Abstract
Cigarette smoking increases susceptibility for microbial infection in respiratory system. However, the underlying molecular mechanism(s) is not fully elucidated. Here we report that cigarette smoking extract (CSE) increases bacterial load in lung epithelial cells via downregulation of the ubiquitin-specific protease 25 (USP25)/histone deacetylase 11 (HDAC11) axis. CSE treatment decreases HDAC11 at protein level in lung epithelial cells without significant changes of its transcription. Concomitantly, CSE treatment accelerates a ubiquitin-specific protease USP25 ubiquitination and degradation. Coimmunoprecipitation studies showed that USP25 associated with HDAC11. USP25 catalyzes deubiquitination of HDAC11, which regulates HDAC11 protein stability. CSE-mediated degradation of USP25 thereafter reduces HDAC11 at the protein level. Interestingly, CSE-downregulated USP25/HDAC11 axis increases the bacterial load of Pseudomonas aeruginosa in lung epithelial cells. These findings suggest that CSE-downregulated USP25 and HDAC11 may contribute to high susceptibility of bacterial infection in the cigarette smoking population.
Keywords: chronic obstructive pulmonary disease, cigarette smoke extract, histone deacetylase 11, lung epithelial cell, Pseudomonas aeruginosa, ubiquitin-specific protease 25
INTRODUCTION
Cigarette smoking is a severe public health concern that affects millions of people worldwide. Cigarette smoking is the etiological cause of many deleterious diseases in the respiratory system including chronic obstructive pulmonary diseases (COPD), lung cancer, and acute microbial infections. Many studies have demonstrated that cigarette smoking increased susceptibility for microbial infection in respiratory systems. An active tobacco user has a higher risk of viral or bacterial respiratory infection, and the patient’s severity of airflow limitation is correlated with the degree of pulmonary inflammation (36, 52, 67). In addition, ample evidence show that bacterial infections are the major causes linked with the exacerbations of COPD (41). Exacerbation of COPD is a prominent feature of COPD that increases patients’ mortality, deteriorates patients’ life quality, and raises economic burden of the society (42). Few remedies could effectively reverse the COPD exacerbation, except for the routine antisymptomatic management to mitigate the patient’s discomfort (60). Pseudomonas aeruginosa, a Gram-negative bacillus, is an opportunistic but important pathogen causing both acute and chronic infections in susceptible populations. It has been reported that P. aeruginosa implicated in the pathogenesis of COPD and was a cause of acute exacerbation of COPD (18). Moreover, P. aeruginosa–mediated exacerbation in COPD patients linked to poor prognosis and higher mortalities (18, 39). Many reports showed that cigarette smoking compromised both the innate and adaptive immune function of the lung and was strongly correlated to the ongoing airway inflammation, as well as lung emphysema (1, 13, 23, 36). However, the molecular mechanism of how P. aeruginosa selectively invades the vulnerable smoking-related COPD patient is not fully understood.
Histone deacetylases (HDACs) are a group of enzymes that catalyze the deacetylation of histone and nonhistone proteins. HDACs are exclusively involved in a broad range of life processes including gene transcription, cell death, proliferation, development, and stress or immune response (15, 49). HDACs are categorized into four major classes based on their structure, phylogeny, and function. Class I (HDAC1, HDAC2, HDAC3, HDAC8), Class II (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, HDAC10), and Class IV (HDAC11) are zinc-dependent enzymes, while Class III HDACs (SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, SIRT7) are NAD+ (nicotine adenine dinucleotide)-dependent enzymes. Among them, HDAC11 is cloned and characterized as the only member of Class IV HDACs and the smallest one of the HDAC family (15, 49). Uniquely in the primary sequence, HDAC11 contains only the catalytic core region for deacetylation shared by both Class I and II HDACs without other domains (17). A recent study reported that HDAC11 cleaved long-chain acyl modifications on lysine side chain (38). HDAC11 knockout mice are resistant to high-fat diet–induced obesity and metabolic syndrome, suggesting that HDAC11 is a major player in regulation of metabolism (54). HDAC11 associates with BRD2 (Bromodomain Containing 2) to regulate the critical gene regulatory elements of BRD2, which is important to the process of brown adipose tissue differentiation and thermogenesis (2). HDAC11 plays an essential role in neutrophil biology and regulates OX40L (tumor necrosis factor superfamily member 4) expression in Hodgkin lymphoma (8, 46). Upregulation of HDAC11 is correlated with suppression of myoblast differentiation, which is MyoD (a helix-loop-helix muscle-specific transcription factor) dependent (9). Strikingly, HDAC11 is an important regulatory factor of immune response. HDAC11 exerts diverse and contradictory functions in regulation of immunity by targeting cytokine secretion, B cell biology, and T cell activation (33, 59). However, there is a rare amount of reports exploring the function of HDAC11 in the pathogenesis of COPD. Protein poly-ubiquitination is a reversible posttranslational modification. Deubiquitination enzymes competently remove ubiquitin moieties from the targeted proteins (37). Ubiquitin-specific protease 25 (USP25) is a deubiquitination enzyme that belongs to the ubiquitin-specific protease (USP) subfamily, located at 21q11.2 of the human genome (56). USP25 is linked to the tumorigenesis of many cancers, which include breast cancer, non–small cell lung cancer, hepatocellular carcinoma, and colorectal carcinoma (11, 14, 32, 62). USP25 is also the type 1 interferon responding gene. USP25 expression is upregulated in viral infection, as well as in LPS treatment (30, 45, 65). Our recent study shows that USP25 may stabilize an acetyltransferase HBO1, previously known as an important enzyme in DNA replication licensing, to regulate inflammatory gene transcription in monocytes (35). Nevertheless, the role of USP25 in COPD is the least studied.
The respiratory airway is an open system, continuously exposed to various external microbial pathogens. Lung epithelial cells, coordinating with macrophages or neutrophils, constitute a unique role in host defense against microbial infection. Crucially as the first physiological barrier, lung epithelial cells may regulate microbial invasion, colonization, or replication. In this study, we identified that cigarette smoke extract downregulated ubiquitin-specific protease USP25 to trigger degradation of a histone deacetylase, HDAC11, in lung epithelial cells. Reduced USP25/HDAC11 increases the bacterial load of P. aeruginosa, which may contribute to the recurrence of bacterial infections usually seen in COPD exacerbation.
Experimental Procedures
Cell line and reagents.
Human BEAS-2B cells were cultured in HITES medium (500 mL DMEM/F-12, 2.5 mg insulin, 2.5 mg transferrin, 2.5 mg sodium selenite, 2.5 mg transferrin, 10 μM hydrocortisone, 10 μM β-estradiol, 10 mM HEPES, and 2 mM l-glutamine) supplemented with 10% fetal bovine serum (FBS) as previously described (31). Human primary small airway epithelial cells were purchased from ATCC and maintained with airway epithelial cell basal medium supplemented with bronchial epithelial cell growth kit following the instruction of the manufacturer (ATCC). Cells were maintained in a 37°C incubator in the presence of 5% CO2. HDAC11 antibody (cat. no. sc-390737) and USP25 antibody (cat. no. sc-398414) were purchased from Santa Cruz Biotechnology. V5 antibody (cat. no. P/N 46-0705), pcDNA3.1D-His-V5-TOPO cloning kit (cat. no. K490001) and Escherichia coli Top10 competent cells (cat. no. C404006) were purchased from Invitrogen. β-Actin antibody (cat. no. A5441) and leupeptin (cat. no. L2884) were from Sigma Aldrich. P. aeruginosa strain (PAO1, 10145) was purchased from ATCC. Protein A/G Agarose (cat. no. 20422) and Completed protease inhibitor cocktail (cat. no. 88266) were from Pierce. MG132 (cat. no. F1101) was a product of UBPBio. Cycloheximide (CHX; cat. no. ALX-380–269-G001) and ubiquitin aldehyde (cat. no. BML-UW8450-0050) were products of Enzo Life Science. RNeasy mini kit (cat. no. 74104) was purchased from QIAGEN. High-Capacity RNA-to-DNA kit (cat. no. 4387406) and SYBR select Master Mix (cat. no. 4472942) for quantitative PCR were purchased from Applied Biosystems. QuikChange II XL site-directed mutagenesis kit (cat. no. 200522) was purchased from Agilent Technologies. Phusion High-Fidelity DNA Polymerase kit (cat. no. M0530L) was purchased from New England Biolabs.
Plasmid and Small Interfering RNA Transfection
Small interfering RNA (siRNA) or plasmids were transfected into BEAS-2B cells using Nucleofection II system (Amaxa Biosystem, Gaithersburg, MD) as previously described (66). siRNA against USP25 or HDAC11 and Scrambled-siRNA (cat. no. 51-01-14-03) were purchased from Integrated DNA Technology. Briefly, 1 × 106 cells were homogenously suspended in 100 μL of electrotransfection buffer (1× PBS, 20 mM HEPES) and mixed well with 10 pM of siRNA or 1 μg of plasmids in each cuvette. Preset program T-013 was executed for electroporation. HITES medium (2 mL) was immediately added to each cuvette after the electroporation. After 72 h incubation, transfected cells were used for analysis or further assay.
