Abstract
Acute kidney injury (AKI), a serious complication in critically ill patients, is associated with poor clinical outcomes. We explored the hypothesis that β2-microglobulin (β2-MG) is an independent indicator of AKI development and outcomes in patients with intracerebral hemorrhage (ICH) in the neurosurgical intensive care unit (NICU).
Patients with ICH (n = 403) admitted to the NICU of Zhongnan Hospital, Wuhan University, between January 1, 2015 and December 31, 2016 were prospectively enrolled in this single-center, observational study. The primary outcome was the incidence of AKI, secondary outcomes were in-hospital mortality and 1-year mortality (from time of admission).
The overall AKI incidence, in hospital, was 35.2%; patients were diagnosed with stage 1 (22.1%), 2 (5.7%), and 3 (7.4%) AKI. β2-MG levels predicted AKI with an area under the curve of 0.712 (95% confidence interval [CI], 0.652–0.772) and a cut-off of 2026.85 μg/L (sensitivity, 57.5%; specificity, 79.4%). Compared with the group having lower β2-MG values, the group with higher values (β2-MG >2123.50 μg/L) had significantly higher risks of AKI (odds ratio, 2.606; 95% CI, 1.315–5.166), in-hospital mortality (hazard ratio [HR], 2.548; 95% CI, 1.318–4.924), and 1-year mortality (HR, 3.161; 95% CI, 1.781–5.611) in adjusted analyses.
β2-MG levels predict AKI development and outcomes in patients with ICH in the NICU.
Keywords: β2-microglobulin, acute kidney injury, intracerebral hemorrhage, prognosis
1. Introduction
Intracerebral hemorrhage (ICH) is a critical disease, and acute kidney injury (AKI) is a common and serious disorder in critically ill patients that confers a high risk of chronic kidney disease (CKD) that may progress to end-stage renal disease (ESRD) and death.[1–4] A few studies have suggested that AKI is a more common complication in patients with ICH than in those with other types of stroke. However, the data relating AKI risk with ICH are limited.[5,6] Despite increased knowledge regarding the management of critically ill patients, the major obstacle to improving the clinical prognoses of patients with ICH and AKI is the inability to recognize patients at high risk of developing AKI and then to provide early-stage interventions. Despite its limitations, serum creatinine levels remain the clinical parameter used to define AKI. In fact, a rise in the serum creatinine level may not truly reflect kidney damage due to the many confounding factors, such as age, sex, muscle mass, and others. In early-stage AKI, nephron damage may precede changes in serum creatinine levels[7–10]; therefore, a new biomarker that predicts early-stage kidney injury is needed.
β2-microglobulin (β2-MG) is an 11.8 kDa, nonglycosylated polypeptide that is present in all nucleated cells[11,12] and is not affected by sex or age.[13,14] As a low molecular-weight protein, β2-MG is released into circulation at a constant rate, freely filtered by the glomeruli, and completely reabsorbed and catabolized in the renal tubules. These properties may make it an ideal endogenous biomarker for estimating the glomerular filtration rate.[13,15,16] Several studies found that the β2-MG level is a novel, early predictor of AKI in critically ill children,[17] renal allograft patients,[18] and liver transplant patients.[19] Furthermore, clinical studies have investigated β2-MG as a useful biomarker for predicting poor outcomes in patients with kidney (eg, ESRD) and cardiovascular diseases.[20–22]
Thus, the present study attempted to characterize the risk association between β2-MG levels and the incidence of AKI in patients with ICH in the neurosurgical intensive care unit (NICU). We hypothesized that β2-MG is an independent indicator of AKI in patients with ICH and suggests patient prognoses.
2. Materials and methods
2.1. Study design
This single-center, prospective, observational study included all patients, 18 to 90 years old, experiencing spontaneous, nontraumatic ICH admitted to the NICU of Zhongnan Hospital, Wuhan University, between January 1, 2015 and December 31, 2016. All patients who remained in the NICU for >48 hours and demonstrated symptom onset within 24 hours were enrolled. We excluded patients with histories of kidney transplantation, pre-existing hemodialysis or peritoneal dialysis, and missing serum creatinine data.
This study was approved by the hospital's ethics committee, and informed consent was obtained from each study participant.
2.2. Definitions
The kidney disease improving global outcomes (KDIGO) 2012 creatinine criteria were used to define and stage the severity of AKI.[23] The estimated glomerular filtration rate (eGFR) was determined using the baseline plasma creatinine level and calculated using the modification of diet in renal disease formula[24]; this equation is clinically valuable for estimating GFRs in adult patients with stable kidney status.[25] Baseline serum creatinine levels were measured on the first day of hospitalization as was each patient's acute physiology and chronic health evaluation II (APACHE II) score, widely used to describe the severity of disease and evaluate the prognoses of critically ill patients.[26] CKD was diagnosed if the patient had an eGFR of <60 mL/min/1.73 m2) for >3 months before hospital admission, according to the Clinical Practice Guideline for Chronic Kidney Disease.[27] Brain hemorrhages, occurring at >2 sites, were detected using computed tomography. Anemia was defined as hemoglobin levels <120 g/L (males) or <110 g/L (females).
