Abstract
Resistance to inhibitors of cholinesterase 8A (Ric-8A) is a prominent nonreceptor GEF and a chaperone of G protein α-subunits (Gα). Recent studies shed light on the structure of Ric-8A, providing insights into the mechanisms underlying its interaction with Gα. Ric-8A is composed of a core armadillo-like domain and a flexible C-terminal tail. Interaction of a conserved concave surface of its core domain with the Gα C-terminus appears to mediate formation of the initial Ric-8A/GαGDP intermediate, followed by the formation of a stable nucleotide-free complex. The latter event involves a large-scale dislocation of the Gα α5-helix that produces an extensive primary interface and disrupts the nucleotide-binding site of Gα. The distal portion of the C-terminal tail of Ric-8A forms a smaller secondary interface, which ostensibly binds the switch II region of Gα, facilitating binding of GTP. The two-site Gα interface of Ric-8A is distinct from that of GPCRs, and might have evolved to support the chaperone function of Ric-8A.
Keywords: GPCR, Ric-8A/Ric8A, GBA-motif, G proteins, GEF, chaperone, signal transduction
Graphical Abstract
Structures of resistance to inhibitors of cholinesterase 8A (Ric-8A) protein and the models of its complexes with G protein α-subunits have advanced understanding of the biological mechanisms of this prominent Gα GEF and chaperone. The mechanism underlying the Ric-8A GEF function has emerged and is very distinct from that of G protein-coupled receptors

1. Introduction: GPCR-mediated activation of G proteins occurs at a distance
In a classical pathway, heterotrimeric G proteins (Gαβγ) mediate transduction of a myriad of extracellular signals from G protein-coupled receptors (GPCRs) in the plasma membrane to various intracellular effectors that define cellular responses. In this pathway, agonist binding to a GPCR at the cell surface causes conformational changes in the receptor thereby creating a G-protein binding crevice on its cytoplasmic face. Interaction with GPCRs catalyzes the release of GDP from Gα subunits leading to a transient complex between the receptor and nucleotide-free Gαβγ. This complex is disrupted by binding of GTP to Gα, which triggers dissociation of Gα from Gβγ and GPCR. Thus, two signaling entities are produced, Gα and Gβγ, both of which are capable of regulating different effector proteins.[1–3] By virtue of the reaction that GPCRs catalyze, they are G-protein guanine nucleotide exchange factors (GEFs). The ability of Gαβγ to form a stable complex with GPCRs in the absence of GTP enabled extensive research into its structure, culminating in solution of structures of several of such complexes at the atomic level.[4–11] These structures reveal common conformational changes in Gα subunits leading to GDP release.
Gα subunits are made up of two domains, the Ras-like (RD) and the α-helical domain (HD) (Fig. 1A). The nucleotide-binding site lies in a deep cleft at the interface between the two domains, and the contacts are made primarily by the RD; the HD serves as a lid over the bound nucleotide. The RD domain features a six-stranded β-sheet (β1-β6) with five intervening α-helices (α1-α5) and connecting loops.[12, 13] GPCR engages Gαβγ at two primary sites of Gα, the C-terminal α5 helix and the N-terminal αN-β1 loop[4]. The former interaction results in an outward translation with rotation of the α5 helix. This largest GPCR-induced conformational change in Gα allows α5 to dock into a cavity between transmembrane helices in the activated receptor.[4] Furthermore, together with the Gα core, the α5-helix of Gα contains key selectivity determinants for the GPCR/G-protein coupling.[1, 14, 15] While strictly conserved Gα α5-helix residues ensure a stereotypical activation mechanism for all G proteins, Gα family-specific residues within Gα α5 provide for the selectivity of G-protein activation by numerous GPCRs.[14] A notable aspect of the GPCR-induced GDP-release is that the nucleotide-binding site is located on the RD surface that is opposite and remote to the receptor-binding surface of Gα. Thus, the GPCR-induced conformational changes are propagated through the RD of Gα.[16] The Gα α5-helix is the main conduit of the GPCR-induced disruption of the guanine ring binding loop β6-α5.[1, 3, 4, 17] In addition, the movement of the α5-helix causes rearrangement of the interfaces between α5, α1 and β2-β3 thereby destabilizing the phosphate-binding P-loop, β1-α1.[3, 16, 18] More directly, the disruption of the P-loop occurs through a conformational change transmitted via the β1-strand from the second receptor contact site of Gα, the αN-β1 loop. Although the structural changes in the P-loop appear relatively small based on the crystal structures,[4] the hydrogen-deuterium exchange (HDX) studies reveal large GPCR-induced exchange increases in β1, the P-loop, and α1, suggesting significant dynamic disordering of this Gα region.[19] This observation highlights the significance of the receptor interactions with the Gα αN-β1 loop and provides a rationale for the requirement of Gβγ in efficient G-protein/GPCR coupling as αN is constrained in place by its interaction with Gβ. The disruption of the guanine nucleotide binding site is accompanied by weakening of the RD/HD interface and separation of the RD and HD, in part, because of severed contacts of the α1 helix with the αF in the HD.[3, 20, 21] While domain separation is not sufficient to cause GDP release, it is necessary to allow the nucleotide to escape from the binding pocket.[20]
Figure 1.
A: Key structural elements of Gα. GDP-bound Gα (PDB ID 1TAG) is shown in cartoon representation. The RD and HD are shown in cyan and blue, respectively. The switch I, II, and III regions (S1-S3) are colored wheat, orange, and olive, respectively. The P-loop, the β6-α5, and the α5-helix are shown in green, magenta, and grey, respectively. GDP is shown as sticks.