Cloning and Mutagenesis
pCMV6-Entry/HDAC11 plasmids (cat. no. MR205242) and pCMV6-XL5/USP25 plasmids (cat. no. SC115248) were from Origene. Open reading frame of HDAC11 in pCMV6- Entry/HDAC11 plasmid was cloned into pcDNA3.1D-His-V5-TOPO plasmid using PCR-based approaches as previously described (35). PCR was executed using Phusion High-Fidelity DNA Polymerase kit. Mutagenesis was introduced by using a QuikChange II XL site-directed mutagenesis kit according to the manufacturer’s instructions. The accuracy of site-directed mutagenesis was confirmed by Sanger sequencing provided by GENEWIZ. The primers used in the subcloning of HDAC11, site-directed mutagenesis, and DNA sequencing are listed in Table 1. An online tool was used for the primers design for mutagenesis studies (https://www.agilent.com/store/primerDesignProgram.jsp).
Table 1.
List of primers used for HDAC11 subcloning, DNA sequencing, and site-directed mutagenesis
| Primer Sequence | |
|---|---|
| Subcloning | |
| HDAC 11-forward | 5′-CACCATGCCTCACGCAACACAGCT-3′ |
| HDAC11-reverse | 5′-AGGCACAGCACAGGAAAGCA-3′ |
| DNA sequencing | |
| T7 | 5′-TAATACGACTCACTATAGGG-3′ |
| HDAC11 ORF 610-627 | 5′-TACAACCGCCACATCTAC-3′ |
| Site-directed mutagenesis | |
| K14R-forward | 5′-GGGCCAGCGTCTCTCTGGTACATGCTGGTA-3′ |
| K14R-reverse | 5′-TACCAGCATGTACCAGAGAGACGCTGGCCC-3′ |
| K33R-forward | 5′-GGGGTGCAGCCTCTCCAGGCCCATGAAG-3′ |
| K33R-forward | 5′-CTTCATGGGCCTGGAGAGGCTGCACCCC-3′ |
| K41R-forward | 5′-GATCACCTTGCCCCATCTCCCAGCATCAAAGGG-3′ |
| K41R-reverse | 5′-CCCTTTGATGCTGGGAGATGGGGCAAGGTGATC-3′ |
| K44R-forward | 5′-GGAAGTTGATCACCCTGCCCCATTTCCCAGC-3′ |
| K44R-reverse | 5′-GCTGGGAAATGGGGCAGGGTGATCAACTTCC-3′ |
| K50R-forward | 5′-AGCTTCTCTTCTCTCAGGAAGTTGATCACCTTGCCC-3′ |
| K50R-reverse | 5′-GGGCAAGGTGATCAACTTCCTGAGAGAAGAGAAGCT-3′ |
| K53R-forward | 5′-TCGGACAGCAGCCTCTCTTCTTTCAGGAAGTTGATC-3′ |
| K53R-reverse | 5′-GATCAACTTCCTGAAAGAAGAGAGGCTGCTGTCCGA-3′ |
| K84R-forward | 5′-CTATCTCAACGAGCTGAGGTGGTCCTTTGTGGTGG-3′ |
| K84R-reverse | 5′-CCACCACAAAGGACCACCTCAGCTCGTTGAGATAG-3′ |
| K109R-forward | 5′-GGCCTCAGCACCCTCCTCTGCACAAGG-3′ |
| K109R-reverse | 5′-CCTTGTGCAGAGGAGGGTGCTGAGGCC-3′ |
| K126R-forward | 5′-CCACAGCCAGCCTCCCTGCCATGATGGTGC-3′ |
| K126R-reverse | 5′-GCACCATCATGGCAGGGAGGCTGGCTGTGG-3′ |
| K163R-forward | 5′-CGCGTTCAAACAGGAACCTGATAGCCAGTGTGATG-3′ |
| K163R-reverse | 5′-CATCACACTGGCTATCAGGTTCCTGTTTGAACGCG-3′ |
| K196R-forward | 5′-ATGTATACTCGCCTGTCACCCATGAAGTCTCGCTCA-3′ |
| K196R-reverse | 5′-TGAGCGAGACTTCATGGGTGACAGGCGAGTATACAT-3′ |
| K216R-forward | 5′-TGATGGCCTCTCTAGCAAAGCGATCCCCAGG-3′ |
| K216R-reverse | 5′-CCTGGGGATCGCTTTGCTAGAGAGGCCATCA-3′ |
| K222R-forward | 5′-CTCCAATTCCACCCTCCGCCTGATGGCCT-3′ |
| K222R-reverse | 5′-AGGCCATCAGGCGGAGGGTGGAATTGGAG-3′ |
| K237R-forward | 5′-TTCCTCTCCACCCTCTCCAGATATTCCTCATCTTCT-3′ |
| K237R-reverse | 5′-AGAAGATGAGGAATATCTGGAGAGGGTGGAGAGGAA-3′ |
| K280R-forward | 5′-ACCACTTCATCCCTCCTCACAATGCCCGCTG-3′ |
| K280R-reverse | 5′-CAGCGGGCATTGTGAGGAGGGATGAAGTGGT-3′ |
| K306R-forward | 5′-GCTGTGCGCCTCTGGTACCCACCCGAG-3′ |
| K306R-reverse | 5′-CTCGGGTGGGTACCAGAGGCGCACAGC-3′ |
| K41R K44R-forward | 5′-GGAAGTTGATCACCCTGCCCCATCTCCCAGCATCAAAGGG-3′ |
| K41R K44R-reverse | 5′-CCCTTTGATGCTGGGAGATGGGGCAGGGTGATCAACTTCC-3′ |
| K50R K53R-forward | 5′-CGGACAGCAGCCTCTCTTCTCTCAGGAAGTTGATCACCTTGC-3′ |
| K50R K53R-reverse | 5′-GCAAGGTGATCAACTTCCTGAGAGAAGAGAGGCTGCTGTCCG-3′ |
HDAC, histone deacetylase; ORF, open reading frame.
Immunoblotting and Coimmunoprecipitation
Immunoblotting and coimmunoprecipitation (co-IP) were conducted as previously described (66). Briefly, cells were harvested and lysed with an appropriate amount of lysis buffer [1× Tris-buffered saline (PH 8.0), 1:500 protease inhibitor cocktail, 0.5% of Triton X-100, 0.5% of SDS]. Whole-cell lysates were obtained by sonication and precleared with centrifugation. The proteins were separated with SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% (wt/vol) nonfat milk in Tris-buffered saline and probed with primary antibody and corresponding secondary antibody. The images were visualized with chemiluminescent reagents and acquired by a Kodak image station (in vivo professional 4000 system). For immunoprecipitation, 1 mg of cell lysates (1× PBS, 1% Tween 20, and protease inhibitors) were mixed with a specific primary antibody overnight at 4 °C. Protein A/G-agarose beads (40 μL) was added to the mixture for 2 h at 4°C. The beads were washed three times with the wash buffer (1× PBS, 0.5% Tween 20) and boiled with the loading dye as the precipitates were subjected to immunoblotting as described above. To inhibit deubiquitinating enzyme activity, ubiquitin aldehyde (5 μM) was used as an additive of cell lysis buffer in V5-Ub co-IP experiment (34).
Quantitative RT-PCR
RNeasy Mini kit was used for total RNA extraction according to the manufacturer’s instructions. High-Capacity RNA-to-DNA kit was used to perform reverse transcription reaction according to the protocol provided by the manufacturer. Total RNA (1 μg) was used for reverse transcription reaction. Then the cDNA templates, SYBR select Master Mix, and specific primers were mixed together in a 20 μL system for the following PCR analysis in a CFX96 Real-Time PCR Detection System (Bio-Rad) according to the manufacturer’s recommendations. The comparative Ct method was used for the presentation of gene expression. The primers used in quantitative RT-PCR (qRT-PCR) are listed below: HDAC11 forward primer: 5′-CCAGACAGGAGGAACCATAA-3′; HDAC11 reverse primer: 5′-TCCGCATAGGCACAGAA-3′; USP25 forward primer: 5′-AATGTGATTGATCTCACT GG-3′; USP25 reverse primer: 5′-TCATCAGTTATTCCAGTCTCC-3′; GAPDH forward primer: 5′-GGCATGGACTGTGGTCATGA-3′; GAPDH reverse primer: 5′-TTCACCATGGAGAAGGC −3′.
Cigarette Smoking Extract Preparation
Research-grade cigarettes (3R4F) were from the University of Kentucky. Cigarette smoke extracts (100%; CSE) were prepared by combusting one filter-free cigarette and dissolving the smoke in serum-free HITES medium based on published method (53). Briefly, 10 mL of serum-free cell culture media was sucked into a 60-mL syringe device with a tip (1 mL) attached to it as an adapter to hold the cigarette. One whole cigarette was used to generate smoke. 50 mL smoke was sucked into 10 mL serum-free media and mixed with the medium by vigorously shaking (30 s per round), then replaced with another fresh 50 mL smoke for next shaking round, which made the total shaking time 6 min. Then the smoked media (10 mL) was filtered using a 0.22 μm filter to exclude any microorganisms and insoluble particles and transferred to a closed sterile tube as 100% CSE for the subsequent assay.