2.3. Data collection
The collected patient data included patient demographics (age, sex), pre-existing co-morbidities (diabetes, hypertension, coronary artery disease, chronic kidney disease), Glasgow Coma Scale and APACHE II scores (determined using validated methods and data from the admission physical exams), the site(s) of ICH-related bleeding determined using computed tomography (basal ganglia, frontal cortex, temporal lobe, parietal lobe, thalamus, multiple brain hemorrhages), serum creatinine values recorded upon NICU admission and routinely until discharge, blood samples upon NICU admission and before the start of treatment, and clinical outcomes (length of NICU stay, AKI occurrence, in-hospital mortality, 1-year mortality).
2.4. Biomarker measurements
Venous blood samples were obtained in plasma collection tubes; β2-MG concentrations were then determined in the central laboratory of Zhongnan Hospital, using a solid-phase, 2-site, chemiluminescent immunometric assay (Siemens Healthcare, Erlangen, Germany). The diagnostic ranges were established by the manufacturer, with serum β2-MG levels of 1000 to 3000 μg/L considered normal. The laboratory investigators were blinded to patient outcomes.
2.5. Outcomes
The primary outcome was the occurrence of AKI, diagnosed using the KDIGO criteria. Secondary outcomes were in-hospital mortality and all-cause mortality between admission and the 1-year follow up. The post-admission complications that were tracked included blood-stream infections, pulmonary infections, wound infections, need for mechanical ventilation, deep venous thrombosis, and death.
2.6. Statistical analysis
We used classification and regression trees (CART) and Chi-square automatic interaction detection (CHAID) to divide patients into 2 groups according to their β2-MG values and clinical outcomes. Continuous variables are presented as means and standard deviations or as medians and interquartile ranges. These variables were analyzed using 2-sample t tests or Mann–Whitney U-tests. Categorical variables, expressed as frequencies and percentages, were compared using the Chi-square test, as appropriate. The associations between β2-MG levels and clinical outcomes were first evaluated using a receiver operating characteristic (ROC) curve analysis to obtain a β2-MG cut-off value. To explore the predictive value of the β2-MG level for predicting AKI and mortality, we calculated the area under the curve (AUC) and recorded the sensitivity and specificity of the value and its corresponding 95% confidence interval (CI). The odds ratio (OR) and 95% CI were calculated using multivariable logistic regression analyses to assess the impact of β2-MG levels on AKI prediction. A univariate Cox logistic regression was used to determine mortality risk factors and identify the statistically significant variables for inclusion in the multivariable Cox proportional hazards analysis. This analysis was used to detect the independent indicators of NICU patient prognoses. The Kaplan–Meier survival analysis and log-rank tests were used to determine the association of β2-MG levels with 1-year mortality. In this study, we analyzed β2-MG levels as both continuous and categorical variables. A 2-tailed P-value <.05 was considered statistically significant; all analyses were performed using SPSS for Windows, version 22.0 (SPSS, Chicago, IL).
3. Results
3.1. Patient characteristics
Within the study period, 447 adult patients with ICH were screened for inclusion into this study. After applying the exclusion criteria, 403 patients (237 men, 166 women; median age, 59 [IQR, 50–69] years) were ultimately included in the analyses (Fig. 1). The locations of the ICH lesions were the basal ganglia (170 patients, 42.2%), brain lobe (82 patients, 20.3%), thalamus (51 patients, 12.7%), multiple brain locations (34 patients, 8.4%), brainstem (29 patients, 7.2%), intraventricular area (19 patients, 4.7%), cerebellum (18 patients, 4.2%).
Figure 1.
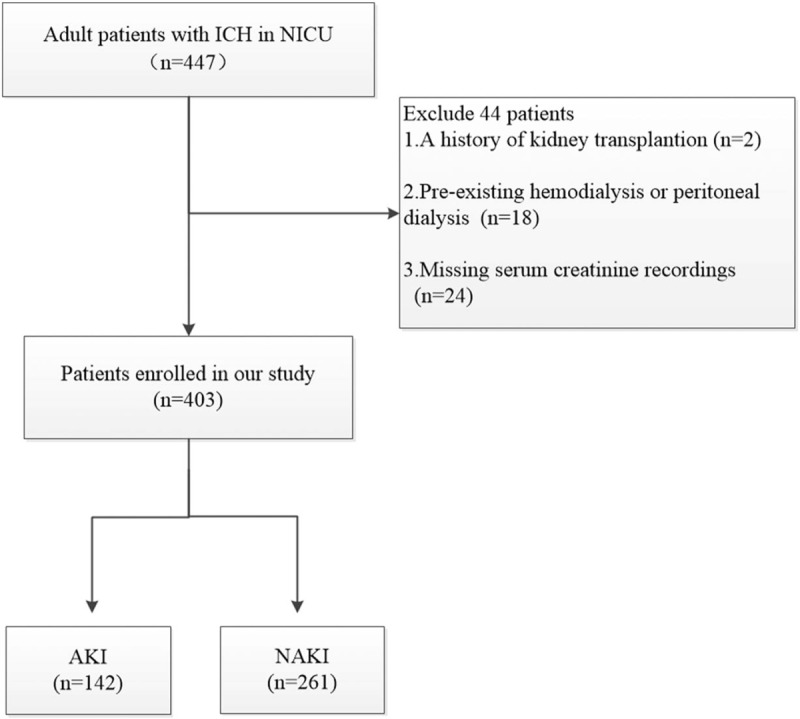
Flow chart for patient enrollment in the study. AKI = acute kidney injury, ICH = intracerebral hemorrhage, NICU = neurosurgical intensive care unit.