B, C. Interactions of the Gα α5-helix with β2AR. B: In the proposed intermediate complex, β2AR interacts with the α5-helix residues R389 and E392 of Gαs that are surface exposed in the GsGDP heterotrimer (PDB ID 6E67). C: In the fully coupled nucleotide-free state, translation and rotation of the Gαs α5-helix switches the interface to residues H387 and Y391 on the opposite side of α5 (PDB ID 3SN6). D: KB-752 and GIV/Girdin stabilize Gα conformation with low affinity for GDP. KB-752 (orange) and the GBA motif of GIV/girdin (magenta) bind between the switch II region (β3-α2 loop/α2-helix, grey) and the α3-helix of Gαi causing the switch II region to move away from the α3 helix and the nucleotide-binding site. GDP is shown as sticks (overlay, PDB IDs 1Y3A -green, 6MHF - cyan). E, F. Interactions of the α5-helix of transducin-α (Gαt) with rhodopsin and Ric-8A. E: The side of the α5-helix of Gαt interfacing with activated rhodopsin corresponds to that in the fully coupled nucleotide-free state GPCR/G-protein complex (PDB ID 6OYA). F: The side of Gαt α5-helix that interacts with Ric-8A is similar to that for the interaction with GPCRs in the fully coupled nucleotide-free state (PDB ID 6N85). The orientation of the α5-helix in (F) is similar to that in (E) as indicated by the positions of the labeled side chains.
Recent studies using time-resolved HDX and hydroxyl-radical footprinting indicated that the transition of Gs to a stable nucleotide-free GPCR-bound state with a large outward movement of the Gαs α5-helix is relatively slow and it likely occurs via an intermediate complex with bound GDP.[22] This intermediate complex appears to be facilitated by the β2-adrenergic receptor (β2AR) contacts with the C-terminal E392 and R389 of Gαs, which are replaced by the contacts with H387 and Y391 in the stable nucleotide-free complex (Fig. 1BC).[23] Gαs E392 and R389 are surface exposed in heterotrimeric Gs and available for the initial coupling with β2AR.[23] The nucleotide-binding site may already be destabilized in the intermediate complex due to disruption of the interactions between the C-terminal portion of the α5 helix and the αN-β1 loop in Gαs, thus promoting further transition to a stable nucleotide-free complex conformation.[22, 23] Yet another intermediate state termed non-canonical (NC) has been structurally described for the complex neurotensin receptor 1 with heterotrimeric Gi.[24] In the NC state, the α5-helix of Gαi rotated and translated similarly to the conformational change in the canonical (C) fully active state, however, the rotational orientation of Gαi and the α5-helix/receptor contacts are different from those in the C state. The NC state allows GDP release, but the nucleotide-binding site is less dynamic in NC compared to the C state, suggesting that the NC to C transition facilitates GTP-binding.[24] It remains to be seen how common the NC-like intermediates are among different GPCR/G-protein pathways.
2. GBA-motif proteins leverage the Gα switch regions
GPCR-induced G-protein signaling is tightly controlled by various modulators that either attenuate or augment the signal transduction. Proteins with two types of structural motifs, RGS domains and GoLoco/GPR motifs, negatively regulate G-protein signaling acting as GTPase activating proteins (GAPs) and GDP dissociation inhibitors (GDIs), respectively.[25–28] The interfaces and mechanisms of action of RGS and GoLoco/GPR regulators have been elucidated at the molecular level.[29, 30] Growing evidence implicates nonreceptor GEFs for Gα subunits as important positive regulators of G-protein signaling.[31] Prominent among these are proteins containing Gα-binding and activating motifs (GBA) and the resistance to inhibitors of cholinesterase 8 (Ric-8) proteins.[32–36] The existence of a family of GBA-motif proteins was initially recognized based on the motif’s homology to a GαGDP-conformation selective peptide, KB-752, identified in a phage display screen.[37] KB-752 was found to enhance the nucleotide exchange rate on Gαi subunits, but not on Gαo, possibly due to the high spontaneous exchange rate of the latter.[37] Subsequently, KB-752 was shown to interact with GαsGDP, however, here it acted as a GDI rather than a GEF.[38] GIV, Gα-interacting vesicle-associated protein, is a prototypical GBA-motif protein that modulates a variety of signaling networks at the plasma membrane and in different intracellular compartments.[32, 39] Mirroring the opposing effects of KB-752 on Gα subunits, GIV displayed GEF activity towards Gαi and GDI-like activity with respect to Gαs.[40] Accordingly, GIV and other members of the family, including Calnuc/Nucleobindin 1 and 2 (NUCB1 and NUCB2), Daple, PLCd4, and GBAS-1 are also referred to as guanine exchange modulators (GEMs).[32, 41, 42] The structure of the complex of GαiGDP with KB-752 revealed the principal basis for the GEF activity of GBA-motif proteins.[37] Biochemical studies of the GαiGDP/GIV-GEM complex, and more recently, its crystal structure further elucidated the underlying GEF mechanism.[43–45] Both, KB-752 and the GBA motif of GIV bind to the hydrophobic pocket between the switch II region (β3-α2 loop/α2-helix) and the α3-helix of Gαi in a similar manner causing changes in the switch regions of Gα that stabilize low-affinity states for GDP and enhance GDP dissociation (Fig. 1D). In particular, the switch II region upon binding of KB-752 is displaced away from the α3-helix and the GDP-binding site.[37, 45] This displacement is opposite to the movement of switch II upon binding of GTPγS (Fig. 1D). On the other hand, KB-752 induces the switch I region to move closer to the GDP-binding site, which disrupts a salt bridge that it makes with the P-loop (R178/E44) that has been shown to stabilize the HD/RD interface and bound GDP.[37]
In addition to the changes in the switch regions, GBA proteins appear to cause more wide-spread changes in Gα that are not evident from the crystal structures. NMR studies indicated strong GIV-induced perturbations in the β1-strand, the P-loop (β1-α1), and the β2/β3 strands that are adjacent to switches I and II. [44] The increased dynamics of the β2/β3 strands in the Gα complex with GIV are also supported by molecular dynamics (MD) simulations.[45] Thus, the interaction network between β2-β3, α1, and α5 may also be destabilized by GBA-motif protein, as it has been shown in the case of GPCR-induced GDP release.