Bacterial Infection and Detection
For bacterial infection, 1 × 106 cells were prepared in six-well plates. P. aeruginosa was propagated for 1 h in 10 mL tryptic soy broth (TSB) at 37°C with shaking at 200 revolutions/min. The optical density at 600 nm wavelength (OD600) value was measured to estimate the number of bacteria [OD600 = 1 corresponds to ~109 CFU (colony-forming unit)/mL]. P. aeruginosa (~2 × 107 CFU/mL) was added to each well and incubated for 90 min at 37°C in 5% CO2. Then, the supernatants were aspirated and replaced with fresh 2 mL of HITES medium and gentamicin (100 μg/mL). After 1 h of bacteria killing at 37°C in 5% CO2, the supernatants were removed entirely by aspiration and the cells were washed with PBS three times for the subsequent bacteria counting using drop plate method or 16S rRNA-target qRT-PCR. For the drop plate method, 1 mL of cell lysis buffer (0.5% Triton X-100 in PBS) was added to each well. The cell lysates, containing the internalized bacteria, were subjected to gradient dilution (1:10, 1:100, 1:1,000, and 1:10,000) for the following inoculation to the TSB agar plate. After 16 h of incubation, the results of CFU were obtained. The approach of qRT-PCR for detection of 16S rRNA has been described above. The following primers targeting 16S rRNA of P. aeruginosa were listed below: forward 5′-CAAAACTACTGAGCTAGAGTACG-3′; reverse 5′-TAAGATCTCAAG GATCCCAACGGC-3′.
Animal Studies
The mouse experiments were conducted complying with the Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine. Male and female C57BL/6J mice (Jackson Laboratory) at the age of 10 wk with a weight of 20 g were exposed to cigarette smoke for 4 wk (4 cigarettes/day, 5 days/wk, n = 3–7). The mice were maintained in a pathogen-free barrier facility with a 12-h light/dark cycle in Plexiglas cages (four mice per cage) and free access to sterilized water and irradiated pellet food. Animal health, weight, and overall behavior were managed throughout the experiment. After smoke exposure, animals (n = 6) were euthanized, and the lysate of lung tissues was used for immunoblotting analysis.
Statistical Analysis
Online Web Statistical Calculators (https://astatsa.com) were used for corresponding statistical analysis such as one-way ANOVA with post hoc Tukey’s honestly significant difference (HSD) or Bonferroni and Holm multiple comparisons. P < 0.05 indicates statistical significance.
RESULTS
CSE Decreased HDAC11 Protein Levels in BEAS-2B Cells
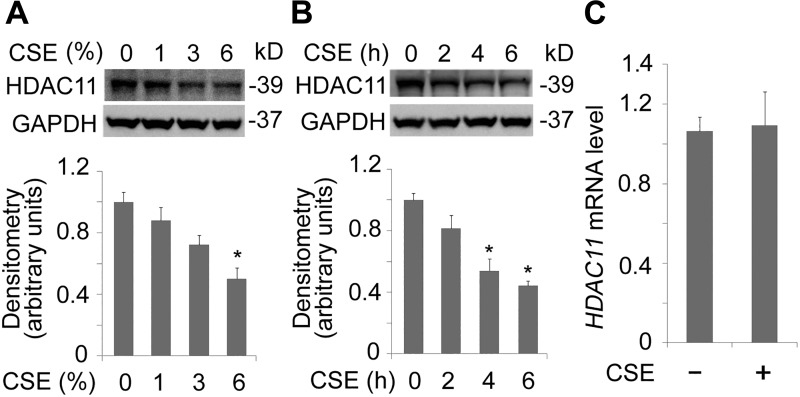
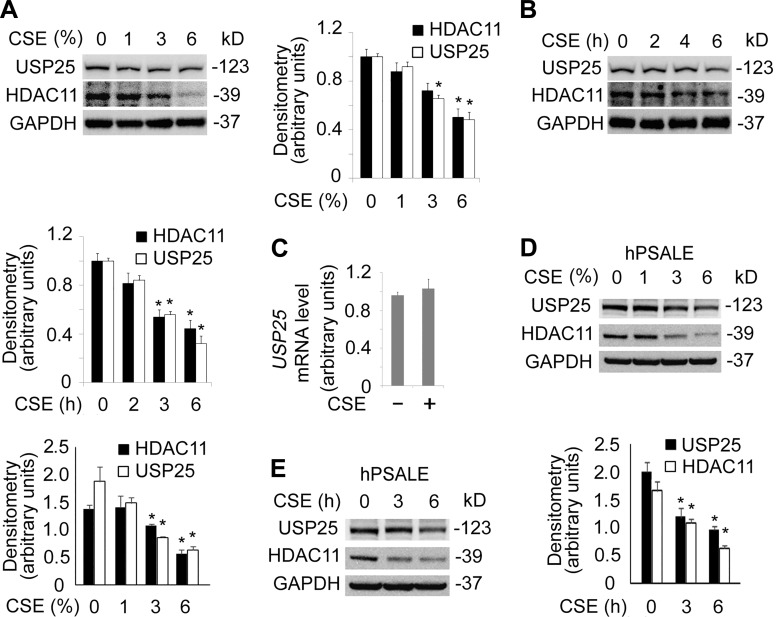
Increasing data show that cigarette smoking impairs the immune system (44, 51). Since it has been reported that HDAC11 was an important immune modulator, we were intrigued to understand its function in epithelial cells (8, 59, 64). We observed that CSE altered HDAC11 protein levels in human lung bronchial epithelial BEAS-2B cells (Fig. 1A). Results from immunoblotting analysis indicated that CSE decreased HDAC11 proteins, 3% of CSE treatment caused significant decrease in HDAC11 protein levels over a time period of 6 h (Fig. 1A). To confirm this observation, we have conducted a time course study. CSE with a concentration of 6% markedly decreased HDAC11 protein levels at 4 h. CSE deregulated HDAC11 in a time-dependent manner (Fig. 1B). Next, we checked whether the mRNA of HDAC11 was regulated under CSE exposure. qRT-PCR results showed that HDAC11 mRNA levels were not significantly affected by CSE treatment (Fig. 1C). These data indicate that HDAC11 protein was possibly turned over after protein translation.
Fig. 1.
Cigarette smoke extract (CSE) decreased histone deacetylase 11 (HDAC11) protein levels in BEAS-2B cells. A: BEAS-2B cells were treated with CSE in a range of concentrations as indicated for 6 h. Cell lysates were subjected to immunoblotting for HDAC11 or GAPDH. Densitometry results are plotted in the lower panel. B: BEAS-2B cells were treated with CSE (6%) at diverse time points. Cell lysates were analyzed with HDAC11 or GAPDH immunoblotting. Densitometry results of the blots are plotted in the lower panel. C: total RNA was extracted from untreated and CSE-treated BEAS-2B cells (6% for 6 h). HDAC11 mRNA levels were determined with quantitative RT-PCR. Data represent n = 3 separate experiments. Graphs show means ± SD. *P < 0.05. qRT-PCR, quantitative RT-PCR.
HDAC11 was Unstable and Degraded via Proteasome System
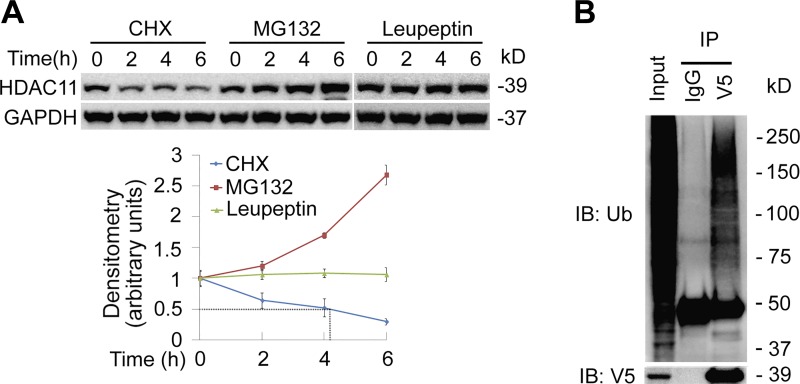
Proteins destined to be turned over were generally polyubiquitinated and subsequently proteolyzed via proteasome. To test whether HDAC11 was unstable, we treated BEAS-2B cells with cycloheximide (CHX) to inhibit protein synthesis. Immunoblotting results showed that HDAC11 is a labile protein with a half-life ~4 h in BEAS-2B cells (Fig. 2A, left and lower panels). We observed substantial accumulation of HDAC11 protein by MG132 (a reversible proteasome inhibitor) but not leupeptin (a lysosome inhibitor), suggesting that degradation of HDAC11 was mainly via proteasomal but not a lysosomal pathway in BEAS-2B cells (Fig. 2A, middle, right, and lower panels). To test whether HDAC11 was ubiquitinated, V5-tagged wild-type (WT) HDAC11 was introduced into Beas-2B cells and anti-V5 immunoprecipitants were analyzed with ubiquitin via immunoblotting. Results showed that HDAC11 was polyubiquitinated (Fig. 2B). These results indicated that HDAC11 was an unstable protein with a half-life around 4 h.