3.2. β2-MG as a predictor of AKI
In the study, AKI developed in 142 patients (35.2%), including 89 (22.1%) with stage 1 AKI, 23 (5.7%) with stage 2, and 30 (7.4%) with stage 3 disease. The patients were grouped according to the presence/absence of AKI. Patients with AKI had higher levels of serum creatinine, blood glucose, blood urea nitrogen, uric acid, and β2-MG; they also demonstrated higher leukocyte numbers, a higher mean arterial pressure, and lower serum bicarbonate concentrations. AKI was also more frequent in those with histories of chronic kidney disease or diabetes. In the AKI group, compared with the non-AKI group, the admission APACHE II scores were markedly higher while the Glasgow Coma Scale scores were lower. AKI was more likely to occur in patients with infections and in those who had undergone surgeries or used loop diuretics. Compared with those with AKI, patients without AKI were more frequently exposed to contrast media; the histories of mannitol exposure were similar between the groups (Table 1).
Table 1.
Baseline characteristics of AKI and NAKI patients.
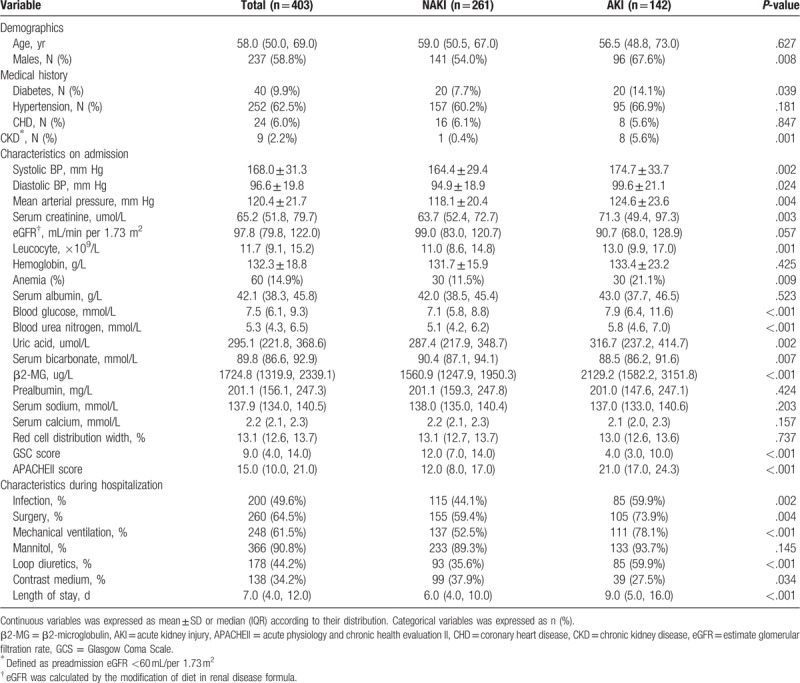
Figure 2 shows that as β2-MG levels increased, so did the risk of developing AKI. The relationship between AKI incidence and serum β2-MG levels was further investigated using multivariate regression. Higher circulating levels of β2-MG were associated with increased AKI risk, after adjusting for other variables. CART and CHAID were used to divide patients with ICH into 2 groups, according to their β2-MG values. Compared with the group with β2-MG levels ≤2123.50 μg/L, the prevalence of AKI (OR, 3.024; 95% CI, 1.504–6.079; P = .002) was higher in the group with β2-MG levels >2123.50 μg/L (Table 3). Furthermore, the β2-MG level measured on the first day of admission was an independent indicator of AKI when we analyzed the β2-MG level as a continuous variable in the regression model (OR, 1.068; 95% CI, 1.029–1.109; P < 001) (Table 2). In an attempt to predict AKI, we used the ROC curve analysis to calculate the optimal β2-MG cut-off; the cut-off value was determined to be 2026.85 μg/L (AUC, 0.712; 95% CI, 0.652–0.772), with a sensitivity of 57.5% and specificity of 79.4%. In the current study, the best cut-off value for the admission APACHE II score for predicting AKI was 17.5 (AUC, 0.823; 95% CI, 0.781–0.864) (Fig. 3A).