Overall, the interfaces and mechanisms of GPCR- and GBA-dependent nucleotide exchange are markedly different. GPCRs interact with the C- and N-terminal sites of Gα complexed with Gβγ and cause strong allosteric disruptions of the remote nucleotide-binding site. A GPCR/G protein interface enables fast and efficient nucleotide exchange and signaling. GBA-motif proteins interact directly with the switch II region allosterically linked to the bound GDP. The GBA/Gα interface is smaller, and GBA-motif proteins are less efficient GEFs compared to GPCRs. Nonetheless, some of the structural consequences of GPCR and GBA binding are seemingly common, i. e. destabilizations of the Gα P-loop, the RD hydrophobic core and the RD/HD interface.[45] The cellular activity of GEMs is controlled by the exposure of GBA-motifs and/or their phosphorylation. GBA-motifs are often localized in intrinsically disordered regions and are available for interactions with Gα depending on the conformations of the neighboring protein regions.[46] Consistent with the crystal structure of the Gα/GIV-GBA complex, phosphorylation of the N-terminal and C-terminal Ser residues of the GIV-GBA motif enhances and diminishes its GEF activity, respectively.[45, 47, 48]
3. Biochemical and structural advances towards understanding the biology of Ric-8A
3.1. Ric-8 proteins: hugging Gα tightly
Similar to the GBA-motif proteins and unlike GPCRs, Ric-8 (synonyms Ric8, synembryn) proteins interact with monomeric Gα subunits. Ric-8 stimulates nucleotide release from GαGDP, leading to the formation of a stable intermediate complex of Ric-8 and nucleotide-free Gα. Binding of GTP to Gα complexed with Ric-8 disrupts the complex and liberates GαGTP, thereby underlying the GEF activity of Ric-8[49]. Invertebrates possess a single Ric-8 isoform capable of interacting with all families of Gα subunits.[33, 50] Vertebrate genomes encode two isoforms, each with specificity for a subset of Gα subunits. The Ric-8A isoform is more promiscuous and can bind Gαi, Gαq, and Gα12/13.[49] The Ric-8B isoform is selective for Gαs.[49, 51, 52] The ability of Ric-8 proteins to positively regulate G-protein signaling was initially attributed to their GEF activity.[49, 53] However, growing evidence points to an additional chaperone function of Ric-8. In studies of mouse ES cells lacking Ric-8A, newly synthesized Gα subunits failed to associate with membranes and were rapidly degraded, and in cell-free translation systems, Ric-8A was required for the expression of properly folded Gα subunits.[54, 55] Furthermore, co-expression of Ric-8A or Ric-8B with Gα subunits in HEK293 cells and insect cells led to significant elevations in the expression of Gα subunits.[56] As a chaperone, Ric-8 has been proposed to bind nascent partially unfolded Gα, to help properly position the RD and HD, and to facilitate formation of the nucleotide-binding site, thereby enabling the first-time GTP-binding event.[57] Mechanistically, the two activities of Ric-8 may be quite similar, as the Ric-8/Gα complex underlying the GEF activity may parallel the folding intermediate in biosynthesis of Gα.[57]
Ric-8 isoforms lack any sequence similarity to other proteins. The lack of homology and structural relatedness between Ric-8, GPCRs, and GBA-motif proteins suggests that the mechanisms whereby these GEFs cause nucleotide exchange on Gα are fundamentally different, yet superficial parallels have also emerged from biochemical studies. Ric-8A and GPCRs both interact with the C-termini of Gα which is essential for the coupling selectivity and transmission of the activation signals, and both destabilize the HD/RD interface facilitating nucleotide release.[58–60] An HDX study of the Ric-8A/Gα complex indicated that a broad surface of Gα interfaces with Ric-8A. In addition to the two Gα sites involved in GPCR coupling, the C-terminus/α5-helix and the αN-β1 loop, the switch I, and to a greater extent, switch II regions become protected on binding of Ric-8A.[59] In contrast, segments that directly surround the nucleotide binding site, the P-loop, and the guanine base interacting β5-αG loop/αG region revealed increased rates of HDX exchange indicative of a protein backbone destabilization. The observed deprotection extended to the scaffolding β1, β4, and β5 strands not previously reported for the G-protein/GPCR complexes.[59] Thus, the interface of Gα with Ric-8 appears to be more extensive than that with GPCRs, and it incorporates sites utilized by GPCRs as well as by GBA-motif proteins (the switch II region). As a result, Ric-8 may cause greater conformational perturbations in Gα compared to GPCRs.
3.2. The armadillo-like scaffold of Ric-8A is inherently unstable
For a long period of time, Ric-8A escaped attempts to determine its structure by means of X-ray crystallography. A probable reason for the “reluctance” of Ric-8 to form well-diffracting crystals became apparent following the recent solution of its structure.[60, 61] The structure of the active nearly full length Ric-8A1–492 revealed two principal domains of Ric-8A: an armadillo-like core (residues about 1–426) and an unstructured C-terminal tail (residues 427–492).[60] The electron density for the C-terminal tail of Ric-8A was missing in the structure of a shorter fragment Ric-8A1–452 as well.[61] The armadillo-like core of Ric-8A is a mixture of canonical ARM repeats (three α-helices h1-h3, short h1 runs nearly perpendicular to a hairpin formed by h2 and h3) and non-canonical repeats lacking h1[60], also described as ARM-related HEAT repeats.[61] The tandem packing of ARM/HEAT repeats produces a right-handed ribbon-like superhelix featuring a concave surface formed by the h3 α-helices from repeats 2–8.[60] It turned out that the truncated protein Ric-8A1–426 comprising the armadillo core alone, likely owing to its mixed nature, is thermally unstable (Tm 36.5 °C) and does not bind the C-terminal α5 helix of Gα.[60] In contrast, Ric-8A1–452 regained the stability and the Gα α5 binding capacity of Ric-8A.[60] Thus, the stability of Ric-8A and ability to interact with Gα critically depend on the intramolecular interactions between the proximal portion of the C-terminal tail (residues 427–452) and the armadillo-core domain. Ric-8A427–452 is a strongly acidic segment that is highly conserved in Ric-8 proteins (Fig. 2). It has been shown to interact with a highly basic and conserved area on the C-terminal portion of the Ric-8A core domain.[60] Crosslinking constraints and the SAXS profile of Ric-8A guided the modeling of the proximal portion of the C-terminal tail Ric-8A, and MD simulations of the ensuing model of Ric-8A1–452 provided support for the stabilization effect of the intramolecular interactions.[60]
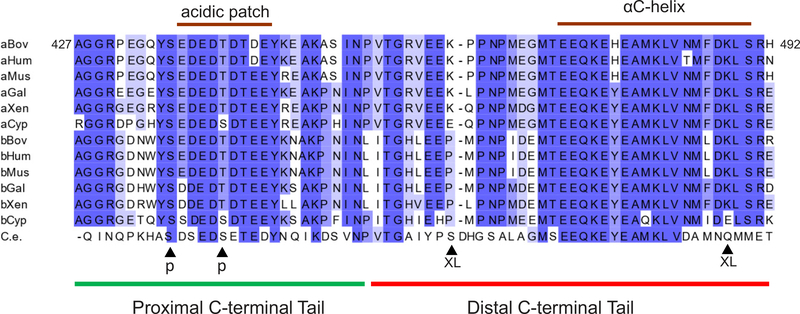
Figure 2.
