Abstract
Background:
Traditional Chinese Medicine (TCM) has gradually drawn the attention of clinicians as an alternative choice for insomniacs and TCM Yangxin Anshen Therapy (TYAT) is a crucial therapy of treating insomniacs. The purpose of this study was to evaluate the efficacy and safety of TYAT for insomnia.
Methods:
Seven electronic databases were searched from inception to July 2019. Two authors independently identified Randomized Controlled Trials (RCTs), extracted data and assessed risk of bias by Cochrane risk bias assessment tool. Comprehensive meta-analysis was conducted with the Review Manager for eligible and appropriate studies.
Results:
Fourteen trials (1549 participants) were finally included in this study. The included studies were of moderate-to-high quality. Twelve trials reported the specific methods of random sequence generation, and 4 of them used the allocation concealment. Blinding of participants and personnel were used in 7 studies, and blinding of outcome assessment was performed in 3 studies. The main meta-analysis showed:
-
1.
TYAT was superior to placebo from the point view of PSG parameters, Pittsburgh Sleep Quality Index (PSQI) scale, TCM curative efficacy, and PSQI curative efficacy.
-
2.
TYAT was not inferior to benzodiazepines from the point view of PSG parameters, PSQI scale, TCM curative efficacy, and PSQI curative efficacy.
-
3.
In terms of PSQI scale and PSQI curative efficacy, there were no significant differences between TYAT and nonbenzodiazepine hypnotics in the treatment of insomnia.
-
4.
The clinical application of TYAT was relatively safe.
Conclusion:
TYAT is an effective alternative therapy for insomnia, and its clinical application appears safe. The conclusions of this paper have a certain reference value for further research and clinical practice.
Trial registration number:
PROSPERO CRD 42019135115.
Keywords: insomnia, meta-analysis, systematic review, Traditional Chinese Medicine, Yangxin Anshen Therapy
1. Introduction
Insomnia is a subjective experience characterized by difficulty falling asleep, impaired sleep maintenance, early awakening, decreased sleep quality, and reduced sleep time along with daytime dysfunction.[1,2] The proportion of people suffering from insomnia in China has reached as high as 15%, which is lower than that in Western countries, but similar to that in other Asian countries.[3–5] More noteworthy than that is insomnia has a bearing on increased risk of hypertension,[6,7] diabetes,[8–10] chronic kidney disease,[11,12] and dementia.[13] Besides, in terms of economic costs, insomnia also imposes a huge burden on social welfare.[14]
At present, clinical medications for insomniacs in China mainly include benzodiazepine drugs, non-benzodiazepine drugs, melatonin receptor agonists, orexin receptor antagonist, and the antidepressant drugs with hypnotic effects.[2] Among them, benzodiazepines are still one of the most commonly used drugs in clinical treatment of insomnia. Benzodiazepines are the most frequently medication for insomnia in the Korean.[15] Based on the analysis of data from 22 hospitals in Beijing, China, the results showed that benzodiazepine receptor agonists was the most widely used drug, accounting for 58.49% of prescriptions, of which benzodiazepine drugs accounted for 38.64% and nonbenzodiazepine drugs accounted for 19.85%.[16] However, adverse reactions of these drugs restrict their clinical application.[1,2,17] Take benzodiazepines as an example, the use of it increases the risk of Alzheimer's disease.[18]
TYAT (tranquilizing the mind by nourishing the heart therapy, named Yangxin Anshen in Chinese pinyin) is one of the crucial therapeutic principles for insomnia in TCM which contains a series of Chinese herbal prescriptions with the effect of nourishing the heart and tranquilizing the mind. In the past meta-analysis,[19,20] all Chinese herbal medicines have been regarded as the same treatment method, which is inconsistent with the practice of TCM diagnosis and treatment, resulting in a high degree of heterogeneity and conclusions with limited significance. The purpose of this article was to summarize and analyze the clinical effect of TYAT on insomnia by means of high-quality researches.
2. Methods
2.1. Protocol register
This protocol of systematic review and meta-analysis has already been registered on the PROSPERO platform (https://www.crd.york.ac.uk/PROSPERO/) with an assigned registration number CRD42019135115. The detailed protocol[21] has been published, so this article will not repeat the methods of collecting and analyzing data.
2.2. Ethics
In view of the fact that this is a secondary study based on published literature, no further ethical approval is required.
3. Results
3.1. Literature search and screening results
A total of 3457 articles were retrieved according to the search strategy and no additional articles were identified by manual search. Of these, 682 were retrieved by CBM, 710 by CNKI, 879 by VIP, 923 by Wanfang, 57 by PubMed, 115 by EMBASE, and 91 by CENTRAL. A total of 1505 duplicates were found. After that, 1665 literature were excluded through the title and abstract, including 50 animal experiments, 21 reviews, 284 combined with other diseases, 131 clinical experiences, 480 non-RCTs, 11 protocols, and 688 other therapies. For a further step, the authors screened the remaining 287 full-texts for identification, and 246 articles were removed with reasons: 2 protocols, 107 non-RCT, 13 non-fixed prescriptions, 91 utilizing other therapies, and 33 combined with other diseases. The resulting of 41 articles were then subjected to Cochrane risk of bias assessment, of which 27 studies were excluded because of a high risk of bias (less than 4 scores), and 14 studies[22–35] were included in the meta-analysis finally.
3.2. Quality evaluation of included studies
The Cochrane bias risk score of the included studies ranged from 4 to 6. The included articles were all RCTs, and all mentioned randomly grouping, of which 12 studies described the specific method of randomization, but the remaining 2[32,33] studies did not mention the explicit randomization method. Four studies[22,25,26,30] described specific methods of allocation concealment, such as central randomization, envelopes. Seven studies[22,25,26,30–32,34] used a double-blind protocol. Three studies[22,26,30] performed a blinded evaluation of study outcomes. Selective reporting of study results and other sources of bias were not identified in the included studies. The bias summary and the bias for each study show as below (Figs. 1 and 2).
Figure 1.