Figure 2.
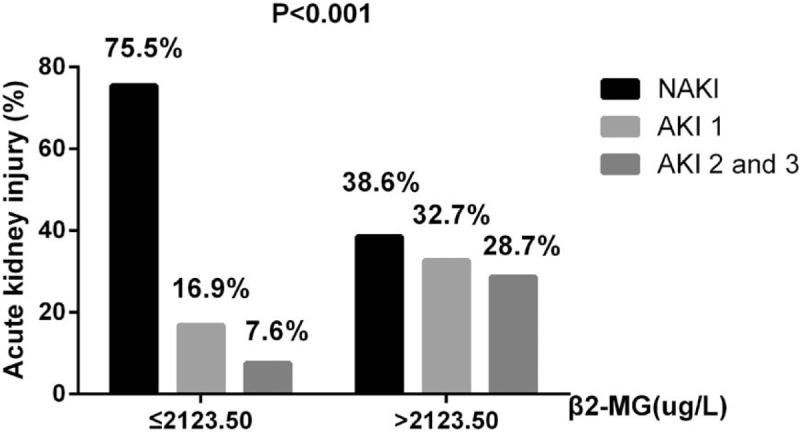
The prevalance of AKI in ICH patients, grouped according to the β2-MG level below or above of 2123.5 ug/L. AKI = acute kidney injury, ICH = intracerebral hemorrhage.
Table 3.
Univariate and multivariate logistic regression analysis of the ability of β2-MG to predict AKI when analyzed as a categorical variable.
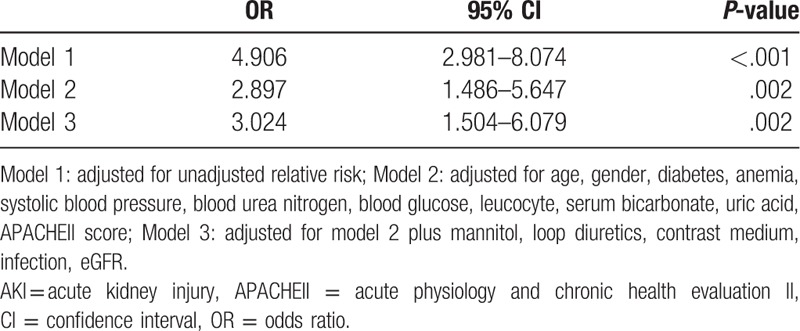
Table 2.
Univariate and multivariate logistic regression analysis of the ability of β2-MG to predict AKI when analyzed as a continuous variable.
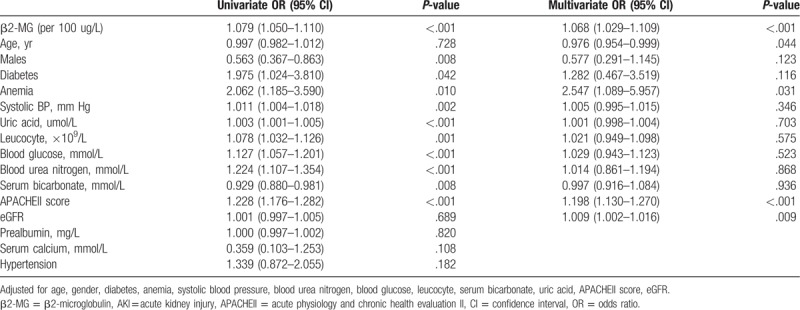
Figure 3.
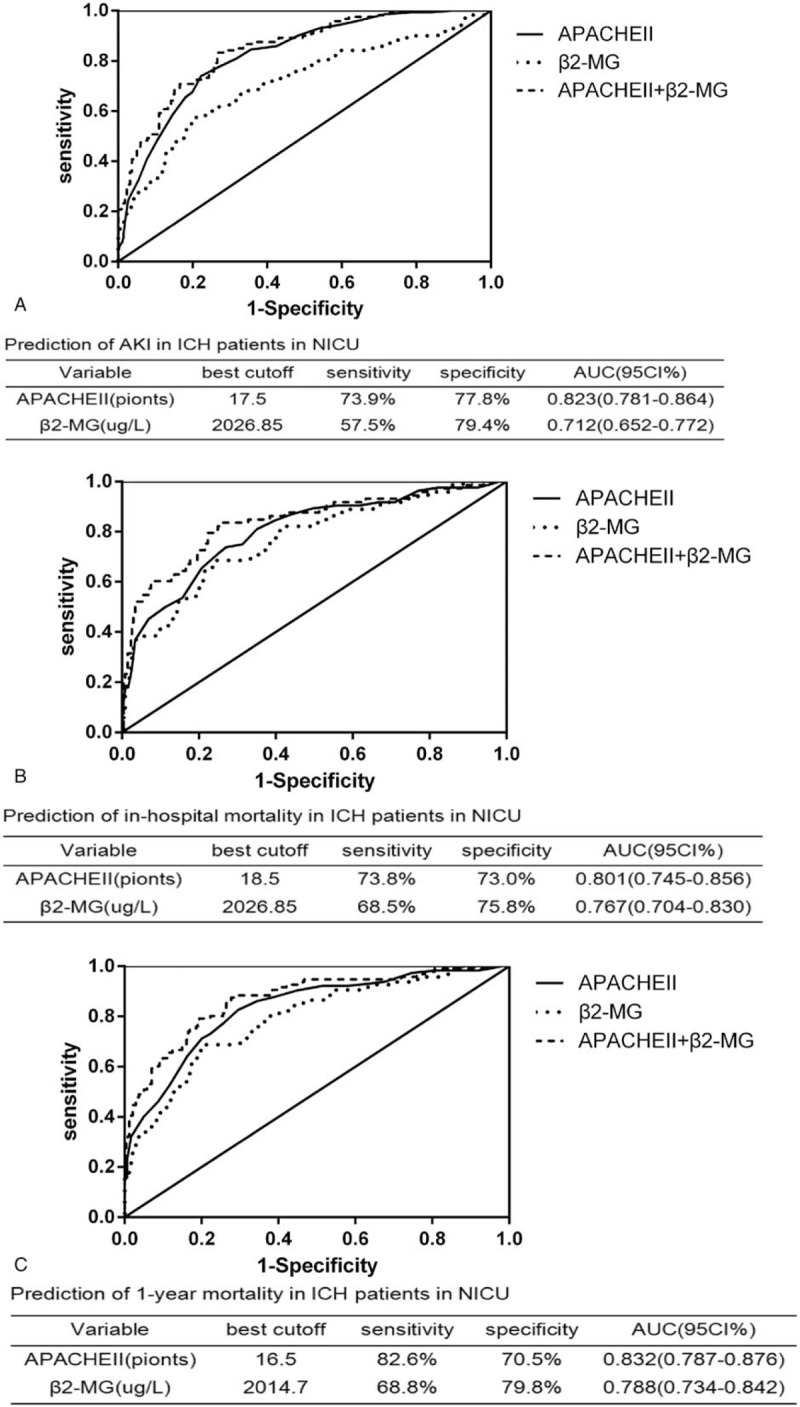
ROC analysis of β2-MG, APACHE II score, and β2-MG plus APACHE II score for AKI detection (A), in-hospital mortality (B) and 1-yr mortality (C) in all ICH patients in NICU. AKI = acute kidney injury, APACHEII = acute physiology and chronic health evaluationn ICH = intracerebral hemorrhage, NICU = neurosurgical intensive care unit, ROC = receiver operating characteristic.
3.3. β2-MG as a predictor of mortality
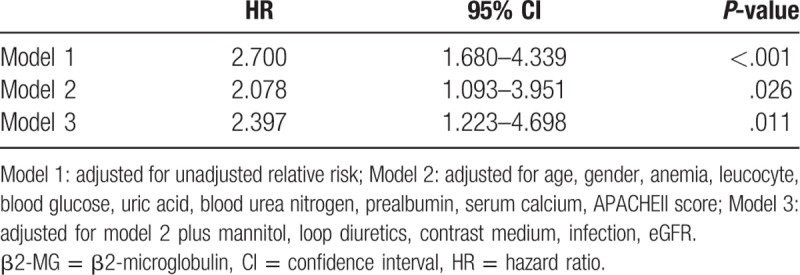