Critical elements within the C-terminal tail of Ric-8A are highly conserved. Sequence alignment of C-terminal regions of Ric-8A (a) and Ric-8B (b) proteins corresponding to residues 427–492 of bovine Ric-8A. The segments with the highest conservation are: (1) the acidic patch within the proximal C-terminal tail that is involved in the stabilizing intramolecular interaction with the conserved basic surface of the armadillo-core domain, and (2) the αC-helix from the distal C-terminal tail that is predicted to interact with the switch II/α3-helix of Gα. Arrows indicated the sites of phosphorylation by the CK2 kinase (p) and the positions corresponding to K462 and K488 involved in crosslinking to Gαi (XL). aBov/bBov, bovine NP_001015627/XP_010803593; aHum/bHum, human NP_001273063/ NP_001338293; aMus/bMus, murine NP_444424/ NP_898995; aGal/bGal, chicken NP_001026345/XP_416305; aXen/bXen, Xenopus tropicalis NP_989159/ XP_012815216; aCyp/bCyp, Cyprinus carpio XP_018918733/XP_018939971; C.e., C. elegans NP_001023561.
Interestingly, two residues within the acidic segment, S435 and T440, are phosphorylated by the CK2 kinase resulting in potentiation of Ric-8A GEF activity (Fig. 2).[62] Apparently, the phosphorylation sites augment the stabilizing intramolecular electrostatic interactions in Ric-8A, which (a) allosterically enhance the binding of Gα α5 helix to the core domain of Ric-8A, and (b) may help positioning the distal C-terminus of Ric-8A for the interaction with the Gα switch II region.[63] Serendipitously, in the crystal lattice of the complex of Ric-8A1–492 with MBP-Gαt327–350, the Ric-8A core is stabilized by contacts between its positively charged region and the negatively charged domain of MBP, enabling solution of this structure.[60] Likewise, phosphorylation of Ric-8A1–452 was required to obtain its diffracting crystals.[61]
3.3. Interaction of Ric-8A with Gα: the critical role of the Gα α5 helix
The crystal structure of the complex of Ric-8A1–492 with MBP-Gαt327–350 revealed an extensive interaction site of the Gα C-terminus on the concave surface of Ric-8A[60]. Residues Gαt335–346 form an α-helix, whereas Gαt347–350 is in an extended conformation. Mutational analysis of the interface residues from both Gα and Ric-8A confirmed that the same interface is utilized for the Ric-8A interaction with the full-length Gα.[60] Remarkably, the side of Gαt α5-helix that interacts with Ric-8A is similar to that for the interaction with GPCRs in the fully coupled nucleotide-free state (Fig. 1CEF). [4, 5, 11, 60] This side of α5 is not available in GαGDP for the initial recognition by Ric-8A. However, the opposite side of α5 is available. Furthermore, just 11 C-terminal residues of Gα are sufficient for strong interaction with Ric-8A, and the structurally unconstrained C-terminal Gαt F350 forms an extensive interaction network with Ric-8A.[60] In crystal structures of GαtGDP, residues Gα325–340 comprise an α5 helix, but the C-terminal 8–10 residues lack a regular secondary structure or electron density[64, 65]. Thus, we hypothesize that the initial interaction of Gαt with Ric-8A involves few C-terminal residues of Gαt, and it induces an extension of the α5-helix to residues about 341–346. In an early transitional state, Ric-8A would engage with the side of the α5-helix that has been implicated in the potential intermediate complex of Gs with the β2AR.[23] A model for such an early intermediate complex of GαGDP with Ric-8A, approximated by superimposition and rotation of the α5-helix, indicates little or no steric overlap between the proteins (Fig. 3).
Figure 3.
Model of a potential early-intermediate complex of GαGDP with Ric-8A. The model was generated by superimposition of the α5-helix residues Gαt335–340 from GαtGDP (PDB 1TAG) and the Ric-8A1–492/MBP-Gαt327–350 structure (PDB 6N85), followed by rotation of 180° about the α-helix axis. This rotation switches the Ric-8A-interacting side of the Gαt α5-helix to that paralleled by the proposed intermediate Gs/β2AR complex [23]. Ric-8A – green, the RD and HD of GαtGDP are in orange and grey, respectively, the α5-helix of Gαt – magenta. Gα residues from the interface with GoLoco/GPR-motif proteins (based on PDB ID 4G5Q) are highlighted in cyan.
A protein complex similar to the initial complex of Ric-8A with GαGDP may also be formed between Ric-8A and GαGDP bound to GoLoco/GPR-motif proteins[28] such as mPINS/LGN and AGS3. GoLoco/GPR proteins and Ric8A both play important roles in Gα-regulated positioning of mitotic spindle during cell division, but the interplay between these proteins in this process is poorly understood.[66–68] Ric-8A has been shown to catalyze nucleotide exchange on Gα bound to AGS3 by forming a transient ternary complex Ric-8A•GaGDP•AGS3.[69] Such a ternary complex is possible since the GoLoco/GPR binding site of Gα[30, 70] is unperturbed in the initial complex (Fig. 3).