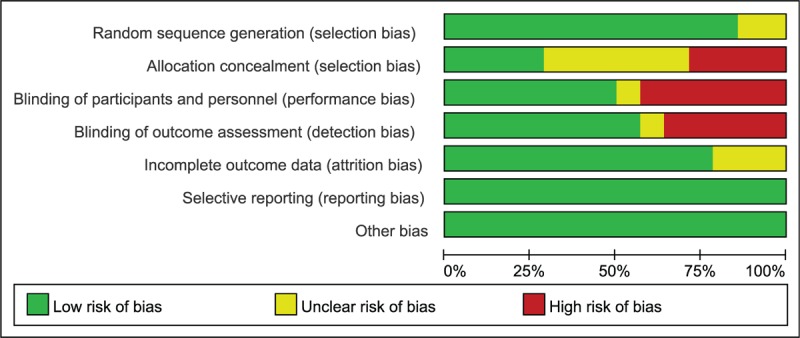
Risk of bias graph in included studies.
Figure 2.
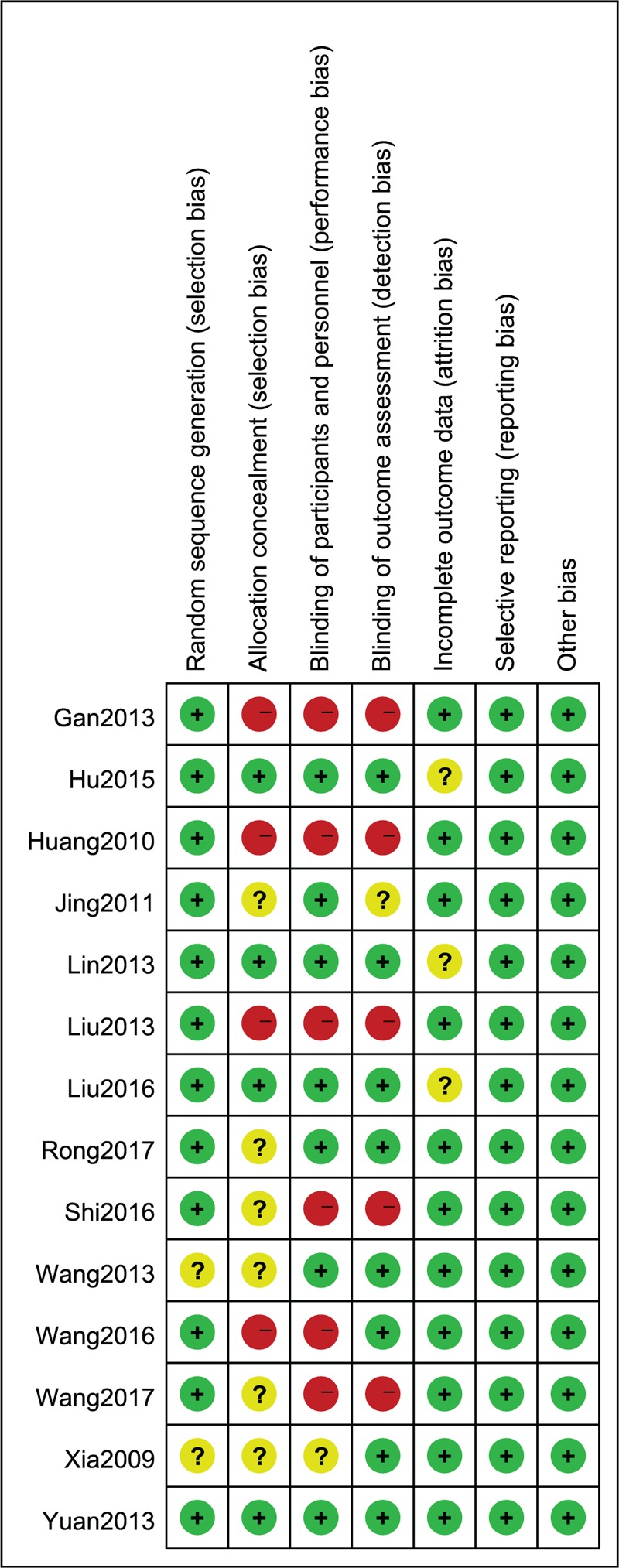
Risk of bias summary in included studies.
3.3. Literature characteristics
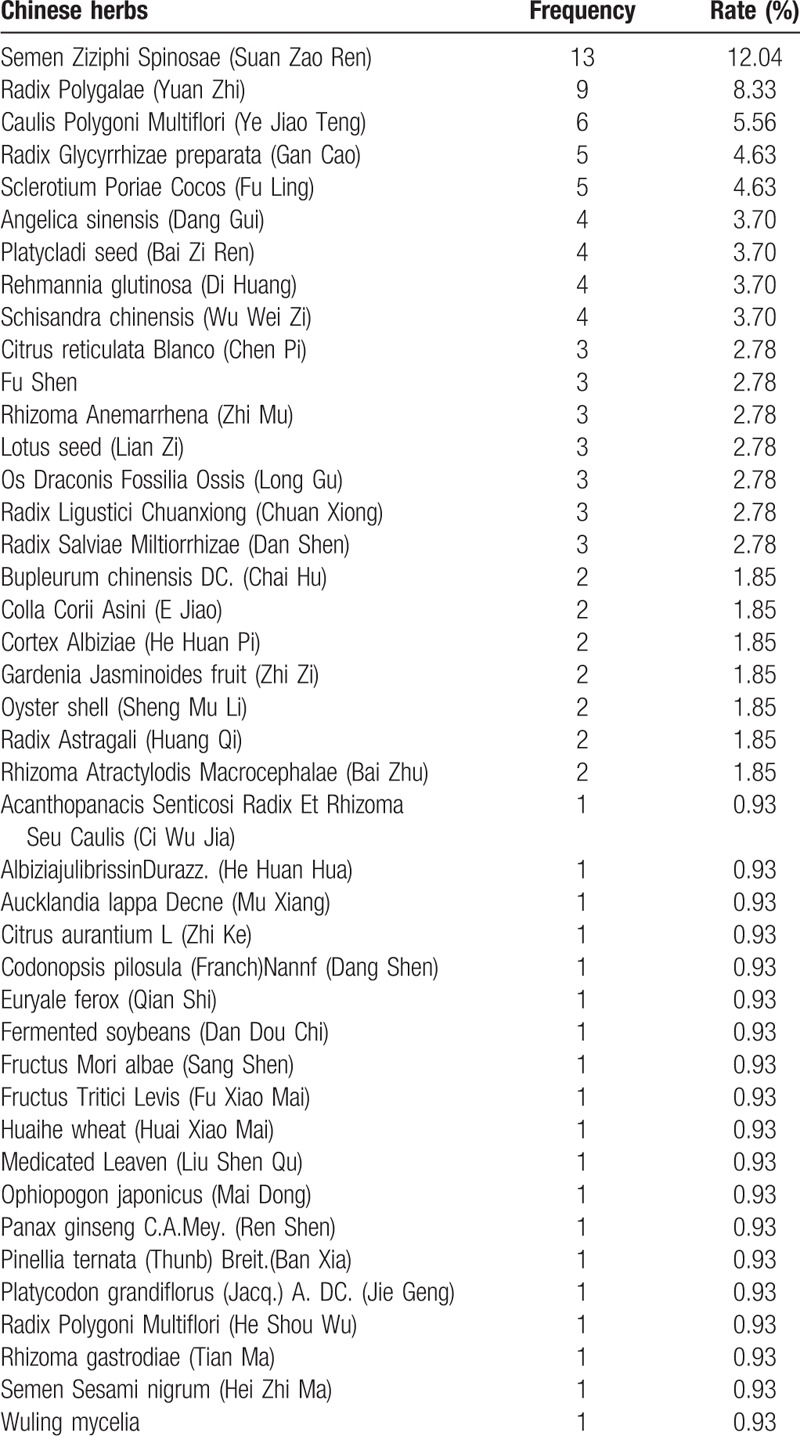
Table 1 presents characteristics table extracted from the 14 articles included. Three[26,30,33] of them were published in English journals, and the left 11 were published in Chinese. The 14 studies incorporated into the meta-analysis involved a total of 1549 patients accurately diagnosed with insomnia (778 in the experimental group and 771 in the control group). The sample of patients ranged from 47 to 198, and all studies were