During their hospitalizations, 84 (20.8%) patients died, including 31 (7.7%) in the β2-MG ≤2123.50 μg/L group and 53 (13.1%) in the β2-MG >2123.50 μg/L group. A univariate Cox proportional hazards analysis revealed that high β2-MG levels significantly increased the probability of in-hospital mortality. Further, high β2-MG levels were closely associated with in-hospital mortality, remaining significant after adjusting for age, sex, anemia, leukocyte count, blood glucose level, uric acid level, blood urea nitrogen level, prealbumin level, serum calcium level, and APACHE II scores (HR, 1.010; 95% CI, 1.001–1.020; P = .045) (Table 4). The in-hospital mortality risk in the high β2-MG group remained higher than that in the low β2-MG group even when β2-MG was analyzed as a categorical variable (HR, 2.397; 95% CI, 1.223–4.698; P = .011) (Table 5). The AUCs for the APACHE II and β2-MG ROC curves for predicting of in-hospital mortality were 0.801 (95% CI, 0.745–0.856) and 0.767 (95% CI, 0.704–0.830), respectively (Fig. 3B).
Table 4.
Cox proportional hazards analysis of the ability of β2-MG to predict in-hospital mortality when analyzed as a continuous variable.

Table 5.
Cox proportional hazards analysis of the ability of β2-MG to predict in-hospital mortality when analyzed as a categorical variable.