Nearly all of the Ric-8 residues that interact with the Gα α5-helix are strongly conserved across the Ric-8A and Ric-8B isoforms suggesting that the Gα α5-helix residues rather than Ric-8 residues encode the selectivity of the protein coupling. Indeed, residues at two positions, corresponding to I340 and N343 of Gαt, were shown to be important for the interaction selectivity.[60]
3.4. Formation of a stable nucleotide-free Ric-8A/Gα complex involves large conformational changes in Gα
The exact position of the Gα α5 with respect to Ric-8A allowed modeling of the stable nucleotide-free complex of Gα with Ric-8A.[60] When modeled according to the position of the α5-helix, all known conformations of Gα produce extensive steric overlap with Ric-8A. However, the clashes are significantly less severe when the GPCR-bound conformation of Gα is used for modeling.[60] Thus, Gα at minimum undergoes transition to a GPCR-bound like conformation, but Ric-8A and/or Gα must wrestle one another for additional space by inducing further conformational changes. Several initial indications pointed to possible conformational changes in Ric-8A. The structure of Ric-8A (Fig. 4A) and MD simulations show higher conformational flexibility of the N- and C-terminal parts of Ric-8A,[60] which has also been reported for other armadillo or HEAT repeat proteins.[71–73] An “open” unwind conformation of the Ric-8A core was generated using the steered MD simulations (SMD), and force applied to the C-terminal part of Ric-8A involved in clashes with Gα (Fig. 4B).[60] Not only could the “open” conformation of Ric-8A accommodates Gα; it was also consistent with the SAXS profile of Ric-8A1–452 (Fig. 4C).[60] Parsimonious models of Ric-8A1–452 and Ric-8A1–492 in complex with minimal Gα (miniGα) and full-length Gα have been proposed based on the GPCR-bound conformations of Gα and an assumed “open” conformation of Ric-8A.[60] However, an attempt to validate these models using SAXS analysis of the Ric-8A1–492/miniGαi complex revealed strong disagreement between the theoretical and experimental SAXS profiles.[63] This disagreement prompted us to investigate the possibility that Ric-8A induces more widespread and profound conformational changes in Gα compared to those caused by GPCRs. The SMD simulation was then designed to mimic potential Ric-8A-induced changes in miniGα, and the resulting miniGα conformations were assessed in models of the Ric-8A/miniGα complex.[63] Notably, a number of similar miniGα conformations have been identified with minimal clash scores and good agreement with the experimental SAXS data. The key and striking feature of these Gα conformations is a large hinge-like dislocation of the Gα α5-helix, whereby it is displaced from the hydrophobic β-sheet core of Gα (Figs. 5, 6).[63] The Gα β-sheet core then packs against the conserved C-terminal end of the Ric-8A armadillo domain. The resulting model of the Ric-8A1–452/Gαi complex is satisfying as it agrees well with the biochemical evidence for the extensive interface between these proteins (Figs. 5, 6).[59, 63] The model predicts the interface that is larger than the Gα interface with GPCRs. The entire Gα α5-helix, the β6/α5-loop, the β6 and possibly β5 strands abut the C-terminal part of the Ric-8A core domain. In addition, the αN-β1 loop, the β1-β3 strands, and to a lesser extent, the switch II region wrap around portions of the proximal C-terminal tail of Ric-8A (Figs. 5, 6).[63] Hence, the model is consistent with destabilization of the Gα scaffolding β-sheet observed in the complex with Ric-8A.[59]
Figure 4.
Hypothetical “open” conformation of Ric-8A based on the conformational flexibility of the C-terminal part of the Ric-8A armadillo core domain. A: The structure of apo Ric-8A (PDB ID 6N86). The backbone is shown as a tube with a diameter correlated to the B-factor of the structure. The higher B-factors for the N- and C-terminal portions of Ric-8A1–423 indicate higher conformational flexibility. B: Overlay of the apo Ric-8A structure (green) and the “open” conformation of the Ric-8A core domain (orange), which resulted from the SMD simulation with force applied to the Ric-8A region that clashed with Gα[60]. The “open” conformation of Ric-8A would allow the avoidance of clashes with Gα in a Ric-8A/Gα complex modelled using Gα in a GPCR-bound conformation. C: The “open” conformation of the Ric-8A is not excluded by the SAXS analysis of apo Ric-8A. Experimental SAXS data for Ric-8A1–452 (black)[60]. Theoretical SAXS profile calculated for the original model of apo Ric-8A1–452 fits the SAXS data with a χ2 value of 1.82. The SMD model of apo Ric-8A1–452 with “open” conformation fits the SAXS data with a χ2 value of 1.64.
Figure 5.
Conformational changes in the distal C-terminal tail of Ric-8A and the C-terminal α5-helix of Gα based on the solution structure of the Ric-8A/miniGα complex. A: A model of apo Ric-8A1–492 based on the Ric-8A structure and the SAXS analysis indicates an extended conformation of the distal C-terminal tail (cyan)[60]. B: Experimental SAXS data for apo Ric-8A1–492 (black curve) (radius of gyration, Rg 34.0 Å). The theoretical SAXS profile for the model in (A) (red curve) fits the data with χ2 = 1.51. Inset: The pairwise distance distribution function P(r) indicates an elongated molecule with a maximum dimension Dmax of ~127 Å[60]. C: A model of the Ric-8A1–492/miniGαi complex based on the Ric-8A structure and a miniGαi conformation derived from the SMD simulation with force applied to the α5-helix of mini Gαi[63]. The model indicates a conformational change whereby the distal C-tail of Ric-8A becomes immobilized by the interaction with the switch II/α3-helix of Gα, and a large-scale re-arrangement of the Gα α5-helix. The model disfavors the “open” conformation of Ric-8A in the complex with Gα. D: Experimental SAXS data for the Ric-8A1–492/miniGαi complex (black curve) (radius of gyration, Rg 32.3 Å). The theoretical SAXS profile for the model in (C) fits the data with χ2 = 1.71. Inset: The pairwise distance distribution function P(r) indicates a molecule with a maximum dimension Dmax of ~107 Å[63].
Figure 6.
GPCR and Ric-8A footprints on Gα subunits. A: GPCR-interacting surface of Gαi based on the structure of the rhodopsin/Gαiβγ complex (PDB ID 6CMO). The Gαi backbone is shown as a green cartoon. The surfaces of the RD and HD domains are shown in light grey and dark grey, respectively. The Gαi surface from the interface with rhodopsin (residues within 5 Å of GPCR) is colored orange. B: Putative Ric-8A-interacting surface of Gαi based on the model of the Ric-8A/Gαi complex[63]. The Gαi surface from the primary and secondary interfaces with Ric-8A (residues within 5 Å of Ric-8A) are colored orange and yellow, respectively.