undertaken in China. The experimental group was given TYAT, while the control group was given the placebo, benzodiazepines, or non-benzodiazepine hypnotics. Treatment duration varied from 2 to 6 weeks. Four articles[22,28,30,31] reported the follow-up time, which was 7–14 days. The authors have listed the components of prescriptions used in each literature in Table 2 and briefly analyzed the frequency of each component (see Table 3).
Table 1.
Basic characteristic of the included studies.
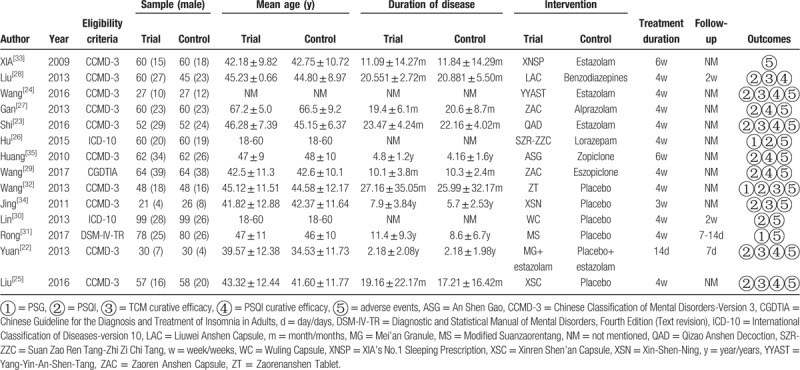
Table 2.
The ingredients of each prescription.
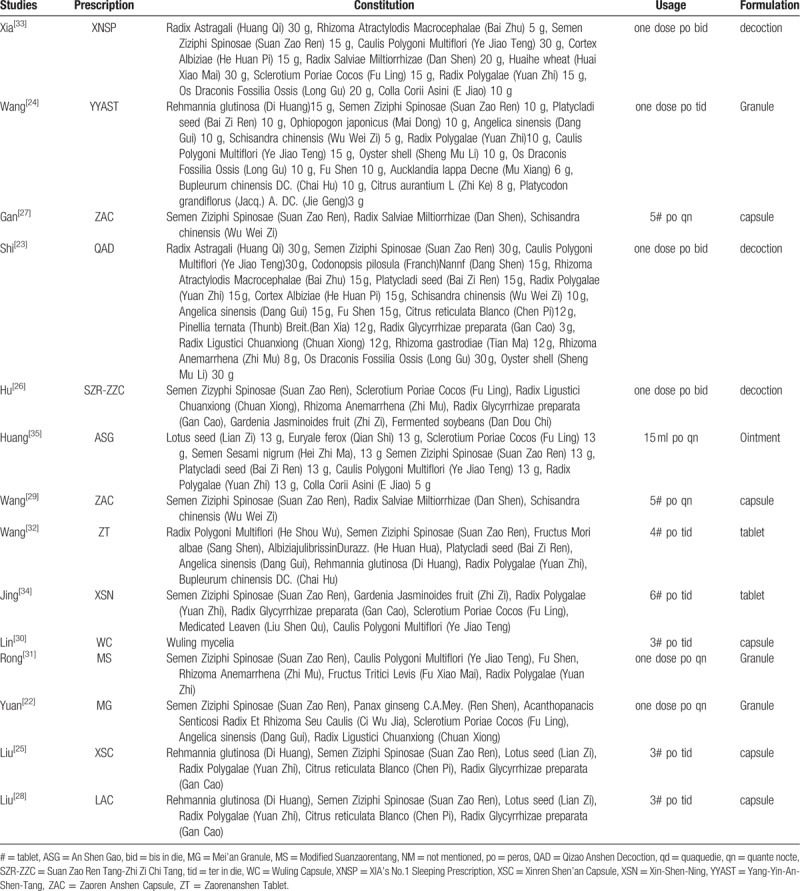
Table 3.
Frequencies of usage and distribution in TCM.