The median follow-up time for the overall cohort was 9.1 (range, 3.4–11.2) months, with a total of 115 patients (28.5%) dying within 1 year of NICU admission. The Cox proportional hazards analysis of 1-year mortalities is presented in Tables 6 and 7. The 1-year mortality rate was significantly higher among patients with high β2-MG levels than in those with low β2-MG levels (HR, 1.014; 95% CI, 1.005–1.022; P = .001). Further, the β2-MG level was a meaningful prognostic indicator of 1-year mortality (HR, 2.815; 95% CI, 1.568–5.051; P = .001), with a cut-off of 2014.7 μg/L being useful for predicting 1-year mortality (AUC, 0.788; 95% CI, 0.734–0.842) (Fig. 3C). The Kaplan–Meier survival curves, stratified by β2-MG level and AKI stage, are presented in Figure 4, further demonstrating the ability of the β2-MG level to predict the 1-year mortality of patients with ICH in the NICU. The 1-year mortality rate was higher in patients with stage 2–3 AKI than in those without AKI or with stage 1 disease (log rank, P < .001) (Fig. 4A); patients in the low β2-MG group also had a significantly lower 1-year mortality rate (log rank, P < .001) (Fig. 4B). Figure 4C further reveals the additive effect on 1-year mortality risk, for all patients, when the factors are combined. Patients who developed AKI and had an β2-MG value >2123.50 μg/L had the highest 1-year mortality.
Table 6.
Cox proportional hazards analysis of the ability of β2-MG to predict 1-yr mortality when analyzed as a continuous variable.
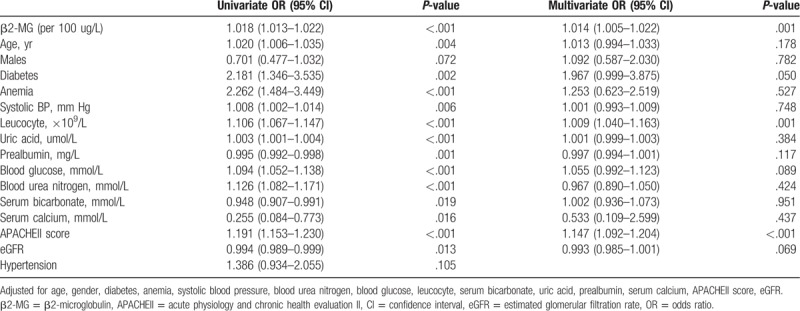
Table 7.
Cox proportional hazards analysis of the ability of β2-MG to predict 1-yr mortality when analyzed as a categorical variable.
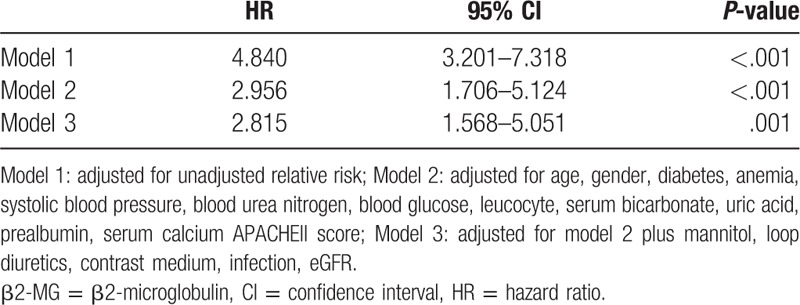
Figure 4.
Kaplan–Meier plots for cumulative 1-yr survival according to the presence of AKI (A), β2-MG category (B) and their combination (C), respectively. AKI = acute kidney injury.
4. Discussion
In this study of 403 patients with ICH, AKI was a frequent complication of those in the NICU (incidence, 35.2%). George et al reported, in a prospective study of 2155 patients with acute, first-ever stroke, reported an AKI incidence of 27%.[5] Moreover, Burgess et al, using the AKI Network criteria to diagnose AKI, recently reported a prospective study that indicated that 31% of patients with ICH developed AKI while hospitalized.[28] The differences in the reported AKI incidences, between the studies, may be related to the sample sizes, AKI diagnostic criteria, stroke types, and so on.