Fig. 2.
Histone deacetylase 11 (HDAC11) protein was unstable and degraded through proteasome system. A: BEAS-2B cells were treated with the protein synthesis inhibitor cycloheximide (CHX; 40 μM), proteasome inhibitor MG132 (40 μM), or lysosome inhibitor leupeptin (20 μM) at various time points as indicated. Cell lysates were subjected to HDAC11 or GAPDH immunoblotting analysis. Densitometry results are plotted in the lower panel. B: wild-type pcDNA3.1D-His-V5/HDAC11 plasmids were introduced into Beas-2B cells for 48 h. Cell lysates were applied to V5 immunoprecipitation (IP) and the immunoprecipitants were analyzed with ubiquitin and V5 immunoblotting (IB) analysis. Results are representative of n = 3 experiments.
HDAC11 was Polyubiquitinated at K50 and K280
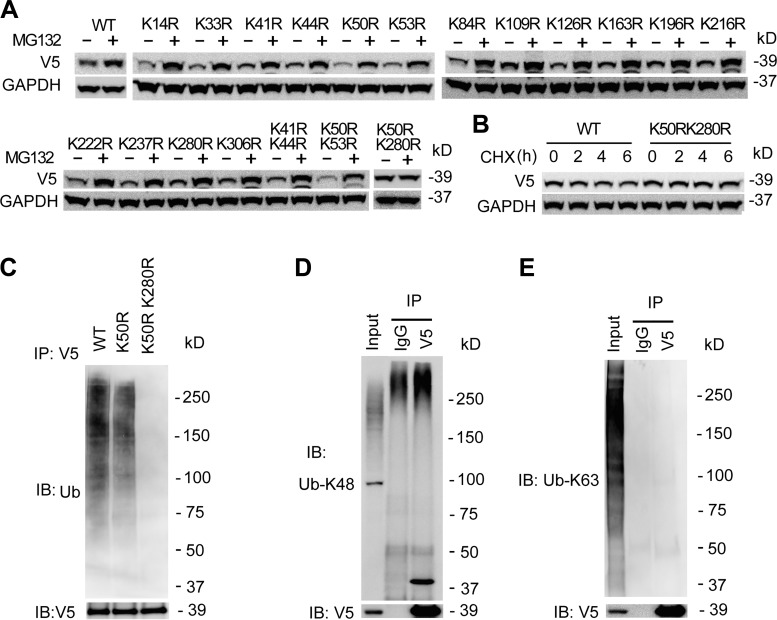
Protein ubiquitination occurs in the α-amino group side chain of lysine residues. To obtain more detail of the HDAC11 degradation, we have constructed HDAC11 mutants by replacement of lysine with arginine. WT or mutant HDAC11 plasmids were delivered in BEAS-2B cells, and the cells were treated with MG132 for 6 h. Among the mutants, the protein accumulated in WT HDAC11 and all single mutants upon MG132 treatment, suggesting that there were multiple ubiquitination sites within HDAC11 for proteasomal degradation (Fig. 3A). Studies from mass spectrometry indicated that HDAC11 was ubiquitinated at multiple sites (K33, 50, 84, 237, 280, and 306) (55, 57), thus we screened the ubiquitination sites with double mutations. Among the double mutants, a mutant K50RK280R was less accumulated compared with that of K41R/K44R and K50RK53R mutants, suggesting that both K50 and K280 participated in the process of ubiquitination in Beas-2B cells (Fig. 3A). CHX studies showed that the K50RK280R mutant appeared to have a prolonged half-life compared with the WT HDAC11, (Fig. 3B). In addition, K50RK280R mutant showed a lower level of ubiquitination (Fig. 3C). Further observations showed that ubiquitin K48-linkage, not the K63-linkage, contributed to the ubiquitin-HDAC11 association (Fig. 3, D and E). In all, these data indicated that both K50 and K280 were the potential ubiquitin-accepting sites within HDAC11 protein that mediated its proteasomal degradation.
Fig. 3.
Histone deacetylase 11 (HDAC11) protein was polyubiquitinated at K50 and K280. A: wild-type (WT) pcDNA3.1D-His-V5/HDAC11 plasmids or the mutants were delivered into BEAS-2B cells using electroporation as indicated. After 48 h, the cells were treated with MG132 for 2 h. The lysates were analyzed using V5 and GAPDH immunoblotting. B: WT or K50RK280R mutant HDAC11 plasmids were delivered into BEAS-2B cells. After 48 h, the cells were treated with cycloheximide (CHX) at four time points. The cell lysates were analyzed using V5 and GAPDH immunoblotting. C: WT, K50R, and K50RK280R HDAC11 plasmids were delivered into BEAS-2B cells. After 48 h, the cell lysates were subjected to V5 immunoprecipitation (IP) followed by ubiquitin (Ub) and V5 immunoblotting (IB). D and E: WT HDAC11 plasmids were delivered into BEAS-2B cells for 48 h. Equal amounts of cell lysates were subjected to V5 immunoprecipitation. Immunoblotting analysis used Ub-K48 linkage antibody (D) or Ub-K63 linkage antibody (E) separately. Results were representative of n = 3 experiments.
USP25 Associated with and Deubiquitinated HDAC11
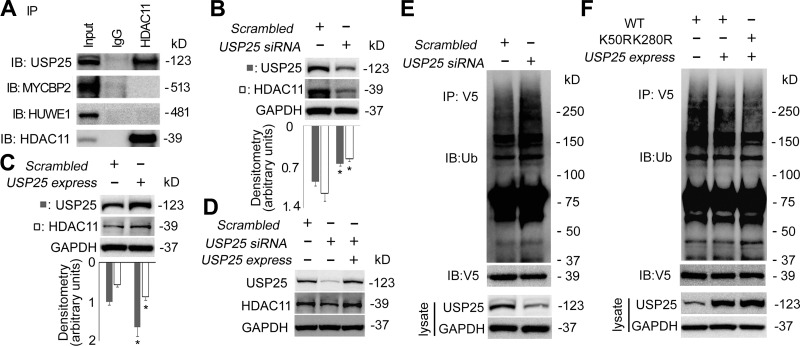
In general, polyubiquitinated proteins were quickly subjected to subsequent degradation; however, this process was exquisitely controlled and polyubiquitination was reversible. A family of enzymes, referred to as deubiquitinating enzymes, catalyzed this reversible process by removing the polyubiquitin moieties to regulate the degrading-protein pool (37). To study whether HDAC11 was regulated by deubiquitinating enzymes, we conducted immunoprecipitation and identified that USP25 was associated with HDAC11 (Fig. 4A). However, results from our co-IP studies were unable to identify two E3 ubiquitin ligases (MYCBP2 and HUWE1) that were reported to be associated with HDAC11 (29), possibly due to the huge molecular weight of the E3 ubiquitin ligases or distinct cell lines. To further elucidate the presumption that USP25 protein was a stabilizing contributor to HDAC11 protein, we knocked down or overexpressed USP25 in Beas-2B cells and observed whether USP25 affected HDAC11 protein stability. Immunoblotting results showed that HDAC11 protein level was highly correlated to USP25 protein level. Silence of USP25 led to a decrease of HDAC11 as the overexpression of USP25 increased HDAC11 at protein level (Fig. 4, B and C). Ectopic expression of USP25 rescued the USP25 siRNA-led decrease of HDAC11 protein to an untreated level, suggesting that USP25 specifically regulated the protein stability of HDAC11 (Fig. 4D). In addition, silence of USP25 by siRNA increased the ubiquitination level of HDAC11 protein (Fig. 4E). Overexpression of USP25 protein led to less ubiquitination of WT HDAC11 protein at a comparable level of that in K50RK280R double mutant, suggesting the deubiquitination mainly happened at these two sites (Fig. 4F). Overall, these data indicated that the USP25 mediated HDAC11 deubiquitination to modulate HDAC11 stability.
Fig. 4.
Ubiquitin-specific protease 25 (USP25) interacted with and deubiquitinated histone deacetylase 11 (HDAC11). A: BEAS-2B cell lysates were coimmunoprecipitated (co-IP) with HDAC11 antibody. The immunoprecipitants were analyzed with USP25, MYCBP2, HUWE1, and HDAC11 immunoblotting (IB) as indicated. B: BEAS-2B cells were transfected with scrambled-siRNA or USP25-siRNA for 72 h. Cells were harvested and immunoblotting analyses were conducted with the indicated antibodies. C: BEAS-2B cells were transfected with empty or pCMV6-XL5/USP25 plasmids for 72 h. Cell lysates were analyzed by immunoblotting with indicated antibodies. D: BEAS-2B cells were transfected with scrambled, USP25-siRNA, or USP25-siRNA plus pCMV6-XL5/USP25 plasmids for 72 h. Cell lysates were analyzed by immunoblotting with indicated antibodies. E: wild-type (WT) pcDNA3.1D-His-V5/HDAC11 plasmids with Scrambled-siRNA or USP25 siRNA were codelivered into BEAS-2B cells for 72 h. Cell lysates were subject of anti-V5 co-IP, and the immunoprecipitants were subjected to immunoblotting analysis as indicated. Part of the cell lysates were analyzed with immunoblotting as indicated. F: WT or K50RK280R mutant pcDNA3.1D-His-V5/HDAC11 plasmids were codelivered with or without pCMV6-XL5/USP25 plasmids into BEAS-2B cells for 72 h. Cell lysates were subjected to anti-V5 co-IP or immunoblotting to check the expression of USP25. The immunoprecipitants were analyzed with ubiquitin or V5 immunoblotting. Results are representative of n = 3 experiments.