3.4. Clinical efficacy
3.4.1. TYAT vs placebo
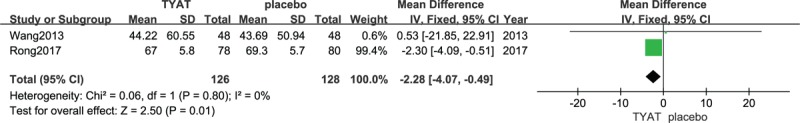
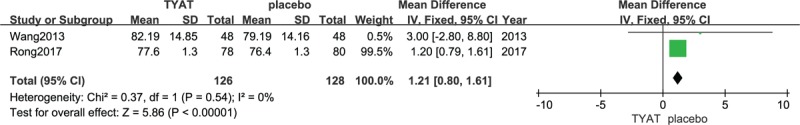
Two studies[31,32] reported the improvement of PSG after treatment, which mainly included 4 parameters: total sleep time (TST), sleep efficiency (SE), sleep of latency (SOL), and wake time after sleep onset (WASO). The results of heterogeneity analysis for the 4 parameters were: P = .35, I2 = 0%; P = .54, I2 = 0%; P = .71, I2 = 0%; P = .80, I2 = 0%. Therefore, we used a fixed effect model. The results (mean difference (MD) = 5.16, 95% confidence interval (CI): 3.09,7.23, P < .00001; MD = 1.21, 95% CI:0.80, 1.61, P < .00001;MD = −2.87, 95% CI:−4.12, −1.62, P < .00001; MD = −2.28, 95% CI:−4.07, −0.49, P = .01) of the meta-analysis suggested that the clinical efficacy of TYAT, according to PSG parameters, was significantly better than that of placebo in treating insomnia (See Figs. 3–6).
Figure 3.
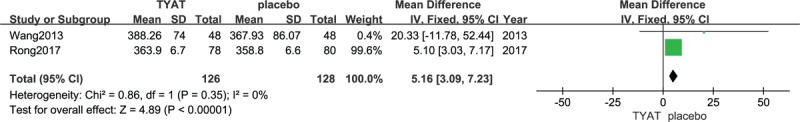
Forest plot of comparison: TYAT vs Placebo, outcome: TST.
Figure 6.
Forest plot of comparison: TYAT vs Placebo, outcome: WASO.
Figure 4.
Forest plot of comparison: TYAT vs Placebo, outcome: SE.
Figure 5.
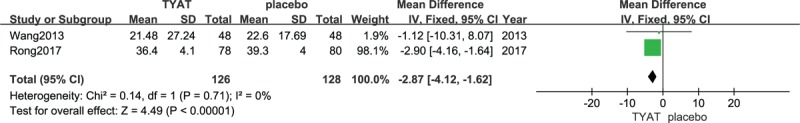
Forest plot of comparison: TYAT vs Placebo, outcome: SOL.
Five studies[22,25,30,32,34] reported the improvement of PSQI scale after treatment. Results of heterogeneity analysis were: P < .00001, I2 = 90%, which meant a pronounced heterogeneity existed among studies. In the study of Lin,[30] the intervention of the treatment group was “Wuling Capsule”, which belongs to the extract of Chinese herbal medicine, and the experimental intervention of the others belongs to the TCM compound prescription. Thus, we excluded this study, which may be the source of heterogeneity, and subgroup analysis was performed according to the duration of treatment. We used a fixed effect model, depending on the results of the heterogeneity analysis (P = .80, I2 = 0%; P = .90, I2 = 0%). Upon the results of meta-analysis (MD = −2.00, 95% CI: −2.93, −1.07, P < .0001; MD = −4.05, 95% CI: −4.86, −3.23, P < .00001), the clinical efficacy of TYAT in the treatment of insomnia was significantly better than placebo. Moreover, with the extension of the course of treatment, the efficacy difference between 2 groups was seemly more and more significant (Fig. 7).
Figure 7.
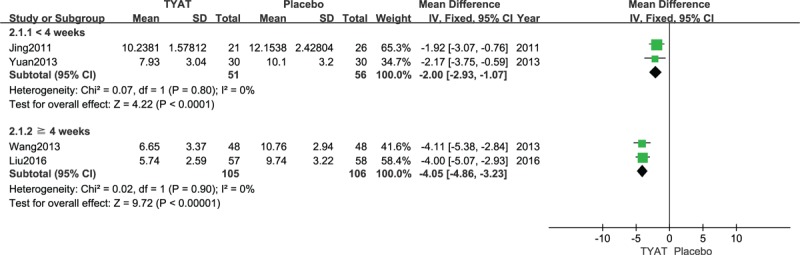
Forest plot of comparison: TYAT vs Placebo, outcome: PSQI.
Two studies[25,34] reported the improvement of TCM curative efficacy after treatment, and the results of heterogeneity analysis were: P = .68, I2 = 0%. Therefore, we used a fixed effect model. Meta-analysis results (risk ratio (RR) = 1.99,95% CI: 1.53, 2.58, P < .00001) suggested that: TCM curative efficacy of TYAT for insomnia was significantly better than that of placebo (see Fig. 8).
Figure 8.
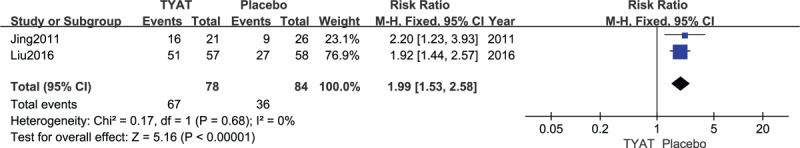
Forest plot of comparison: TYAT vs Placebo, outcome: TCM curative efficacy.
Two studies[22,25] reported the improvement of PSQI curative efficacy after the intervention. Results of heterogeneity analysis were P = .02, I2 = 81%. There was great heterogeneity between studies, therefore, we only did a qualitative analysis. As showed in the results of the 2 studies, PSQI curative efficacy of insomnia patients treated with TYAT was superior to that of placebo.
3.4.2. TYAT vs benzodiazepines
Only 1 study reported changes in PSG after treatment. From the results of Hu study,[26] we found that TYAT could significantly prolong TST and slow wave sleep (SWS), and significantly shorten SOL and WASO. Moreover, TYAT was superior to lorazepam in prolonging the duration of SWS and shortening the duration of WASO. The other 2 items were not significantly different.
Five studies [23,24,26–28] reported the improvement of PSQI scale after treatment, and the results of the heterogeneity test (P < .00001, I2 = 95%) suggested a high heterogeneity existed among studies. Therefore, only qualitative descriptive analysis was implemented. Three studies [24,27,28] concluded that there was no significant difference between TYAT and benzodiazepines. Another 2[23,26] concluded that there was a significant difference between 2 groups in improving the PSQI scale and that TYAT was superior to benzodiazepines.