AKI, a common complication after ischemic stroke and ICH, is strongly associated with poor prognoses, including short- and long-term increases in post-stroke mortality.[29,30] Therefore, there is an urgent need to find a reliable method for the early identification of patients with a high AKI risk. Unlike acute coronary syndrome, where biomarkers (such as troponin) for early diagnoses are available, biomarkers predicting AKI are lacking. Despite its unreliability, the serum creatinine level remains the most commonly used indicator of an AKI diagnosis, at present.[31] Hence, this study investigated β2-MG as an alternative and perhaps better predictor of AKI. The major finding of this study is that serum β2-MG levels, upon admission, are related to AKI development in hospitalized patients with ICH. For every 100 μg/L increase in serum β2-MG concentration, the prevalence of AKI increased by 6.4%. Further, the serum β2-MG concentration may be an early and useful biomarker for predicting AKI; a β2-MG cut-off level of 2026.85 μg/L may be advantageous for the early detection of AKI in patients with low serum creatinine values. Herrero-Morin et al also suggested that the serum β2-MG level may be a useful and novel biomarker, with greater diagnostic accuracy than serum creatinine, for detecting AKI in critically ill children.[17] Similar to our findings, cleaved urinary β2-MG may be a potential marker for determining early tubular injury in Balkan endemic nephropathy and aristolochic acid nephropathy.[32]
The serum β2-MG level, measured at discharge, was proven to be a novel biomarker for predicting mortality and graft loss in a retrospective study of 2190 patients who underwent primary kidney transplantation.[20] Studies have shown that the serum β2-MG level is a strong, independent biomarker that is predictive of cardiovascular events and mortality in stable kidney transplant recipients[33] and in adults with CKD.[21] Supported by these reports, our study also shows that serum β2-MG levels predict the prognoses of patients with ICH. Patients with ICH categorized into the β2-MG level >2123.50 μg/L group had a 2-fold higher risk of in-hospital mortality than did patients in the lower β2-MG group, after adjusting for other clinical indices. Moreover, the 1-year mortality risk in the high β2-MG group was also higher than in the lower β2-MG group. Thus, this prospective study suggested that serum β2-MG levels, measured upon NICU admission, are predictive of both in-hospital and 1-year mortality, with AUCs of 0.767 and 0.788, respectively. A survival analysis, conducted 1 year after NICU admission, further indicated that serum β2-MG levels could predict the 1-year mortality of the patients in this observational study.
We also found that the APACHE II score, calculated for each patient during the first 24 hours after NICU admission, was strongly associated with the development of AKI, in-hospital mortality, and 1-year mortality for patients with ICH. The APACHE II score is a well-known and important tool for accurately predicting critically ill patient outcomes; numerous studies have verified the score as an independent predictor of AKI in critically ill patients.[34,35] The present study also found that the combined APACHE II score and serum β2-MG level provides a better prediction of AKI, in-hospital mortality, and 1-year mortality in the target patient population than either value, alone.
To our knowledge, this is the first study that has explored the potential association between serum β2-MG levels and the risks for AKI and all-cause mortality in NICU patients with ICH. Our study has several strengths, including its prospective design (prespecified protocol, analytical methods, surgical standards, and diagnostic criteria). The study also has some limitations. First, it was a single-center, observational study that recruited only 403 patients with ICH. A multi-center study, involving a larger study population is warranted to evaluate the value of β2-MG levels for predicting the incidence of AKI in NICU patients with ICH, as well as their overall prognosis. Second, only serum creatinine, and not urine output (particularly affected by the loop diuretics used by 44.2% of our patients), was used to diagnose AKI; thus, we may have underestimated the incidence of AKI. However, our study showed an AKI incidence that was consistent with a recent prospective study.[28] Third, the temporal changes in serum and urinary β2-MG levels were not determined. Several studies have reported that urinary β2-MG levels are an early and promising biomarker of AKI and may predict the prognoses of patients with idiopathic membranous nephropathy or immunoglobulin A nephropathy.[36,37] Furthermore, we did not evaluate the risks for ESRD or new cardiovascular events in patients with ICH and who developed AKI while hospitalized.
5. Conclusion
This prospective study showed that the serum β2-MG level, measured upon NICU admission, maybe an early and promising biomarker of AKI and adverse outcomes in patients with ICH in the NICU. Further research should confirm this finding, which may help clinicians take timely action to improve patient outcomes.
Acknowledgments
We are indebted to the medical staff in the Department of Neurosurgery, Zhongnan Hospital, Wuhan University.
Author contributions
Conceptualization: Rui Wang, Hong He.
Data curation: Rui Wang, Hua Shui.
Formal analysis: Hongtao Hu.
Writing – original draft: Rui Wang, Hua Shui.
Writing – review and editing: Shuang Hu.
Footnotes
Abbreviations: β2-MG = β2-microglobulin, AKI = acute kidney injury, APACHE II = acute physiology and chronic health evaluation II score, AUC = area under curve, CART = classification and regression tress, CHAID = Chi-square automatic interaction detection, CI = confidence intervals, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, ESRD = end-stage kidney disease, ICH = intracerebral hemorrhage, KDIGO = kidney disease improving global outcomes, NICU = neurosurgical intensive care unit, OR = odds ratio, ROC = receiver operating characteristic curve.