3.5. The distal C-terminal tail interacts with Gα, potentially promoting binding of GTP
Similar to the proximal portion, the distal portion of the C-terminal tail of Ric-8A is highly conserved in Ric-8 proteins, suggesting its significance in the protein’s function (Fig. 2). Indeed, the distal C-tail is important for the GEF activity of Ric-8A. Various degrees of impairment of the GEF activity have been reported for the truncated Ric-8A1–452 construct, but a preponderance of the data suggests that it is a very poor GEF compared to the full-length Ric-8A or Ric-8A1–492.[59–61, 69] Moreover, a potential Gα-binding site has been proposed for residues Ric-8A455–470.[59] Nucleotide exchange in Gα subunits is limited by GDP release, not by binding of GTP (or GTPγS). Likewise, the GEF activity of Ric-8A is not limited by the nucleotide binding, since the reported rates for the Ric-8-catalyzed GDP-release and GTPγS-binding are similar.[49, 58] However, while Ric-8A1–452 retains substantial capacity to catalyze GDP release, its GEF activity appears to be limited by the rate of GTPγS binding.[58] Binding of GTPγS to a preformed nucleotide-free complex of Gα with Ric-8A1–452 was shown to be strongly impaired as well.[59] From the Ric-8A1–452/Gα model[63] we infer that disorganization of the Gα nucleotide-binding site in the Ric-8A/Gα complex is so significant, that unless Ric-8A453–492 assists the binding of GTP, the latter reaction and GαGTP release from the complex limit the GEF activity of Ric-8A.
The interaction of the distal portion of the C-terminal tail of Ric-8A with Gα is further supported by the experimental SAXS profiles of Ric-8A1–492 alone and in complex with miniGα. The scattering profiles suggest that residues 453–492 assume an extended conformation in apo Ric-8A1–492 and a more compact conformation in the protein complex indicative of immobilization of the C-terminal tail (Fig. 5).[60, 63] Furthermore, no intramolecular crosslinks involving residues 453–492 were identified for apo Ric-8A1–492, but two intermolecular crosslinks with miniGαi were detected for Ric-8A K462 and K488 in the protein complex (Fig. 2).[60]
The crosslinking constraints facilitated FloppyTail modeling of the distal portion of the C-terminal tail of Ric-8A in complex with miniGα. Secondary structure prediction indicates that the N-terminal segment of Ric-8A453–492 is largely unstructured while the C-terminal residues 471–489 form an α-helix (Fig. 2). Remarkably, the favored cluster of FloppyTail models featured the C-terminal α-helix of Ric-8A (αC) interacting with the groove between the switch II region and α3-helix of Gα (Figs. 5, 6).[63] This conformation-sensitive surface mediates interaction of GαGDP with GBA- or GoLoco/GPR-motif proteins as well as binding of effector proteins to GαGTP.[2, 30, 45]
Hypothetically, the αC-helix can help to destabilize bound GDP by a mechanism similar to that of GBA-motif proteins. However, this seems to be unnecessary, since Ric-8A1–452 is capable of catalyzing GDP release. We propose that the main contribution of the distal C-terminal tail is to promote the binding of GTP to Gα by stabilizing its GTP-bound conformation. Such a mechanism would imply preferential binding of αC to GαGTP as if αC were an “effector”. How can the preference of αC for GαGTP be reconciled with the latter release from Ric-8A? The secondary interface between the distal C-terminal tail of Ric-8A and Gα is small, and insufficient alone for a stable protein-protein complex (Fig. 6). Binding of GTP would disrupt the main interface via stabilization of the nucleotide binding loops, the Gα β-sheet core, and retraction of the α5-helix. The role of the αC-helix as a helping hand in the binding of GTP is particularly attractive when one considers the Gα chaperone activity of Ric-8A as its primary function.
4. Conclusions and future perspectives
Recent studies have provided structural clues to the mechanism of activity of Ric-8 proteins, a prominent group of nonreceptor GEFs and chaperones for Gα subunits. As it has been anticipated from biochemical analyses, the Gαβγ/GPCR and Gα/Ric-8 complexes and the activation mechanisms differ in critical aspects, notwithstanding the fact that the latter interface cannot accommodate heterotrimeric Gαβγ.[60] One well-known and striking difference is the relative inefficiency of Ric-8 proteins as GEFs compared to GPCRs. In the prototypical GPCR-mediated phototransduction cascade, a single molecule of photoexcited rhodopsin R* can activate up to 80 molecules of transducin per second[74]. The rates of Ric-8A-catalyzed nucleotide exchange vary from 0.1 to about 0.3 min−1 depending on a type of Gα subunit,[49] i. e. at least two-orders of magnitude lower than that for R*. Agonist-bound GPCRs are likely such powerful GEFs, because they favor preexisting G-protein states with low affinity for GDP, such as with a position of the α5-helix away from the nucleotide binding site or with disrupted contacts between the α5-helix and the αN-β1 loop.[20, 23] These conformations are rare in GαGDP, but they become dominant in stable or intermediate G-protein/GPCR complexes.