Three studies[23,24,28] reported the improvement of TCM curative efficacy after treatment, and the consequences of heterogeneity analysis were: P = .25, I2 = 27%. Therefore, we implemented a fixed effect model. The results of meta-analysis (RR = 1.09, 95%CI: 0.97, 1.22, P = .15) showed that there was no significant difference in TCM curative efficacy of TYAT for insomnia compared with benzodiazepines (see Fig. 9).
Figure 9.
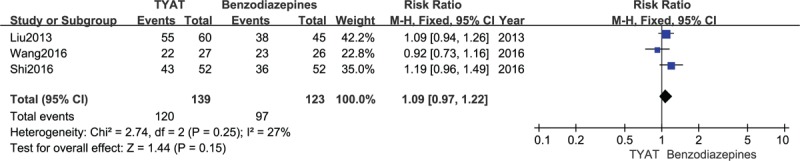
Forest plot of comparison: TYAT vs Benzodiazepines, outcome: TCM curative efficacy.
Four studies[23,24,27,28] reported the improvement of PSQI curative efficacy after treatment, and the results of heterogeneity analysis were P = .41, I2 = 0%. Therefore, we adopted a fixed effect model. The results of meta-analysis (RR = 1.05, 95%CI: 0.95,1.16, P = .30) showed that there was no significant difference in PSQI curative efficacy of TYAT for insomniacs compared with benzodiazepines (see Fig. 10).
Figure 10.
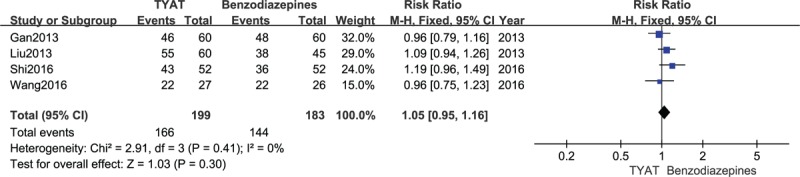
Forest plot of comparison: TYAT vs Benzodiazepines, outcome: PSQI curative efficacy.
3.4.3. TYAT versus non-benzodiazepine hypnotics
Two studies[29,35] reported the improvement of PSQI scale after treatment, whose results of heterogeneity analysis were: P < .00001, I2 = 99%. Therefore, we employed a random effect model. There was no significant difference between TYAT and non-benzodiazepines in improving PSQI scores based on the results of meta-analysis (MD = −1.16, 95%CI: −3.80,1.49, P = .39) (see Fig. 11).
Figure 11.
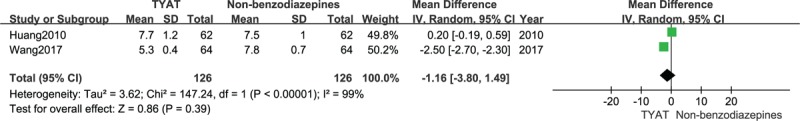
Forest plot of comparison: TYAT vs Non-benzodiazepine, outcome: PSQI.
Two studies[29,35] reported the improvement of PSQI curative efficacy after treatment. In line with the results of heterogeneity analysis (P = .006, I2 = 87%), a random effect model was adopted. There was no significant difference in PSQI curative efficacy between TYAT and non-benzodiazepines for insomniacs on the basis of meta-analysis (RR = 1.08, 95%CI:0.86, 1.36, P = .52) (see Fig. 12).
Figure 12.
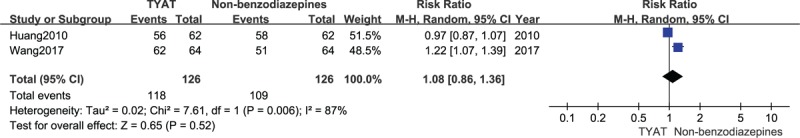
Forest plot of comparison: TYAT vs Non-benzodiazepine, outcome: PSQI curative efficacy.
3.5. Adverse events
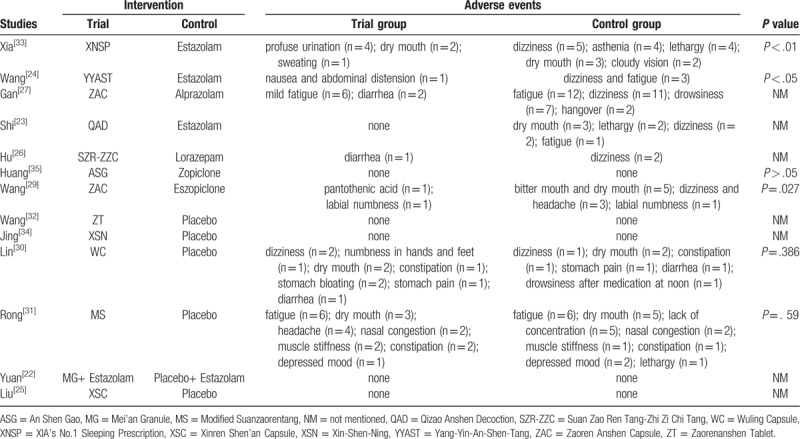
Thirteen out of 14 studies reported adverse events. Adverse events of TYAT were mainly manifested as fatigue, dry mouth, abdominal distension, diarrhea, constipation, and so on. The main adverse events of benzodiazepines were fatigue, dry mouth, dizziness, drowsiness, hangover, and other symptoms. The adverse events of non-benzodiazepines were mainly dry mouth, bitter mouth, dizziness, numbness of lips, and so on. Adverse events reported in all recruited studies were mild, and no life-threatening adverse events were found. Specific adverse events for each study were summarized in Table 4.
Table 4.
Adverse events in the included studies.
4. Discussion
4.1. Summary of evidence
-
1.
The TYAT was superior to placebo in improving PSG parameters, PSQI scale, TCM curative efficacy, and PSQI curative efficacy.
-
2.
In the light of objective PSG parameters, PSQI scale, TCM curative efficacy or PSQI curative efficacy, there was no significant difference between TYAT and benzodiazepines.