CSE Promoted USP25 Ubiquitination and Degradation
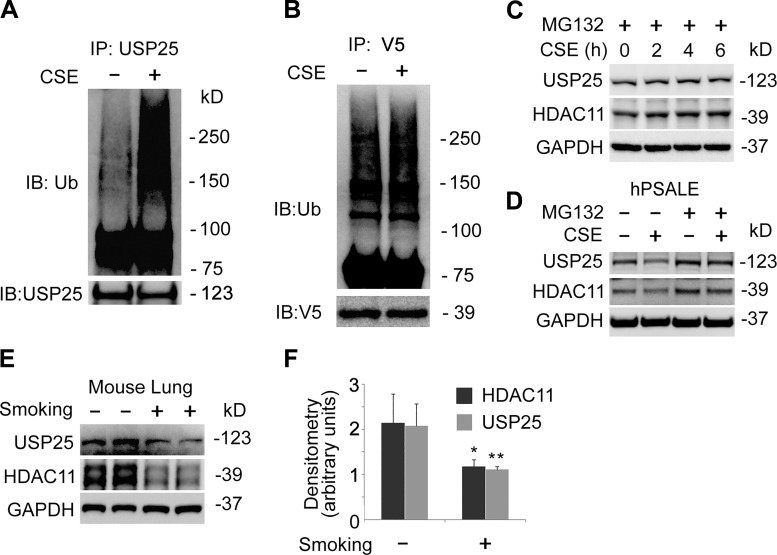
To determine if USP25 contributed to a CSE-mediated decrease of the HDAC11 protein, we treated the cells with CSE and analyzed the cell lysate with immunoblotting. Results showed that USP25 was unstable in a CSE concentration-dependent and time-dependent manner (Fig. 5, A and B). Meanwhile, USP25 mRNA was not significantly affected by CSE treatment, suggesting that CSE-reduced USP25 occurred possibly at posttranslational level (Fig. 5C). To verify our observation in the BEAS-2B cells, we observed that CSE decreased both USP25 and HDAC11 at the protein levels in both concentration and time course studies in human primary small airway epithelial cells (Fig. 5, D and E). Notably, CSE decreased more HDAC11 compared with USP25, suggesting CSE may have regulated HDAC11 protein via USP25. Further observations demonstrated that USP25, as well as HDAC11 protein, was polyubiquitinated in CSE-treated BEAS-2B cells (Fig. 6, A and B). Blockade of the proteasome degradation pathway with the proteasome inhibitor, MG132, protected USP25 and HDAC11 proteins from CSE-mediated degradation (Fig. 6C). We observed similar results in human primary small airway epithelial cells as well (Fig. 6D). In addition, we observed that both USP25 and HDAC11 proteins were downregulated in smoking mouse lung tissues (Fig. 6, E and F). These data suggested that CSE degraded USP25, as well as HDAC11, in lung epithelial cells.
Fig. 5.
Cigarette smoke extract (CSE) destabilized histone deacetylase 11 (HDAC11) and ubiquitin-specific protease 25 (USP25) proteins. A: BEAS-2B cells were treated with CSE at various concentrations as indicated. Cells lysates were analyzed with USP25, HDAC11, or GAPDH immunoblotting. The densitometry results were plotted in the lower panel. B: BEAS-2B cells were treated with CSE at four time points as indicated. Cells lysates were analyzed by USP25, HDAC11, or GAPDH immunoblotting. Densitometry results are plotted in the lower panel. C: total RNA was extracted from untreated and CSE-treated BEAS-2B cells (6% for 6 h). USP25 mRNA levels were determined with quantitative RT-PCR. D and E: human primary small airway epithelial cells were treated with CSE in concentration (D) and time courses (E). Cell lysates were analyzed with immunoblotting as indicated. Results are representative of n = 3 experiments. hPSALE, human primary small airway lung epithelium.
Fig. 6.
Cigarette smoke extract (CSE) increased ubiquitination of histone deacetylase 11 (HDAC11) and ubiquitin-specific protease 25 (USP25) proteins. A: Beas-2B cells were treated with or without CSE (6% for 6 h) and cell lysates were subjected to USP25 co-IP, followed by ubiquitin and USP25 immunoblotting (IB: Ub). B: pcDNA3.1D-His-V5/HDAC11 plasmids were delivered to BEAS-2B cells for 48 h followed by CSE treatment (6% for 6 h). Cell lysates were subjected to V5 coimmunoprecipitation (IP), followed by ubiquitin and V5 immunoblotting. C: BEAS-2B cells were treated with MG132 and CSE, as indicated. Cells lysates were immunoblotting analyzed as indicated. D: human primary small airway epithelial cells were treated with CSE and MG132, as indicated. Cell lysates were analyzed with immunoblotting as indicated. E and F: proteins were extracted from nonsmoking or smoking mouse lung (n = 6) and subjected to USP25, HDAC11, or GAPDH immunoblotting. Representative immunoblotting results (E) and densitometric results of the immunoblotting (F) are shown. Results are representative of n = 3 experiments except for E and F n = 6 experiments. hPSALE, human primary small airway lung epithelium. *P < 0.05 and **P < 0.01.
CSE Regulated P. Aeruginosa Load via USP25/HDAC11 Axis in Beas-2B Cells
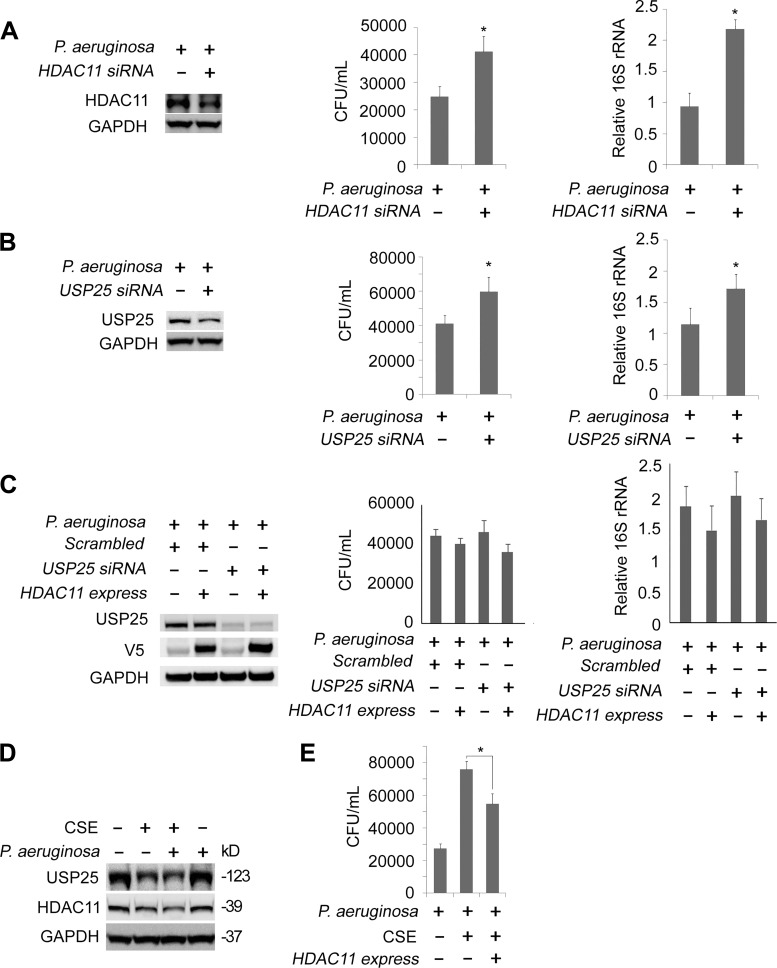
Clinical studies and observation in mouse models indicated that cigarette smoking increased the susceptibility of bacterial infections in the lungs (1, 12, 19, 25). We theorized that CSE may impact P. aeruginosa load in lung epithelial cells via the function of the USP25/HDAC11 axis. For starters, we tested whether the silencing of HDAC11 affected the CSE-induced internalized P. aeruginosa load. HDAC11 was successfully silenced in Beas-2B cells by the siRNA (Fig. 7A, left panels). We challenged the cells with P. aeruginosa and observed a higher CFU value and higher 16S rRNA level in HDAC11-silenced cells compared with the untreated cells (Fig. 7A, middle and right panels). We observed similar results in USP25 depleted cells (Fig. 7B, left, middle, and right panels). Moreover, USP25 silence-mediated augment of bacterial load was reversed by ectopic expression of HDAC11 (Fig. 7C). Finally, we tested whether the ectopic express of HDAC11 affected CSE-induced internalized P. aeruginosa load. P. aeruginosa infection did not substantially affect CSE-mediated decrease of HDAC11 and USP25 at protein levels (Fig. 7D). Consistent with our above observations, ectopic expression of the HDAC11 partially reduced the CSE-mediated bacterial infection, which might be explained by an impaired capability of clearance (Fig. 7E). These results suggested that the USP25/HDAC11 axis participated in the controlling of P. aeruginosa load in CSE-treated BEAS-2B cells.