[20] The structural rearrangements in Gα induced by Ric-8, particularly in the C-terminal α5-helix, appear to be much greater compared to the changes induced by GPCRs. A conformation of Gα similar to the one in complex with Ric-8A is not expected to be adopted by folded GαGDP alone even on a long time-scale. A slow conformational transition from GαGDP to the Ric-8A-bound Gα likely involves intermediate complexes and may constrain the GEF activity of Ric-8A. A structure of the Ric-8/Gα complex at atomic resolution will help to understand such a transition. However, an incompletely folded intermediate in biosynthesis of Gα may parallel to a degree the Ric-8A-bound conformation of Gα, thus making Ric-8 an efficient chaperone. Another aspect of Ric-8A function awaiting atomic resolution is: how does Ric-8A help partially unfolded Gα to bind GTP? GTP-binding to the Gα/Ric-8 complex appears to be slow and only barely faster than the Ric-8-induced GDP release. This perhaps is due to a severe disorganization of the Gα nucleotide-binding site in complex with Ric-8. To aid binding of GTP to Gα, Ric-8 recruits the distal C-terminal tail, which forms a smaller secondary interface with Gα. Curiously, this secondary interface involves the same conformation-sensitive “effector” area of Gα that GBA-motif proteins target to promote GDP release. Future studies will also clarify which activity, GDP-release/GEF or chaperone, is the principal function of Ric-8 proteins. Although likely mechanistically similar, the GDP-release activity of Ric-8 is irrelevant to its function as a Gα chaperone. If Ric-8 proteins evolved as chaperones of Gα subunits, the role of the main Ric-8/Gα interface is to stabilize partially unfolded Gα intermediate, while the secondary interface helps organize the GTP-binding site.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1 EY-12682 to N.O.A.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
References
- [1].Oldham WM, Hamm HE, Nat. Rev. Mol. Cell Biol 2008, 9, 60. [DOI] [PubMed] [Google Scholar]
- [2].Sprang SR, Chen Z, Du X, Adv. Protein Chem 2007, 74, 1. [DOI] [PubMed] [Google Scholar]
- [3].Hilger D, Masureel M, Kobilka BK, Nat. Struct. Mol. Biol 2018, 25, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK, Nature 2011, 477, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kang Y, Kuybeda O, de Waal PW, Mukherjee S, Van Eps N, Dutka P, Zhou XE, Bartesaghi A, Erramilli S, Morizumi T, Gu X, Yin Y, Liu P, Jiang Y, Meng X, Zhao G, Melcher K, Ernst OP, Kossiakoff AA, Subramaniam S, Xu HE, Nature 2018, 558, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carpenter B, Nehme R, Warne T, Leslie AG, Tate CG, Nature 2016, 536, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Draper-Joyce CJ, Khoshouei M, Thal DM, Liang YL, Nguyen ATN, Furness SGB, Venugopal H, Baltos JA, Plitzko JM, Danev R, Baumeister W, May LT, Wootten D, Sexton PM, Glukhova A, Christopoulos A, Nature 2018, 558, 559. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Nafria J, Nehme R, Edwards PC, Tate CG, Nature 2018, 558, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H, Schertler GFX, Granier S, Weis WI, Dror RO, Manglik A, Skiniotis G, Kobilka BK, Nature 2018, 558, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SGB, Christopoulos G, Coudrat T, Danev R, Baumeister W, Miller LJ, Christopoulos A, Kobilka BK, Wootten D, Skiniotis G, Sexton PM, Nature 2017, 546, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gao Y, Hu H, Ramachandran S, Erickson JW, Cerione RA, Skiniotis G, Mol. Cell 2019, 75, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Noel JP, Hamm HE, Sigler PB, Nature 1993, 366, 654. [DOI] [PubMed] [Google Scholar]
- [13].Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR, Science 1994, 265, 1405. [DOI] [PubMed] [Google Scholar]
- [14].Flock T, Hauser AS, Lund N, Gloriam DE, Balaji S, Babu MM, Nature 2017, 545, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okashah N, Wan Q, Ghosh S, Sandhu M, Inoue A, Vaidehi N, Lambert NA, Proc. Natl. Acad. Sci. U. S. A 2019, 116, 12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mahoney JP, Sunahara RK, Curr. Opin. Struct. Biol 2016, 41, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Natochin M, Moussaif M, Artemyev NO, Neurochem J. 2001, 77, 202. [DOI] [PubMed] [Google Scholar]
- [18].Kaya AI, Lokits AD, Gilbert JA, Iverson TM, Meiler J, Hamm HE, J. Biol. Chem 2016, 291, 19674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL Jr., Sunahara RK, Nature 2011, 477, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dror RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, Arlow DH, Philippsen A, Villanueva N, Yang Z, Lerch MT, Hubbell WL, Kobilka BK, Sunahara RK, Shaw DE, Science 2015, 348, 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van Eps N, Preininger AM, Alexander N, Kaya AI, Meier S, Meiler J, Hamm HE, Hubbell WL, Proc. Natl. Acad. Sci. U. S. A 2011, 108, 9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Du Y, Duc NM, Rasmussen SGF, Hilger D, Kubiak X, Wang L, Bohon J, Kim HR, Wegrecki M, Asuru A, Jeong KM, Lee J, Chance MR, Lodowski DT, Kobilka BK, Chung KY, Cell 2019, 177, 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu X, Xu X, Hilger D, Aschauer P, Tiemann JKS, Du Y, Liu H, Hirata K, Sun X, Guixa-Gonzalez R, Mathiesen JM, Hildebrand PW, Kobilka BK, Cell 2019, 177, 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kato HE, Zhang Y, Hu H, Suomivuori CM, Kadji FMN, Aoki J, Krishna Kumar K, Fonseca R, Hilger D, Huang W, Latorraca NR, Inoue A, Dror RO, Kobilka BK, Skiniotis G, Nature 2019, 572, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dohlman HG, Thorner J, J. Biol. Chem 1997, 272, 3871. [DOI] [PubMed] [Google Scholar]
- [26].Siderovski DP, Willard FS, Int. J. Biol. Sci 2005, 1, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Natochin M, Lester B, Peterson YK, Bernard ML, Lanier SM, Artemyev NO, J. Biol. Chem 2000, 275, 40981. [DOI] [PubMed] [Google Scholar]
- [28].Blumer JB, Lanier SM, Mol. Pharmacol 2014, 85, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tesmer JJ, Berman DM, Gilman AG, Sprang SR, Cell 1997, 89, 251. [DOI] [PubMed] [Google Scholar]
- [30].Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP, Nature 2002, 416, 878. [DOI] [PubMed] [Google Scholar]