-
3.
In terms of the PSQI scale and PSQI curative efficacy, there was no significant difference between TYAT and non-benzodiazepine hypnotics in the treatment of insomnia.
-
4.
The clinical application of TYAT was comparatively safe for individuals with insomnia.
4.2. Summary of major herbals
Among the prescriptions concerned in this study, 42 Chinese herbal medicines were used to treat insomnia. Among them, Semen Ziziphi Spinosae (Suan Zao Ren) was used most frequently, totaling 13 times, which means that it was used in almost every study. Following it were Radix Polygalae (Yuan Zhi), Caulis Polygoni Multiflori (Ye Jiao Teng), Radix Glycyrrhizae Preparata (Gan Cao), Sclerotium Poriae Cocos (Fu Ling), Angelica sinensis (Dang Gui), Platycladi seed (Bai Zi Ren), Rehmannia glutinosa (Di Huang), Schisandra chinensis (Wu Wei Zi), Radix Salviae Miltiorrhizae (Dan Shen), and so forth.
Suan Zao Ren is a relatively safe hypnotic drug for insomniacs, whose secondary metabolites have been proved in vitro and in vivo researches to produce the effect of sedation and hypnosis by regulating GABAergic activity and 5-HT system.[36] In clinical research, Zhou et al[37] collected and analyzed the high-quality RCTs on the treatment of insomnia with formulations containing Suan Zao Ren, and found that it is an effective replacement therapy for insomniacs. Tanshinones may be a sedative hypnotic active substance extracted from Dan Shen, which may be more suitable for insomniacs accompanied by cardiovascular diseases for its cardiovascular activity.[38] Besides, Dan Shen and Suan Zao Ren can play a significant synergistic role in reducing latent sleep and prolonging TST.[38] It is worth noting that Dan Shen appeared 3 times in total in enclosed 14 formulations and Suan Zao Ren was simultaneously used in 3 formulations containing Dan Shen. Yunfeng Li team discovered that Yuanzhi-1, a triterpenoid saponin component extracted from Yuan Zhi, may potentially become a new triple reuptake inhibitor because of its antidepressant-like activity.[39,40] Insomnia was largely bound up with a higher risk of depression and insomniacs were more than twice as likely to suffer from depression as non-insomniacs.[41] Ye Jiao Teng, Yuan Zhi, Suan Zao Ren, Fu Ling, Gan Cao, Dang Gui, Bai Zi Ren, Di Huang, and Wu Wei Zi were frequently applied to treating insomnia in TCM.[42,43] Among them, Ye Jiao Teng and Suan Zao Ren which are categorized as Anshen herbals in TCM are designated as Sovereign or Minister herbals, but Fu Ling and Gan Cao used as an Assistant or Courier herbal have no definite effect of sedation.[42] Wu Wei Zi and its extracts can produce effective sedative and hypnotic bioactivity and antidepressant-like activity, the mechanism of sedative and hypnotic bioactivity mainly associating with the serotonergic and GABAergic system.[44–48]
4.3. Limitations
-
1.
The retrieved studies were only those published in Chinese or English at present, and there may be unpublished negative conclusions, which may bring about potential publication bias. In addition, because the number of studies in each subgroup was less than 10, the funnel plot was not implemented to evaluate the publication bias in our study.
-
2.
According to the assessment of Cochrane risk bias, we did recognize that there were some methodological limitations which may have an impact on the reliability of the results. The authors noted that appropriate allocation concealment was only used in 4 studies, and adequate randomization methods in 12 studies. The poor status of randomization may have contributed to the low methodological quality or low reporting quality, which may have inflated the clinical efficacy evaluation.
-
3.
Although double blinding is encouraged in RCTs, it is difficult to practice it for herbal intervention because of the different shape, smell, taste, and administration of herbal from pharmacotherapy[20]. Considering this challenge, it is understandable that double blinding protocol was implemented in merely 7 studies. Insufficient blinding was also the cause of overestimation of the effect size, which cannot be ignored.
-
4.
There were certain differences in the diagnose criteria, interventions, dosage, duration of medication, and the judgment of efficacy, which may cause heterogeneity in this meta-analysis. To make matters worse, clinical, and methodological heterogeneity could not be well settled by subgroup analysis.
-
5.
Due to the relatively short observation period, the long-term efficacy of TYAT could not be further assessed. Arguably, however, the long-term treatment of insomnia using hypnotics is clinically relevant because insomnia typically returns following withdrawal.[49]
-
6.
Nowadays, PSG is the most advanced instrument for the diagnosis of many sleep disorders. In terms of accuracy, the PSG is the best method, reporting the most complete and precise information.[50] The fact is, however, that only 3 studies chose PSG as the outcome measure of the intervention.
4.4. Implications for future research
In light of the above limitations, many implications arose out of related research. Firstly, we are required to refine the trial protocol and conduct more high-quality, multi-center, large-sample RCTs. Before that, the prospective registration of clinical trials was needed. Registries can identify potential problems before research begins, such as allocation concealment, randomization methods, and blind methods, thereby improving the quality of clinical trials. Secondly, syndrome differentiation is a unique diagnostic method for the classification of diverse individual pathological states under the guidance of TCM theory, which can be regarded as a further stratification of diseases.[51] Treatment based on syndrome differentiation is the key to enhance clinical efficacy. In TCM, different syndromes correspond to different therapeutic principles and prescriptions. Therefore, comprehensive searches and appraisals of the evidence for different therapeutic principles and TCM prescriptions are necessary for the future. Thirdly, prolonging the trial period appropriately and improving the follow-up protocol, so that we can observe the long-term efficacy and safety of TYAT. Finally, PSG is of great significance to the study of sleep. In future relevant research, investigators should consider PSG as one of the outcome measurements as far as possible.
5. Conclusion
In summary, TYAT is an effective alternative therapy for insomnia, and its clinical application is relatively safe. Due to the uneven quality of the included literature, further multicenter, large-sample, high-quality clinical trials are still needed for validation in the future. Only in this way are we able to obtain a more decisive conclusion about the clinical efficacy and safety of TYAT for insomnia.
Author contributions
Conceptualization: Ping Wang and Feizhou Li.
Data curation: Feizhou Li and Bo Xu.