Fig. 7.
Cigarette smoke extract (CSE) destabilized USP25/histone deacetylase 11 (HDAC11) to modulate bacterial load in BEAS-2B cells. A: scrambled-siRNA or HDAC11-siRNA was delivered to BEAS-2B cells for 72 h. Cells were infected with P. aeruginosa for 90 min and subjected to Gentamicin Protection Assay. The efficacy of HDAC11 knockdown was checked by immunoblotting (left). The numbers of colony-forming units (CFU) and relative 16S rRNA levels of P. aeruginosa are shown (middle and right). B: scrambled-siRNA or ubiquitin-specific protease 25 (USP25)-siRNA were delivered to BEAS-2B cells for 72 h. Cells were subjected to P. aeruginosa infection followed by Gentamicin Protection Assay as in A. The efficacy of HDAC11 knockdown was checked by immunoblotting (left). The numbers of CFU and relative 16S rRNA levels of P. aeruginosa are illustrated (middle and right). C: scrambled-siRNA or USP25-siRNA were codelivered with or without wild-type HDAC11 plasmids into BEAS-2B cells. Cells were subjected to P. aeruginosa infection followed by Gentamicin Protection Assay as in A. The efficacy of USP25 knockdown and HDAC11 express were checked by immunoblotting (left). The numbers of CFU and relative 16S rRNA levels of P. aeruginosa are illustrated (middle and right). D: BEAS-2B cells were treated with or without CSE for 3 h followed by P. aeruginosa infection for another 90 min, as indicated. Cells lysates were analyzed with USP25, HDAC11, or GAPDH immunoblotting. E: BEAS-2B cells were predelivered with or without HDAC11 plasmids for 48 h followed by CSE treatment or P. aeruginosa infection, as indicated. The numbers of CFU are illustrated. Values are means ± SD. *P < 0.05. Results are representative of n = 3 experiments.
DISCUSSION
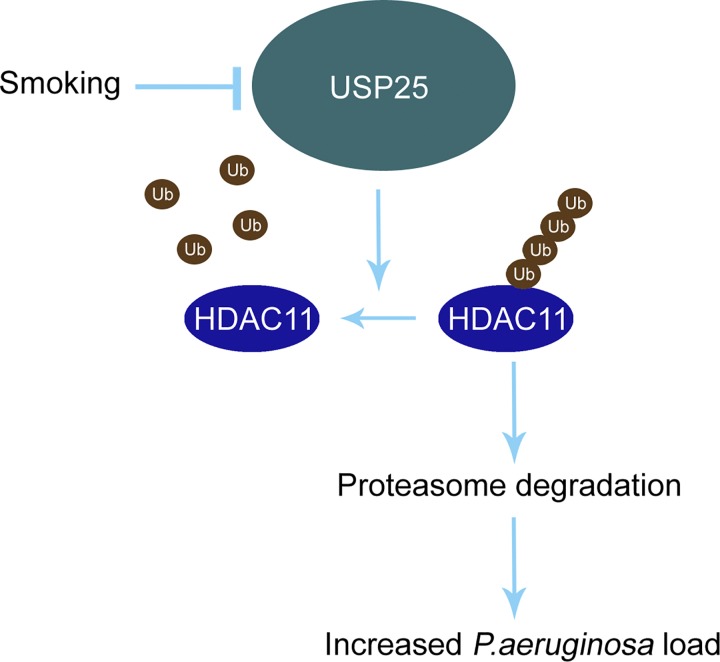
In this study, we identified that 1) CSE reduced an unstable protein HDAC11 in BEAS-2B cells, 2) USP25 interacted with HDAC11 and deubiquitinated HDAC11 at sites K50 and K280, 3) CSE-mediated USP25 polyubiquitination and subsequent degradation, and 4) CSE impaired defensive abilities to P. aeruginosa invasion via USP25/HDAC11 signaling in BEAS-2B cells (Fig. 8). Many studies indicated that cigarette smoking increased the susceptibility of microbial infections in lower respiratory airway. Meanwhile, recurrent infections may have elicited dysregulated inflammation reactions that compromised the clearance of exotic pathogens, which may have contributed to the higher morbidity of cigarette smoking–related COPD (7, 39, 47, 48). It has been noticed that cigarette smoke affected the clearance and inflammation of the P. aeruginosa infection. So far, several factors were linked to this process. Reduced levels of hBD-2 (β defensin-2) in pharyngeal washing fluid and sputum from patients with acute pneumonia were identified in current or former smokers that resulted in the smoke-induced inhibition of epithelial host defense against bacterial infections (25). Smoke exposure reduced the fatty acid binding protein 5 (FABP5) gene in C. elegans and it was lower in smoker COPD compared with nonsmoker COPD (16, 21), promoting the P. aeruginosa infection. Grumelli (22) reported that choline triggered exacerbation of COPD in patients infected with P. aeruginosa. Except for epithelial cells, several studies reported that cigarette smoke may have impacted bacterial clearance in alveolar macrophages in COPD mouse models (5, 24). Among them, NRF2 (nuclear erythroid factor 2), TFEB (transcription factor EB), and oxygen (3, 43, 58) may have affected macrophage-derived bacterial clearance following cigarette exposure. Here, we investigated the potential molecular mechanism that cigarette smoke increased the susceptibility of bacterial infections via the USP25/HDAC11 pathway. It has been known that epigenetic changes were involved in the defensive action to bacterial invasion. There were complex mechanisms that the histone acetyltransferase coordinated with the histone deacetylase to tune the expression of inflammatory genes by regulating accessibility of the chromatin structure (50). Accelerated turnover of HDACs may have disrupted the expression of inflammatory genes that leads to hyper- or hyposensitivity to xenobiotics, which were linked to unexpected damage of healthy tissues and an increased amount of intracellular bacteria (20). A clinical study showed that HDAC activity was decreased in lungs from patients with COPD compared with healthy nonsmokers (28). Among the decreased HDAC enzymes, it is believed that HDAC2 played a pivotal role in provoking inflammatory gene transcription in COPD patients that accelerated and worsened the progress of exacerbation in COPD (4). In consistency, results from our cellular and mouse experiments demonstrated that HDAC11 protein was downregulated in a smoking mouse compared with a nonsmoking mouse.
Fig. 8.
Cigarette smoke extract (CSE) modulated P. aeruginosa load via ubiquitin-specific protease 25 (USP25)/histone deacetylase 11 (HDAC11) axis. HDAC11 protein level was stringently regulated by ubiquitin proteasomal degradation system. Smoking enhanced polyubiquitination and thereafter, degradation of USP25. Lower USP25 protein level compromised the stability of HDAC11, which led to increments of P. aeruginosa load in Beas-2B cells.
How HDAC11 protein is reprogrammed in pathophysiological settings is largely unknown, though our study indicated that HDAC11 was a labile protein with a half-life of ~4 h. HDAC11 was polyubiquitinated, and our evidence from site-directed mutagenesis studies suggested that HDAC11 was ubiquitinated at both K50 and K280. Polyubiquitinated HDAC11 was degraded via proteasomal machinery. The E3 ubiquitin ligase(s) for HDAC11 polyubiquitination is yet to be studied. Affinity purification studies reported that two E3 ubiquitin ligases (MYCBP2 and HUWE1) were associated with HDAC11 in T cells (29). However, results from our co-IP studies were unable to confirm this observation, possibly due to the huge molecular weight of the E3 ubiquitin ligases or due to the use of distinct cell lines. USP25 interacted with and removed ubiquitin moieties from HDAC11, thereby stabilizing the protein from degradation. Cigarette smoke promoted USP25 ubiquitination and proteasomal degradation, enhancing the degradation of HDAC11. Reduced USP25/HDAC11 increased the bacterial load in P. aeruginosa–infected lung epithelial cells, suggesting that the USP25/HDAC11 axis may have played an important role in increased susceptibility to bacterial infections in COPD patients.