- [31].Oner SS, Maher EM, Gabay M, Tall GG, Blumer JB, Lanier SM, J. Biol. Chem 2013, 288, 3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aznar N, Kalogriopoulos N, Midde KK, Ghosh P, Bioessays 2016, 38, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tall GG, Recept J. Signal Transduct. Res 2013, 33, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB, Proc. Natl. Acad. Sci. U. S. A 1996, 93, 12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller KG, Emerson MD, McManus JR, Rand JB, Neuron 2000, 27, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coleman BD, Marivin A, Parag-Sharma K, DiGiacomo V, Kim S, Pepper JS, Casler J, Nguyen LT, Koelle MR, Garcia-Marcos M, Mol. Biol. Evol 2016, 33, 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Johnston CA, Willard FS, Jezyk MR, Fredericks Z, Bodor ET, Jones MB, Blaesius R, Watts VJ, Harden TK, Sondek J, Ramer JK, Siderovski DP, Structure 2005, 13, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Johnston CA, Ramer JK, Blaesius R, Fredericks Z, Watts VJ, Siderovski DP, FEBS Lett. 2005, 579, 5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garcia-Marcos M, Ghosh P, Farquhar MG, Proc. Natl. Acad. Sci. U. S. A 2009, 106, 3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gupta V, Bhandari D, Leyme A, Aznar N, Midde KK, Lo IC, Ear J, Niesman I, Lopez-Sanchez I, Blanco-Canosa JB, von Zastrow M, Garcia-Marcos M, Farquhar MG, Ghosh P, Proc. Natl. Acad. Sci. U. S. A 2016, 113, E5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maziarz M, Broselid S, DiGiacomo V, Park JC, Luebbers A, Garcia-Navarrete L, Blanco-Canosa JB, Baillie GS, Garcia-Marcos M, J. Biol. Chem 2018, 293, 16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garcia-Marcos M, Kietrsunthorn PS, Wang H, Ghosh P, Farquhar MG, J. Biol. Chem 2011, 286, 28138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Garcia-Marcos M, Ghosh P, Farquhar MG, J. Biol. Chem 2015, 290, 6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Opakua AI, Parag-Sharma K, DiGiacomo V, Merino N, Leyme A, Marivin A, Villate M, Nguyen LT, de la Cruz-Morcillo MA, Blanco-Canosa JB, Ramachandran S, Baillie GS, Cerione RA, Blanco FJ, Garcia-Marcos M, Nat Commun 2017, 8, 15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kalogriopoulos NA, Rees SD, Ngo T, Kopcho NJ, Ilatovskiy AV, Sun N, Komives EA, Chang G, Ghosh P, Kufareva I, Proc. Natl. Acad. Sci. U. S. A 2019, 116, 16394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lin C, Ear J, Midde K, Lopez-Sanchez I, Aznar N, Garcia-Marcos M, Kufareva I, Abagyan R, Ghosh P, Mol. Biol. Cell 2014, 25, 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bhandari D, Lopez-Sanchez I, To A, Lo IC, Aznar N, Leyme A, Gupta V, Niesman I, Maddox AL, Garcia-Marcos M, Farquhar MG, Ghosh P, Proc. Natl. Acad. Sci. U. S. A 2015, 112, E4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lopez-Sanchez I, Garcia-Marcos M, Mittal Y, Aznar N, Farquhar MG, Ghosh P, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tall GG, Krumins AM, Gilman AG, J. Biol. Chem 2003, 278, 8356. [DOI] [PubMed] [Google Scholar]
- [50].Wilkie TM, Kinch L, Curr. Biol 2005, 15, R843. [DOI] [PubMed] [Google Scholar]
- [51].Nagai Y, Nishimura A, Tago K, Mizuno N, Itoh H, J. Biol. Chem 2010, 285, 11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Von Dannecker LE, Mercadante AF, Malnic B, Neurosci J. 2005, 25, 3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chan P, Gabay M, Wright FA, Tall GG, J. Biol. Chem 2011, 286, 19932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gabay M, Pinter ME, Wright FA, Chan P, Murphy AJ, Valenzuela DM, Yancopoulos GD, Tall GG, Sci Signal 2011, 4, ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chan P, Thomas CJ, Sprang SR, Tall GG, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chan P, Gabay M, Wright FA, Kan W, Oner SS, Lanier SM, Smrcka AV, Blumer JB, Tall GG, J. Biol. Chem 2011, 286, 2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Papasergi MM, Patel BR, Tall GG, Mol. Pharmacol 2015, 87, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Thomas CJ, Briknarova K, Hilmer JK, Movahed N, Bothner B, Sumida JP, Tall GG, Sprang SR, PLoS One 2011, 6, e23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kant R, Zeng B, Thomas CJ, Bothner B, Sprang SR, Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Srivastava D, Gakhar L, Artemyev NO, Nat Commun 2019, 10, 3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zeng B, Mou TC, Doukov TI, Steiner A, Yu W, Papasergi-Scott M, Tall GG, Hagn F, Sprang SR, Structure 2019, 27, 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Papasergi-Scott MM, Stoveken HM, MacConnachie L, Chan PY, Gabay M, Wong D, Freeman RS, Beg AA, Tall GG, Sci Signal 2018, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Srivastava D, Artemyev NO, J. Biol. Chem 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lambright DG, Noel JP, Hamm HE, Sigler PB, Nature 1994, 369, 621. [DOI] [PubMed] [Google Scholar]
- [65].Singh G, Ramachandran S, Cerione RA, Biochemistry 2012, 51, 3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Willard FS, Kimple RJ, Siderovski DP, Annu. Rev. Biochem 2004, 73, 925. [DOI] [PubMed] [Google Scholar]
- [67].Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, Siderovski DP, Gonczy P, Cell 2004, 119, 219. [DOI] [PubMed] [Google Scholar]
- [68].Hampoelz B, Knoblich JA, Cell 2004, 119, 453. [DOI] [PubMed] [Google Scholar]
- [69].Thomas CJ, Tall GG, Adhikari A, Sprang SR, J. Biol. Chem 2008, 283, 23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jia M, Li J, Zhu J, Wen W, Zhang M, Wang W, J. Biol. Chem 2012, 287, 36766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bujalowski PJ, Nicholls P, Barral JM, Oberhauser AF, FEBS Lett. 2015, 589, 123. [DOI] [PubMed] [Google Scholar]
- [72].Zhang Z, Lin K, Gao L, Chen L, Shi X, Wu G, Biochem. Biophys. Res. Commun 2011, 412, 732. [DOI] [PubMed] [Google Scholar]
- [73].Kappel C, Zachariae U, Dolker N, Grubmuller H, Biophys. J 2010, 99, 1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Leskov IB, Klenchin VA, Handy JW, Whitlock GG, Govardovskii VI, Bownds MD, Lamb TD, Pugh EN Jr., Arshavsky VY, Neuron 2000, 27, 525. [DOI] [PubMed] [Google Scholar]