Formal analysis: Feizhou Li and Tong Zhang.
Investigation: Feizhou Li and Bo Xu.
Methodology: Ziyu Song and Yanhua Chen.
Project administration: Ping Wang.
Resources: Ziyu Song and Yanhua Chen.
Software: Feizhou Li and Tong Zhang.
Supervision: Ping Wang, Heyuan Shi and Ling Liu.
Validation: Ping Wang, Heyuan Shi and Ling Liu.
Visualization: Feizhou Li
Writing – original draft: Feizhou Li, Bo Xu, Ziyu Song, Tong Zhang and Yanhua Chen.
Writing – review & editing: Ping Wang, Heyuan Shi and Ling Liu.
Feizhou Li orcid: 0000-0002-9465-1001
Bo Xu orcid: 0000-0001-9262-6059
Ping Wang orcid: 0000-0001-8049-2495.
Footnotes
Abbreviations: CI = confidence interval, MD = mean difference, PSQI = Pittsburgh Sleep Quality Index, RCTs = randomized controlled trials, RR = risk ratio, SE = sleep efficiency, SOL = sleep of latency, SWS = slow wave sleep, TCM = Traditional Chinese Medicine, TST = total sleep time, TYAT = TCM Yangxin Anshen Therapy, WASO = wake time after sleep onset.
How to cite this article: Li F, Xu B, Shi H, Zhang T, Song Z, Chen Y, Liu L, Wang P. Efficacy and safety of TCM yangxin anshen therapy for insomnia: A systematic review and meta-analysis. Medicine. 2020;99:8(e19330).
FL and BX contributed equally to this work as co-first authors.
This study was supported by National Key R&D Program of China (NO.2018YFC1705600). This study was supported by the General Program of National Natural Science Foundation of China (No. 81573865).
All data were extracted from the original literature and can be obtained through the corresponding author or the first author.
The authors declare that this study does not have any conflict of interest.
References
- [1].Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:307–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sleep Disorder Section, Neurology Branch, Chinese Medical Association. Chinese Guideline for the Diagnosis and Treatment of Insomnia in Adults (2017 edition). Chin J Neurol 2018;51:324–35. [Google Scholar]
- [3].Cao XL, Wang SB, Zhong BL, et al. The prevalence of insomnia in the general population in China: a meta-analysis. PloS One 2017;12:e0170772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nowicki Z, Grabowski K, Cubala WJ, et al. Prevalence of self-reported insomnia in general population of Poland. Psychiatr Pol 2016;50:165–73. [DOI] [PubMed] [Google Scholar]
- [5].van de Straat V, Bracke P. How well does Europe sleep? A cross-national study of sleep problems in European older adults. Int J Public Health 2015;60:643–50. [DOI] [PubMed] [Google Scholar]
- [6].Pepin JL, Borel AL, Tamisier R, et al. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev 2014;18:509–19. [DOI] [PubMed] [Google Scholar]
- [7].Thomas SJ, Calhoun D. Sleep, insomnia, and hypertension: current findings and future directions. J Am Soc Hypertens 2017;11:122–9. [DOI] [PubMed] [Google Scholar]
- [8].Zhu B, Hershberger PE, Kapella MC, et al. The relationship between sleep disturbance and glycaemic control in adults with type 2 diabetes: an integrative review. J Clin Nurs 2017;26:4053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin CL, Chien WC, Chung CH, et al. Risk of type 2 diabetes in patients with insomnia: a population-based historical cohort study. Diabetes Metab Res Rev 2018;34: [DOI] [PubMed] [Google Scholar]
- [10].Anothaisintawee T, Reutrakul S, Van Cauter E, et al. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev 2016;30:11–24. [DOI] [PubMed] [Google Scholar]
- [11].Sasaki S, Yoshioka E, Saijo Y, et al. A prospective cohort study of insomnia and chronic kidney disease in Japanese workers. Sleep Breath 2018;22:257–65. [DOI] [PubMed] [Google Scholar]
- [12].Li J, Huang Z, Hou J, et al. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrol 2017;12:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev 2018;40:4–16. [DOI] [PubMed] [Google Scholar]
- [14].Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- [15].Lee MH, Choi JW, Lee J, et al. Trends in prescriptions for sedative-hypnotics among Korean adults: a nationwide prescription database study for 2011-2015. Soc Psychiatry Psychiatr Epidemiol 2019;54:477–84. [DOI] [PubMed] [Google Scholar]
- [16].Mintao Z, Wei Z, Tingting Z, et al. Analysis of drug use in elderly patients with insomnia from 22 hospitals in Beijing. Clin Medicat J 2017;15:57–60. [Google Scholar]
- [17].Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12. [DOI] [PubMed] [Google Scholar]
- [18].Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer's disease: case-control study. BMJ 2014;349:g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yeung WF, Chung KF, Poon MM, et al. Chinese herbal medicine for insomnia: a systematic review of randomized controlled trials. Sleep Med Rev 2012;16:497–507. [DOI] [PubMed] [Google Scholar]
- [20].Ni X, Shergis JL, Guo X, et al. Updated clinical evidence of Chinese herbal medicine for insomnia: a systematic review and meta-analysis of randomized controlled trials. Sleep Med 2015;16:1462–81. [DOI] [PubMed] [Google Scholar]
- [21].Li F, Wang X, Song Z, et al. Efficacy and safety of traditional Chinese medicine yangxin anshen therapy for insomnia: a protocol of systematic review and meta-analysis. Medicine 2019;98:e16945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yuan C, Chen Y, Li J, et al. Adjuvant treatment of insomnia of liver-yin deficiency pattern by “Mei’an Granule”: A randomized double-blind and placebo-controlled trial. Shanghai J Tradition Chin Med 2013;47:57–9. +71. [Google Scholar]
- [23].Shi C, Sun N. Effect of Qizao Anshen Decoction on the sleep quality of nsomnia patients with syndrome of deficiency of both heart and spleen. China Medical Herald 2016;13:146–9. [Google Scholar]