Among the HDAC family members, the biological function of HDAC11 is the least studied. Recent studies linked HDAC11 with gene transcription, development, metabolism, and immunity. It appeared that HDAC11 played distinct and contradictory roles in the regulation of immunity (63). HDAC11 targeted an anti-inflammatory cytokine, IL-10, at the promoter region to negatively regulate IL-10 transcription in antigen-presenting macrophages under LPS stimulation (33, 59). HDAC11 played an essential role in neutrophil biology, such as how high expression of HDAC11 in neutrophils contributed to neutrophil activation (46). A recent study using KO techniques suggested that null of HDAC11 increased the proliferation of T cells and expression of interferon-γ, tumor necrosis factor α, and T cell activation–related transcript factors, eomesodermin, and TBX21 (61). In addition, adoptive transfer of HDAC11 KO T cells markedly reduced tumor burden in a murine B cell lymphoma model. Our study suggested that HDAC11 linked to bacterial invasion in lung epithelial cells. Depletion of HDAC11 by siRNA increased bacterial load in lung small airway epithelial Beas-2B cells. Lung epithelial cells, as the first defensive barrier, may have contributed to increased inflammation, caused by bacterial infections in COPD. Respiratory ducts are an open system exposed to numerous bacterial pathogens, and P. aeruginosa is known to be internalized in lung epithelial cells (6). The molecular mechanisms of bacterial invasion to epithelial cells are complex and could be classified in several steps. At first contact, bacteria adhere to the surface of the epithelial cell using their flagella. The bacteria are then internalized or penetrate the cellular membrane. Finally, the bacteria may survive by escaping the clearance mechanism from host cells and replicate in the cells (6, 27). In a healthy human being, a decent inflammatory reaction would be orchestrated, and bacteria would be eradicated both inside and outside of the epithelial cells. Our data suggested that USP25 and HDAC11 may have participated in this process by modulation of the intracellular bacteria load, thus affecting COPD advance. Cigarette smoking may have damaged the innate immunity of epithelial cells by downregulation of USP25 and HDAC11, which impaired their normal function against bacterial colonization. That resulted in P. aeruginosa proliferation, causing persistent unresolved inflammation. HDAC family members including type I subfamily member HDAC4 are known to modulate host immunity in microbial infections. How the USP25/HDAC11 axis affected the intracellular bacteria burden is still opaque. Latest studies reported that HDAC11 regulated a crucial cytokine type 1 interferon-mediated signaling in epithelial cells to control viral infections (10). Type 1 interferon is known to be an important factor against viral and bacterial infection. HDAC11 catalyzed a new posttranslational modification, which is defatty-acylation of SHMT2 to regulate type 1 interferon. A similar study reported that HDAC11 may have affected the gene transcription of interferon-stimulated genes, IFITM3 and ISG15, thus modulating the microbial infection (40). These reports are in line with our findings that HDAC11 may be crucial in high susceptibility of bacterial infection in the cigarette smoking population.
GRANTS
This work was supported in part by National Institutes of Health National Heart, Lung, and Blood Institute R01 Grants HL125435 and HL142997 (to C. Zou) and US Department of Veterans Affairs Grants 5I01CX000105-06 and CX001048 (to T. Nyunoya).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.Z. conceived and designed research; C.L. and Y.L. performed experiments; C.L. and T.L. analyzed data; C.L., Y.L., T.N., and C.Z. interpreted results of experiments; C.L. prepared figures; C.L. drafted manuscript; Y.L., T.L., T.N., and C.Z. edited and revised manuscript; C.L., Y.L., T.L., T.N., and C.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of C. Long: Dept. of Minimally Invasive Surgery, the Second Xiangya Hospital, Central South University, Changsha, Hunan 41001, China.
REFERENCES
- 1.Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis 4: 12, 2008. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagchi RA, Ferguson BS, Stratton MS, Hu T, Cavasin MA, Sun L, Lin YH, Liu D, Londono P, Song K, Pino MF, Sparks LM, Smith SR, Scherer PE, Collins S, Seto E, McKinsey TA. HDAC11 suppresses the thermogenic program of adipose tissue via BRD2. JCI Insight 3: e120159, 2018. doi: 10.1172/jci.insight.120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain WG, Tripathi A, Mandke P, Gans JH, D’Alessio FR, Sidhaye VK, Aggarwal NR. Low-dose oxygen enhances macrophage-derived bacterial clearance following cigarette smoke exposure. J Immunol Res 2016: 1, 2016. doi: 10.1155/2016/1280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol 71: 451–464, 2009. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 5.Basilico P, Cremona TP, Oevermann A, Piersigilli A, Benarafa C. Increased myeloid cell production and lung bacterial clearance in mice exposed to cigarette smoke. Am J Respir Cell Mol Biol 54: 424–435, 2016. doi: 10.1165/rcmb.2015-0017OC. [DOI] [PubMed] [Google Scholar]
- 6.Bauman SJ, Kuehn MJ. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol 9: 26, 2009. doi: 10.1186/1471-2180-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 12, Suppl 2: S169–S175, 2015. doi: 10.1513/AnnalsATS.201503-126A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buglio D, Khaskhely NM, Voo KS, Martinez-Valdez H, Liu YJ, Younes A. HDAC11 plays an essential role in regulating OX40 ligand expression in Hodgkin lymphoma. Blood 117: 2910–2917, 2011. doi: 10.1182/blood-2010-08-303701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byun SK, An TH, Son MJ, Lee DS, Kang HS, Lee EW, Han BS, Kim WK, Bae KH, Oh KJ, Lee SC. HDAC11 inhibits myoblast differentiation through repression of MyoD-dependent transcription. Mol Cells 40: 667–676, 2017. doi: 10.14348/molcells.2017.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J, Sun L, Aramsangtienchai P, Spiegelman NA, Zhang X, Huang W, Seto E, Lin H. HDAC11 regulates type I interferon signaling through defatty-acylation of SHMT2. Proc Natl Acad Sci USA 116: 5487–5492, 2019. doi: 10.1073/pnas.1815365116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing T, Dong L, Tang E, Yang H. Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res Treat 104: 21–30, 2007. doi: 10.1007/s10549-006-9393-7. [DOI] [PubMed] [Google Scholar]
- 12.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stämpfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 170: 1164–1171, 2004. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]
- 13.Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect 67: 169–184, 2013. doi: 10.1016/j.jinf.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, Arai Y, Takahashi H, Shirakihara T, Nagasaki M, Shibuya T, Nakano K, Watanabe-Makino K, Tanaka H, Nakamura H, Kusuda J, Ojima H, Shimada K, Okusaka T, Ueno M, Shigekawa Y, Kawakami Y, Arihiro K, Ohdan H, Gotoh K, Ishikawa O, Ariizumi S, Yamamoto M, Yamada T, Chayama K, Kosuge T, Yamaue H, Kamatani N, Miyano S, Nakagama H, Nakamura Y, Tsunoda T, Shibata T, Nakagawa H. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 44: 760–764, 2012. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 15.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 17: 195–211, 2007. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 16.Gally F, Chu HW, Bowler RP. Cigarette smoke decreases airway epithelial FABP5 expression and promotes Pseudomonas aeruginosa infection. PLoS One 8: e51784, 2013. doi: 10.1371/journal.pone.0051784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem 277: 25748–25755, 2002. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Vidal C, Almagro P, Romaní V, Rodríguez-Carballeira M, Cuchi E, Canales L, Blasco D, Heredia JL, Garau J. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J 34: 1072–1078, 2009. doi: 10.1183/09031936.00003309. [DOI] [PubMed] [Google Scholar]
- 19.Garmendia J, Morey P, Bengoechea JA. Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur Respir J 39: 467–477, 2012. doi: 10.1183/09031936.00061911. [DOI] [PubMed] [Google Scholar]