How to cite this article: Wang R, Hu H, Hu S, He H, Shui H. β2-microglobulin is an independent indicator of acute kidney injury and outcomes in patients with intracerebral hemorrhage. Medicine. 2020;99:8(e19212).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury incritically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- [2].Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet 2013;382:170–9. [DOI] [PubMed] [Google Scholar]
- [3].Lai CF, Wu VC, Huang TM, et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care 2012;16:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis 2016;67:742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tsagalis G, Akrivos T, Alevizaki M, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol 2009;4:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saeed F, Adil MM, Piracha BH, et al. Acute renal failure worsens in-hospital outcomes in patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2015;24:789–94. [DOI] [PubMed] [Google Scholar]
- [7].Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43:1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jia HM, Zheng Y, Huang LF, et al. Derivation and validation of plasma endostatin for predicting renal recovery from acute kidney injury: a prospective validation study. Crit Care 2018;22:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 2014;25:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parikh CR, Puthumana J, Shlipak MG, et al. Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berggard I, Bearn AG. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem 1968;243:4095–103. [PubMed] [Google Scholar]
- [12].Woitas RP, Stoffel-Wagner B, Poege U, et al. Low-molecular weight proteins as markers for glomerular filtration rate. Clin Chem 2001;47:2179–80. [PubMed] [Google Scholar]
- [13].Jovanovic D, Krstivojevic P, Obradovic I, et al. Serum cystatin C and beta2-microglobulin as markers of glomerular filtration rate. Ren Fail 2003;25:123–33. [DOI] [PubMed] [Google Scholar]
- [14].Filler G, Priem F, Lepage N, et al. Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 2002;48:729–36. [PubMed] [Google Scholar]
- [15].Bianchi C, Donadio C, Tramonti G, et al. Reappraisal of serum beta2-microglobulin as marker of GFR. Ren Fail 2001;23:419–29. [DOI] [PubMed] [Google Scholar]
- [16].Amighi J, Hoke M, Mlekusch W, et al. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke 2011;42:1826–33. [DOI] [PubMed] [Google Scholar]
- [17].Herrero-Morin JD, Malaga S, Fernandez N, et al. Cystatin C and beta2-microglobulin: markers of glomerular filtration in critically ill children. Crit Care 2007;11:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schaub S, Wilkins JA, Antonovici M, et al. Proteomic-based identification of cleaved urinary beta2-microglobulin as a potential marker for acute tubular injury in renal allografts. Am J Transplant 2005;5:729–38. [DOI] [PubMed] [Google Scholar]
- [19].Hei ZQ, Li XY, Shen N, et al. Prognostic values of serum cystatin C and beta2 microglobulin, urinary beta2 microglobulin and N-acetyl-beta-D-glucosaminidase in early acute renal failure after liver transplantation. Chin Med J (Engl) 2008;121:1251–6. [PubMed] [Google Scholar]
- [20].Astor BC, Muth B, Kaufman DB, et al. Serum beta2-microglobulin at discharge predicts mortality and graft loss following kidney transplantation. Kidney Int 2013;84:810–7. [DOI] [PubMed] [Google Scholar]
- [21].Foster MC, Coresh J, Hsu CY, et al. Serum beta-trace protein and beta2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 2016;68:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Astor BC, Shafi T, Hoogeveen RC, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 2012;59:653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- [24].Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- [25].National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39: 2 Suppl 1: S1–266. [PubMed] [Google Scholar]
- [26].Del BC, Morelli A, Bassein L, et al. Severity scores in respiratory intensive care: APACHE II predicted mortality better than SAPS II. Respir Care 1995;40:1042–7. [PubMed] [Google Scholar]
- [27].KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–50. [DOI] [PubMed] [Google Scholar]
- [28].Burgess LG, Goyal N, Jones GM, et al. Evaluation of acute kidney injury and mortality after intensive blood pressure control in patients with intracerebral hemorrhage. J Am Heart Assoc 2018;7:e008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khatri M, Himmelfarb J, Adams D, et al. Acute kidney injury is associated with increased hospital mortality after stroke. J Stroke Cerebrovasc Dis 2014;23:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zorrilla-Vaca A, Ziai W, Connolly ES, Jr, et al. Acute kidney injury following acute ischemic stroke and intracerebral hemorrhage: a meta-analysis of prevalence rate and mortality risk. Cerebrovasc Dis 2018;45:1–9. [DOI] [PubMed] [Google Scholar]
- [31].Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med 2004;30:33–7. [DOI] [PubMed] [Google Scholar]
- [32].Stefanovic V, Djukanovic L, Cukuranovic R, et al. Beta2-microglobulin and alpha1-microglobulin as markers of Balkan endemic nephropathy, a worldwide disease. Ren Fail 2011;33:176–83. [DOI] [PubMed] [Google Scholar]
- [33].Foster MC, Weiner DE, Bostom AG, et al. Filtration markers, cardiovascular disease, mortality, and kidney outcomes in stable kidney transplant recipients: the FAVORIT trial. Am J Transplant 2017;17:2390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fuhrman DY, Kane-Gill S, Goldstein SL, et al. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16-25 years of age treated in an adult intensive care unit. Ann Intensive Care 2018;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hu Y, Liu H, Fu S, et al. Red blood cell distribution width is an independent predictor of AKI and mortality in patients in the coronary care unit. Kidney Blood Press Res 2017;42:1193–204. [DOI] [PubMed] [Google Scholar]
- [36].van den Brand JA, Hofstra JM, Wetzels JF. Low-molecular-weight proteins as prognostic markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2011;6:2846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shin JR, Kim SM, Yoo JS, et al. Urinary excretion of beta2-microglobulin as a prognostic marker in immunoglobulin A nephropathy. Korean J Intern Med 2014;29:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