- [24].Anhui University of Chinese Medicine, Wang D. The Research on the Treatment of Fire Excess from Yin Deficiency of Senile Insomnia with Yang-Yin-An-Shen-Tang. 2016. [Google Scholar]
- [25].Liu F, Jiang Y, Chang C, et al. Multicenter, Randomized, Double Blind and Placebo Parallel Controlled Trial for Xinren Shen’an capsule in treating insomnia of fire excess due to Yin-deficiency with phlegm syndrome. J Tradition Chin Med 2016;57:1934–8. [Google Scholar]
- [26].Hu LL, Zhang X, Liu WJ, et al. Suan zao ren tang in combination with zhi zi chi tang as a treatment protocol for insomniacs with anxiety: A randomized parallel-controlled trial. Evid-based Complement Alternat Med 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gan J, Tian G, Qin G. Study on efficacy of zaoren anshen capsules in treating senile insomnia and changes in its hemorheology. China J Chin Materia Medica 2013;38:273–5. [PubMed] [Google Scholar]
- [28].Liu J, Liu T, Ou Y. Clinical Observation on Liuwei Anshen capsule for treating insomnia on 60 cases. China Pharmaceut 2013;22:84–5. [Google Scholar]
- [29].Wang J, Shen W, Yao X, et al. Analysis of clinical value of Zaoren Anshen capsule in improving sleep. Asia-Pacific Tradition Med 2017;13:137–8. [Google Scholar]
- [30].Lin Y, Wang XY, Ye R, et al. Efficacy and safety of Wuling capsule, a single herbal formula, in Chinese subjects with insomnia: a multicenter, randomized, double-blind, placebo-controlled trial. J Ethnopharmacol 2013;145:320–7. [DOI] [PubMed] [Google Scholar]
- [31].Rong R, Zhong J, Lin S, et al. Efficacy and safety of modified suanzaorentang on primary insomnia: a double-blind randomized placebo-controlled study. Chin Mental Health J 2017;31:606–13. [Google Scholar]
- [32].Wang S, Liu J, Ma G, et al. Clinical study of Zaorenanshen tablet treating anhypnia. Hebei J Tradition Chin Med 2013;35:1217–9. [Google Scholar]
- [33].Xia CY, Xia CY, Deng SP, et al. The XIA's No. 1 sleeping prescription for the treatment of insomnia of the deficiency type: a clinical observation of 60 cases. J Tradition Chin Med 2009;29:211–5. [DOI] [PubMed] [Google Scholar]
- [34].Jing X. Randomized and controlled study on Xin-Sheng-Ning slice in treatment of insomnia patients of 48 cases with heart-spleen deficiency and disturbance of deficiency-heat inside the body. Chengdu Univ Tradition Chin Med 2011. [Google Scholar]
- [35].Huang Y, Yang B, Chen Y, et al. Clinical observation on 124 cases of Insomnia caused by heart and spleen blood deficiency treated with An Shen Gao. Strait Pharmaceut J 2010;22:169–71. [Google Scholar]
- [36].Shergis JL, Ni X, Sarris J, et al. Ziziphus spinosa seeds for insomnia: a review of chemistry and psychopharmacology. Phytomedicine 2017;34:38–43. [DOI] [PubMed] [Google Scholar]
- [37].Zhou QH, Zhou XL, Xu MB, et al. Suanzaoren formulae for insomnia: updated clinical evidence and possible mechanisms. Front Pharmacol 2018;9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fang X, Hao JF, Zhou HY, et al. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine 2010;17:75–80. [DOI] [PubMed] [Google Scholar]
- [39].Jin ZL, Gao N, Li XR, et al. The antidepressant-like pharmacological profile of Yuanzhi-1, a novel serotonin, norepinephrine and dopamine reuptake inhibitor. Eur Neuropsychopharmacol 2015;25:544–56. [DOI] [PubMed] [Google Scholar]
- [40].Jin ZL, Gao N, Zhang JR, et al. The discovery of Yuanzhi-1, a triterpenoid saponin derived from the traditional Chinese medicine, has antidepressant-like activity. Prog Neuropsychopharmacol Biol Psychiatry 2014;53:9–14. [DOI] [PubMed] [Google Scholar]
- [41].Li L, Wu C, Gan Y, et al. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry 2016;16:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yeung WF, Chung KF, Poon MM, et al. Prescription of chinese herbal medicine and selection of acupoints in pattern-based traditional chinese medicine treatment for insomnia: a systematic review. Evid Based Complement Alternat Med 2012;2012:902578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Singh A, Zhao K. Treatment of Insomnia with traditional Chinese herbal medicine. Int Rev Neurobiol 2017;135:97–115. [DOI] [PubMed] [Google Scholar]
- [44].Zhu H, Zhang L, Wang G, et al. Sedative and hypnotic effects of supercritical carbon dioxide fluid extraction from Schisandra chinensis in mice. J Food Drug Anal 2016;24:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yan T, Xu M, Wu B, et al. The effect of Schisandra chinensis extracts on depression by noradrenergic, dopaminergic, GABAergic and glutamatergic systems in the forced swim test in mice. Food Funct 2016;7:2811–9. [DOI] [PubMed] [Google Scholar]
- [46].Zhang C, Zhao X, Mao X, et al. Pharmacological evaluation of sedative and hypnotic effects of schizandrin through the modification of pentobarbital-induced sleep behaviors in mice. Eur J Pharmacol 2014;744:157–63. [DOI] [PubMed] [Google Scholar]
- [47].Zhang C, Mao X, Zhao X, et al. Gomisin N isolated from Schisandra chinensis augments pentobarbital-induced sleep behaviors through the modification of the serotonergic and GABAergic system. Fitoterapia 2014;96:123–30. [DOI] [PubMed] [Google Scholar]
- [48].Huang F, Xiong Y, Xu L, et al. Sedative and hypnotic activities of the ethanol fraction from Fructus Schisandrae in mice and rats. J Ethnopharmacol 2007;110:471–5. [DOI] [PubMed] [Google Scholar]
- [49].Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675–700. [DOI] [PubMed] [Google Scholar]
- [50].Ibáñez V, Silva J, Cauli O. A survey on sleep assessment methods. PeerJ 2018;6:e4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].M J, C Z, G Z, et al. Traditional chinese medicine zheng in the era of evidence-based medicine: a literature analysis. Evid-Based Complement Alternat Med 2012;409568. [DOI] [PMC free article] [PubMed] [Google Scholar]