- 20.Grabiec AM, Potempa J. Epigenetic regulation in bacterial infections: targeting histone deacetylases. Crit Rev Microbiol 44: 336–350, 2018. doi: 10.1080/1040841X.2017.1373063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green RM, Gally F, Keeney JG, Alper S, Gao B, Han M, Martin RJ, Weinberger AR, Case SR, Minor MN, Chu HW. Impact of cigarette smoke exposure on innate immunity: a Caenorhabditis elegans model. PLoS One 4: e6860, 2009. doi: 10.1371/journal.pone.0006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grumelli S. Choline triggers exacerbations of chronic obstructive pulmonary disease in patients infected with Pseudomonas aeruginosa. Curr Respir Med Rev 12: 167–174, 2016. doi: 10.2174/1573398X12999160506104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, Jerome JA, Gregory AD, Mallampalli RK. Cigarette smoke destabilizes NLRP3 protein by promoting its ubiquitination. Respir Res 18: 2, 2017. doi: 10.1186/s12931-016-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 3: 78ra32, 2011. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, Schroeder JM, Vogelmeier C, Group RBCS; R Bals for the CAPNETZ Study Group . Suppression of pulmonary innate host defence in smokers. Thorax 64: 144–149, 2009. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa JK, Norris A, Bangera MG, Geiss GK, van ’t Wout AB, Bumgarner RE, Lory S. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc Natl Acad Sci USA 97: 9659–9664, 2000. doi: 10.1073/pnas.160140297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967–1976, 2005. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 29.Joshi P, Greco TM, Guise AJ, Luo Y, Yu F, Nesvizhskii AI, Cristea IM. The functional interactome landscape of the human histone deacetylase family. Mol Syst Biol 9: 672, 2013. doi: 10.1038/msb.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasparkova J, Kostrhunova H, Novakova O, Křikavová R, Vančo J, Trávníček Z, Brabec V. A photoactivatable platinum (IV) complex targeting genomic DNA and histone deacetylases. Angew Chem Int Ed Engl 54: 14478–14482, 2015. doi: 10.1002/anie.201506533. [DOI] [PubMed] [Google Scholar]
- 31.Lai Y, Li J, Li X, Zou C. Lipopolysaccharide modulates p300 and Sirt1 to promote PRMT1 stability via an SCFFbxl17-recognized acetyldegron. J Cell Sci 130: 3578–3587, 2017. doi: 10.1242/jcs.206904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Tan Q, Yan M, Liu L, Lin H, Zhao F, Bao G, Kong H, Ge C, Zhang F, Yu T, Li J, He X, Yao M. miRNA-200c inhibits invasion and metastasis of human non-small cell lung cancer by directly targeting ubiquitin specific peptidase 25. Mol Cancer 13: 166, 2014. doi: 10.1186/1476-4598-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian ZR, Xu YF, Wang XB, Gong JP, Liu ZJ. Suppression of histone deacetylase 11 promotes expression of IL-10 in Kupffer cells and induces tolerance following orthotopic liver transplantation in rats. J Surg Res 174: 359–368, 2012. doi: 10.1016/j.jss.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Lear T, Zhao Y, Zhao J, Zou C, Chen BB, Mallampalli RK. F-box protein Fbxl18 mediates polyubiquitylation and proteasomal degradation of the pro-apoptotic SCF subunit Fbxl7. Cell Death Dis 6: e1630, 2015. doi: 10.1038/cddis.2014.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long C, Lai Y, Li J, Huang J, Zou C. LPS promotes HBO1 stability via USP25 to modulate inflammatory gene transcription in THP-1 cells. Biochim Biophys Acta Gene Regul Mech 1861: 773–782, 2018. doi: 10.1016/j.bbagrm.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol 192: 5226–5235, 2014. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mevissen TET, Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu Rev Biochem 86: 159–192, 2017. doi: 10.1146/annurev-biochem-061516-044916. [DOI] [PubMed] [Google Scholar]
- 38.Moreno-Yruela C, Galleano I, Madsen AS, Olsen CA. Histone deacetylase 11 Is an ε-N-myristoyllysine hydrolase. Cell Chem Biol 25: 849–856.e8, 2018. doi: 10.1016/j.chembiol.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 853–860, 2008. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 40.Nutsford AN, Galvin HD, Ahmed F, Husain M. The Class IV human deacetylase, HDAC11, exhibits anti-influenza A virus properties via its involvement in host innate antiviral response. Cell Microbiol 21: e12989, 2019. doi: 10.1111/cmi.12989. [DOI] [PubMed] [Google Scholar]
- 41.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 173: 1114–1121, 2006. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 42.Pavord ID, Jones PW, Burgel PR, Rabe KF. Exacerbations of COPD. Int J Chron Obstruct Pulmon Dis 11: 21–30, 2016. doi: 10.2147/COPD.S85978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pehote G, Bodas M, Brucia K, Vij N. Cigarette smoke exposure inhibits bacterial killing via TFEB-mediated autophagy impairment and resulting phagocytosis defect. Mediators Inflamm 2017: 1, 2017. doi: 10.1155/2017/3028082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget 8: 268–284, 2017. doi: 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Y, Zhao Y, Lin D, Xu X, Zhu Q, Yao J, Shu HB, Zhong B. The Type I interferon-IRF7 axis mediates transcriptional expression of Usp25 gene. J Biol Chem 291: 13206–13215, 2016. doi: 10.1074/jbc.M116.718080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahakian E, Chen J, Powers JJ, Chen X, Maharaj K, Deng SL, Achille AN, Lienlaf M, Wang HW, Cheng F, Sodré AL, Distler A, Xing L, Perez-Villarroel P, Wei S, Villagra A, Seto E, Sotomayor EM, Horna P, Pinilla-Ibarz J. Essential role for histone deacetylase 11 (HDAC11) in neutrophil biology. J Leukoc Biol 102: 475–486, 2017. doi: 10.1189/jlb.1A0415-176RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos S, Marin A, Serra-Batlles J, de la Rosa D, Solanes I, Pomares X, López-Sánchez M, Muñoz-Esquerre M, Miravitlles M. Treatment of patients with COPD and recurrent exacerbations: the role of infection and inflammation. Int J Chron Obstruct Pulmon Dis 11: 515–525, 2016. doi: 10.2147/COPD.S98333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sethi S. Infection as a comorbidity of COPD. Eur Respir J 35: 1209–1215, 2010. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 49.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6: a018713, 2014. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol 32: 335–343, 2011. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2: 372–377, 2002. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 52.Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health 15: 1033, 2018. doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sumanasekera WK, Tran DM, Sumanasekera TU, Le N, Dao HT, Rokosh GD. Cigarette smoke adversely affects functions and cell membrane integrity in c-kit+ cardiac stem cells. Cell Biol Toxicol 30: 113–125, 2014. doi: 10.1007/s10565-014-9273-6. [DOI] [PubMed] [Google Scholar]
- 54.Sun L, Marin de Evsikova C, Bian K, Achille A, Telles E, Pei H, Seto E. Programming and regulation of metabolic homeostasis by HDAC11. EBioMedicine 33: 157–168, 2018. doi: 10.1016/j.ebiom.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan F, Lu L, Cai Y, Wang J, Xie Y, Wang L, Gong Y, Xu BE, Wu J, Luo Y, Qiang B, Yuan J, Sun X, Peng X. Proteomic analysis of ubiquitinated proteins in normal hepatocyte cell line Chang liver cells. Proteomics 8: 2885–2896, 2008. doi: 10.1002/pmic.200700887. [DOI] [PubMed] [Google Scholar]
- 56.Valero R, Marfany G, González-Angulo O, González-González G, Puelles L, Gonzàlez-Duarte R. USP25, a novel gene encoding a deubiquitinating enzyme, is located in the gene-poor region 21q11.2. Genomics 62: 395–405, 1999. doi: 10.1006/geno.1999.6025. [DOI] [PubMed] [Google Scholar]
- 57.Vasilescu J, Smith JC, Ethier M, Figeys D. Proteomic analysis of ubiquitinated proteins from human MCF-7 breast cancer cells by immunoaffinity purification and mass spectrometry. J Proteome Res 4: 2192–2200, 2005. doi: 10.1021/pr050265i. [DOI] [PubMed] [Google Scholar]
- 58.Vij N, Chandramani-Shivalingappa P, Van Westphal C, Hole R, Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol 314: C73–C87, 2018. doi: 10.1152/ajpcell.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, Pinilla-Ibarz J, Seto E, Sotomayor EM. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol 10: 92–100, 2009. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 370: 786–796, 2007. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods DM, Woan KV, Cheng F, Sodré AL, Wang D, Wu Y, Wang Z, Chen J, Powers J, Pinilla-Ibarz J, Yu Y, Zhang Y, Wu X, Zheng X, Weber J, Hancock WW, Seto E, Villagra A, Yu XZ, Sotomayor EM. T cells lacking HDAC11 have increased effector functions and mediate enhanced alloreactivity in a murine model. Blood 130: 146–155, 2017. doi: 10.1182/blood-2016-08-731505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu D, Liu J, Fu T, Shan B, Qian L, Pan L, Yuan J. USP25 regulates Wnt signaling by controlling the stability of tankyrases. Genes Dev 31: 1024–1035, 2017. doi: 10.1101/gad.300889.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanginlar C, Logie C. HDAC11 is a regulator of diverse immune functions. Biochim Biophys Acta Gene Regul Mech 1861: 54–59, 2018. doi: 10.1016/j.bbagrm.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Yuan L, Chen X, Cheng L, Rao M, Chen K, Zhang N, Meng J, Li M, Yang LT, Yang PC, Wang X, Song J. HDAC11 regulates interleukin-13 expression in CD4+ T cells in the heart. J Mol Cell Cardiol 122: 1–10, 2018. doi: 10.1016/j.yjmcc.2018.07.253. [DOI] [PubMed] [Google Scholar]
- 65.Zhong B, Liu X, Wang X, Liu X, Li H, Darnay BG, Lin X, Sun SC, Dong C. Ubiquitin-specific protease 25 regulates TLR4-dependent innate immune responses through deubiquitination of the adaptor protein TRAF3. Sci Signal 6: ra35, 2013. doi: 10.1126/scisignal.2003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou C, Synan MJ, Li J, Xiong S, Manni ML, Liu Y, Chen BB, Zhao Y, Shiva S, Tyurina YY, Jiang J, Lee JS, Das S, Ray A, Ray P, Kagan VE, Mallampalli RK. LPS impairs oxygen utilization in epithelia by triggering degradation of the mitochondrial enzyme Alcat1. J Cell Sci 129: 51–64, 2016. doi: 10.1242/jcs.176701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol 307: L205–L218, 2014. doi: 10.1152/ajplung.00330.2013. [DOI] [PubMed] [Google Scholar]