolism, and glycemic control (2HPG), but failed to improve insulin sensitivity (HOMA-IR) in prediabetes. A recent meta-analysis showed that vitamin D supplementation improved glycemic measures and insulin sensitivity and might be useful as part of a preventive strategy for type 2 diabetes.[21] However, the recent meta-analysis[21] included adults at risk for type 2 diabetes, including prediabetes, overweight, or obesity. Additionally, unlike several studies including known diabetes, our study was performed on the patients with prediabetes. The greatest benefits were found in populations most at risk for early disease.
Our meta-regression analysis showed that ages, serum levels of 25(OH) vitamin D at baseline, doses of vitamin D, and follow-up durations were not responsible for heterogeneity across studies. It is important to note that vitamin D supplementation may play a role in the prevention of type 2 diabetes in high-risk populations, however, type 2 diabetes is a multifactorial disease. Larger studies are needed to further evaluate the glycemic effects of vitamin D treatment especially in patients with vitamin D deficiency.
Some limitations were showed in the present study. Firstly, regarding the associations between serum levels of 25(OH) vitamin D and risks of prediabetes, there were a limited number of studies, potentially limiting statistical power. More large-scale studies might be performed to explore the associations between serum levels of 25(OH) vitamin D and risks of prediabetes. Secondly, regarding the clinical benefit of vitamin D treatment in patients with prediabetes, the amount of included studies was limited to explore the sources of heterogeneities.
5. Conclusions
In conclusion, the study supported that low serum levels of 25(OH) vitamin D increased the risk of prediabetes. In addition, vitamin D supplementation improves impaired glucose tolerance in prediabetes. However, more large-scale clinical trials are essential to explore the association between vitamin D and prediabetes (Supplementary references).
Author contributions
Data curation: Lu Yu, Yu Zhai.
Funding acquisition: Shanmei Shen.
Investigation: Lu Yu, Yu Zhai.
Methodology: Yu Zhai.
Supervision: Shanmei Shen.
Writing – original draft: Lu Yu.
Writing – review & editing: Shanmei Shen.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 25(OH) vitamin D = 25-hydroxy (OH) vitamin D, 2HPG = 2-hour oral glucose tolerance test, CI = confidence intervals, FPG = fasting plasma glucose, HbA1c = hemoglobin A1c, HOMA-IR = homeostatic model assessment of insulin resistance, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analysis, RCTs = randomized controlled trials, RR = relative risk, SMD = standardized mean difference.
How to cite this article: Yu L, Zhai Y, Shen S. Association between vitamin D and prediabetes: A PRISMA-compliant meta-analysis. Medicine. 2020;99:8(e19034).
LY and YZ have contributed equally to the manuscript.
Funding: No funding.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ogurtsova K, Da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- [2].Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37: suppl: S81–90. [DOI] [PubMed] [Google Scholar]
- [3].Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- [4].Standards of medical care in diabetes--2013. Diabetes Care 2013;36: suppl: S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mancini GB, Cheng AY, Connelly K, et al. Diabetes for cardiologists: practical issues in diagnosis and management. Can J Cardiol 2017;33:366–77. [DOI] [PubMed] [Google Scholar]
- [6].Lucato P, Solmi M, Maggi S, et al. Low vitamin D levels increase the risk of type 2 diabetes in older adults: a systematic review and meta-analysis. Maturitas 2017;100:8–15. [DOI] [PubMed] [Google Scholar]
- [7].Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complications 2017;31:1115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta AK, Brashear MM, Johnson WD. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care 2011;34:658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care 2011;34:1114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Forouhi NG, Menon RK, Sharp SJ, et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab 2016;18:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davidson MB, Duran P, Lee ML, et al. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013;36:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sollid ST, Hutchinson MY, Fuskevag OM, et al. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 2014;37:2123–31. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao Y, Zheng T, Ran X. Vitamin D and incidence of prediabetes or type 2 diabetes: a four-year follow-Up community-based study. Dis Markers 2018;2018:1926308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gupta AK, Brashear MM, Johnson WD. Low vitamin D levels, prediabetes and prehypertension in healthy African American adults. Nutr Metab Cardiovasc Dis 2012;22:877–82. [DOI] [PubMed] [Google Scholar]
- [17].Qurrat Ul A, Khan DA, Ijaz A, et al. Decreased serum 25-Hydroxycalciferol levels in pre-diabetic adults. J Coll Physicians Surg Pak 2016;26:87–90. [PubMed] [Google Scholar]
- [18].Tian LQ, Shi WQ, Zhou Y, et al. The Association of serum vitamin D deficiency and metabolic risk factors in Chinese adults with prediabetes: a cross-sectional study. J Nutr Sci Vitaminol (Tokyo) 2019;65:211–8. [DOI] [PubMed] [Google Scholar]
- [19].Tsur A, Feldman BS, Feldhammer I, et al. Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care 2013;36:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Angellotti E, Pittas AG. The role of Vitamin D in the prevention of Type 2 diabetes: to D or not to D? Endocrinology 2017;158:2013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mirhosseini N, Vatanparast H, Mazidi M, et al. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc 2018;2:687–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


